Abstract
Mammalian target of rapamycin (mTOR) signaling pathways have been shown to be activated in thyroid cancer. Recent evidences have demonstrated that the antidiabetic agent metformin, an activator of 5′-AMP-activated protein kinase, can impair the proliferation and migration of cancer cells via inhibition of mTOR. However, the underlying mechanisms remain unclear. In this study, we show that metformin can inhibit mTOR pathway to impair growth and migration of the thyroid cancer cell lines. Cyclin D1 and c-Myc are important regulators of cancer cell growth, and we observed that treatment of thyroid cancer cells with metformin reduced c-Myc and cyclin D1 expression through suppression of mTOR and subsequent inhibition of P70S6K1 and 4E-BP1 phosphorylation. Metformin reduced epithelial to mesenchymal transition (EMT) in thyroid carcinoma cells. Moreover, metformin regulated expression of the EMT-related markers E-cadherin, N-cadherin, and Snail. Additionally, knockdown of TSC2, the upstream regulatory molecule of mTOR pathway, or treatment of rapamycin, the mTOR inhibitor, could abolish the effects of metformin to regulate thyroid cancer cell proliferation, migration, EMT, and mTOR pathway molecules. These results indicate that metformin can suppress the proliferation, migration, and EMT of thyroid cancer cell lines by inhibiting mTOR signaling. These findings suggest that metformin and its molecular targets may be useful in thyroid carcinoma therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid cancer is the most common endocrine malignancy, and its incidence has increased in recent decades [1, 2]. It has been estimated that thyroid cancer rates have increased 48.0 % among male individuals and 66.7 % among female individuals from 1973–1977 to 1998–2002 [3]. Dependent upon the origin of neoplastic cells and the various influences that act upon them, thyroid cancer comes in multiple forms, including papillary, follicular, anaplastic, medullary, and other poorly differentiated thyroid cancers [4]. While conventional treatments such as surgery and radioactive iodine therapy are generally effective, persistent disease causes significantly increased morbidity and mortality [5]. Various molecular alterations have been clearly identified in the development of thyroid cancer, and multiple signaling pathways are involved in thyroid tumorigenesis and the progression of thyroid cancer. In particular, the MAPK and mammalian target of rapamycin (mTOR) pathways have been shown to be activated in thyroid cancer [6–8]. Further attention to the molecular pathogenesis of thyroid cancer could uncover more effective treatment strategies for thyroid cancer.
Metformin (N,N-dimethylbiguanide) is an oral biguanide used in the clinical management of type 2 diabetes mellitus [9, 10]. The primary effects of metformin are to suppress hepatic glucose production, reduce gluconeogenesis, and increase use of glucose by body tissues [11]. In addition to its antidiabetic role, metformin has been reported to depress tumor growth in various cancers [12–14], including thyroid cancer. Previous studies have demonstrated that metformin can inhibit the proliferation of multiple, thyroid cancer cell lines [15–17]. Metformin is an activator of AMP-activated protein kinase (AMPK), and activation of AMPK induces inhibition of the mTOR pathway. This mechanism has been implicated in metformin-mediated inhibition of cancer cell growth [18]. However, in thyroid cancer cells, the precise molecular mechanisms and downstream effectors underlying the actions of metformin remain largely unknown. In addition to unrestricted proliferation, cancer cells are capable of distant metastasis, and metformin has been found to significantly inhibit cell metastasis in lung adenocarcinoma [19], breast cancer [20], ovarian cancer [21], and melanoma [22]. However, whether metformin can depress metastasis of thyroid cancer cells by regulating the mTOR pathway remains unresolved and is worthy of further exploration.
In this study, we demonstrate that metformin impairs the proliferation, invasion, and migration of thyroid cancer cells and that these effects of metformin require involvement of the mTOR pathway. We also investigate the metformin-mediated suppression of tumor growth in a nude mouse model. More importantly, we describe a novel mechanism by which metformin inhibits thyroid cancer cell metastasis by suppressing epithelial-to-mesenchymal transition (EMT) through downregulation of the mTOR pathway.
Materials and methods
Cell culture and reagents
The human SW579 and TT thyroid cancer lines were purchased from the American Type Culture Collection (ATCC; Rockville, MD, USA). Cells were maintained in RPMI1640 medium (Gibco, Grand Island, NY, USA) supplemented with heat-inactivated 10 % fetal bovine serum (FBS; HyClone Laboratories, Inc., Logan, UT, USA) in a humidified atmosphere of 95 % air plus 5 % CO2 at 37 °C. Antibodies used were anti-cyclin D1, anti-c-Myc, and anti-GAPDH (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA); anti-E-cadherin, anti-N-cadherin, and anti-Snail (BD Biosciences San Diego, CA, USA); anti-AMPKα, anti-p-AMPKα (Thr172), anti-mTOR, anti-p-mTOR (Ser2448), anti-p-S6K1(Thr389), and anti-p-4E-BP1 (Thr37/46) (Cell Signaling Technology, Inc., Danvers, MA, USA). Metformin and rapamycin were obtained from Sigma-Aldrich (St. Louis, MO, USA). Transforming growth factor (TGF)-β1 was obtained from R&D Systems, Inc. (Minneapolis, MN, USA).
Transfection
For transfection, cells were seed in six-well plates and were transfected with expression for TSC2 siRNA (GenePharma, Shanghai, China) and control siRNA (GenePharma, Shanghai, China) using the Lipofectamine 2000 Reagent (Invitrogen; Carlsbad, CA, USA) according to the manufacturer’s protocol.
Western blot analysis
Cells were lysed in RIPA lysis buffer for 30 min at 4 °C then mixed with SDS buffer. Samples were separated using 10 % SDS-PAGE and transferred onto PVDF membranes (Millipore UK Ltd., Consett, UK). Non-specific binding was blocked with 0.1 % Tween-20 in TBS (TBS-T) containing 5 % skim milk powder for 1 h. Membranes were then incubated overnight with the primary antibodies in TBS-T at 4 °C. Following three 5-min washes with TBS-T, blots were incubated with HRP-linked anti-mouse or anti-rabbit secondary antibodies for 1 h at room temperature. After three further washes, bands were visualized using ECL reaction reagent (Amersham Pharmacia Biotech, Shinjuku-ku, Tokyo, Japan).
Cell growth, colony formation, migration, and invasion assays
Cell growth, colony formation, migration, and invasion assays following treatment of cells with metformin were performed as previously reported [23].
In vivo tumor growth and metastasis
SW579-Luc thyroid cancer cells were washed and resuspended in PBS. Subsequently, a uniform suspension containing 1 × 107 cells in 100 μL PBS was injected into the left armpit or lateral tail vein of 6-week-old male nude mice (Vital river Inc. Beijing, China), which were then randomly sorted into two groups (n = 4). The treatment was initiated with either the vehicle alone (control group) or metformin treatment at a concentration of 100 mg/kg and administered once a day. In vivo photon emissions from SW579-Luc cells in mammary glands were detected and photographed using an IVIS200 imaging system (Chinese PLA General Hospital, Beijing, China) on the 45th day. Tumor size was detected with Vernier calipers, and tumor volume was calculated using the formula volume = (longest diameter × short diameter2)/2.
Statistics
Results were expressed as the mean ± standard deviation (SD) from three independent assays. The differences between groups were analyzed using a Student’s t test or the χ 2 test of variance. Statistical calculations were performed using SPSS 17.0. Statistical significant was considered when P < 0.05.
Results
Metformin represses activation of mTOR and activates AMPK
Given that metformin can upregulate p-AMPK and suppress p-mTOR in many cancers, we investigated whether this also occurred in SW579 cells. SW579 cells were treated with increasing concentrations of metformin in a dose-dependent manner. Western blot analysis showed that metformin was associated with dose-dependent activation of AMPK phosphorylation and inhibition of mTOR phosphorylation, especially at 10-mM concentration. No significant change of the total mTOR level was observed (Fig. 1a). These results indicate that metformin activates AMPK and inhibits mTOR phosphorylation level in thyroid cancer cells, specifically.
Metformin suppresses the mTOR pathway in thyroid cancer cells
As the growth inhibitory effects of metformin have been attributed to downregulation of mTOR signaling, we next examined whether metformin represses activation of downstream targets of mTOR. Western blot analysis revealed that treatment of SW579 and TT cell lines with metformin led to reduced phosphorylation of the two mTOR kinase targets S6K1 and 4E-BP1, and levels of the mTOR signal downstream effectors c-Myc and cyclin D1 were decreased (Fig. 1b). But the total protein levels of S6K1 and 4E-BP1 were not changed (Fig. 1b).
Metformin suppresses the proliferation, migration, and invasion of thyroid carcinoma cells
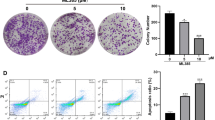
To determine the effect of metformin on thyroid cancer cells, we performed cell proliferation and colony formation assays using SW579 and TT cell lines. Treatment with metformin reduced the proliferation and colony-forming activity of these cell lines (Fig. 2a–d). Upregulation of p-AMPK and downregulation of p-mTOR were observed following metformin treatment of thyroid cancer cells (Fig. 2a, c). Soft agar experiments revealed that metformin suppressed anchorage-independent SW579 cell proliferation (Fig. 2g). Together, these results suggest that metformin can inhibit thyroid cancer cell proliferation.
Metformin suppresses thyroid cancer cell growth, migration, and invasion in vitro. a, c SW579 and TT cells were treated with or without 10 mM metformin and cell numbers were measured at indicated times. The representative immunoblot depicts the effect of metformin on p-AMPK and p-mTOR expression. b, d Colony development in SW579 and TT cells 3 weeks following treatment with or without 10 mM metformin. Representative images of colonies in plates (left panel). e, f Wound-healing assays were conducted in SW579 and cells treated with or without 10 mM metformin. Scale bar: 100 μm. g Colony formation by SW579 cells in soft agar 4 weeks following treatment with or without 10 mM metformin. Representative images show soft agar colonies. Scale bar: 100 μm. h Matrigel invasion assay in SW579 cells treated with or without 10 mM metformin. Representative invasive cells stained with crystal violet (left panels). Scale bar: 100 μm. All values shown are mean ± SD of triplicate measurements and have been repeated three times with similar results (*P < 0.01 versus corresponding control)
Next, the influence of metformin on the migration and invasion of thyroid cancer cells was assessed. Metformin inhibited the migration of both SW579 and TT cells (Fig. 2e, f) and decreased the invasion of SW579 cells (Fig. 2h).
Metformin inhibits thyroid cancer cell tumor growth in nude mice
To confirm the role of metformin on tumor growth in vivo, SW579-Luc cells were injected into the left armpit of 6-week-old male nude mice. Tumors were measured daily from days 4–45 following injection. Metformin markedly suppressed tumor growth (Fig. 3a, b). Furthermore, at day 45, post-implantation tumors were visualized using the IVIS200 imaging system, again indicating that treatment of metformin significantly inhibited tumor growth (Fig. 3a). These results confirm that metformin can modulate tumor cell growth in vivo. But for the metastasis, we observed no significant difference between metformin minus and plus groups (Supplementary Fig. 1).
Metformin inhibits thyroid cancer cell tumor growth in vivo. a, b Nude mice were injected SW579-Luc cells and treated with either the vehicle alone or metformin (100 mg/kg) via oral gavage for 45 days. a Representative in vivo photon emissions of SW579-Luc cells is shown. b Tumors were measured with Vermier calipers at indicated times (mean ± SD; n = 4). *P < 0.01
Metformin inhibits thyroid cancer cell proliferation, migration, and invasion through regulation of mTOR signaling
As is well known, metformin inhibited mTOR pathway through activation of TSC2. To assess whether metformin-induced inhibition of cyclin D1 and c-Myc resulted from inhibition of mTOR, we transfected SW579 cells with TSC2 siRNA or control siRNA and treated or untreated with metformin, respectively. The result showed that TSC2 knockdown could activate mTOR pathway and upregulate the expression of c-Myc and cyclin D1, while TSC2 knockdown abolished the ability of metformin to suppress c-Myc, cyclin D1, and other mTOR pathway molecules (Fig. 4a).
Metformin inhibits thyroid cancer cell proliferation, migration, and invasion via TSC2-mediated inhibition of the mTOR pathway. a SW579 cells were transfected with TSC2 siRNA or control siRNA prior to treatment with or without 10 mM metformin as indicated. Immunoblot analysis of mTOR signaling and downstream effectors in SW579 cells was shown. b Cell proliferation was then assessed at the specified times. c Wound-healing assays and d invasion assays for SW579 cells treated with or without 10 mM metformin after knockdown TSC2 expression. Scale bar: 100 μm. All values shown are mean ± SD of triplicate measurements and have been repeated three times with similar results (*P < 0.01 versus corresponding control)
Next, to determine whether metformin influenced the phenotype of thyroid cancer cell through mTOR pathway, we transfected SW579 cells with TSC2 siRNA or control siRNA and treated or untreated with metformin. The results showed that metformin inhibited the proliferation of SW579 cells (Fig. 4b). Interestingly, knockdown of TSC2 abolished the ability of metformin to regulate cell proliferation (Fig. 4b). There was a similar trend in the migration and invasion of SW579 cells treated with metformin after knockdown of TSC2 expression (Fig. 4c, d).
To further confirm the important role of mTOR in metformin mediating its effects, SW579 cells were exposed to rapamycin, the mTOR inhibitor, prior to treatment with metformin and performed the experiments. As expected, inhibition of mTOR signaling by rapamycin abolished the ability of metformin to suppress growth, migration, invasion, and mTOR pathway molecules (Supplementary Fig. 2A, 2B, 2C, and 2D). These results indicate that metformin inhibits thyroid cancer cell proliferation, migration, and invasion through the suppression of mTOR signaling. In addition, we confirmed that the inhibitory effect of rapamycin were more obvious than metformin in all our experiments designed for testing the proliferation, migration, and invasion ability of cells.
Metformin impairs EMT through inhibition of mTOR activation
EMT plays an important role in cancer cell invasion and metastasis and is subject to regulation by TGF-β1 and mTOR. To test whether metformin has an effect on EMT through mTOR pathway, we first observed the phenotype following treatment of metformin in SW579 and TT cells compared with treatment of TGF-β1. As expected, treatment of metformin inhibited morphologic changes form a polarized epithelial phenotype, which caused an elongated fibroblastoid phenotype as treatment of TGF-β1 (Fig. 5a, b), indicating that metformin suppressed EMT. Furthermore, metformin upregulated the epithelial marker E-cadherin and downregulated the mesenchymal markers N-cadherin and Snail following inhibition of the mTOR pathway (Fig. 5a, b). These results indicated that metformin may repress thyroid cancer progression and metastasis via regulation of EMT.
Metformin inhibits thyroid cancer cell EMT by downregulating the mTOR pathway. a, b Morphological changes in SW579 and TT cells following treatment with TGF-β1 (10 ng/ml) or metformin (left panel) and immunoblot analysis of EMT marker expression following metformin treatment (right panel). c Morphological changes of SW579 cells following treatment with or without metformin after knockdown TSC2 expression for indicated groups. d Immunoblot analysis of SW579 cells following treatment with or without metformin after knockdown TSC2 expression for indicated groups. Scale bar: 100 μm
Knockdown TSC2 prior to metformin treatment could abolish the effects of EMT (Fig. 5c, d). Moveover, inhibition of mTOR pathway by rapamycin observed the similar results (Supplementary Fig. 3A and 3B). These data indicate that metformin inhibited EMT through regulation of mTOR signaling.
Discussion
Metformin has been shown to possess an anti-cancer effect over and above its understood role as an antidiabetic agent. However, the mechanisms by which metformin inhibits thyroid cancer growth and metastasis remain poorly understood. This study demonstrates that metformin inhibits cell growth, migration, and invasion of thyroid cancer and that these activities require the participation of mTOR. This report is the first to describe metformin-mediated suppression of c-Myc and EMT marker expression though increased AMPK phosphorylation and subsequent depressed mTOR pathway signaling. These results provide a novel insight into the mechanisms underlying metformin-mediated inhibition of cancer development and progression.
The increasing incidence [1] and high metastatic rate [24] of thyroid cancer has led to great interest in identifying the mechanisms underlying the growth and metastasis of thyroid cancer cells. Several studies have described important molecular events in the pathogenesis of thyroid cancer, and at the tumor formation stage, various genetic mutations appear to play an important role. Mutation of the BRAF-V600E gene occurs in approximately 45 % of papillary thyroid cancers [25], while mutation of RAS [8, 26] and ALK [27] genes are often identified. These mutations drive activation of mTOR pathway and lead to thyroid tumorigenesis [4]. AMPK pathway has also been known to have cross-talk with mTOR. In this study, we observed that treatment with metformin can apparently activate the AMPK pathway in a dose-dependent manner, leading to the inhibition of cyclin D1 and c-Myc expression through downregulated p-mTOR. As proto-oncogenes, cyclin D1 and c-Myc play critical roles in tumor cell growth, migration, and invasion [28–31]. Concurrent with reducing the expression of cyclin D1 and c-Myc, metformin inhibited growth, migration, and invasion in thyroid cancer cell lines. Based on these results, metformin can inhibit thyroid cancer cell development and progression by reducing the expression of key proto-oncogenes.
mTOR signaling plays a critical role in human cancer [32]. It can be induced by abnormal activation of upstream signaling involving protein kinase B/AKT and extracellular signal-regulated kinase (ERK) [33]. In addition to these kinases, AMPK can regulate mTOR signaling by promoting TSC1/2 complex activation [32]. In this study, we have demonstrated that metformin, as an activator of AMPK, depresses mTOR protein phosphorylation in thyroid cancer cells. Our data therefore reveal a novel mechanism for the inhibition of cyclin D1 and c-Myc expression by metformin though depression of the mTOR pathway.
EMT underlies cancer cell invasion and metastasis and is a key event in the progression of many cancers. Earlier studies have demonstrated that a variety of molecules can induce EMT, including transforming growth factor beta [34], epidermal growth factor [34], AKT [23], and ERK [23]. Metformin can also influence EMT by acting upon these EMT-regulating pathways and has been reported to inhibit transforming growth factor beta-induced EMT [35, 36] and interleukin-6-induced EMT [19, 37]. Metformin can also inhibit miRNA-induced EMT and regulate transcriptional control to inhibit EMT. Recently, a new mechanism of AMPK-mediated regulation of EMT through modulation of the Akt-MDM2-Foxo3a signaling axis has also been described [38]. Taken together, these results indicate an important role for metformin in the regulation of EMT. However, metformin-mediated changes to thyroid cancer cell phenotype and depression of EMT marker expression at the post-transcriptional level have been rarely reported. In this study, we have demonstrated that treatment with metformin alters the phenotype of thyroid cancer cells from a mesenchymal to an epithelial phenotype as characterized by increased expression of epithelial markers and decreased expression of mesenchymal markers. Investigation of the mechanisms underlying these changes identified that the expression of EMT markers is different. Metformin inhibits EMT via repression of mTOR-mediated enhancement of protein expression through regulation of ribosomal activity. However, the inhibitory effect of metformin on tumor metastasis did not occur in vivo compared with tumor growth in vivo (Fig. 3a–c, and Supplementary Fig. 1). The main reason may be due to different ways of metformin administration. Some experimental studies utilized intraperitoneal injection to administer metformin [12, 18, 19, 21], in order to get relatively high plasma concentration of metformin. However, metformin is usually taken orally on clinic. In our study, metformin was adopted for mice by oral gavage which was closer to the actual condition of clinical administration. The insufficient metformin plasma concentration of mice may fail to inhibit the metastasis of thyroid cancer cells compared with that of in vitro experiment. There are still other possible reasons, including different metastatic ability of the tumor cells or inadequate sample size. Nevertheless, the exact effect of metformin in tumor metastasis still needs further investigation.
In conclusion, our study demonstrated that metformin can inhibit thyroid cancer cell proliferation, migration, invasion, and EMT through activation of AMPK and subsequent suppression of mTOR. These findings suggest that metformin could be a potential clinical drug for the treatment of thyroid carcinoma.
References
Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–7.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, et al. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control. 2009;20:525–31.
Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–99.
Liu D, Xing J, Trink B, Xing M. BRAF mutation-selective inhibition of thyroid cancer cells by the novel MEK inhibitor RDEA119 and genetic-potentiated synergism with the mTOR inhibitor temsirolimus. Int J Cancer. 2010;127:2965–73.
Ciampi R, Knauf JA, Kerler R, Gandhi M, Zhu Z, Nikiforova MN, et al. Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J Clin Invest. 2005;115:94–101.
Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006;6:292–306.
Liu Z, Hou P, Ji M, Guan H, Studeman K, Jensen K, et al. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab. 2008;93:3106–16.
Crandall JP, Knowler WC, Kahn SE, Marrero D, Florez JC, Bray GA, et al. Diabetes prevention program research, the prevention of type 2 diabetes. Nat Clin Pract Endocrinol Metab. 2008;4:382–93.
Witters LA. The blooming of the French lilac. J Clin Invest. 2001;108:1105–7.
Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74.
Kato K, Gong J, Iwama H, Kitanaka A, Tani J, Miyoshi H, et al. The antidiabetic drug metformin inhibits gastric cancer cell proliferation in vitro and in vivo. Mol Cancer Ther. 2012;11:549–60.
Lea MA, Pourat J, Patel R, des Bordes C. Growth inhibition of colon cancer cells by compounds affecting AMPK activity. World J Gastrointest Oncol. 2014;6:244–52.
Rattan R, Giri S, Hartmann LC, Shridhar V. Metformin attenuates ovarian cancer cell growth in an AMP-kinase dispensable manner. J Cell Mol Med. 2011;15:166–78.
Chen G, Xu S, Renko K, Derwahl M. Metformin inhibits growth of thyroid carcinoma cells, suppresses self-renewal of derived cancer stem cells, and potentiates the effect of chemotherapeutic agents. J Clin Endocrinol Metab. 2012;97:E510–20.
Cho SW, Yi KH, Han SK, Sun HJ, Kim YA, Oh BC, et al. Therapeutic potential of metformin in papillary thyroid cancer in vitro and in vivo. Mol Cell Endocrinol. 2014;393:24–9.
Klubo-Gwiezdzinska J, Jensen K, Costello J, Patel A, Hoperia V, Bauer A, et al. Metformin inhibits growth and decreases resistance to anoikis in medullary thyroid cancer cells. Endocr Relat Cancer. 2012;19:447–56.
Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–12.
Zhao Z, Cheng X, Wang Y, Han R, Li L, Xiang T, et al. Metformin inhibits the IL-6-induced epithelial-mesenchymal transition and lung adenocarcinoma growth and metastasis. PLoS One. 2014;9:e95884.
Vazquez-Martin A, Oliveras-Ferraros C, Cufi S, Del Barco S, Martin-Castillo B, Lopez-Bonet E, et al. The anti-diabetic drug metformin suppresses the metastasis-associated protein CD24 in MDA-MB-468 triple-negative breast cancer cells. Oncol Rep. 2011;25:135–40.
Wu B, Li S, Sheng L, Zhu J, Gu L, Shen H, et al. Metformin inhibits the development and metastasis of ovarian cancer. Oncol Rep. 2012;28:903–8.
Cerezo M, Tichet M, Abbe P, Ohanna M, Lehraiki A, Rouaud F, et al. Metformin blocks melanoma invasion and metastasis development in AMPK/p53-dependent manner. Mol Cancer Ther. 2013;12:1605–15.
Xu X, Fan Z, Kang L, Han J, Jiang C, Zheng X, et al. Hepatitis B virus X protein represses miRNA-148a to enhance tumorigenesis. J Clin Invest. 2013;123:630–45.
Madani A, Jozaghi Y, Tabah R, How J, Mitmaker E. Rare metastases of well-differentiated thyroid cancers: a systematic review. Ann Surg Oncol. 2014;22(2):460–6.
Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–62.
Abubaker J, Jehan Z, Bavi P, Sultana M, Al-Harbi S, Ibrahim M, et al. Clinicopathological analysis of papillary thyroid cancer with PIK3CA alterations in a Middle Eastern population. J Clin Endocrinol Metab. 2008;93:611–8.
Murugan AK, Xing M. Anaplastic thyroid cancers harbor novel oncogenic mutations of the ALK gene. Cancer Res. 2011;71:4403–11.
Cai X, Hu X, Cai B, Wang Q, Li Y, Tan X, et al. Metformin suppresses hepatocellular carcinoma cell growth through induction of cell cycle G1/G0 phase arrest and p21CIP and p27KIP expression and downregulation of cyclin D1 in vitro and in vivo. Oncol Rep. 2013;30:2449–57.
Dai M, Al-Odaini AA, Fils-Aime N, Villatoro MA, Guo J, Arakelian A, et al. Cyclin D1 cooperates with p21 to regulate TGFbeta-mediated breast cancer cell migration and tumor local invasion. Breast Cancer Res. 2013;15:R49.
Marfil V, Blazquez M, Serrano F, Castell JV, Bort R. Growth-promoting and tumourigenic activity of c-Myc is suppressed by Hhex. Oncogene. 2014. doi:10.1038/onc.2014.240.
Zhou W, Feng X, Ren C, Jiang X, Liu W, Huang W, et al. Over-expression of BCAT1, a c-Myc target gene, induces cell proliferation, migration and invasion in nasopharyngeal carcinoma. Mol Cancer. 2013;12:53.
Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22.
Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci. 2011;36:320–8.
Mimeault M, Batra SK. Interplay of distinct growth factors during epithelial mesenchymal transition of cancer progenitor cells and molecular targeting as novel cancer therapies. Ann Oncol. 2007;18:1605–19.
Cufi S, Vazquez-Martin A, Oliveras-Ferraros C, Martin-Castillo B, Joven J, Menendez JA. Metformin against TGFbeta-induced epithelial-to-mesenchymal transition (EMT): from cancer stem cells to aging-associated fibrosis. Cell Cycle. 2010;9:4461–8.
Lee JH, Kim JH, Kim JS, Chang JW, Kim SB, Park JS, et al. AMP-activated protein kinase inhibits TGF-beta-, angiotensin II-, aldosterone-, high glucose-, and albumin-induced epithelial-mesenchymal transition. Am J Physiol Renal Physiol. 2013;304:F686–97.
Li L, Han R, Xiao H, Lin C, Wang Y, Liu H, et al. Metformin sensitizes EGFR-TKI-resistant human lung cancer cells in vitro and in vivo through inhibition of IL-6 signaling and EMT reversal. Clin Cancer Res. 2014;20:2714–26.
Chou CC, Lee KH, Lai IL, Wang D, Mo X, Kulp SK, et al. AMPK reverses the mesenchymal phenotype of cancer cells by targeting the Akt-MDM2-Foxo3a signaling axis. Cancer Res. 2014;74:4783–95.
Acknowledgments
The authors wish to thank Dr. JT Dou for his help and support.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Additional information
Baiyu Han, Hanzhi Cui, and Lei Kang contributed equally to this work. The 264 Hospital of PLA Chinese and PLA General Hospital also contributed equally to this work
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
(PDF 34 kb)
Supplementary Fig. 2
(PDF 801 kb)
Supplementary Fig. 3
(PDF 487 kb)
Rights and permissions
About this article
Cite this article
Han, B., Cui, H., Kang, L. et al. Metformin inhibits thyroid cancer cell growth, migration, and EMT through the mTOR pathway. Tumor Biol. 36, 6295–6304 (2015). https://doi.org/10.1007/s13277-015-3315-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3315-4