Abstract
Annexin A1 (ANXA1) belongs to the annexin superfamily of proteins, which contribute to the pathological consequence and sequelae of most serious human diseases. Recent studies have reported diverse roles of ANXA1 in various human cancers; however, its involvement in human hepatocellular carcinoma (HCC) still remains controversial. To investigate the expression pattern of ANXA1 in HCC tissues and evaluate its associations with tumor progression and patients’ prognosis, immunohistochemistry was performed using 160 pairs of formalin-fixed and paraffin-embedded cancerous and adjacent non-cancerous tissues from patients with HCC. Then, the associations between ANXA1 expression, clinicopathological characteristics, and prognosis of HCC patients were statistically evaluated. In vitro migration and invasion assays of siRNA-targeted ANXA1-transfected cells were further performed. As a result, the expression levels of ANXA1 protein in HCC tissues were significantly higher than those in adjacent non-cancerous tissues (P < 0.001). High ANXA1 expression was closely correlated with advanced TNM stage (P = 0.001) and high Edmondson grade (P = 0.02). Then, univariate and multivariate analyses showed that the status of ANXA1 expression was an independent predictor for overall survival of HCC patients. Furthermore, knockdown of ANXA1 by transfection of siRNA–ANXA1 could suppress the migration and invasion abilities of HCC cells in vitro. Collectively, these findings offer the convincing evidence that ANXA1 may play an important role in HCC progression and can be used as a molecular marker to predict prognosis and a potential target for therapeutic intervention of HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC), the fifth (seventh) common cancer in men (women), has been the third leading cause of cancer-related deaths worldwide [1]. Because of its extremely unfavorable prognosis, the deaths and newly diagnosed cases each year are almost equal. Currently, hepatic resection is the most common treatment modality for HCC and one of the most effective interventions for achieving long-term survival [2]. In addition to this, transplantation and radiofrequency ablation were both potential curative methods for HCC [3]. However, the majority of patients with this malignancy are diagnosed at an advanced stage with underlying liver dysfunction, which makes curative treatments not effective or feasible due to tumor spread [4]. Only 10–20 % of tumors are resectable at the time of diagnosis, and the 5-year survival is poor even compared with other gastrointestinal malignancies [5]. Therefore, it is of great importance to seek optimal biomarkers for early-stage diagnosis, for predicting survival, and for evaluating treatment efficiency.
Annexin A1 (ANXA1) belongs to the annexin superfamily of calcium- and phospholipid-binding proteins and has been detected in miscellaneous organisms, including vertebrates, invertebrates, and plants [6]. It is an endogenous mediator of the anti-inflammatory effects of glucocorticoids through the inhibition of phospholipase A2, including arachadonic acid [7]. Functionally, ANXA1 has been reported to be involved in intracellular signaling, cell growth, and cell differentiation [8]. Growing evidence suggest that it contributes to the pathological consequence and sequelae of most serious human diseases, including cancers. As a potential marker for malignant progression, ANXA1 expression levels have been observed to be upregulated in melanoma, breast cancer, pancreatic cancer, and colorectal cancer [9–13]. In contrast, reduced ANXA1 expression levels have been found in esophageal cancer, gastric cancer, bile duct cancer, and prostate cancer [14–17]. However, its expression pattern in human HCC still remains controversial. Immunohistochemistry analysis of Suo et al. [18] observed the upregulated expression of ANXA1 protein in 20 HCC tissues compared to non-tumor tissues, but Hongsrichan et al. [19] found that the expression of ANXA1 protein was negative in all histological patterns for HCC. To confirm the expression pattern of ANXA1 in HCC tissues and evaluate its associations with tumor progression and patients’ prognosis, immunohistochemistry was performed using 160 pairs of formalin-fixed and paraffin-embedded cancerous and adjacent non-cancerous tissues from patients with HCC. Then, the associations between ANXA1 expression, clinicopathological characteristics, and prognosis of HCC patients were statistically evaluated. In vitro migration and invasion assays of siRNA-targeted ANXA1-transfected cells were further performed.
Materials and methods
Patients and tissue samples
This study was authorized by the Research Ethics Committee of Fuzhou General Hospital and People’s Hospital Affiliated to Fujian University of Traditional Chinese Medicine, China. All patients agreed to the procedure and signed consent forms. All specimens were handled and made anonymous according to the ethical and legal standards.
This was a retrospective study based on archived materials. A total of 160 pairs of formalin-fixed and paraffin-embedded cancerous and adjacent non-cancerous tissues from patients with HCC were surgically obtained between 2000 and 2008 in Fuzhou General Hospital and People’s Hospital Affiliated to Fujian University of Traditional Chinese Medicine, China. The patients were selected according to the following criteria: (1) primary HCC and (2) previously untreated and with surgery as the first treatment. Thus, data analysis in this series would reflect actual impact of the tumor biology on the clinical outcome. There were 130 men and 30 women ranging in age from 20 to 80 years (median age 60 years). Tumor differentiation was defined according to the Edmondson grading system [20], and tumor stage was performed according to the sixth edition of the tumor-node-metastasis (TNM) classification of the International Union against Cancer. Liver function was assessed using Child–Pugh classification. The patient characteristics, including patients’ age, gender, levels of preoperative alpha-fetoprotein (AFP), status of hepatitis B e antigen (HBeAg), cirrhosis, tumor size, number of tumor nodule, tumor capsula, vascular invasion, Edmondson grade, Child–Pugh grade, and TNM stage, are summarized in Table 1.
The survival information from the postoperative follow-up of all 160 HCC patients was received by telephone or mail. The median follow-up time was 26 months (range 2–72 months). The overall survival was defined as the interval from the end of treatment to death due to any cause or to the date of last contact.
Cell culture
Human HCC cell lines HepG2 and BEL-7402 were purchased from American Tissue Type Collection (Manassas, VA). All cells were cultured in RPMI-1640 medium supplemented with 10 % fetal calf serum (GIBCO) in a humidified atmosphere of 5 % CO2 at 37 °C.
Immunohistochemical staining for ANXA1 protein
Tissue sections (4 μm thick) were deparaffinized with xylene, rehydrated, and subjected to microwave antigen retrieval in citrate buffer (pH 6.0) for 20 min. Endogenous peroxidase was quenched with 3 % hydrogen peroxide for 10 min. The sections were then incubated with rabbit anti-annexin A1 antibody (1:1,000 dilution; Abcam, Cambridge, UK) at 4 °C overnight. The slides were incubated for 30 min with donkey anti-rabbit IgG polyclonal antibody, biotin conjugated (1:200 dilution; Abcam, Cambridge, UK) after being washed with Tris-buffered NaCl solution for 30 min. Then, the sections were washed with phosphate-buffered saline (PBS) for three times and further incubated using the PV-9000 Polymer Detection System (GBI Labs, Mukilteo, WA, USA) and color reacted with 3,3′-diaminobenzidine (DAB) solution (Zhongshan, Beijing, China) as a chromogen.
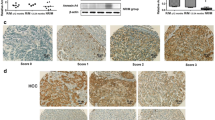
Immunostainings were evaluated by two experienced pathologists who were blinded to the clinical data reviewed the stained tissue sections, using the semiquantitative scoring system linking the staining intensity with the percentage of positive cells, according to the previous studies [21, 22]. The intensity was classified as follows: 0, negative staining; 1, weak staining; 2, moderate staining; and 3, strong staining. The percent of positive cells was judged followed by Remmele W’s criteria [23]: (1) 0–25 %; (2) 26–50 %; (3) 51–75 %; and (4) >75 %. A final immunohistochemical score was achieved by multiplying the intensity and the percent of positive cells. In order to classify all 160 HCC patients into high ANXA1 expression and low ANXA1 expression groups, a cutoff value for ANXA1 expression levels was chosen on the basis of a measure of heterogeneity with the log-rank test statistic with respect to overall survival.
RNA interference of ANXA1
Human HCC cell lines HepG2 and BEL-7402 were transfected with scrambled control (si-con) and human ANXA1-specific siRNA (si-ANXA1), which were purchased from Bioneer (Daejeon, Korea), during the logarithmic growth phase using Lipofectamine 2000 liposome (Invitrogen Co., Carlsbad, CA, USA) according to the manufacturers’ instruction. Cells were verified and used for analysis 48 h after transfection.
Western blot analysis
Human HCC cell lines HepG2 and BEL-7402 were harvested 48 h after transient transfection, and Western blot analysis was performed to detect the expression levels of ANXA1 protein. Standard Western blotting was performed using a rabbit anti-annexin A1 antibody in a 1:1,000 dilution (Abcam, Cambridge, UK) and a donkey anti-rabbit IgG polyclonal antibody (Abcam, Cambridge, UK). Equal protein sample loading was monitored by probing the same membrane filter with an anti-β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, USA), which was used as an internal control for the normalization of candidate proteins. Protein expression was assessed by enhanced chemiluminescence and exposure to chemiluminescent film (Pierce Biotechnology).
Cell migration and invasion assay
The migration abilities of human HCC cell lines HepG2 and BEL-7402 transfected with ANXA1 siRNA (si-ANXA1) and scrambled control (si-con) were tested in Corning transwell insert chambers. Briefly, 48 h after transfection, cells that were resuspended in 200 μl serum-free 1,640 medium were placed into the upper chamber of the insert without Matrigel. Medium with 5 % FBS was added into the lower chambers as a chemoattractant. After 24 h of incubation, cells remaining on the upper membrane were carefully removed. Cells that had migrated through the membrane were manually counted at 200× magnification from ten different fields of each filter.
The invasion abilities of human HCC cell lines HepG2 and BEL-7402 transfected with ANXA1 siRNA (si-ANXA1) and scrambled control (si-con) were tested in Matrigel coated cell culture chambers (8 μm pore size, Millipore, Billerica, MA, USA). Briefly, cells were transfected and cultured to confluence or near (>90 %) confluence in 24-well dishes. Then, cells that were resuspended in 200 μl serum-free 1,640 medium were placed into the upper chamber of the insert with Matrigel. Medium with 5 % FBS was added into the lower chambers as a chemoattractant. After 24 h of incubation, cells remaining on the upper membrane were carefully removed. Cells that had invaded through the membrane were manually counted at 200× magnification from ten different fields of each filter.
All assays were conducted in triplicate, and the mean values were calculated.
Statistical analysis
All statistic analyses were performed by SPSS 17.0 (SPSS Inc, Chicago, IL, USA). Continuous data were expressed as mean ± standard deviation (SD). Comparison among different groups was analyzed using paired samples t tests or Mann–Whitney U tests as appropriate. Fisher’s exact test and Chi-square test were performed to evaluate the associations between ANXA1 expression and different clinicopathological characteristics. Univariate and multivariate analyses were performed by Kaplan–Meier method and Cox proportional hazards regression model. Differences were considered statistically significant when P was <0.05.
Results
Expression of ANXA1 protein is upregulated in human HCC tissues
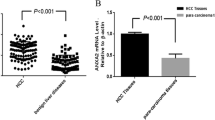
Immunostaining of ANXA1 protein was mainly localized in the cytoplasm of tumor cells with varying staining intensity as shown in Fig. 1a, but was seen faintly or with no staining in hepatocytes of adjacent non-cancerous tissues as shown in Fig. 1b. The statistical analysis showed that the mean immunohistochemical scores of ANXA1 protein in HCC and adjacent non-cancerous tissues were, respectively, 3.86 (range 2.0–6.04) and 1.92 (range 0.79–3.72). Thus, the expression level of ANXA1 protein was significantly higher in HCC tissues than in adjacent non-cancerous tissues (P < 0.001; Fig. 1c).
Based on the measure of heterogeneity with the log-rank test statistic with respect to overall survival, an optimal cutoff value (3.87) was identified: the low ANXA1 expression group (expression level lower than the cutoff value; mean expression value 3.00, n = 70, 43.75 %) and the high ANXA1 expression group (expression level higher than the cutoff value; mean expression value 4.53, n = 90, 56.25 %).
Increased expression of ANXA1 protein associates with aggressive progression of HCC patients
Table 1 summarized the association of ANXA1 protein expression with clinicopathological parameters of HCC patients. High ANXA1 expression was closely correlated with advanced TNM stage (P = 0.001, Table 1) and high Edmondson grade (P = 0.02, Table 1). However, ANXA1 immunoreactivity showed no significant correlation with patients’ age, gender, levels of preoperative AFP, status of HBeAg, cirrhosis, tumor size, number of tumor nodule, tumor capsula, vascular invasion, and Child–Pugh grade (all P > 0.05, Table 1).
Increased expression of ANXA1 protein predicts poor prognosis in HCC patients
To evaluate the prognostic value of ANXA1 protein immunostaining with overall survival of HCC patients, clinical follow-up was performed for all 160 patients in the current study. In the univariate analysis, we found a significant difference in patients’ overall survival between low ANXA1 expression and high ANXA1 expression groups (P < 0.001; Fig. 2; Table 2). In addition to ANXA1 expression, tumor size (P = 0.01), vascular invasion (P = 0.02), Edmondson grade (P = 0.001), and TNM stage (P < 0.001) were also correlated with overall survival of HCC patients (Table 2). In the multivariate analysis using the Cox proportional hazards model with significant parameters in univariate analysis, we further found that ANXA1 expression (P = 0.008), tumor size (P = 0.03), Edmondson grade (P = 0.01), and TNM stage (P = 0.006) were independent prognostic factors for overall survival in HCC patients (Table 2).
Kaplan–Meier analyses for the correlation between annexin A1 (ANXA1) expression and survival. The overall survival of HCC patients with high and low expression is shown. Log-rank test showed that patients with high ANXA1 expression had a significantly poorer overall survival versus patients with low ANXA1 expression (P < 0.001)
Knocking down ANXA1 expression suppresses the migration and invasion potentials of HCC cell lines
Since the increased expression of ANXA1 protein was associated with aggressive tumor progression and poor prognosis in patients with HCC as mentioned above, we hypothesized that ANXA1 might influence the malignant phenotypes of HCC cells. We succeeded establishing two ANXA1-silencing HCC cell lines: HepG2-siANXA1 and BEL-7402-siANXA1. As shown in Fig. 3, the expression levels of ANXA1 protein in two transfected HCC cell lines were both significantly lower than those in the control cells (both P < 0.001). In addition, the results shown in Fig. 4 indicated that the migratory potentials of si-ANXA1-transfected HepG2 and BEL-7402 cells were both dramatically reduced, and suppression of ANXA1 in both cell lines with si-ANXA1 also inhibited the cell invasion abilities in the Matrigel substrate (all P = 0.01).
Relative expression of annexin A1 (ANXA1) protein in hepatocellular carcinoma (HCC) cell lines HepG2 and BEL-7402 transfected with ANXA1 siRNA (si-ANXA1) and negative control siRNA (si-con). a Representative bolts showing expression of ANXA1 in HepG2 and BEL-7402 transfected with ANXA1 siRNA (si-ANXA1) and negative control siRNA (si-con). Expression of β-actin was used as a loading control. b Statistical analysis showed that the expression levels of ANXA1 protein in two transfected HCC cell lines were both significantly lower than those in the control cells (both P < 0.001)
Knocking down annexin A1 (ANXA1) expression inhibits hepatocellular carcinoma (HCC) cells’ migration and invasion in vitro. a, b Transwell migration assay and Matrigel invasion assay of HCC cell HepG2 transfected with si-ANXA1 and si-con. c Migratory activity of si-ANXA1-transfected HepG2 and BEL-7402 cells was dramatically reduced, and suppression of ANXA1 in both cell lines with si-ANXA1 also decreased invasion in the Matrigel substrate. **P = 0.001 when comparison with control cells
Discussion
HCC remains incurable to most of patients and recurrences after surgical treatment occur in approximately 70 % of the patients within 5 years [24]. Although several molecular factors and histological features have been reported to be associated with the progression and prognosis of HCC, more effective biomarkers are necessary for the early-stage diagnosis and the accurate prediction of the clinical outcome of HCC patients. In the current study, we detected the subcellular localization and the expression pattern of ANXA1 protein in a large cohort of HCC and adjacent non-cancerous tissues. Most primary HCC tissues showed strongly positive immunostaining of ANXA1 protein in cytoplasm of malignant cells with significant upregulation of ANXA1 expression relative to normal control tissues. Our data are consistent with the previous findings of Suo et al. but different from those of Hongsrichan et al. [19]. The reasons for the difference may include different genetic backgrounds and a larger study cohort (160 vs. 46). In addition, the relationship between clinicopathological features and ANXA1 expression showed that ANXA1 expression was positively correlated with TNM stage and Edmondson grade (both P < 0.05). To evaluate the prognostic value of ANXA1 expression in HCC patients, we divided them into two subgroups (high ANXA1 expression and low ANXA1 expression) and compared outcome between the two groups. The Kaplan–Meier survival analysis revealed that patients with high ANXA1 expression had a significantly shorter overall survival than those with low ANXA1 expression. In the multivariate analysis, we observed that ANXA1 expression, together with some traditional prognostic factors (tumor size, Edmondson grade, and TNM stage) were independent risk factors in the prognosis of HCC patients. To the best of our knowledge, this is the first study which demonstrated that ANXA1 may play an important role in the progression of HCC, and it may be used as a candidate prognostic biomarker for this disease.
The regulations of cell migration and invasion are crucial for maintaining cellular homeostasis, and their loss is a basic feature of cancer cells. Recent studies have reported that ANXA1 may function as either a negative or a positive regulator of malignant phenotypes of various cancer cells. For example, downregulation of ANXA1 expression with specific ANXA1 siRNA in human breast cancer cell line MDA-MB-231 led to decreased cancer cell migration and invasion [10]; Ang et al. [25] observed that silencing of ANXA1 with specific ANXA1 siRNA could reverse the estrogen-dependent proliferation as well as growth arrest of MCF-7 breast cancer cells; Boudhraa et al. [9] reported that in a B16Bl6 spontaneous metastasis model, a siRNA-induced decrease in tumoral ANXA1 expression significantly reduced tumoral MMP2 activity and number of lung metastases, and in human melanoma cell lines, ANXA1 level was positively correlated with in vitro invasion capacity, whereas normal melanocytes contained low ANXA1 levels; and Gao et al. [11] indicated that the forced ANXA1 expression in gastric cancer cells resulted in cell growth inhibition. Since the biological functions of ANXA1 in HCC are incompletely understood, in the current study, we knocked down the ANXA1 expression and investigated its effects on the biological behavior. ANXA1 knockdown in HepG2 and Bel-7402 cells contributed to inhibit the migration and invasion of cells, which suggested that ANXA1 might be associated with metastatic events in HCC cells. These findings are also consistent with observations in other human cancers mentioned above. But more research about the exact mechanism is needed.
In conclusion, our results offer the convincing evidence that ANXA1 may play an important role in HCC progression and can be used as a molecular marker to predict prognosis and a potential target for therapeutic intervention of HCC.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35–50.
Thomas MB, Zhu AX. Hepatocellular carcinoma: the need for progress. J Clin Oncol. 2005;23:2892–9.
Bosch FX, Ribes J, Borràs J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–85.
El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–27.
Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–71.
Guo C, Liu S, Sun MZ. Potential role of Anxa1 in cancer. Future Oncol. 2013;9:1773–93.
Bist P, Shu S, Lee H, Arora S, Nair S, Lim JY, Dayalan J, Gasser S, Biswas SK, Fairhurst AM, Lim LH. Annexin-A1 regulates TLR-mediated IFN-β production through an interaction with TANK-binding kinase 1. J Immunol. 2013;191:4375–82.
Boudhraa Z, Rondepierre F, Ouchchane L, Kintossou R, Trzeciakiewicz A, Franck F, Kanitakis J, Labeille B, Joubert-Zakeyh J, Bouchon B, Perrot JL, Mansard S, Papon J, Dechelotte P, Chezal JM, Miot-Noirault E, Bonnet M, D’Incan M, Degoul F. Annexin A1 in primary tumors promotes melanoma dissemination. Clin Exp Metastasis. 2014;31:749–60.
Kang H, Ko J, Jang SW. The role of annexin A1 in expression of matrix metalloproteinase-9 and invasion of breast cancer cells. Biochem Biophys Res Commun. 2012;423:188–94.
Gao Y, Chen Y, Xu D, Wang J, Yu G. Differential expression of ANXA1 in benign human gastrointestinal tissues and cancers. BMC Cancer. 2014;14:520.
Su N, Xu XY, Chen H, Gao WC, Ruan CP, Wang Q, Sun YP. Increased expression of annexin A1 is correlated with K-ras mutation in colorectal cancer. Tohoku J Exp Med. 2010;222:243–50.
Sheu MJ, Li CF, Lin CY, Lee SW, Lin LC, Chen TJ, Ma LJ. Overexpression of ANXA1 confers independent negative prognostic impact in rectal cancers receiving concurrent chemoradiotherapy. Tumour Biol. 2014;35:7755–63.
Rossi AF, Duarte MC, Poltronieri AB, Valsechi MC, Jorge YC, de-Santi Neto D, Rahal P, Oliani SM, Silva AE. Deregulation of annexin-A1 and galectin-1 expression in precancerous gastric lesions: intestinal metaplasia and gastric ulcer. Mediators Inflamm. 2014;2014:478138.
Zhang ZQ, Li XJ, Liu GT, Xia Y, Zhang XY, Wen H. Identification of annexin A1 protein expression in human gastric adenocarcinoma using proteomics and tissue microarray. World J Gastroenterol. 2013;19:7795–803.
Han G, Tian Y, Duan B, Sheng H, Gao H, Huang J. Association of nuclear annexin A1 with prognosis of patients with esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:751–9.
Jorge YC, Mataruco MM, Araújo LP, Rossi AF, de Oliveira JG, Valsechi MC, Caetano A, Miyazaki K, Fazzio CS, Thomé JA, Rahal P, Oliani SM, Silva AE. Expression of annexin-A1 and galectin-1 anti-inflammatory proteins and mRNA in chronic gastritis and gastric cancer. Mediators Inflamm. 2013;2013:152860.
Suo A, Zhang M, Yao Y, Zhang L, Huang C, Nan K, Zhang W. Proteome analysis of the effects of sorafenib on human hepatocellular carcinoma cell line HepG2. Med Oncol. 2012;29:1827–36.
Hongsrichan N, Rucksaken R, Chamgramol Y, Pinlaor P, Techasen A, Yongvanit P, Khuntikeo N, Pairojkul C, Pinlaor S. Annexin A1: a new immunohistological marker of cholangiocarcinoma. World J Gastroenterol. 2013;19:2456–65.
Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503.
Jiang SS, Weng DS, Wang QJ, Pan K, Zhang YJ, Li YQ, Li JJ, Zhao JJ, He J, Lv L, Pan QZ, Xia JC. Galectin-3 is associated with a poor prognosis in primary hepatocellular carcinoma. J Transl Med. 2014;12:273.
Cao Z, Fu B, Deng B, Zeng Y, Wan X, Qu L. Overexpression of Chemokine (C-X-C) ligand 1 (CXCL1) associated with tumor progression and poor prognosis in hepatocellular carcinoma. Cancer Cell Int. 2014;14:86.
Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–40.
El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–73.
Ang EZ, Nguyen HT, Sim HL, Putti TC, Lim LH. Annexin-1 regulates growth arrest induced by high levels of estrogen in MCF-7 breast cancer cells. Mol Cancer Res. 2009;7:266–74.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, Y., Lin, G., Fang, W. et al. Increased expression of annexin A1 predicts poor prognosis in human hepatocellular carcinoma and enhances cell malignant phenotype. Med Oncol 31, 327 (2014). https://doi.org/10.1007/s12032-014-0327-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0327-7