Abstract
The findings of associations between interleukin-8 (IL-8) polymorphisms and risk of oral cancer are controversial. We conducted a meta-analysis on the basis of data from all published studies to provide evidence of the current understanding of the genetic association with oral cancer. Eligible studies were identified by means of an electronic search of PubMed, Elsevier, ScienceDirect, EMBASE, EBSCO, and CBM databases for studies published up to March 2013. In accordance with the inclusion and exclusion criteria, a total of six eligible studies were included in the pooled analyses. In the overall analysis, we did not observe any significant associations between the IL-8-251A>T polymorphism and oral cancer risk under any of the genetic models (all P > 0.05). In the stratified analysis by ethnicity, Caucasian individuals with genotype AA had a higher risk of oral cancer under the dominant model (OR = 1.35, 95 % CI 1.09–1.67, P = 0.006). This meta-analysis indicated that the IL-8-251A>T polymorphism was not associated with the susceptibility of oral cancer, while individuals in the Caucasian population with genotype AA had a higher risk of oral cancer under the dominant model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral cancer refers to cancer that originates in the head and neck region. Oral squamous cell carcinoma (OSCC), which is a common malignant cancer in the head and neck region, is the eighth most frequent neoplasm in humans according to the worldwide cancer incidence ranking [1]. The development of OSCC is a multifactorial and multistep process. Several risk factors have been identified, such as alcohol and tobacco consumption [2], betel quid mastication [3], and human papillomavirus infection [4]. Inflammatory cytokines and anti-inflammatory cytokines, such as interleukin (IL)-1, IL-8, and IL-6 [5, 6], have recently been proved to be correlated with oral cancer through multiple and often controversial pathways. Genetic polymorphisms of some inflammatory and anti-inflammatory cytokines have been found to be associated with the risk of oral cancer [7, 8].
Interleukin 8, one of a chemokine superfamily of structurally and functionally related inflammatory cytokines, was discovered in 1987 [9, 10]. As an important inflammatory chemokine, IL-8 is involved in enhancing early host defense responses. The overexpression of IL-8 has been found in various human cancers, such as non-small cell lung carcinoma [11], breast tumors [12], gastric carcinoma [13], and hepatocellular carcinoma [14]. The IL-8 gene, located on human chromosome 4, is composed of four exons, three introns, and a proximal promoter region [15]. The gene coding of IL-8 exhibits several functional polymorphisms, of which 15 have been characterized thus far [16]. A common single nucleotide polymorphism is located at position −251 of IL-8 in the transcription start site. Studies have shown that the IL-8-251A>T polymorphism affected IL-8 transcriptional activity or protein expression, both in vivo and in vitro [17, 18]. Therefore, it could be presumed that the IL-8-251A allele increases the risk of developing cancer through elevation of its expression of IL-8.
In 2007, Vairaktaris et al. [19] published the first study indicating that the A/T heterozygotes of IL-8-251A>T had a twofold greater risk of developing OSCC. Since then, the potential associations of IL-8 polymorphisms with the risk of OSCC have been explored in several studies; however, the results of such associations were contradictory and inconclusive. Campa et al. [20] investigated the role of the polymorphisms of genes involved in inflammation in the risk of cancers of the upper aerodigestive tract, and they reported that the IL-8-251A>T polymorphism was not associated with the risk of OSCC. However, in 2010, Kietthubthew et al. [21] reported that the IL-8-251A>T polymorphism decreased the risk of OSCC. Generally, the inconsistent results were likely due to small sample sizes and low statistical power. A quantitative synthesis of accumulated data from different studies might provide strong evidence of the correlation between genetic polymorphisms and diseases. Therefore, we conducted a meta-analysis on the basis of data from all published studies.
Methods
Search strategy
Eligible studies were identified by means of an electronic search of the PubMed, Elsevier, ScienceDirect, EMBASE, EBSCO, and CBM databases for studies published up to March 2013. The primary search included the following key terms: “oral cancer,” “oral squamous cell carcinoma,” “polymorphism,” and “interleukin-8.” The search focused on human studies and was restricted to English and Chinese language papers. We also searched reference lists of reviews and reviewed retrieved articles again to trace other relevant publications at the same time.
Inclusion and exclusion criteria
Eligible studies included in the current meta-analysis had to meet all of the following explicit inclusion criteria: (1) the association between the IL-8 polymorphism and oral cancer risks was explored; (2) the study was designed using the methodology of a case–control study; (3) the study provided the size of the sample and sufficient data (i.e., genotype distributions of both cases and controls were available) for estimating an odds ratio (OR) with a 95 % confidence interval (CI) or information to help infer the results reported in the paper; and (4) carcinoma cases were diagnosed by histopathology. The exclusion criteria were as follows: (1) reviews and use of overlapping data published by the same first author, (2) studies with insufficient information (e.g., genotype frequency or number was not reported; histopathological diagnosis of cancer was not confirmed), and (3) studies not designed as case/control or cohort studies.
Data extraction
The following information was independently summarized from each included study: first author, publication year, origin country, ethnicity of study population, genotyping method, source of controls, and genotype distribution in cases and controls. To minimize bias and improve reliability, two researchers compared the results of all the included studies for accuracy and discussed any discrepancies before reaching an agreement. If the researchers could not reach a consensus, the disagreement was resolved by a third author.
Methodological quality assessment
Three reviewers independently evaluated the quality of the selected studies by scoring them according to a set of predetermined criteria (Table 1), which were modified from a previous meta-analysis of molecular association studies [22–25]. The range of the scores was 0–12, with higher scores indicating better quality. Disagreements were resolved by discussion.
Statistical analysis
The strength of the association between IL-8 and oral cancer risk was estimated by ORs with 95 % CIs. The pooled ORs were calculated, including (1) the AT genotype versus the TT genotype, (2) the AA genotype versus the TT genotype, (3) TT + AT genotypes versus the AA genotype (the dominant model), and (4) the TT genotype versus AT + AA genotypes (the recessive model). A chi-square-based Q test and inconsistency index I 2 [26, 27] were used to check the heterogeneity among the different studies. A P value ≥0.10 on the Q test indicated that there was no heterogeneity among the studies. Generally, we considered an I 2 value <25 % as indicator of mild heterogeneity, I 2 values ranging from 25 to 50 % as moderate heterogeneity, and I 2 values >50 % as strong heterogeneity. When the Q test showed the existence of notable heterogeneity (P < 0.10 and/or I 2 > 50 %), the pooled OR estimate of each study was calculated by the random-effects model (DerSimonian and Laird method) [28]; otherwise, the fixed-effects model (Mantel and Haenszel method) was conducted [29]. To investigate whether publication bias might affect the validity of the estimates, funnel plots were constructed, with an asymmetric plot suggesting possible publication bias. Funnel plot asymmetry was assessed using Begg's test and Egger's test, which is a linear-regression approach for measuring funnel-plot asymmetry on the natural logarithmic scale of the OR [30, 31]. All statistical tests were two-sided, and P < 0.05 was considered an indicator of significance, except where specifically noted. All of the statistical tests were performed with STATA version 10.0 (Stata Corporation, College Station, TX).
Results
Characteristics of studies
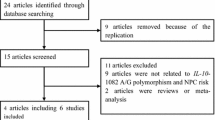
Six reports that addressed the role of IL-8-251 polymorphism in oral cancer risk were included in this meta-analysis [20, 21, 32–35]. A flowchart depicting the study selection process is shown in Fig 1. Two of the studies [19, 33] investigated the same population; only the more recent one was included [33]. The characteristics and genotype frequency distribution of these studies are shown in Table 2. Of the six studies, four investigated Asians and two investigated Caucasians. The controls were hospital-based in five of the studies and population-based in one. Four studies described oral cancers and two focused on tongue cancers; only one study described laryngopharynx cancer. Four studies used genetic DNA from blood samples to detect genotypes and two studies used tissue samples; a total of four genotyping methods were used. The genotype distribution of the controls in all of the studies was consistent with the Hardy–Weinberg equilibrium (HWE), with the exception of the study by Vairaktaris et al. (2008) [33] on oral cancer.
Meta-analysis results
The results for association strength between the IL-8-251A>T polymorphism and the susceptibility to oral cancers are shown in Table 3. In the overall analysis, we did not observe any significant associations between the IL-8-251A>T polymorphism and oral cancer risk under any of the genetic models (all P > 0.05) (Figs. 2, 3, 4, and 5).
Cancer site, source of controls, DNA sample, genotype method, and HWE test were taken into consideration for subgroup analysis. In the stratified analysis by ethnicity, Caucasian individuals with genotype AA had a higher risk of oral cancer under the dominant model (OR = 1.35, 95 % CI 1.09–1.67, P = 0.006) (Table 3; Fig. 6), but this association was not present in the Asian populations (Table 3). Studies using hospital-based controls and those using population-based controls were analyzed separately; however, none of the studies, regardless of source of controls, showed significant association (Table 3). Furthermore, when we conducted stratified analyses according to cancer site, DNA sample, genotype method, and HWE test, no significant associations were found in any genetic models (all P > 0.05) (Table 3). There was a statistically higher risk of oral cancer in the one non-HWE study, by Vairaktaris et al. [33]; however, this result lacked reliability due to the estimation of effect size from a single study.
Publication bias
Funnel plots and Egger's test were used to assess publication bias. The funnel plot results showed no apparent evidence of publication bias (Fig. 7). There was also no significant difference in the Egger's test for the allelic genetic model, suggesting a low probability of publication bias in the present meta-analysis (T = 1.26, P = 0.256).
Discussion
The potential role of the IL-8-251A>T polymorphism as a determinant of oral cancer risk was investigated using six published case–control studies. Ever since the IL-8 polymorphism was found to occur frequently in human populations, many studies have been conducted and published on the polymorphism and the risk of cancer. The first study, published in 2007, revealed that the A/T heterozygotes of IL-8-251A>T displayed a twofold greater risk of developing OSCC [19]. After that, several investigators duplicated this work in different populations. However, no consensus has been reached regarding the correlation between IL-8-251A>T polymorphism and oral risk, even within the same population. Considering that a single study might be too underpowered to detect the effect of the gene polymorphism on cancer, especially when the sample size is relatively small, we performed a meta-analysis of all eligible studies to derive a more precise estimation of the association of the IL-8 polymorphism with oral cancer risk.
However, a meta-analysis for this same polymorphism had been published by Wang et al. [36], which we read with great interest. In Wang's study, which included six case–control studies, the conclusions indicate that it provided strong evidence that the AA and AT genotypes of the IL-8-251A>T polymorphism were associated with an increased risk of oral cancer (OR = 1.23, 95 % CI 1.03–1.46, P = 0.025; OR = 1.25, 95 % CI 1.07–1.47, P = 0.006, respectively). However, the data reported by Wang et al. [36] for the study by Campa et al. [20] do not seem in line with the data provided by Campa et al. [20] in their original publication. The numbers reported by Wang et al. [36] for TT, AT, and TT in the controls are 241, 468, and 189, but after carefully studying the data presented by Campa et al. [20], the frequencies we retrieved were 197, 370, and 158. The data reported by Wang et al. [36] for the study by Vairaktaris et al. [33] also do not seem in line with the data provided by Vairaktaris et al. [33] in their original publication. The numbers reported by Wang et al. [36] for TT, AT, and TT in the controls are 74, 84, and 0, but after carefully studying the data presented by Vairaktaris et al. [33], the frequencies we retrieved were 84, 72, and 0. Furthermore, the data reported by Wang et al. [36] for the study by Hu et al. [35] do not seem in line with the data provided by Hu et al. [35] in their original publication. The numbers reported by Wang et al. [36] for TT, AT, and TT in the cases are 42, 51, and 16; interestingly enough, after carefully studying the data presented by Hu et al. [35], the frequencies we retrieved were 54, 67, and 21.
The pooled results of the current meta-analysis indicated that the IL-8-251A>T polymorphism was not associated with a risk of oral cancer under any of the genetic models. Afterwards, we conducted several subgroup analyses and found that IL-8-251A>T polymorphism, in a stratified analysis by ethnicity, was statistically related with elevated cancer risks. This finding indicates that the genetic variant in IL-8-251A>T might have a crucial role in the susceptibility to oral cancer in the Caucasian population. Our findings are not in accordance with the results published previously by Wang et al. [36]; in their study, a marginally significant association was found between the IL-8-251A>T polymorphism and oral cancer. The reason for this different result might be the erroneous data used in the meta-analysis by Wang et al. [36]. Nonetheless, considering the limited number of published studies available in the present meta-analysis, our results should be interpreted cautiously.
In summary, this meta-analysis indicated that the IL-8-251A>T polymorphism was not associated with the susceptibility to oral cancer, while in the Caucasian population, individuals with genotype AA had a higher risk of oral cancer under the dominant model. To confirm our findings, further large-scale case–control and population-based association studies are required. In particular, studies on gene–gene and gene–environment interactions are needed to identify the possible roles of IL-8-251A>T in oral cancer.
References
Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359(11):1143–54.
Biolchini F, Pollastri G, Figurelli S, et al. Carcinogen metabolism, DNA damage repair, and oral head and neck squamocellular carcinoma (HNSCC): a review. Minerva Stomatol. 2005;54(7–8):405–14.
Sharan RN, Mehrotra R, Choudhury Y, et al. Association of betel nut with carcinogenesis: revisit with a clinical perspective. PLoS One. 2012;7(8):e42759. PMCID: 3418282.
Joseph AW, D'Souza G. Epidemiology of human papillomavirus-related head and neck cancer. Otolaryngol Clin North Am. 2012;45(4):739–64.
Chen MF, Wang WH, Lin PY, et al. Significance of the TGF-beta1/IL-6 axis in oral cancer. Clin Sci (Lond). 2012;122(10):459–72. PMCID: 3393047.
Cheng YA, Shiue LF, Yu HS, et al. Interleukin-8 secretion by cultured oral epidermoid carcinoma cells induced with nicotine and/or arecoline treatments. Kaohsiung J Med Sci. 2000;16(3):126–33.
Liu CJ, Wong YK, Chang KW, et al. Tumor necrosis factor-alpha promoter polymorphism is associated with susceptibility to oral squamous cell carcinoma. J Oral Pathol Med. 2005;34(10):608–12.
Vairaktaris E, Yiannopoulos A, Vylliotis A, et al. Strong association of interleukin-6–174 G>C promoter polymorphism with increased risk of oral cancer. Int J Biol Markers. 2006;21(4):246–50.
Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705.
Taub DD, Oppenheim JJ. Chemokines, inflammation, and the immune system. Ther Immunol. 1994;1(4):229–46.
Chen JJ, Yao PL, Yuan A, et al. Upregulation of tumor interleukin-8 expression by infiltrating macrophages: its correlation with tumor angiogenesis and patient survival in non-small cell lung cancer. Clin Cancer Res. 2003;9(2):729–37.
Green AR, Green VL, White MC, et al. Expression of cytokine messenger RNA in normal and neoplastic human breast tissue: identification of interleukin-8 as a potential regulatory factor in breast tumors. Int J Cancer. 1997;72(6):937–41.
Kitadai Y, Haruma K, Sumii K, et al. Expression of interleukin-8 correlates with vascularity in human gastric carcinomas. Am J Pathol. 1998;152(1):93–100. PMCID: 1858127.
Akiba J, Yano H, Ogasawara S, et al. Expression and function of interleukin-8 in human hepatocellular carcinoma. Int J Oncol. 2001;18(2):257–64.
Mukaida N, Shiroo M, Matsushima K. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol. 1989;143(4):1366–71.
Hull J, Rowlands K, Lockhart E, et al. Haplotype mapping of the bronchiolitis susceptibility locus near IL8. Hum Genet. 2004;114(3):272–9.
Hull J, Thomson A, Kwiatkowski D. Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax. 2000;55(12):1023–7. PMCID: 1745668.
Ohyauchi M, Imatani A, Yonechi M, et al. The polymorphism interleukin 8–251 A/T influences the susceptibility of Helicobacter pylori-related gastric diseases in the Japanese population. Gut. 2005;54(3):330–5. PMCID: 1774396.
Vairaktaris E, Yapijakis C, Serefoglou Z, et al. The interleukin-8 (−251A/T) polymorphism is associated with increased risk for oral squamous cell carcinoma. Eur J Surg Oncol. 2007;33(4):504–7.
Campa D, Hashibe M, Zaridze D, et al. Association of common polymorphisms in inflammatory genes with risk of developing cancers of the upper aerodigestive tract. Cancer Causes Control. 2007;18(4):449–55.
Kietthubthew S, Wickliffe J, Sriplung H, et al. Association of polymorphisms in proinflammatory cytokine genes with the development of oral cancer in Southern Thailand. Int J Hyg Environ Health. 2010;213(2):146–52.
Thakkinstian A, McEvoy M, Minelli C, et al. Systematic review and meta-analysis of the association between {beta} 2-adrenoceptor polymorphisms and asthma: a HuGE review. Am J Epidemiol. 2005;162(3):201–11.
Guo J, Jin M, Zhang M, et al. A genetic variant in miR-196a2 increased digestive system cancer risks: a meta-analysis of 15 case–control studies. PLoS One. 2012;7(1):e30585. PMCID: 3265498.
Gao LB, Pan XM, Li LJ, et al. RAD51 135G/C polymorphism and breast cancer risk: a meta-analysis from 21 studies. Breast Cancer Res Treat. 2011;125(3):827–35.
Camargo MC, Mera R, Correa P, et al. Interleukin-1beta and interleukin-1 receptor antagonist gene polymorphisms and gastric cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev: Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2006;15(9):1674–87.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. PMCID: 192859.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–48.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Liu CM, Yeh CJ, Yu CC, et al. Impact of interleukin-8 gene polymorphisms and environmental factors on oral cancer susceptibility in Taiwan. Oral Dis. 2012;18(3):307–14.
Vairaktaris E, Yapijakis C, Serefoglou Z, et al. Gene expression polymorphisms of interleukins-1 beta, -4, -6, -8, -10, and tumor necrosis factors-alpha, -beta: regression analysis of their effect upon oral squamous cell carcinoma. J Cancer Res Clin Oncol. 2008;134(8):821–32.
Shimizu Y, Kondo S, Shirai A, et al. A single nucleotide polymorphism in the matrix metalloproteinase-1 and interleukin-8 gene promoter predicts poor prognosis in tongue cancer. Auris Nasus Larynx. 2008;35(3):381–9.
Hu YPLB, Su T, Cheng J, Zhao W, Yang HD. IL-8–251 single nucleotide polymorphism in the recurrence of squamous cell carcinoma of tongue. J Pract Stomatol. 2012;28(3):328–32.
Wang Z, Wang C, Zhao Z, et al. Association between -251A>T polymorphism in the interleukin-8 gene and oral cancer risk: a meta-analysis. Gene. 2013;522(2):168–76.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yang, L., Zhu, X., Liang, X. et al. Association of IL-8-251A>T polymorphisms with oral cancer risk: evidences from a meta-analysis. Tumor Biol. 35, 9211–9218 (2014). https://doi.org/10.1007/s13277-014-2193-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2193-5