Abstract
Hepatic annexin A2 (ANXA2) orchestrates multiple biologic processes and clinical symptoms and plays a key role in development, metastasis, and drug resistance of lethal hepatocellular carcinoma (HCC). However, the prognostic significance of ANXA2 for HCC has not been elucidated up to now. In this study, ANXA2 was frequently found to be up-regulated in HCC tissues compared with benign liver disease (BLD) tissues, which was consistent with the results in serum samples and tissue specimens of patients with HCC. Furthermore, ANXA2 expression was significantly correlated with differentiated degree, intrahepatic metastasis, portal vein thrombus, and tumor node metastasis (TNM) staging. More importantly, increased ANXA2 level was first confirmed to be closely associated with shortened overall survival of HCC (χ 2 = 12.872, P = 0.005) and identified as an independent prognostic factor (hazard ratio 1.338, 95 % confidence interval (CI) 1.013 ~ 1.766, P = 0.040), suggesting that ANXA2 up-regulation might represent an acquired metastasis phenotype of HCC, help to screen out high-risk population for HCC, or more effectively treat a subset of postsurgical HCC patients positive for ANXA2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, hepatocellular carcinoma (HCC) has become the third leading cause of cancer-related deaths with poor prognosis [1], and the 5-year survival rate of HCC is merely 7 % [2]. HCC is highly lethal because of its aggressive metastasis and an advanced stage at the time of diagnosis [3, 4]. Therefore, the early diagnosis is the only hope for curative therapies before metastasis of HCC is finally conquered [5], but the most important thing is to effectively screen out high-risk population for HCC according to prognostic factor [6]. With the increasing understanding of tumor biology of HCC, many new prognostic biomarkers of HCC [7, 8] have been successively identified by clinical and/or pathological staging systems [9, 10]. The identification of these genetic and epigenetic abnormalities in HCC is likely to improve the prognostic prediction of HCC patients and identify specific subgroups of tumor.
One of these prognostic factors recently described is annexin A2 (ANXA2), a calcium-dependent phospholipid-binding member of the annexin family [11, 12]. ANXA2 is first identified as a phosphorylation substrate of v-Src [13] and involved in diverse biological functions including vesicle trafficking, differentiation, apoptosis, calcium-dependent signaling transduction, actin rearrangement, cell motility, and growth regulation [14, 15]. ANXA2 dysregulation has been reported to correlate with the occurrence, development, and prognosis, especially metastasis of many cancers [6]. ANXA2 is up-regulated and phosphorylated [16] as the membrane receptor for β2GP I [17] and identified as a discriminative serological candidate in early HCC with a good diagnostic value [18, 19]. Furthermore, ANXA2 is found to interact with HAb18G/CD147 [20], promote invasion and migration of HCC cells via the activation of RhoA–Rac1 pathways and regulation of CD147-harboring microvesicles shedding from tumor cells [21], and induce resistance to 5-fluorouracil by regulating β-catenin and cyclin D1 expression [22]. However, the relationship between ANXA2 expression and prognosis of patients with HCC has not been reported so far.
In this study, on the basis of our previous evaluation, the diagnostic value of ANXA2 for HCC [18], ANXA2 over-expression was firstly confirmed in serum samples and tissue specimens of patients with HCC as well as ANXA2 messenger RNA (mRNA) up-regulation in HCC tissues. Sequentially, a scientific and reasonable four-level grading system was established for ANXA2 staining in HCC tissue microarray (TMA) so as to define the cutoff value. Furthermore, the correlation of ANXA2 expression with clinicopathologic features of HCC patients was analyzed. Finally, univariate and multivariable analysis was carried out on Cox regression model in order to screen out significant prognostic variables of overall survival according to patient follow-up data.
Materials and methods
Ethics statement
The study was approved by the Institutional Review Ethics Committee (No: 2012(1)) of Affiliated Hospital of Nantong University, Jiangsu Province, China. The diagnosis of HCC, cirrhosis, and viral hepatitis was based on the criteria proposed by the Ministry of Health of the People’s Republic of China [23] and the Chinese National Viral Hepatitis Meeting [24], respectively.
Blood samples
We evaluated 115 cases of HCC and 74 cases of benign liver disease (BLD) patients who were treated at the Affiliated Hospital of Nantong University, Nantong, China. The age of HCC patients ranged from 25 to 81 years old (median, 48.3 years), including 88 men and 27 women. BLD patients were consisted of 30 cases of liver cirrhosis, 24 cases of chronic hepatitis, and 20 cases of acute hepatitis. All the cases were diagnosed by biochemical tests, viral histology, and B-ultrasonic examination. Five milliliters of blood were collected with heparin in the morning and separated sera at once. Alpha-fetoprotein (AFP) level was detected by radiological method. Prior written informed consents were obtained from all patients according to the World Medical Association Declaration of Helsinki [25].
Liver specimens
Thirty pairs of fresh HCC tissues and self-matched para-carcinoma tissue specimens after surgery in Affiliated Hospital of Nantong University, Jiangsu Province, China were respectively collected with prior written informed consents [25] and immediately frozen in liquid nitrogen for total RNA extraction and ANXA2 mRNA detection. And, total 84 cases of HCC and 59 cases of BLD formalin-fixed paraffin-embedded (FFPE) liver tissue blocks were prepared and used for tissue microarray (TMA). No informed consent (written or verbal) was obtained for use of retrospective tissue samples from the patients in the study, most of whom were deceased, since the consent was not deemed necessary by the ethics committee [26]. The serum samples, the frozen tissues used for mRNA analysis, and FFPE tissues used for TMA respectively belonged to different, random cohort of patients.
Enzyme-linked immunosorbent assay
The level of serum ANXA2 was detected by using a human ANXA2 enzyme-linked immunosorbent assay (ELISA) kit (USCN Life Science Inc., Wuhan, China) according to the manufacturer’s instructions. One hundred microliters of serum samples or standard was separately added into each well, and then, 100 μL of detection reagent A was added and incubated for 1 h at 37 °C. Subsequently, 100 μL of detection reagent B was added and incubated for 0.5 h at 37 °C. Then, 90 μL of substrate solution was added and incubated for 25 min at 37 °C. Finally, 50 μL of stop solution was added to each well, and absorbance was read at 450 nm. During the procedure, washing the plate was according to the ELISA routine method.
Real-time quantitative polymerase chain reaction
Total RNA was isolated from 50 mg of liver tissue, using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The integrity of the total RNA was examined by 1 % agarose gel electrophoresis, the quantity was determined based on absorbance at 260 nm (A 260), and the purity was analyzed based on the absorbance ratio at 260 and 280 nm (A 260/280) (Bio-Rad SmartSpec™ plus, USA). The ANXA2 complementary DNA (cDNA) was synthesized from 1 μg of total RNA using First Strand cDNA Synthesis Kit (Fermentas, Canada) according to the manufacturer’s instructions.
The real-time quantitative polymerase chain reaction (qPCR) was run on an Applied Biosystems StepOne™ real-time PCR system according to the manufacturer’s recommendations. The reaction solution contained 25 μL 2× SYBR Premix Ex Taq (TaKaRa, Japan), 2 μL primer mix, 1 μL 50× ROX Reference Dye I, 4 μL cDNA, and 18 μL deionized water to make a total volume of 50 μL. ANXA2 primers were as follows: forward, 5′-TGAGCGGGATGCTTTGA AC-3′, and; reverse, 5′-ATCCTGTCTCTGTGCATTGCTG-3′; β-actin primers were as follows: forward, 5′-ATTGCCGACAGGATG CAGA-3′, and reverse, 5′-GAGTA CTTGCGCTCAGGAGGA-3′ used as an internal control [27], while no template control (H2O) was included in each reaction run. The optimized PCR conditions were as follows: 1 cycle at 95 °C for 2 min; 40 cycles of 95 °C for 10 s, 62 °C for 1 min; and final extension at 60 °C for 15 s. The relative quantitative analysis was performed by comparison of the 2−∆∆Ct values.
Tissue microarray construction
As previously described [28], TMA was generated using the manual Tissue Microarray System Quick-Ray (UT06, UNITMA, Korea) in the Department of Clinical Pathology, Affiliated Hospital of Nantong University (Jiangsu Province, China). Specifically, core tissue biopsies (2 mm in diameter) were taken from individual FFPE blocks, cut into 4-μm sections, and placed on super frost-charged glass microscope slides to generate TMA slides. In total, four TMAs were made for immunohistochemistry analysis.
Immunohistochemistry
Immunohistochemistry (IHC) analysis was performed as previously described [28]. Tissue sections were deparaffinized and rehydrated through graded alcohols. Endogenous peroxidase activity was blocked by incubation in 3 % H2O2. Antigen retrieval was carried out with 0.01 M citrate buffer pH 6.0 and microwave heat induction. ANXA2 was detected by a diluted mouse-anti-human ANXA2 monoclonal antibody (1:500, Santa Cruz Biotechnology, USA). Reactions were detected with Envision™ peroxidase kit (Dako, Carpinteria, CA, USA). Color development was accomplished by incubating with 3,3′-diaminobenzidine plus (Dako, Carpinteria, CA, USA), counterstained with hematoxylin, dehydrated through graded alcohols, cleared in xylene, and coverslipped with permanent mounting media.
Immunohistochemistry evaluation
All sections were scored in a semiquantitative manner according to the method described previously [18], which reflects both the intensity and percentage of cell staining. Staining intensity was scored as follows: 0 (no staining), 1 (weak staining), 2 (moderate staining), and 3 (strong staining). The percentage of ANXA2-positive cells was also classified as four categories where 1 (0 ~ 25 %), 2 (25 ~ 50 %), 3 (51 ~ 75 %), and 4 (>75 %). The product of the intensity and percentage scores was used as the final ANXA2 staining score. The cutoff point for a statistically significant ANXA2 expression score in terms of survival was set using the X-tile software program (The Rimm Lab, Yale University; http://www.tissuearray.org/rimmlab) and defined the ANXA2 staining using a four-level grading system as follows: negative (−): (0 ~ 4), weakly positive (+): (4 ~ 6), moderately positive (++): (6 ~ 9), and strongly positive: (9 ~ 12). Staining was scored independently by the two individuals who were blinded to each other’s findings and the clinical characteristics of these sections.
Statistical analysis
The comparison between mean was analyzed with Student’s t test. The comparison of ranked data was evaluated with rank-sum test. Survival curves were calculated using the Kaplan–Meier method, and differences in survival were determined by the log-rank analysis. Factors shown to be of prognostic significance with the univariate Cox regression model were subsequently investigated with the multivariate Cox regression model. P < 0.05 was considered significant. The statistical data were obtained using an SPSS software package (SPSS 13.0 Inc, Chicago, IL, USA).
Results
Over-expression of serum ANXA2 in hepatocellular carcinoma patients
The levels of serum ANXA2 expression in patients with HCC (n = 115) or BLD (n = 74) including liver cirrhosis and chronic or acute hepatitis are shown in Fig. 1a. Circulating ANXA2 levels were the normal distribution among these cases, and significantly higher expression (t = 10.32, P < 0.001) was found in the HCC group (24.82 ± 8.18 ng/mL) more than those (12.80 ± 7.21 ng/mL) in the BLD group.
Serum ANXA2 level in patients with HCC or benign liver diseases and ANXA2 mRNA expression in HCC and their para-carcinoma tissues. a ELISA showed that the expression level of serum ANXA2 was normally distributed and apparently higher in 115 cases of HCC group than in 74 cases of benign liver disease (BLD) samples, including 30 cases of liver cirrhosis, 24 cases of chronic hepatitis, and 20 cases of acute hepatitis (24.82 ± 8.18 vs 12.80 ± 7.21 ng/mL, t = 10.32, P < 0.001). b qPCR indicated that the level of ANXA2 mRNA was significantly higher in 30 cases of HCC tissues than self-matched para-carcinoma tissues (1.00 ± 0.04 vs 0.43 ± 0.10, t = 12.45, P < 0.001)
Up-regulation of ANXA2 messenger RNA expression in hepatocellular carcinoma tissues
The analysis of ANXA2 mRNA expression in 30 pairs of HCC and their surrounding tissues by qPCR technology is shown in Fig. 1b. The level of ANXA2 mRNA (0.43 ± 0.10) was significantly lower (t = 12.45, P < 0.001) in the para-carcinoma tissues than that (1.00 ± 0.04) in the HCC tissues with exuberant hepatic nucleic acid metabolism and abnormal ANXA2 mRNA transcription.
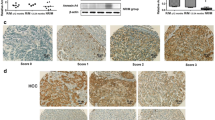
Increased expression and distribution of ANXA2 in hepatocellular carcinoma
Representative images of hepatic ANXA2 staining by immunohistochemical analysis are shown in Fig. 2. The staining of positive ANXA2 was predominantly localized to the cellular membrane and cytoplasm in the HCC tissues; some localization was detected in the nucleus. However, only a few positive staining was found in the BLD tissues. The summary of hepatic ANXA2 staining in HCC or BLD tissues by the TMA analysis is shown in Table 1. The incidence of positive ANXA2 staining was 73.81 % (62 of 84) in the HCC tissues and 35.59 % (21 of 59) in the BLD tissues. Significant difference of liver ANXA2 staining (Z = 5.237, P < 0.001) was presented between HCC and BLD tissues, and the data were consistent with serum ANXA2 expression or hepatic ANXA2 mRNA transcription in HCC patients.
Representative tissue microarray-based immunohistochemistry images of different ANXA2 staining level. A four-level grading system was used to evaluate ANXA2 immunohistochemical staining in HCC and benign liver diseases by tissue microarray. ANXA2 staining score was the product of the intensity and percentage scores. The cutoff point for a statistically significant ANXA2 expression score in terms of survival was set using the X-tile software program and defined as follows: negative (−): (0–4), weakly positive (+): (4–6), moderately positive (++): (6–9), and strongly positive (9–12). The staining in HCC and benign liver diseases tissues was scored independently by the two individuals who were blinded to each other’s findings and the clinical characteristics of these sections (left panel, ×40, bar 500 μm; right panel, ×400, bar 50 μm)
Clinicopathologic characteristics of ANXA2 expression in hepatocellular carcinoma
The clinicopathologic features of hepatic ANXA2 expression in HCC patients are shown in Table 2. The increasing ANXA2 expression was significantly correlated with intrahepatic metastasis (Z = 2.288, P = 0.022), portal vein thrombus (Z = 2.972, P = 0.003), and tumor node metastasis (TNM) staging (Z = 2.251, P = 0.024). In contrary, no significant correlation was found between ANXA2 expression and other clinical variables of the patients, such as sex, age, tumor size, AFP level, HBV infection, liver cirrhosis, and tumor nodes. In particular, significant difference was presented between well/moderate differentiation group and poor differentiation group (Z = 2.157, P = 0.031, data not shown). Taken together, ANXA2 expression was elevated along with the occurrence of intrahepatic metastasis and portal vein thrombus, the decline of differentiated degree, and TNM staging.
Univariate and multivariate analyses of prognostic variables
The univariate and multivariable analysis of prognostic variables for 5-year survival rate of HCC is shown in Table 3. The results of univariate Cox regression analyses for all variables showed that up-regulated ANXA2 was a significant prognostic factor for HCC (hazard ratio 1.567, 95 % confidence interval (CI) 1.209–2.030, P = 0.001). Kaplan–Meier survival analysis of ANXA2 further confirmed that increasing ANXA2 level was significantly associated with shortened overall survival of HCC, which was gradually decreased with the increase of ANXA2 expression (Fig. 3a, χ 2 = 12.872, P = 0.005). Survival curve corresponding to different ANXA2 level showed a good distinction without too much overlap, presenting the success of score standard for evaluating the ANXA2 staining and the reliability of ANXA2 as a prognostic factor.
Kaplan–Meier survival analysis of HCC patients (log-rank test). a Increased ANXA2 expression was significantly associated with shortened survival of HCC patients (χ 2 = 12.872, P = 0.005). Five-year overall survival rate of HCC patients is gradually decreased with the increase of ANXA2 expression. b Overall survival rate in HCC patients with poorly differentiated tumors was significantly lower than that with moderately to highly differentiated tumors (χ 2 = 46.305, P < 0.001). C Overall survival rate in HCC patients with multiple tumor nodes was significantly lower than that with single tumor node (χ 2 = 7.707, P = 0.005). d Overall survival rate in HCC patients with intrahepatic metastasis was significantly lower than that without intrahepatic metastasis (χ 2 = 8.534, P = 0.003). e Overall survival rate in HCC patients with portal vein tumor thrombus was significantly lower than that without portal vein tumor thrombus (χ 2 = 19.988, P < 0.001). f Overall survival rate in HCC patients with tumor TNM stages III–IV was significantly lower than that with tumor TNM stages I–II (χ 2 = 44.188, P < 0.001)
Besides, differentiated grading (hazard ratio 0.342, 95 % CI 0.206–0.567, P < 0.001), tumor nodes (hazard ratio 0.348, 95 % CI 0.196–0.619, P < 0.001), intrahepatic metastasis (hazard ratio 3.134, 95 % CI 1.785–5.503, P < 0.001), portal vein tumor thrombus (hazard ratio 5.621, 95 % CI 2.381–13.268, P < 0.001), and TNM staging (hazard ratio 8.119, 95 % CI 4.466–14.760, P < 0.001) were all significantly associated with the patient survival. Kaplan–Meier survival curves revealed that the patients with well-differentiated grading (χ 2 = 46.305, P < 0.001), single tumor node (χ 2 = 7.707, P = 0.005), and early TNM stage (χ 2 = 44.188, P < 0.001), without intrahepatic metastasis (χ 2 = 8.534, P = 0.003) and portal vein tumor thrombus (χ 2 = 19.988, P < 0.001) had a significantly better prognosis (Fig. 3b–f).
Multivariate Cox regression analysis of the same set of HCC (Table 3) further demonstrated that the up-regulation of ANXA2 was found to be an independent poor prognostic marker for the patient’s survival (hazard ratio 1.338, 95 % CI 1.013–1.766, P = 0.040). In addition, intrahepatic metastasis (hazard ratio 0.419, 95 % CI 0.191–0.915, P = 0.029) and tumor TNM staging (hazard ratio 18.243, 95 % CI 7.277–45.734, P < 0.001) were also independent prognostic factors of overall survival.
Discussion
In the present study, ANXA2 was frequently found to be up-regulated in HCC TMA compared with BLD tissues, which was consistent with the results in serum samples and tissue specimens of patients with HCC. According to the four-level grading system, ANXA2 expression was analyzed to be significantly correlated with differentiated degree, intrahepatic metastasis, portal vein thrombus, and TNM staging. Increased ANXA2 expression was confirmed to be closely associated with shortened overall survival of HCC patients for the first time, which was gradually decreased with the increase of ANXA2 expression. Importantly, ANXA2 expression was identified as an independent poor prognostic factor for HCC patients.
ANXA2 is involved in malignant transformation of HCC, which is a multifactor, multistep, multigene, and complicated process [29]. The predominant reason for carcinogenesis can be attributed to down-regulation of tumor suppressor genes by epigenetic inactivation and up-regulation of oncogenes or cancer-promoting genes by epigenetic derepression [30]. ANXA2, as a cancer-promoting factor, is frequently reported to be significantly up-regulated in most types of cancer including HCC, colorectal cancer, breast cancer, pancreatic cancer, acute promyelocytic leukemia, and renal cell carcinoma and while down-regulated in prostate cancer, esophageal squamous carcinoma, and nasopharyngeal carcinoma and sinonasal adenocarcinoma [31, 32]. Previous papers have shown that ANXA2 was mainly localized in cancer cells, especially in poorly differentiated HCC [33]. ANXA2 expression was up-regulated at both the transcriptional and translational levels in cancerous and noncancerous regions of HCC tissues compared with normal, chronic hepatitis, and cirrhosis liver tissues, and furthermore, ANXA2 was more dramatically expressed in the cancerous portion than in the noncancerous portion of HCC [16, 34]. Similarly, ANXA2 was confirmed to be significantly increased in the serum of HCC compared with the healthy, benign tumors, hepatitis, cirrhosis, controls, and other malignant tumors [18, 19]. Herein, we further confirmed that ANXA2 was distinctly over-expressed in patients with HCC compared with BLD including liver cirrhosis, chronic hepatitis, and acute hepatitis (Figs. 1 and 2, Table 1), which was consistent with previous research results. These results suggest that ANXA2 is distinguish expressed in patients with HCC or BLD, and increased ANXA2 plays an important role in the malignant transformation process leading to HCC.
ANXA2 promotes metastasis of HCC, although the underlying molecular mechanisms need yet to be uncovered. Previous research suggested that ANXA2 directly induced plasminogen conversion to plasmin, thereby leading to activation of metalloproteinases, degradation of extracellular matrix components, and promotion of neoangiogenesis [32, 35]. But, the recently accumulated evidence suggests that it is ANXA2/S100A10 complex, rather than ANXA2 monomer, that plays as the plasminogen receptor and directly combines with plasminogen and tissue plasminogen activator (tPA) so as to regulate plasmin generation. Actually, ANXA2 is found to interact with a variety of binding protein, not only S100A10, and collectively mediate the metastasis of multiple tumors [13]. ANXA2 was further confirmed to enhance migration of HCC cells in vitro via interaction with HAb18G/CD147 [20], pancreatic cancer cells via combining with S100A6 [36], and breast cancer cells via binding to tPA [35] and promote invasion of HCC cells via activation of RhoA–Rac1 pathways and regulation of CD147-harboring microvesicles shedding from tumor cells [21, 37]. Conversely, ANXA2 depletion was proved to inhibit invasion [27], delay epidermal growth factor receptor (EGFR) endocytic trafficking via cofilin activation, and enhance EGFR signaling and metastasis formation [38]. In this study, metastatic potential of HCC cells promoted by ANXA2 was further confirmed. The increased ANXA2 expression level was found to be significantly correlated with differentiated degree, intrahepatic metastasis, portal vein thrombus, and TNM staging (Table 2), while the latter three characteristics were closely related to HCC metastasis. That is to say, elevation of ANXA2 expression in HCC tissues with metastasis further confirmed that ANXA2 up-regulation promotes HCC metastasis. Actually, our previous studies have confirmed that ANXA2 silencing inhibited invasion and migration potential of hepatoma cells [27]. Without doubt, the molecular mechanisms underlying HCC metastasis promoted by ANXA2 still need to do further going and systematic research.
Increased ANXA2 is correlated with poor prognosis of HCC patients. Previous research has shown that ANXA2 over-expression is correlated with poor prognosis of human gastric carcinoma [39], conventional renal cell carcinoma [40], nonsmall cell lung cancer [11, 41], esophageal squamous cell carcinoma [42], urothelial carcinoma [43], multiple myeloma [44], and colorectal cancer [12] and predicts rapid recurrence after surgery of endometrial cancer [45] and in pancreatic cancer patients undergoing gemcitabine-adjuvant chemotherapy [46]. ANXA2 down-regulation is associated with recurrence and poor prognosis of prostate cancer [47] and oral squamous cell carcinoma [48]. However, although dysregulation of ANXA2 expression predicts adverse prognosis of patients with a wide variety of malignant tumors according to a systematic review and meta-analysis [49], there is increasing evidence that ANXA2 is a differential diagnostic tissue and serum marker for HCC [13]; the relationship between ANXA2 expression and prognosis of HCC patients has not been previously reported. Our results firstly confirmed that ANXA2 up-regulation was found to predict poor prognosis of HCC patients. Higher ANXA2 expression in HCC tissues was significantly associated with inferior survival independently of conventional prognostic factors (Fig. 3, Table 3). It is worth mentioning that 5-year survival rate of HCC patients is gradually decreased with the increase of ANXA2 expression.
In conclusion, the data demonstrated that ANXA2 expression was frequently up-regulated in HCC tissues; increased ANXA2 might represent an acquired metastasis phenotype for HCC. More importantly, this is firstly confirmed that ANXA2 acted as a new adverse independent prognostic factor and could be developed as a useful biomarker for HCC by a series of further independent and retrospective studies, so as to help doctors to screen out high-risk population for HCC and more effectively treat a subset of postsurgical HCC patients with positive for ANXA2.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- ANXA2:
-
Annexin A2
- BLD:
-
Benign liver diseases
- TMA:
-
Tissue microarray
- FFPE:
-
Formalin-fixed paraffin-embedded
- ELISA:
-
Enzyme-linked immunosorbent assay
- PBS:
-
Phosphate-buffered saline
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- IHC:
-
Immunohistochemistry
- SD:
-
Standard deviation
- tPA:
-
Tissue plasminogen activator
- AFP:
-
Alpha-fetoprotein
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29.
Nguyen VT, Law MG, Dore GJ. Hepatitis B-related hepatocellular carcinoma: epidemiological characteristics and disease burden. J Viral Hepat. 2009;16(7):453–63.
Ichikawa T, Sano K, Morisaka H. Diagnosis of pathologically early HCC with EOB-MRI: experiences and current consensus. Liver Cancer. 2014;3(2):97–107.
Shoreibah MG, Bloomer JR, McGuire BM, Massoud OI. Surveillance for hepatocellular carcinoma: evidence, guidelines and utilization. Am J Med Sci. 2014;347(5):415–9.
Sun H, Zhu MS, Wu WR, Shi XD, Xu LB. Role of anti-angiogenesis therapy in the management of hepatocellular carcinoma: the jury is still out. World J Hepatol. 2014;6(12):830–5.
Wang CY, Lin CF. Annexin A2: its molecular regulation and cellular expression in cancer development. Dis Markers. 2014;2014:308976.
Chaiteerakij R, Addissie BD and Roberts LR. Update on biomarkers of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2013.
Zhao YJ, Ju Q, Li GC. Tumor markers for hepatocellular carcinoma. Mol Clin Oncol. 2013;1(4):593–8.
Jour G, Gullet A, Liu M, Hoch BL. Prognostic relevance of Federation Nationale des Centres de Lutte Contre le Cancer grade and MDM2 amplification levels in dedifferentiated liposarcoma: a study of 50 cases. Mod Pathol. 2015;28(1):37–47.
Llovet JM, Bruix J. Prospective validation of the Cancer of the Liver Italian Program (CLIP) score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology. 2000;32(3):679–80.
Luo CH, Liu QQ, Zhang PF, Li MY, Chen ZC, Liu YF. Prognostic significance of annexin II expression in non-small cell lung cancer. Clin Transl Oncol. 2013;15(11):938–46.
Yang T, Peng H, Wang J, Yang J, Nice EC, Xie K, et al. Prognostic and diagnostic significance of annexin A2 in colorectal cancer. Colorectal Dis. 2013;15(7):e373–81.
Waisman DM. Annexin II, tetramer: structure and function. Mol Cell Biochem. 1995;149–150:301–22.
Hedhli N, Falcone DJ, Huang B, Cesarman-Maus G, Kraemer R, Zhai H, et al. The annexin A2/S100A10 system in health and disease: emerging paradigms. J Biomed Biotechnol. 2012;2012:406273.
Vedeler A, Hollas H, Grindheim AK, Raddum AM. Multiple roles of annexin A2 in post-transcriptional regulation of gene expression. Curr Protein Pept Sci. 2012;13(4):401–12.
Mohammad HS, Kurokohchi K, Yoneyama H, Tokuda M, Morishita A, Jian G, et al. Annexin A2 expression and phosphorylation are up-regulated in hepatocellular carcinoma. Int J Oncol. 2008;33(6):1157–63.
Gao PJ, Shi Y, Gao YH, Liu YW, Tan Y. The receptor for beta(2)GP I on membrane of hepatocellular carcinoma cell line SMMC-7721 is annexin II. World J Gastroenterol. 2007;13(24):3364–8.
Zhang HJ, Yao DF, Yao M, Huang H, Wu W, Yan MJ, et al. Expression characteristics and diagnostic value of annexin A2 in hepatocellular carcinoma. World J Gastroenterol. 2012;18(41):5897–904.
Sun Y, Gao G, Cai J, Wang Y, Qu X, He L, et al. Annexin A2 is a discriminative serological candidate in early hepatocellular carcinoma. Carcinogenesis. 2013;34(3):595–604.
Zhao P, Zhang W, Tang J, Ma XK, Dai JY, Li Y, et al. Annexin II promotes invasion and migration of human hepatocellular carcinoma cells in vitro via its interaction with HAb18G/CD147. Cancer Sci. 2010;101(2):387–95.
Zhang W, Zhao P, Xu XL, Cai L, Song ZS, Cao DY, et al. Annexin A2 promotes the migration and invasion of human hepatocellular carcinoma cells in vitro by regulating the shedding of CD147-harboring microvesicles from tumor cells. PLoS One. 2013;8(8), e67268.
Wang C, Guo Y, Wang J, Min Z. Annexin A2 knockdown inhibits hepatoma cell growth and sensitizes hepatoma cells to 5-fluorouracil by regulating betacatenin and cyclin D1 expression. Mol Med Rep. 2015;11(3):2147–52.
Ministry of Health of the People’s Republic of C. Updated standards for the diagnosis and treatment of primary liver cancer. Zhonghua Gan Zang Bing Za Zhi. 2012;20(6):419–26.
o Y. [Management of clinical diagnosis, and antiviral therapy for HBV-related cirrhosis]. Zhonghua Gan Zang Bing Za Zhi. 2014;22(5):327-335.
M PN. World Medical Association publishes the Revised Declaration of Helsinki. Natl Med J India. 2014;27(1):56.
Luo RZ, Cai PQ, Li M, Fu J, Zhang ZY, Chen JW, et al. Decreased expression of PTPN12 correlates with tumor recurrence and poor survival of patients with hepatocellular carcinoma. PLoS One. 2014;9(1), e85592.
Zhang HJ, Yao DF, Yao M, Huang H, Wang L, Yan MJ, et al. Annexin A2 silencing inhibits invasion, migration, and tumorigenic potential of hepatoma cells. World J Gastroenterol. 2013;19(24):3792–801.
Wang Q, Ni Q, Wang X, Zhu H, Wang Z, Huang J. High expression of RAB27A and TP53 in pancreatic cancer predicts poor survival. Med Oncol. 2015;32(1):372.
Breuhahn K, Schirmacher P. Reactivation of the insulin-like growth factor-II signaling pathway in human hepatocellular carcinoma. World J Gastroenterol. 2008;14(11):1690–8.
Kwon MJ, Shin YK. Epigenetic regulation of cancer-associated genes in ovarian cancer. Int J Mol Sci. 2011;12(2):983–1008.
Zhang X, Liu S, Guo C, Zong J, Sun MZ. The association of annexin A2 and cancers. Clin Transl Oncol. 2012;14(9):634–40.
Lokman NA, Ween MP, Oehler MK, Ricciardelli C. The role of annexin A2 in tumorigenesis and cancer progression. Cancer Microenviron. 2011;4(2):199–208.
Liu Z, Ling Q, Wang J, Xie H, Xu X, Zheng S. Annexin A2 is not a good biomarker for hepatocellular carcinoma in cirrhosis. Oncol Lett. 2013;6(1):125–9.
Longerich T, Haller MT, Mogler C, Aulmann S, Lohmann V, Schirmacher P, et al. Annexin A2 as a differential diagnostic marker of hepatocellular tumors. Pathol Res Pract. 2011;207(1):8–14.
Sharma M, Ownbey RT, Sharma MC. Breast cancer cell surface annexin II induces cell migration and neoangiogenesis via tPA dependent plasmin generation. Exp Mol Pathol. 2010;88(2):278–86.
Nedjadi T, Kitteringham N, Campbell F, Jenkins RE, Park BK, Navarro P, et al. S100A6 binds to annexin 2 in pancreatic cancer cells and promotes pancreatic cancer cell motility. Br J Cancer. 2009;101(7):1145–54.
Zhao P, Zhang W, Wang SJ, Yu XL, Tang J, Huang W, et al. HAb18G/CD147 promotes cell motility by regulating annexin II-activated RhoA and Rac1 signaling pathways in hepatocellular carcinoma cells. Hepatology. 2011;54(6):2012–24.
de Graauw M, Cao L, Winkel L, van Miltenburg MH, le Devedec SE, Klop M, et al. Annexin A2 depletion delays EGFR endocytic trafficking via cofilin activation and enhances EGFR signaling and metastasis formation. Oncogene. 2014;33(20):2610–9.
Emoto K, Sawada H, Yamada Y, Fujimoto H, Takahama Y, Ueno M, et al. Annexin II overexpression is correlated with poor prognosis in human gastric carcinoma. Anticancer Res. 2001;21(2B):1339–45.
Zimmermann U, Woenckhaus C, Pietschmann S, Junker H, Maile S, Schultz K, et al. Expression of annexin II in conventional renal cell carcinoma is correlated with Fuhrman grade and clinical outcome. Virchows Arch. 2004;445(4):368–74.
Jia JW, Li KL, Wu JX, Guo SL. Clinical significance of annexin II expression in human non-small cell lung cancer. Tumour Biol. 2013;34(3):1767–71.
Ma RL, Shen LY and Chen KN. Coexpression of ANXA2, SOD2 and HOXA13 predicts poor prognosis of esophageal squamous cell carcinoma. Oncol Rep. 2014;PMID: 24626613.
Zhang Q, Zhao Z, Ma Y, Wang H, Ma J, He X, et al. Combined expression of S100A4 and Annexin A2 predicts disease progression and overall survival in patients with urothelial carcinoma. Urol Oncol. 2014;32(6):798–805.
Seckinger A, Meissner T, Moreaux J, Depeweg D, Hillengass J, Hose K, et al. Clinical and prognostic role of annexin A2 in multiple myeloma. Blood. 2012;120(5):1087–94.
Alonso-Alconada L, Santacana M, Garcia-Sanz P, Muinelo-Romay L, Colas E, Mirantes C, Monge M, Cueva J, Oliva E, Soslow RA, Lopez MA, Palacios J, Prat J, Valls J, Krakstad C, Salvesen H, Gil-Moreno A, Lopez-Lopez R, Dolcet X, Moreno-Bueno G, Reventos J, Matias-Guiu X and Abal M. Annexin-A2 as predictor biomarker of recurrent disease in endometrial cancer. Int J Cancer. 2014;PMID: 25219463.
Takano S, Togawa A, Yoshitomi H, Shida T, Kimura F, Shimizu H, et al. Annexin II overexpression predicts rapid recurrence after surgery in pancreatic cancer patients undergoing gemcitabine-adjuvant chemotherapy. Ann Surg Oncol. 2008;15(11):3157–68.
Ding T, Yang L, Wang Y, Yuan J, Chen T, Cai X. Down-regulation of annexin II in prostate cancer is associated with Gleason score, recurrence, metastasis and poor prognosis. Mol Med Rep. 2010;3(5):781–7.
Rodrigo JP, Lequerica-Fernandez P, Rosado P, Allonca E, Garcia-Pedrero JM, de Vicente JC. Clinical significance of annexin A2 downregulation in oral squamous cell carcinoma. Head Neck. 2011;33(12):1708–14.
Liu X, Ma D, Jing X, Wang B, Yang W, Qiu W. Overexpression of ANXA2 predicts adverse outcomes of patients with malignant tumors: a systematic review and meta-analysis. Med Oncol. 2015;32(1):392.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (81401988), the Academic Program Development of Jiangsu Higher Education Institution (PAPD), and the International S&T Cooperation Program of China (2013DFA32150).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Additional information
Haijian Zhang and Min Yao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, H., Yao, M., Wu, W. et al. Up-regulation of annexin A2 expression predicates advanced clinicopathological features and poor prognosis in hepatocellular carcinoma. Tumor Biol. 36, 9373–9383 (2015). https://doi.org/10.1007/s13277-015-3678-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3678-6