Abstract
Background
Current evidence shows that ML385, a new type of nuclear factor erythroid 2-related factor 2 inhibitor, exerts a good inhibitory effect on tumors. However, whether ML385 can regulate the biological behavior of thyroid cancer remains puzzling.
Objectives
We aimed to observe the regulation of ML385 on biological characteristics such as proliferation, apoptosis, migration, and invasion of thyroid tumor cells in vitro and clarify the molecular mechanism of regulating glucose metabolism of tumor cells to lay a foundation for ML385 as a therapeutic tumor drug.
Results
ML385 effectively reduced the viability, proliferation, invasion, migration, glucose consumption, lactate production, adenosine triphosphate level, and extracellular acidification rate of TPC-1 cells and concentration-dependently promoted TPC-1 cell apoptosis, indicating that ML385 inhibited the biological behavior thyroid cancer cell and aerobic glycolysis in vitro. Moreover, the above cellular behaviors were not significantly altered when 2-DG was added to ML385 treatment, suggesting that the inhibition of glycolysis by 2-DG may partially block the effect of ML385 on thyroid cancer cells.
Conclusions
ML385 inhibits the biological behavior of thyroid cancer cells by impairing aerobic glycolysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Global Cancer Statistics 2020 analyzed the incidence and mortality of 36 cancers in 185 countries. Globally, 586 000 cases of thyroid carcinoma have been recorded, which was higher than 567 000 cases in 2018, still ranking ninth in terms of incidence (Chen et al. 2023). The etiology of thyroid carcinoma is unknown, and ionizing radiation is currently the only established risk factor. Smoking, hormones, environmental pollutants, iodine, chemical carcinogens, and gene mutations may be risk factors for thyroid cancer. Among them, iodine deficiency or excessive iodine intake may lead to thyroid cancer (Laha et al. 2020). Although most patients have a good prognosis after treatment, 10–15% of patients with thyroid cancer will have recurrence and metastasis after treatment, which leads to local recurrence and dropping of the overall survival rate to 70–85% in patients with thyroid cancer (Tuttle 2018). The treatment of thyroid carcinoma is mainly based on surgery, thyroid hormone suppression therapy, and drugs. The U.S. Food and Drug Administration and the European Medicines Agency have approved two kinase inhibitors for the treatment of differentiated thyroid cancer, namely, sorafenib and lenvatinib, which are multikinase inhibitors with antiangiogenic properties. In addition, China has completed the phase III trial of lenvatinib, demonstrating that it can improve the prognosis of patients. These drugs are expected to be used in the treatment of advanced thyroid cancer with extensive metastasis, for example, the prognosis of patients with advanced lung metastasis is also improved. However, they still have some side effects such as hypertension, diarrhea, rash, fatigue, weight loss, and stomatitis (Cabanillas et al. 2019). Moreover, they may increase the concentration of thyroid hormones. Therefore, the molecular mechanisms underlying thyroid cancer development must be elucidated, and promising thyroid cancer therapies established.
Aerobic glycolysis, one of the considerable features of tumor metabolic reprogramming, is closely related to the biological characteristics of tumors such as proliferation, metastasis, and drug resistance. Aerobic glycolysis was found in > 90% of tumors and was gradually used in the diagnosis and treatment of various tumors (Ganapathy-Kanniappan and Geschwind 2013). In cancer cells, 60% of adenosine triphosphate (ATP) is derived from aerobic glycolysis, and associated enzymes, metabolites, and related factors are also closely related to tumor development (Abbaszadeh et al. 2020). Aerobic glycolysis may also exist in thyroid cancer (Huang et al. 2022); however, its mechanism of action has not been thoroughly studied.
In recent years, the metabolic reprogramming of thyroid cancer cells and redox homeostasis imbalance caused by a hypoxic microenvironment have gradually become a research hotspot, and the nuclear factor E2-related factor 2 (NRF2) system, one of the centers regulating the cellular oxidative stress response, has attracted increasing attention. NRF2 is one of the most important nuclear transcription factors of antioxidant response in vivo and participates in the maintenance of intracellular redox homeostasis (He et al. 2020). NRF2 is closely related to breast (Zhang et al. 2019a), lung (Sánchez-Ortega et al. 2021), colorectal (Li et al. 2022a), gastric (Farkhondeh et al. 2021), and pancreatic (Cykowiak and Krajka-Kuźniak 2021) cancers. Dai et al. (Dai et al. 2022) revealed that NRF2 knockdown reduced glucose uptake, lactate products, and ATP levels and downregulated the expression of glycolysis-related proteins HK2 and LDHA, which subsequently suppressed the prostate cancer tumor volume and tumor weight in vivo. Recently, NRF2 was discovered to be widely activated in thyroid cancer cells. Silencing NRF2 can materially inhibit its activity and maturity and strikingly weaken its migration and infiltration ability. It can reduce tumorigenicity and improve sensitivity to rivatinib (Gong et al. 2021). This evidence suggests that NRF2 has great potential as a key target in inhibiting glycolysis in thyroid cancer cells, and targeting the glycolysis pathway may be a feasible and effective method to inhibit the progression of thyroid cancer.
ML385 was reported to be a potent inhibitor of the NRF2 pathway, inhibiting NRF2 nuclear translocation. According to Wang et al. (Wang et al. 2022), dexmedetomidine could reduce and alleviate myocardial infarction and improve cardiac function and inflammation by activating the NRF2 pathway, and this protective effect was counteracted by ML385. Liu et al. (Lin et al. 2020) did not observe the protective effect of gallic acid in improving gouty arthritis in mice after blocking NRF2 with ML385 addition. In addition, JI et al. (Ji et al. 2023) used ML385 to intervene in lung squamous cell carcinoma and noted that ML385 enhanced the growth inhibition in lung squamous cell carcinoma via a pan-PI3K inhibitor. However, whether ML385 can regulate the biological behavior and glycolysis of thyroid cancer remains unclear.
Thus, we aimed to observe ML385 regulation on biological characteristics such as proliferation, apoptosis, migration, and invasion of thyroid tumor cells in vitro and clarify the molecular mechanism of regulating glucose metabolism of tumor cells to lay a foundation for ML385 as a therapeutic tumor drug.
Materials and methods
Cell culture and treatment
The human thyroid cancer TPC-1 cell line was obtained from the Shanghai Cell Bank of the Chinese Academy of Sciences and deposited in the Central Laboratory of the First Hospital of Putian City. TPC-1 cells were grown in monolayer adherent Dulbecco’s modified eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and 100 U/mL penicillin G and streptomycin solution in a 5% CO2 incubator at 37 °C and saturated humidity. Logarithmic phase cells were treated with ML385 (TargetMol Chemicals Inc., MA, USA) at different doses (0, 5, and 10 μM) for 24 h. Subsequently, the collected cells were further divided into four groups, namely control, ML385, 2-DG (WARBIO, Nanjing, China), and 2-DG + ML385, with ML385 and 2-DG concentrations of 10 and 15 μM, respectively. Finally, the cells were collected for further cell behavior experiments.
Cell viability assay
In each group, TPC-1 cells (3 × 105 cells/mL) were seeded in 96-well plates (100 μL/well) and cultured for 24 h. Moreover, 10 μL of the Cell Counting Kit-8 (CCK-8) reagent (MLbio, Shanghai, China) was added to each well for detection at 24 h, and incubation was continued for 3 h at 37 °C. The absorption coefficient of each hole at 450-nm wavelength was measured by a microplate meter. The experiment was repeated three times with three compound holes.
Cell proliferation assay
The bottom layer of the agar was prepared as usual and added to a 24-well plate at 0.8 mL/well. After the agar was completely solidified at room temperature, the cells were digested and counted, the density of the cell suspension was adjusted to 1 × 103/mL, and 10 mL of the cell suspension was prepared. The upper layer of the agar was routinely prepared and added to the 24-well plate in which the lower layer had solidified. Each group was set up with four replicate wells according to 0.8 mL/well. After 14 days of culture in vitro with 5% CO2 at 37 °C, the cells were stained with crystal violet. Cells with a cell count of > 50 were observed under a microscope, and the number of clones was calculated.
Cell apoptosis assay
Following drug treatment, the cells of each group were cultured in a 24-well plate for 24 h, and the starting number of cells was 5.0 × 104/well. Then, the cells of each group were collected, and cell apoptosis was evaluated using Annexin V-FITC/PI kit (Absin, Shanghai, China).
Cell invasion assay
In each group, TPC-1 cells were digested with 0.25% trypsin and then added to the DMEM culture medium to prepare the cell suspension (2.5 × 105 cells/mL). The cell suspension was added to the upper chamber of the Matrigel-coated Transwell (200 μL/well) (Corning, USA), the culture medium containing 10% FBS was added to the lower chamber of Transwell (600 μL/well), and the culture was continued for 24 h. After washing with PBS, the plates were fixed with paraformaldehyde for 20 min and stained with 0.1% crystal violet for 10 min. The number of invasive cells was observed by microscopy. Three replicate wells were set up for the experiment, and each well was repeated three times.
Cell migration assay
Cells in a good growth state and logarithmic growth phase were digested and counted, and the density of the cell suspension was adjusted to 6 × 104/mL and seeded into 96-well plates. After the cells were overgrown, a scratch assay was performed using a scratch apparatus. The medium was then changed, the 96-well plate was placed under a microscope, and cells were photographed at 0 h. After 24 h, pictures were taken again to observe cell migration.
Glycolysis assay
The cell culture medium was collected after 24 h of culturing TPC-1 cells using DMEM without phenol red. The extracellular acidification rate (ECAR) was determined by the Seahorse Assay Kit (MLbio). Glucose intake was measured using a Glucose Assay Kit (Abcam, UK). Lactate production was determined using a Lactate Detection Kit (MLbio). ATP levels were evaluated using the CellTiter-Glo luminescent cell viability assay (MLbio).
Western blot assay
In each group, the TPC-1 cells were added with 400 μL of RIPA lysate to extract the total protein, and the protein concentration was checked by the bicinchoninic acid assay. Proteins were separated by polyacrylamide gel electrophoresis and blocked by 5% nonfat milk powder for 2 h. Then, the primary and secondary antibody diluents were incubated, respectively; the reaction conditions for the primary antibody diluent and the anti-diluent were incubation at 4 °C for 24 h and incubation at room temperature for 1 h, respectively. After washing the protein with TBST, enhanced chemiluminescence was added and exposed to develop in a dark room. Finally, ImageJ was used to analyze the grayscale value of each band. Antibody information is listed in Table S1. Three replicate wells were set up, and each well was repeated three times.
Statistical analysis
Experimental data were statistically analyzed using GraphPad version 8.0. All experiments were conducted as triplicate independent experiments, and the one-way analysis of variance was used in the comparison of three or more groups, and a t-test was used between two groups, with P < 0.05 indicating a significant difference.
Results
ML385 suppresses the proliferation and induces the apoptosis of thyroid carcinoma cells
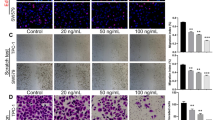
CCK-8 was applied to check the inhibitory effect of ML385 in different concentration gradients (0, 5, and 10 μM) on the activity of TPC-1 cells. Figure 1A displays the chemical structural formula, molecular formula, and molecular weight of ML385. The CCK-8 assay displayed that ML385 concentration- and time-dependently suppressed TPC-1 cell viability, particularly when TPC-1 cells were intervened with 10 μM of ML385 for 24, 48, and 72 h, and the cell viability nearly did not increase. Statistical results revealed that the suppressed effect of ML385 on cell activity was enhanced with increased drug concentration and extended action time, showing dose and time dependence (Fig. 1B). Colony formation assays were also applied to assess the effect of ML385 on TPC-1 cell proliferation. As shown in Fig. 1C, the cell proliferation ability was dose-dependently inhibited under treatment with 5 and 10 μM ML385, as shown by the reduction in the number of cell clones. The apoptosis rates of TPC-1 cells treated with 0, 5, and 10 μM ML385 for 48 h were 4.99 ± 0.93, 14.96 ± 0.81, and 22.89 ± 2.06, respectively, suggesting that ML385 could promote cell apoptosis (Fig. 1D). The expressions of apoptosis-related proteins in TPC-1 cells were further analyzed, and the results showed that the expression levels of apoptosis marker proteins Bcl-2-associated X protein (BAX) and cleaved-caspase3 were remarkably upregulated in TPC-1 cells following ML385 treatment in a dose-dependent manner (Fig. 1E). Therefore, ML385 treatment preeminently repressed thyroid cancer cell proliferation and induced apoptosis.
ML385 constraints the proliferation and induces the apoptosis of thyroid carcinoma cells. A Chemical structure, name, molecular formula, and molecular weight of ML385. B CCK-8 assay measurement of the viability of TPC-1 cells at 24, 48, and 72 h, which were intervened with 0, 5, and 10 μM ML385. C Colony formation measurement of TPC-1 cell proliferation, which was intervened with 0, 5, and 10 μM ML385. D Annexin V/PI double-staining method measurement of the apoptotic ratio of TPC-1 cells, which was intervened with 0, 5, and 10 μM ML385. E Western blotting measurement of the protein levels of BAX and cleaved-caspase3 in TPC-1 cells, which was intervened with 0, 5, and 10 μM ML385. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 vs the 0 μM group, n = 3
ML385 restricts the migration and invasion of thyroid carcinoma cells
Transwell and cell scratch assays are classical methods to verify the effects on tumor cell motility performance, which have the advantages of being reproducible and easy to quantify. Accordingly, Transwell and wound-healing assays were performed to determine the effect of ML385 treatment on TPC-1 cell invasion and migration. The wound-healing test displayed that the average scratch width of the 5 and 10 μM groups was larger than that of the 0 μM group, particularly the 10 μM group 24 h after scratch treatment (Fig. 2A). Further, the Transwell invasion assay demonstrated that ML385 at 5 and 10 μM conspicuously reduced the number of TPC-1 cells crossing the polycarbonate membrane (Fig. 2B). Therefore, the findings indicated that ML385 treatment did seriously affect the invasive and migratory phenotypes of TPC-1 cells, and the suppressed effect was more obvious with the increase in ML385 concentration.
ML385 suppresses the migration and invasion of thyroid carcinoma cells. A Cell scratch assay measurement of TPC-1 cell migration, which was intervened with 0, 5, and 10 μM ML385 (scale bar, 100 μm). B Transwell assay measurement of the invasion ability of TPC-1 cells, which was intervened with 0, 5, and 10 μM ML385 (scale bar, 100 μm). Data are presented as mean ± SEM. **P < 0.01, ***P < 0.001 vs the 0 μM group, n = 3
The glycolysis of thyroid carcinoma cells is impaired following ML385 treatment
Glycolysis, the process of glucose uptake and metabolism, is fundamental to the maintenance of life. Considering that changes in the energy metabolism of tumor cells have a certain effect on tumor cell proliferation, ML385 was used to study the energy metabolism of TPC-1 cells. These cells were treated with 10 μM ML385 under normoxic conditions for 24 h, and the ECAR, lactate and glucose contents, and ATP levels in the supernatant were measured. The ECAR based on the Seahorse assay suggested that the ECAR was preeminently reduced after ML385 addition (Fig. 3A, B). Moreover, ML385 treatment remarkably reduced glucose uptake (Fig. 3C) and lactate production (Fig. 3D) in cells. Finally, ML385 treatment strikingly prevented the level of intracellular ATP production in TPC-1 cells (Fig. 3E). Therefore, ML385 suppressed ECAR, lactate production, glucose consumption, and ATP level in thyroid carcinoma cells, indicating the inhibition of aerobic glycolysis in thyroid carcinoma cells.
The glycolysis of thyroid carcinoma cells is impaired following ML385 treatment. A, B Seahorse assay measurement of ECAR in TPC-1 cells, which was intervened with 10 μM ML385. C Glucose determination kit measurement of the glucose uptake in TPC-1 cells, which was intervened with 10 μM ML385. D Lactic acid detection kit measurement of the relative lactate production in TPC-1 cells, which was intervened with 10 μM ML385. E CellTiter-Glo luminescent cell viability assay measurement of the ATP level in TPC-1 cells, which was intervened with ML385 at 10 μM. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 vs control group, n = 3
Glycolysis inhibition by 2-DG partly blocks the effects of ML385 on thyroid carcinoma cells
2-DG, a glycolysis inhibitor, was included to examine the effect of ML385 on the active phenotype of thyroid cancer cells. 2-DG, as a glycolysis inhibitor, can reduce glycolysis-like ATP content, glucose uptake, and lactate production by inhibiting glycolysis. In this study, 2-DG and ML385 were added to the TPC-1 cell culture medium, either separately or alone, and cell proliferation, apoptosis, invasion, and migration after 24 h were assessed. The results revealed that the proliferation (Fig. 4A), activity (Fig. 4C), migration (Fig. 4E), and invasion (Fig. 4F) ability of TPC-1 cells were significantly decreased following 2-DG treatment, whereas the apoptosis rate (Fig. 4B) and protein levels of BAX and cleaved-caspase3 (Fig. 4D) increased. Interestingly, the above cell behaviors did not change observably after adding 2-DG based on ML385 treatment, suggesting that the inhibition of glycolysis by 2-DG may partially block the effect of ML385 on thyroid cancer cells.
Glycolysis inhibition by 2-DG partly blocks the effects of ML385 on thyroid carcinoma cells. A Clone formation measurement of TPC-1 cell proliferation, which intervened with 10 μM ML385 or/and 15 μM 2-DG. B Annexin V/PI double-staining assay measurement of TPC-1 cell apoptosis, which was intervened with 10 μM ML385 or/and 15 μM 2-DG. C CCK-8 assay measurement of TPC-1 cell viability, which was intervened with 10 μM ML385 or/and 15 μM 2-DG. D Western blotting measurement of the protein levels of BAX and cleaved-caspase3 in TPC-1 cells, which was intervened with 10 μM ML385 or/and 15 μM 2-DG. (E) Cell scratch assay measurement of TPC-1 cell migration, which was intervened with 10 μM ML385 or/and 15 μM 2-DG (scale bar, 100 μm). F The Transwell assay measurement of the invasion ability of TPC-1 cells, which was intervened with 10 μM ML385 or/and 15 μM 2-DG (scale bar, 100 μm). Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, n = 3
Discussion
Thyroid cancer is a common type of endocrine-related malignant tumor, with rapidly increasing incidence in recent years. Recent research on the key molecules regulating papillary thyroid carcinoma has been a hot spot in medical research, which has a high translational significance for the prevention and treatment of postoperative metastasis. Previous studies have found that NRF2 plays a dual regulatory role in tumor occurrence and development, that is, its role is completely different. NRF2 can inhibit the occurrence of a few tumors by inhibiting oxidative stress-induced cell damage and exerting anti-inflammatory effects (Menegon et al. 2016). However, NRF2 activation caused by gene mutation or other reasons has been observed in many cancers, which can facilitate the adaptation of cancer cells to the harsh internal environment and induce metabolic reprogramming, thereby supporting the rapid proliferation and invasion of cancer cells (Schmidlin et al. 2021). Thus, NRF2 is essential for tumor growth once in situ tumors have developed. NRF2 gene expression is increased in thyroid cancer tissues and has a significant tendency to tumorigenesis. This indicates that it can be used as a prognostic marker or therapeutic target. Therefore, the search for methods or drugs that can block abnormal NRF2 expression may be a promising strategy for the treatment of advanced thyroid cancer. ML385 is a potent inhibitor of the NRF2 pathway, which can react with NRF2 to affect the DNA-binding activity of the NRF2–MAFG protein complex. According to Anju Singh et al. (Singh et al. 2016), ML385 exhibited significant antitumor activity in combination with carboplatin by inhibiting NRF2 expression. Zhang et al. (Zhang et al. 2022) found that NRF2 inhibitor ML385 conspicuously inhibited the proliferation activity, invasive phenotype, and tumorigenicity of CRPC cells in vitro, which provided evidence that NRF2 is a key regulator and ML385 is a potential therapeutic option for prostate cancer by targeting NRF2. Of note, Luo et al. (Luo et al. 2022) proposed that ML385 effectively enhanced the anti-anaplastic thyroid cancer effect of pexidartinib in vivo. Therefore, ML385 can constrict the proliferative activity and invasive phenotype of various cancer cells. Tumor cell invasion and metastasis are one of the main causes of cancer recurrence and death in patients with cancer. Therefore, we speculate that ML385 may suppress the proliferation, invasion, migration, and apoptosis of TPC-1 by inhibiting its activity. The results confirmed that ML385 could inhibit the activity, proliferation, invasion, and migration and increased the apoptosis of TPC-1 cells, indicating that ML385 could inhibit the development of thyroid cancer cells in vitro.
Recently, tumor energy metabolism can affect tumor occurrence and development, and other biological events have attracted the attention of many scholars. It is a new research direction in the anticancer field to cut off the abnormal increase in the glycolysis level in tumor cells. The dysregulation of energy metabolism is considered one of the characteristics of cancer (Koppenol et al. 2011; Chelakkot et al. 2023). Biological events such as cell proliferation, invasion, and metastasis require large amounts of energy consumption. As the main end product of aerobic glycolysis, the microenvironment of lactate promotes the infiltration of cancer cells and reduces the antitumor immune function of the body (Li et al. 2022b). The Warburg effect of cancer cells is often accompanied by high levels of metabolite lactate production, glucose consumption, and abnormally active glycolysis (Rabinowitz and Enerbäck 2020). Although large amounts of glucose are consumed during glycolysis, the corresponding amount of ATP produced is not and is much lower than the amount of ATP produced under normal conditions (Bartman et al. 2023). Tumor cells use special metabolic methods to obtain energy and materials, build cell structures, and meet unrestricted cell proliferation. In this study, the concentrations of glucose, lactate, and ATP in the supernatant of TPC-1 cells were detected in vitro, and ML385 treatment of TPC-1 cells effectively reduced glucose consumption, lactate production, ATP levels, and ECAR, which confirmed that ML385 could effectively inhibit aerobic glycolysis of thyroid cancer cells. Subsequently, anaerobic respiration inhibitor 2-DG was employed to investigate whether the inhibition of glycolysis in TPC-1 cells by 2-DG affected the ML385-induced regulation of cell proliferation, apoptosis, invasion, and migration. 2-DG, a glucose analog, targets glucose metabolism to consume the energy of cancer cells, and it can effectively slow the cell growth of specific cancer cells and promote their apoptosis (Zhang et al. 2014). Zhang et al. (Zhang et al. 2019b) found that 2-DG treatment induced apoptosis and inhibited cell proliferation, migration, and invasion by repressing glycolysis in breast cancer cells. Bikas et al. (Bikas et al. 2015) proposed that the introduction of 2-DG increases the sensitivity of thyroid cancer cells to metformin and may represent a novel strategy for the treatment of thyroid cancer. The results of this study confirmed that the above cell behaviors were not significantly changed after 2-DG was added based on ML385 treatment, suggesting that the inhibition of 2-DG on glycolysis may partially block the effect of ML385 on thyroid cancer cells.
In conclusion, this study validated the effect of ML385 on the biological behavior and glycolysis levels of thyroid cancer cells by applying CCK-8, clone formation, Transwell, cell scratch, glucose uptake, and lactate production experiments. ML385 was confirmed to inhibit the proliferation, migration, and invasion of thyroid cancer cells and induce apoptosis, and this phenomenon was achieved by inhibiting glycolysis. Our results provide a new idea for ML385 as a therapeutic drug for thyroid cancer and lay a foundation for further research on ML385 function in thyroid cancer.
Nevertheless, this study has some limitations. First, the specific molecular mechanism by which ML385 affects glycolysis in thyroid cancer cells has not been deeply elucidated. Second, the therapeutic effect of ML385 on thyroid cancer and its effect on glycolysis have not been verified in animal experiments, which are limited to in vitro cell experiments. Finally, this study used a single cell line, and the effect of ML385 on normal thyroid cells and drug-resistant thyroid cancer cell lines was not investigated. We will conduct more research to provide deeper ideas for ML385 as a therapeutic drug for thyroid cancer.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abbaszadeh Z, Çeşmeli S, Biray AÇ (2020) Crucial players in glycolysis: cancer progress. Gene 726:144158
Bartman CR et al (2023) Slow TCA flux and ATP production in primary solid tumours but not metastases. Nature 614:349–357
Bikas A et al (2015) Glucose-deprivation increases thyroid cancer cells sensitivity to metformin. Endocr Relat Cancer 22:919–932
Cabanillas ME, Ryder M, Jimenez C (2019) Targeted therapy for advanced thyroid cancer: kinase inhibitors and beyond. Endocr Rev 40:1573–1604
Chelakkot C, Chelakkot VS, Shin Y, Song K (2023) Modulating glycolysis to improve cancer therapy. Int J Mol Sci. https://doi.org/10.3390/ijms24032606
Chen DW et al (2023) Thyroid cancer. Lancet 401:1531–1544
Cykowiak M, Krajka-Kuźniak V (2021) Role of Nrf2 in pancreatic cancer. Antioxidants (basel). https://doi.org/10.3390/antiox11010098
Dai YQ, Bai Y, Gu J, Fan BY (2022) Stanniocalcin1 knockdown induces ferroptosis and suppresses glycolysis in prostate cancer via the Nrf2 pathway. Neoplasma 69:1396–1405
Farkhondeh T et al (2021) Roles of Nrf2 in gastric cancer: targeting for therapeutic strategies. Molecules. https://doi.org/10.3390/molecules26113157
Ganapathy-Kanniappan S, Geschwind JF (2013) Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer 12:152
Gong Z et al (2021) The knockdown of Nrf2 suppressed tumor growth and increased the sensitivity to lenvatinib in anaplastic thyroid cancer. Oxid Med Cell Longev 2021:3900330
He F, Antonucci L, Karin M (2020) NRF2 as a regulator of cell metabolism and inflammation in cancer. Carcinogenesis 41:405–416
Huang J et al (2022) FTO suppresses glycolysis and growth of papillary thyroid cancer via decreasing stability of APOE mRNA in an N6-methyladenosine-dependent manner. J Exp Clin Cancer Res 41:42
Ji L et al (2023) The NRF2 antagonist ML385 inhibits PI3K-mTOR signaling and growth of lung squamous cell carcinoma cells. Cancer Med 12:5688–5702
Koppenol WH, Bounds PL, Dang CV (2011) Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer 11:325–337
Laha D, Nilubol N, Boufraqech M (2020) New therapies for advanced thyroid cancer. Front Endocrinol (lausanne) 11:82
Li J, Wang D, Liu Y, Zhou Y (2022a) Role of NRF2 in colorectal cancer prevention and treatment. Technol Cancer Res Treat 21:15330338221105736
Li X et al (2022b) Lactate metabolism in human health and disease. Signal Transduct Target Ther 7:305
Lin Y et al (2020) Gallic acid alleviates gouty arthritis by inhibiting NLRP3 inflammasome activation and pyroptosis through enhancing Nrf2 signaling. Front Immunol 11:580593
Luo J et al (2022) Anti-anaplastic thyroid cancer (ATC) effects and mechanisms of PLX3397 (Pexidartinib), a multi-targeted tyrosine kinase inhibitor (TKI). Cancers (basel). https://doi.org/10.3390/cancers15010172
Menegon S, Columbano A, Giordano S (2016) The dual roles of NRF2 in cancer. Trends Mol Med 22:578–593
Rabinowitz JD, Enerbäck S (2020) Lactate: the ugly duckling of energy metabolism. Nat Metab 2:566–571
Sánchez-Ortega M, Carrera AC, Garrido A (2021) Role of NRF2 in lung cancer. Cells. https://doi.org/10.3390/cells10081879
Schmidlin CJ et al (2021) The intricacies of NRF2 regulation in cancer. Semin Cancer Biol 76:110–119
Singh A et al (2016) Small molecule inhibitor of NRF2 selectively intervenes therapeutic resistance in KEAP1-deficient NSCLC tumors. ACS Chem Biol 11:3214–3225
Tuttle RM (2018) Controversial issues in thyroid cancer management. J Nucl Med 59:1187–1194
Wang Z et al (2022) Dexmedetomidine attenuates myocardial ischemia/reperfusion-induced ferroptosis via AMPK/GSK-3β/Nrf2 axis. Biomed Pharmacother 154:113572
Zhang D et al (2014) 2-Deoxy-D-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Lett 355:176–183
Zhang HS et al (2019a) Nrf2 promotes breast cancer cell migration via up-regulation of G6PD/HIF-1α/Notch1 axis. J Cell Mol Med 23:3451–3463
Zhang T et al (2019b) Targeting the ROS/PI3K/AKT/HIF-1α/HK2 axis of breast cancer cells: combined administration of Polydatin and 2-Deoxy-d-glucose. J Cell Mol Med 23:3711–3723
Zhang Y, Xin Z, Dong B, Xue W (2022) Combination of the NRF2 inhibitor and autophagy inhibitor significantly inhibited tumorigenicity of castration-resistant prostate cancer. Comput Math Methods Med 2022:4182401
Acknowledgements
The authors have no acknowledgments.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
WZ and HY conceived the project and designed the experiments. WZ, HY, HL, and HH performed the experiments and analyzed the data. WZ wrote and revised the manuscript. All the authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Corresponding author
Ethics declarations
Conflict of interest
Wentian Zheng declares that they have no conflict of interest. Huan Yang declares that they have no conflict of interest. Hehua Lin declares that they have no conflict of interest. Hanxing Huang declares that they have no conflict of interest.
Ethical approval
This article contains no studies with human participants or animals performed by authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, W., Yang, H., Lin, H. et al. ML385 suppresses the proliferation, migration, and invasion of thyroid carcinoma cells by impairing aerobic glycolysis. Mol. Cell. Toxicol. (2023). https://doi.org/10.1007/s13273-023-00395-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s13273-023-00395-6