Abstract
Oral cancer is a multifactorial disease process and involves complex interactions between gene to gene and gene to environmental factors. Interleukin 8 (IL-8), a pro-inflammatory cytokine, having angiogenic activity with elevated expression in tumor cells, is reported to play an essential role in oral cancer development. This study was conducted with the aim to investigate the role of IL-8 (-A251T) gene polymorphism in susceptibility, progression, and self-reporting pain in oral cancer. The single nucleotide polymorphisms of the IL-8 (-A251T) gene were screened in 300 patients with oral cancer and 300 healthy controls, by polymerase chain reaction-restriction fragment length polymorphism. Genotype and allele frequencies were evaluated by chi-square test and odds ratio (OR) with 95% confidence intervals (CIs) were used to evaluate the strength of associations. The results of the study demonstrated that IL-8 (-A251T) gene polymorphism was significantly associated with susceptibility of oral cancer, whereas its correlation with clinico-pathological status or pain due to oral cancer could not be established. The AT heterozygous (OR 5.31; CI 3.38–8.34; p 0.0001) and AA homozygous (OR 2.89; CI 1.76–4.75; p 0.0001) had a greater risk for oral cancer compared to TT homozygous. Furthermore, significantly increased values of A allele frequencies compared to T allele were observed in all patients (OR 1.56; CI 1.24–1.96; p 0.0002). Tobacco chewing and smoking were also found to influence the development of oral cancer and increased the incidence of pain in oral cancer patients. The findings of this study suggest that the IL-8 (-A251T) gene polymorphism may be associated with increased risk of oral cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral cancer is a frequently encountered malignant cancer in the head and neck region. It is the eight most common type of human malignancies worldwide, with more than 4,260,000 reported cases and ∼128,000 deaths every year (Mallath et al. 2014). In India, the incidence of oral cancer, according to available data, is about 1 million with 600,000–700,000 deaths reported per annum (Mallath et al. 2014). This malignancy is predominant in Indian males (Srivastava et al. 2010). Patients diagnosed with oral cancer have a particularly low 5-year survival rate due to the compounding factors of late detection and lack of effective therapies according to the “American Cancer Society” and the “Online Facts Page of The Oral Cancer Foundation.” Besides high mortality, oral cancer is often associated with difficulty in eating, speech impairment, and general psychological distress (Srivastava et al. 2010). Oral cancer has multistep progression which is influenced by several environmental factors, such as tobacco chewing, smoking, alcohol consumption, and alteration in genes, such as genetic tumor suppressor genes and oncogenes (Liu et al. 2012; Nagaraj et al. 2006). Several studies have observed that the common polymorphisms in angiogenesis, inflammation, and thrombosis-related genes are associated with increased risk for oral cancer as well as cancer-related pain (Dikshit et al. 2012; Reyes-Gibby et al. 2007, 2009).
IL-8 is a member of the chemokine family and is produced by a wide range of cell types like macrophages, neutrophils, epithelial cells, and endothelial cells (Watkins et al. 1995). The IL-8 gene is located on chromosome 4q13-3 at a proximal promoter region (Watkins et al. 1994). IL-8, one of key members of the human α-chemokine subfamily, has been associated with a wide variety of processes, including angiogenesis, tumorigenesis, adhesion, or metastasis of cancer (Li et al. 2009; Shimizu et al. 2008). Some previous studies have reported that the IL-8 (-251)A/T promoter gene polymorphism has significant influence on IL-8 production and is associated with higher risk of oral, prostate, breast, and colorectal cancers (Shimizu et al. 2008; Xue et al. 2012; Wang et al. 2013; McCarron et al. 2002). The frequency of mutant A allele ranges between 18 and 25% in Asians and Europeans (Landi et al. 2003; Savage et al. 2004; Huang et al. 2011). The increased levels of IL-8 have also been found in oral squamous cell carcinoma (OSCC) and ovarian cancers (Savage et al. 2004).
Cytokines have been reported to play a significant role in the pathogenesis of cancer-associated pain (Watanabe et al. 2002; Lokshin et al. 2006). Cytokines are released by activated glial cells in response to any inflammatory process or tissue damage (as in cancer). These cytokines have been reported to cause alterations in perception of cancer pain, either by modified activity of nociceptors or due to hyper-excitability of pain transmitting neurons (Reyes-Gibby et al. 2009; Oh et al. 2001). Therefore, we carried out the present study to evaluate the association of IL-8 (-251A/T) gene polymorphism with risk for oral cancer and its correlation with oral cancer pain.
Materials and Methods
Subjects
This case–control study included 300 diagnosed, previously treated/untreated and confirmed (histological/pathological) oral cancer patients with/without pain, who registered at the department of Surgical Oncology, King George’s Medical University, Lucknow. The control group comprised 300 healthy volunteers who either visited with patients or reported for the general health checkup to our medical university. This study was approved by Institutional Ethical Committee and an informed, written consent, to participate in the study, was obtained from each individual. The clinical information, including age, sex, tobacco chewing, smoking, alcohol consumption and TNM stage, was obtained from patients’ medical charts. “Self-Reported Pain” during the past week was assessed using an 11-point numeric scale (0, ‘‘no pain’’ and 10, ‘‘worst pain’’), whereas visual analog scale (VAS) was used for pain assessment.
Sample Collection and Genomic DNA Extraction
Blood samples were collected in EDTA tubes and genomic DNA was extracted from blood samples using the salting out method (Miller et al. 1988). The DNA was stored at −80°C, until analyzed.
Genotyping of Cytokine Gene Polymorphism
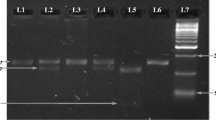
Genotyping of the IL-8 (-A251T) gene polymorphisms was performed by PCR–RFLP. The polymorphism -A251T was typed by using the primer pair F-5′-CCA TCA TGA TAG CAT CTG TA-3′ and R-5′-CCA CAA TTT GGT GAA TTA TTA A-3′. The PCR was carried out in a volume of 20 μl, consisting 40 ng genomic DNA. PCR reaction buffer contained 10 pmol/primers at concentration of 1X, 1X PCR master mixture. PCR amplification was carried out by an initial denaturation at 94°C for 5 min, followed by 35 cycles at 94°C for 30 s, at 59°C for 45 s and at 72°C for 30 s, with a final extension at 72°C for 7 min. After PCR, the product was digested with 1 unit of AseI restriction enzyme, incubated overnight at 37°C and yielded a product 152 + 21 bp (-251A) and 173 bp (-251T). After complete digestion, the fragments were resolved on a 2% agarose gel, stained with ethidium bromide (Fig. 1).
Statistical Analysis
The allele and genotype frequencies of IL-8 (-A251T) gene polymorphisms were compared between oral cancer patients and healthy controls, using the chi-square test. All the demographic and clinical data of the groups concerning age, gender, use of tobacco (either chewing or smoking), alcohol consumption, and clinico-pathological status were analyzed by Fisher’s exact test. Odds ratios (ORs) with 95% confidence interval (CI) were calculated to estimate the strength of association of IL-8 (-A251T) gene polymorphism to the risk of oral cancer. A p-value less than 0.05 was considered significant.
Results
The demographic profile included age, weight, height, gender, relative environmental risk factors, and tumor staging which may contribute to the progression of oral cancer. A total of 300 oral cancer patients [(Male = 202 (67.3%), Female = 98 (32.7%)] with mean age of 47.67 ± 12.67 years, height of 162.34 ± 5.97 cm, and weight of 56.32 ± 8.62 kg, and 300 healthy volunteers [(Male = 193 (64.3%), Female = 107 (35.7%)] with mean age of 43.03 ± 8.49 years, height of 163.25 ± 5.38 cm, and weight of 58.03 ± 7.32 kg were included in this study. The demographic profile of the oral cancer patients and controls is shown in Table 1. In this study, we also observed the influence of tobacco smoking (31.3, 20.7%), chewing (60.7, 46.3%), and alcohol consumption (14.7, 10.0%) in oral cancer patients and controls, respectively (Table 1). The tobacco smoking and chewing were significantly associated (p < 0.05), while alcohol consumption was not associated with oral cancer susceptibility. Oral cancer patients had tumor stages I, II, III, and IV of the disease in 8, 56.33, 33.33, and 2.33% cases, respectively. The tumor T status (≤T2: 70.67%, >T2: 29.33%), lymph node status (N0: 75% and N1 + N2: 25%), metastasis (M0: 98.67% and M1: 1.33%), and cell differentiated grades (grade 1: 10.33% and >grade 1: 89.67%) were also analyzed. Pain was present in 125 (41.7%) patients of oral cancer.
The genotype and allele frequencies of the IL-8 (-A251T) promoter region gene polymorphism among the oral cancer patients and controls are shown in Table 2. The frequencies of the AA, AT, and TT genotypes of IL-8 (-A251T) were 27.7, 37.0, and 35.3% in oral cancer, and 25.7, 63.0, and 11.3% in controls, respectively; on the other hand, the allele frequencies of the A and T were 46.2, 53.8% in oral cancer and 57.2, 42.8% in controls, respectively. There were significant differences in the homozygous AA and homozygous TT genotype (OR 2.89; CI 1.76–4.75; p 0.0001) and homozygous TT and heterozygous AT genotype (OR 5.31; CI 3.38–8.34; p 0.0001) among oral cancer patients and control group. The allele frequencies of the IL-8 (-A251T) gene polymorphism were also significantly correlated with oral cancer (OR 1.56; CI 1.24–1.96; p 0.0002).
We stratified the oral cancer patients into two categories: low-risk oral cancer and high-risk oral cancer groups. Low-risk oral cancer group comprised stage I + II, ≤T2, N0, M0, and grade 1, and high-risk group comprised patients with stage III + IV, > T2, N1 + N2, M1, and >grade 1. Low-risk oral cancer group was taken as reference (Table 3). The frequencies of AA, AT, and TT genotypes were 29.0, 36.3, 34.7% in stage (I + II), 28.4, 36.0, 35.6% in lymph node (N0) and 28.0, 37.2, 34.8 in metastasis (M0) in low-risk oral cancer group and were 25.2, 38.3, 36.5% in stage (III + IV), 25.3, 40.0, 34.7% in lymph node (N1 + N2) and 0.0, 25.0, 75.0% in metastasis (M1) in high-risk oral cancer group, respectively. The frequencies of AA, AT and TT genotypes and A and T allele of the IL-8 (-A251T) did not show significant difference among the two groups.
Associations between IL-8 (-A251T) gene polymorphism and oral cancer pain are shown in Table 4. The frequencies of AA, AT, and TT genotypes of the IL-8 (-A251T) were 27.2, 40.8, 32.0% in patients with painful oral cancer, whereas these were 28.0, 34.3, 37.7%, respectively, in patients with painless oral cancer. The frequencies of A, T allele were 47.6, 52.4% in oral cancer with pain and 45.1, 54.9% in oral cancer without pain. Hence, the frequencies of AA, AT, and TT genotypes and A, T allele of the IL-8 (-A251T) were also not significantly associated with oral cancer pain.
Associations between IL-8 (-A251T) promoter region genetic variations and exposure to related environmental factors on oral cancer susceptibility are shown in Table 5. There were significant differences in the homozygous AA and homozygous TT genotype frequencies of the IL-8 (-A251T) gene polymorphism between oral cancer patients and control groups in smokers (OR 4.63; CI 1.59–11.98; p 0.0057) and tobacco chewers (OR 3.80; CI 1.79–8.07; p 0.0006), whereas allele frequencies of the IL-8 (-A251T) gene polymorphism were also significantly correlated to smoking (OR 1.98; CI 1.24–3.17; p 0.006) and tobacco chewing habit (OR 1.65; CI 1.21–2.26; p 0.0022). The homozygous TT and heterozygous AT genotypes were also significantly different among smokers (OR 5.33; CI 1.937–14.684; p 0.001) and tobacco chewers (OR 8.16; CI 4.09–16.28; p 0.0001). The frequencies of the AA, AT, and TT genotypes and A, T allele of IL-8 (-A251T) were not significantly associated with alcohol consumption (Table 5).
Discussion
IL-8, a pro-inflammatory cytokine produced by tumor cells, macrophages, and other phagocytic cells, has been associated with various cancers due to its angiogenic nature, resulting in angiogenesis, thrombophilia, tumor growth, and metastasis. It also enhances cell proliferation and inhibits DNA repair, leading to DNA damage (Shimizu et al. 2008; Bidwell et al. 1999; Kim et al. 2001). This cytokine also play a crucial role in the cancer-related pain pathogenesis by modifying the function of nociceptors (Reyes-Gibby et al. 2009). The genetic mutations in IL-8 gene may be associated with over expression of IL-8 protein and increased susceptibility to and/or modulation of the risk for oral cancer (Shimizu et al. 2008; Bidwell et al. 1999).
In present study, the IL-8 (-A251T) gene polymorphism was found significantly associated with increased risk of oral cancer. The AA and AT genotype of IL-8 (-A251T) gene polymorphism was found to be significantly associated with increased risk for oral cancer. Moreover, the -251A/A genotype also showed a high risk for oral cancer. The findings of our study are supported by Wang et al. (2013) and Gao et al. (2010), who suggested that the AA and AT genotypes of IL-8 promoter (-251A/T) gene polymorphism were associated with increased risk of oral cancer. Some recent studies have reported the A allele of IL-8 promoter (-251)A/T gene polymorphism to be associated with increase in production of IL-8 and suggested that this may modulate the susceptibility to various malignancies in humans, including oral cancer (Savage et al. 2004; Hull et al. 2000; Kietthubthew et al. 2010; Lee et al. 2005). Contrary to our findings, some previous studies have reported that the IL-8 (-A251T) gene polymorphism was not associated with some cancers such as cervical cancer (Liu et al. 2012), colorectal cancers (Wang et al. 2012), gastric cancer (Savage et al. 2006), and lung cancer (Campa et al. 2005). We did not find any correlation between IL-8 (-A251T) gene polymorphisms and progression of oral cancer. Similarly, Liu et al. (2012) reported that the IL-8 gene polymorphism was not significantly associated with progression of oral cancer (Liu et al. 2012). Contrary to our results, Rafrafi et al. (2013) and Gu and Yang (2011) found that the A allele of IL-8 (-A251T) gene polymorphism was significantly associated with clinico-pathological status, including clinical stage, lymph node, and metastasis of oral cancer.
It may be pointed out that, ours is the first study on the association of IL-8 (-A251T) gene polymorphism with oral cancer-related pain in north Indian population. The actual molecular mechanism by which cytokines influence pain has not yet been established. In the present study, IL-8 gene polymorphisms did not influence the severity of oral cancer pain. Reyes-Gibby et al. (2007, 2009) observed that the -251T/A genotype in the promoter of the IL-8 gene, associated with altered gene expression, influences pain severity in cancer patients. Another study suggested that cytokines, released during tissue damage or inflammation, change the activity of nociceptors, contributing to pain hypersensitivity (Li et al. 2001). IL-8 may produce enhanced sensitivity to pain through actions on cytokine receptors, expressed by nociceptive neurons (Oh et al. 2001). Increased levels of IL-8 are found in patients with chronic pain conditions such back pain, post-herpetic neuralgia, and unstable angina (Kotani et al. 2004). The -251A allele has been reported to be associated with increased IL-8 levels and with severe pain in colorectal adenoma, gastric cancer, and NSCLC (Wang et al. 2012; Gunter et al. 2006). The present study suggests that inflammation-related gene polymorphism does not modulate the severity of oral cancer pain.
In our study, we also explored the correlation of cytokine gene polymorphism in oral cancer patients and controls, with environmental risk factors. The betel chewing, smoking, and alcohol consumptions are well-established factors associated with the risk of oral cancer (Liu et al. 2012). We observed a strong association of AT and AA genotype of IL-8 (-A251T) with increased risk of oral cancer as compared with controls, in smokers and tobacco chewers. The A allele of IL-8 (-A251T) was also associated with an increased risk of oral cancer in smokers and tobacco chewers. Cheng et al. (2000) observed that the consumption of tobacco and betel nut can initiate the production of IL-8 in oral cancer patients. The betel nut may increase the production of c-fos and c-jun proto-oncogene protein levels, whereas tobacco increases nuclear HIF-1a protein level in oral cancer (Lin et al. 2008).
Conclusion
The present study examined the possible association between oral cancer risk and polymorphism of IL-8 (-A251T) gene. The results indicated that the homozygous AA and heterozygous AT genotype of IL-8 (-A251T) gene are significantly associated with increased risk of oral cancer. The A allele of IL-8 -251 polymorphism is also associated with increased risk for oral cancer. This may be due to the higher expression levels of IL-8 protein. In smokers and tobacco chewers, the genotype of IL-8 (-A251T) gene was associated with increased risk of oral cancer. Our results did not indicate any significant correlation between IL-8 (-A251T) gene polymorphisms and the occurrence of pain in oral cancer patients. There was no correlation between this polymorphism and clinico-pathological status of oral cancer or its associated pain. The results of this study suggest that the IL-8 gene may contribute to an inherited predisposition to oral cancer.
References
Bidwell J, Keen L, Gallagher G et al (1999) Cytokine gene polymorphism in human disease: on-line databases. Genes Immun 1:3–19
Campa D, Hung RJ, Mates D et al (2005) Lack of association between 251 T > A polymorphism of IL8 and lung cancer risk. Cancer Epidemiol Biomarkers Prev 14:2457–2458
Cheng YA, Shiue LF, Yu HS, Hsieh TY, Tsai CC (2000) Interleukin-8 secretion by cultured oral epidermoid carcinoma cells induced with nicotine and/or arecoline treatments. Kaohsiung J Med Sci 16:126–133
Dikshit R, Gupta PC, Ramasundarahettige C, Gajalakshmi V, Aleksandrowicz L, Badwe R et al (2012) Cancer mortality in India: a nationally representative survey. Lancet 379:1807–1816
Gao LB, Pan XM, Jia J et al (2010) IL-8 -251A/T polymorphism is associated with decreased cancer risk among population based studies: evidence from a meta-analysis. Eur J Cancer 46:1333–1343
Gu WL, Yang JJ (2011) Expression and clinical significance of serum interleukin-8 level in patients with oral squamous cell carcinoma. Shanghai Kou Qiang Yi Xue 20:78–81
Gunter MJ, Canzian F, Landi S, Chanock SJ, Sinha R, Rothman N (2006) Inflammation-related gene polymorphisms and colorectal adenoma. Cancer Epidemiol Biomarkers Prev 15:1126–1131
Huang Q, Wang C, Qiu LJ, Shao F, Yu JH (2011) IL-8-251A > T polymorphism is associated with breast cancer risk: a meta-analysis. J Cancer Res Clin Oncol 137:1147–1150
Hull J, Thomson A, Kwiatkowski D (2000) Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax 55:1023–1027
Kietthubthew S, Wickliffe J, Sriplung H, Ishida T, Chonmaitree T, Au WW (2010) Association of polymorphisms in proinflammatory cytokine genes with the development of oral cancer in Southern Thailand. Int J Hyg Environ Health 213:146–152
Kim SJ, Uehara H, Karashima T, McCarty M, Shih N, Fidler IJ (2001) Expression of interleukin-8 correlates with angiogenesis, tumorigenicity, and metastasis of human prostate cancer cells implanted orthotopically in nude mice. Neoplasia 3:33–42
Kotani N, Kudo R, Sakurai Y et al (2004) Cerebrospinal fluid interleukin 8 concentrations and the subsequent development of postherpetic neuralgia. Am J Med 116:318–324
Landi S, Moreno V, Gioia-Patricola L et al (2003) Association of common polymorphisms in inflammatory genes interleukin (IL)6, IL8, tumor necrosis factor alpha, NFKB1, and peroxisome proliferator-activated receptor gamma with colorectal cancer. Cancer Res 63:3560–3566
Lee WP, Tai DI, Lan KH et al (2005) The -251T allele of the interleukin-8 promoter is associated with increased risk of gastric carcinoma featuring diffuse-type histopathology in Chinese population. Clin Cancer Res 11:6431–6441
Li A, Varney ML, Singh RK (2001) Expression of interleukin 8 and its receptors in human colon carcinoma cells with different metastatic potentials. Clin Cancer Res 7:3298–3304
Li K, Yao S, Liu S, Wang B, Mao D (2009) Genetic polymorphisms of interleukin 8 and risk of ulcerative colitis in the Chinese population. Clinica Chimica Acta 405:30–34
Lin PY, Yu CH, Wang JT, Chen HH, Cheng SJ, Kuo MY, Chiang CP (2008) Expression of hypoxiainducible factor-1 alpha is significantly associated with the progression and prognosis of oral squamous cell carcinomas in Taiwan. J Oral Pathol Med 37:18–25
Liu CM, Yeh CJ, Yu CC et al (2012) Impact of interleukin-8 gene polymorphisms and environmental factors on oral cancer susceptibility in Taiwan. Oral Dis 18:307–314
Lokshin AE, Winans M, Landsittel D et al (2006) Circulating IL-8 and anti- IL-8 autoantibody in patients with ovarian cancer. Gynecol Oncol 102:244–251
Mallath MK, Taylor DG, Badwe RA, Rath GK, Shanta V, Pramesh CS et al (2014) The growing burden of cancer in India: epidemiology and social context. Lancet Oncol 15:e205–e212
McCarron SL, Edwards S, Evans PR et al (2002) Influence of cytokine gene polymorphisms on the development of prostate cancer. Cancer Res 62:3369–3372
Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215
Nagaraj NS, Beckers S, Mensah JK, Waigel S, Vigneswaran N, Zacharias W (2006) Cigarette smoke condensate induces cytochromes P450 and aldo-keto reductases in oral cancer cells. Toxicol Lett 165:182–194
Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ (2001) Chemokines and glycoprotein 120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci 21:5027–5035
Rafrafi A, Chahed B, Kaabachi S (2013) Association of IL-8 gene polymorphisms with non small cell lung cancer in Tunisia: a case control study. Human Immunol 74:1368–1374
Reyes-Gibby CC, Spitz M, Wu X et al (2007) Cytokine genes and pain severity in lung cancer: exploring the influence of TNF-{alpha}-308 G/A IL6–174G/C and IL8–251T/A. Cancer Epidemiol Biomarkers Prev 16:2745–2751
Reyes-Gibby CC, Shete S, Yennurajalingam S et al (2009) Genetic and non genetic covariates of pain severity in patients with adenocarcinoma of the pancreas: assessing the influence of cytokine genes. J Pain Symptom Manag 38:894–902
Savage SA, Abnet CC, Mark SD et al (2004) Variants of the IL8 and IL8RB genes and risk for gastric cardia adenocarcinoma and esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 13:2251–2257
Savage SA, Hou L, Lissowska J, Chow WH, Zatonski W, Chanock SJ, Yeager M (2006) Interleukin-8 polymorphisms are not associated with gastric cancer risk in a Polish population. Cancer Epidemiol Biomarkers Prev 15:589–591
Shimizu Y, Kondo S, Shirai A, Furukawa M, Yoshizaki T (2008) A single nucleotide polymorphism in the matrix metalloproteinase-1 and interleukin-8 gene promoter predicts poor prognosis in tongue cancer. Auris Nasus Larynx 35:381–389
Srivastava S, Salim N, Robertson MJ (2010) Interleukin-18: biology and role in the immunotherapy of cancer. Curr Med Chem 17:3353–3357
Wang N, Zhou R, Wang C, Guo X, Chen Z, Yang S, Li Y (2012) 251 T/A polymorphism of the interleukin-8 gene and cancer risk: a huge review and meta-analysis based on 42 case–control studies. Mol Biol Rep 39:2831–2841
Wang Z, Wang C, Zhao Z, Liu F, Guan X, Lin X, Zhang L (2013) Association between -251A > T polymorphism in the interleukin-8 gene and oral cancer risk: a meta-analysis. Gene 522:168–176
Watanabe H, Iwase M, Ohashi M, Nagumo M (2002) Role of interleukin-8 secreted from human oral squamous cell carcinoma cell lines. Oral Oncol 38:670679
Watkins LR, Wiertelak EP, Goehler LE, Smith KP, Martin D, Maier SF (1994) Characterization of cytokine-induced hyperalgesia. Brain Res 654:15–26
Watkins LR, Maier SF, Goehler LE (1995) Immune activation: the role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain 63:289–302
Xue H, Liu J, Lin B, Wang Z, Sun J, Huang G (2012) A meta-analysis of interleukin-8 -251 promoter polymorphism associated with gastric cancer risk. PLoS One 7:e28083
Acknowledgments
This work was financially supported by a grant from Indian Council of Medical Research (ICMR) (No: 3/2/2/131/2012/NCD-III).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interests in this article.
Rights and permissions
About this article
Cite this article
Singh, P.K., Chandra, G., Bogra, J. et al. Association of Genetic Polymorphism in the Interleukin-8 Gene with Risk of Oral Cancer and Its Correlation with Pain. Biochem Genet 54, 95–106 (2016). https://doi.org/10.1007/s10528-015-9704-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-015-9704-y