Abstract
Gallbladder carcinoma is a highly aggressive cancer with female predominance. Interindividual differences in the effectiveness of the activation/detoxification of environmental carcinogens and endogenous estrogens may play a crucial role in cancer susceptibility. The present study included 410 patients with carcinoma of the gallbladder (GBC) and 230 healthy subjects. This study examined association of CYP1A1-MspI, CYP1A1-Ile462Val, and CYP1B1-Val432Leu with GBC susceptibility. CYP1A1-MspI [CC] and CYP1A1-Ile462Val [iso/val] genotypes were found to be significantly associated with GBC (p = 0.006 and p = 0.03, respectively), as compared to healthy controls, while CYP1B1-Val432Leu was not associated with GBC. The CYP1A1 haplotype [C-val] showed a significant association with GBC (p = 0.006). On stratification based on gender, the CYP1A1-MspI [CC] genotype showed an increased risk of GBC in females (p = 0.018). In case-only analysis, tobacco users with CYP1A1-MspI [CT] genotypes were at a higher risk of GBC (p = 0.008). Subdividing the GBC patients on the basis of gallstone status, the CYP1A1 haplotype [C-val] imparted a higher risk in patients without stones when compared to controls (p = 0.001). The results remained significant even after applying Bonferroni correction. Multivariate analysis revealed an increased risk of CYP1A1 iso/val and val/val genotypes in GBC patients having BMI >25 (p = 0.021). The CYP1A1 polymorphisms may confer increased risk of GBC, probably due to impaired xenobiotic or hormone metabolism through a gallstone-independent pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carcinoma of the gallbladder (GBC) is the most widespread biliary tract malignancy across the world with one of the highest incidence and mortality rates in northern India (21.5/100,000) [1, 2]. Being a multifaceted disease, numerous genetic variants along with diverse environmental and nutritional factors may act together possibly as disease modifiers. The identification of these risk sets of genetic variants will aid in understanding the pathobiology of the disease.
Epidemiologic studies provide evidence about an individual’s susceptibility to cancer modulation by both genetic as well as environmental factors. Xenobiotic-metabolizing enzymes act as the first line of defense against environmental carcinogens, and most of the cancers arise as a consequence of contact to these carcinogens [3]. The genetic differences in the effectiveness of the activation/detoxification of carcinogens play a vital role in an individual’s susceptibility towards cancer.
The human cytochrome P450 1A1 (CYP1A1) gene, which encodes aryl hydrocarbon hydroxylase, is a phase I enzyme and participates in the metabolism of xenobiotics, i.e., activation of tobacco-related procarcinogens, such as polycyclic aromatic hydrocarbons, nitrosamines, and aromatic amines [4], and a small number of endogenous substrates such as estrogens [5, 6]. Among the different reactions catalyzed by CYP1A1, hydroxylation at a vacant position of an aromatic ring is a well-thought-out hallmark for the initiation of carcinogenesis, through the formation of highly reactive conversion products that can cause oncogenic mutations in experimental animals and humans [7, 8].
A functional genetic variant of CYP1A1 (Ile462Val, rs1048943) polymorphism is located in exon 7 in the heme-binding region of the CYP1A1 gene, near the active site of the enzyme [9]. Another variant, T>C transition (rs4646903), so-called MspI, polymorphism is located in the 3′ noncoding region [10]. Both the polymorphisms are found to contribute towards increased enzymatic activity of CYP1A1 [11]. CYP1B1 is the main cytochrome P450 responsible for the extrahepatic 4-hydroxylation [12]. In the CYP1B1 gene, a nonsynonymous polymorphism, Val432Leu, is caused by a substitution of valine to leucine at codon 432 in exon 3 and is also linked to a higher catalytic activity [13]. Several studies have shown CYP1A1-Ile462Val (rs1048943), CYP1A1-MspI (rs4646903), and CYP1B1-Val432Leu (rs1056836) to be associated with various cancers, including hormone-related cancers [14, 15]. Previously, we had reported CYP1A1-MspI (rs4646903) polymorphism to be associated with tobacco-related risk of GBC but in a smaller sample size [15].
Given the potential role of these genes in various cancers, the present study was planned to evaluate the functional genetic variants in CYP1A1 and CYP1B1 on the risk of gallbladder carcinoma in a large cohort from North India.
Material and methods
Study subjects
The current case-control study included 640 subjects comprising 410 consecutive newly diagnosed GB cancer patients (fine needle aspiration cytology and histopathologically proven), recruited from the clinics of the Department of Surgical Oncology, King George Medical University (KGMU), Lucknow, and the Department of Surgical Gastroenterology, Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), Lucknow, and 230 healthy controls belonging to same ethnicity. Staging of cancer was documented according to the AJCC/UICC staging [16]. The healthy controls were recruited from unrelated individuals of general population. Inclusion criteria for controls were absence of prior history of cancer, precancerous lesions, and absence of gallstones proven by ultrasonography and were frequency-matched to cancer cases on age, gender, and ethnicity. After obtaining informed consent, the subjects were personally interviewed for information on food habits, occupation, and tobacco usage. Ethics approval for the work was granted by the local ethics committee of the institute. The study protocol was approved by the institutional ethical committees of both the institutes, and the authors followed the norms of the World Medical Association’s Declaration of Helsinki. All the participants gave written informed consent for the study.
Genotyping
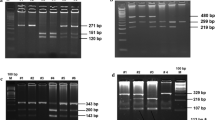
Genomic DNA was isolated from peripheral blood leukocytes according to a standard salting-out method. The polymorphisms were genotyped using the polymerase chain reaction (PCR)-restriction fragment length polymorphism method. As a negative control, PCR mix without DNA sample was used to ensure a contamination-free PCR product. Polymorphisms in CYP1A1 and CYP1B1 were determined by using the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method as described [17]. The digested PCR fragments were separated on polyacrylamide gel, stained with ethidium bromide, and observed under ultraviolet light. Genotyping was performed without knowledge of the case or control status. Ten percent of samples for each genotype were sequenced which showed 100 % concordance. Among control subjects, genotype frequencies for each SNP were examined for deviation from the Hardy-Weinberg equilibrium (HWE) using the χ 2 test.
Statistical analysis
The sample size was calculated using QUANTO 1.1, using minor allele frequency data from HapMap (http://hapmap.ncbi.nlm.nih.gov/). Gene-gene and gene-environment interactions were estimated by the logistic regression model, which included an interaction term as well as variables for genotypes (CYP1A1 and CYP1B1) and potential confounders (age and gender). Statistical analysis was done using SPSS statistical analysis software, version 16.0 (SPSS, Chicago, IL, USA). Statistical analysis of the haplotype estimation and linkage disequilibrium was conducted using the SNPstats software (http://bioinfo.iconcologia.net/snpstats/start.htm). For multiple comparisons, Bonferroni correction was applied. The p-corrected value, i.e., p < 0.025, was taken as significant for subgroup analysis on gender stratification and gallstone status stratification.
Results
Characteristic profile of the study subjects
Among 410 GBC cases and 230 controls, the mean age was 52.32 ± 10.6 and 43.87 ± 11.57 years, respectively. Genomic control method ruled out the possibility of population stratification in our study. Most of the GBC patients were in advanced stages of cancer (stage III and stage IV). In GBC cases, 25 (6 %) had stage II adenocarcinomas, 180 (44.0 %) stage III, and 205 (50 %) stage IV. Among GBC, 31 % of the cases were tobacco users and 37 % of the cases had early age of onset, i.e., <50 years. Gallstones were present in 53 % of GBC. Characteristics of GBC patients and age- and sex-matched controls are given in supplementary Table S1. Several other characteristics of GBC patients are given in supplementary Table S2.
Distribution of studied polymorphisms in controls
The distribution of CYP1A1-MspI (rs4646903), CYP1A1-Ile462Val (rs1048943), and CYP1B1-Val432Leu (rs1056836) polymorphism is shown in Table 1. The observed genotype frequencies of all the studied polymorphisms in controls were in accordance with the Hardy-Weinberg equilibrium (p > 0.05).
Association of CYP1A1-MspI, CYP1A1-Ile462Val, and CYP1B1-Val432Leu polymorphisms with gallbladder cancer
Table 1 shows the risk of gallbladder cancer in relation to each of the SNPs of CYP1A1-MspI, CYP1A1-Ile462Val, and CYP1B1-Val432Leu. On comparing the genotype frequency of CYP1A1-MspI in GBC patients with that of controls, the homozygous variants [CC] and [C] allele showed a statistically significant risk with GBC (p = 0.006; odds ratio (OR) = 3.4; p = 0.003; OR = 1.67). Similarly, the CYP1A1-Ile462Val heterozygous variant [iso/val] genotype and [val] allele also showed a statistically significant risk of developing GBC (p = 0.03; OR = 1.6; p = 0.04; OR = 1.5). On the contrary, no significant difference was observed in the distribution of CYP1B1-Val432Leu polymorphism in any groups both at genotype and allele levels.
Haplotype analysis of CYP1A1-MspI and CYP1A1-Ile462Val in case and control groups
The analysis revealed that the haplotype [C-val] of CYP1A1 was significantly associated with GBC risk (p = 0.006; OR = 2.5). Global haplotype analysis indicated a significant difference between cases and controls based on the distribution pattern of the four haplotypes (p = 0.012) (Table 2).
Association of CYP1A1-MspI, CYP1A1-Ile462Val, and CYP1B1-Val432Leu polymorphisms with gallbladder cancer on stratification on gender
Table 3 shows the risk of gallbladder cancer in relation to each of the SNPs of CYP1A1-Ile462Val, CYP1A1-MspI, and CYP1B1-Val432Leu on the basis of gender. On comparing the genotype frequency distribution of CYP1A1-MspI in GBC female patients with that in female controls, the homozygous variants [CC] and [C] allele showed a statistically significant risk in female patients (p = 0.006; OR = 4.0; p = 0.02; OR = 1.5) and the risk was borderline in the case of males at the allelic level (p = 0.04; OR = 1.9). The results were significant after Bonferroni correction in the female group. On the contrary, no significant differences were observed in the distribution of CYP1A1-Ile462Val and CYP1B1-Val432Leu polymorphisms in any groups both at genotype and allele levels, for developing GBC.
Modulation of risk in the presence or absence of gallstones in GBC patients with CYP1A1 polymorphisms and risk haplotype
Since gallstones were present in 53 % of GBC patients, the cancer cases were stratified on the basis of gallstones. The homozygous variants [CC] and [C] allele of CYP1A1-MspI showed a statistically significant risk in patients without stones (p = 0.001; OR = 4.7; p = 0.001; OR = 1.9) as compared to healthy controls. Similarly, the CYP1A1-Ile462Val heterozygous variant [iso/val] genotype and [val] allele showed a statistically significant risk for GBC among patients without stones (p = 0.01; OR = 1.9; p = 0.01; OR = 1.7). On the contrary, no significant differences were observed in the distribution of CYP1B1-Val432Leu polymorphism in any groups both at genotype and allele levels (Table 4). The haplotype [C-val] of CYP1A1 was significantly associated with GBC risk in patients without stones (p = 0.001; OR = 3.37). The results remained significant after Bonferroni correction. Global haplotype analysis indicated significant differences between cases and controls based on the distribution pattern of the four haplotypes (p = 0.0027). On the contrary, no significant differences were observed in the distribution of the four haplotypes in patients with gallstones as shown in Table 5.
CYP1A1-MspI, CYP1A1-Ile462Val, and CYP1B1-Val432Leu polymorphisms and interaction with tobacco usage, age of onset, diet, and BMI
Because tobacco is a leading risk factor for most cancers, we also stratified our data by tobacco usage in a case-only analysis to explore the modulation of risk for GBC. Genotypes TC and C allele of CYP1A1-MspI were significantly associated with GBC risk in tobacco users (p = 0.008; OR = 1.91; p = 0.004; OR = 1.6) (Table 6), whereas no associations were observed in the other two SNPs.
We also stratified our GBC patients by age of onset, early (<50) versus late (>50), in a case-only analysis to explore the modulation of GBC risk. However, none of the genotypes of the studied polymorphisms showed a statistically significant association with GBC (data not shown). CYP1A1-MspI polymorphism was associated with intake of nonvegetarian diet (OR = 9.1; p = 0.05) (supplementary Table S3). Multivariate analysis revealed an increased risk of the iso/val and val/val genotypes of CYP1A1 with obesity (OR = 6.4; p = 0.021). There were no additional associations between multiparity, earlier age at menarche, higher age at menopause, etc. (data not shown).
Discussion
CYP genes code for major families of cytosolic and endoplasmic reticulum enzymes which catalyze the activation and detoxification of reactive electrophilic compounds including most of the environmental carcinogens (e.g., benzo[a]pyrene). CYP1A1 is a phase I enzyme that regulates the metabolic activation of majority of procarcinogens present in tobacco smoke, for example, aromatic amines and PAHs [4], and the enzyme is also involved in hydroxylation of estrogens [5, 6]. In this case-control study of gallbladder cancer patients, we investigated associations of functionally relevant genetic variants in two genes (CYP1A1, CYP1B1) encoding key proteins of the xenobiotic metabolic pathway with GBC risk. Our results showed CYP1A1-MspI and CYP1A1-Ile462Val (rs1048943) genetic variations to be significantly associated with predisposition to GBC, but no association was found for CYP1B1-Val432Leu polymorphism in conferring risk for GBC. Subgroup analysis revealed association of CYP1A1-MspI polymorphism with overall female cancer patients as well as GBC patients without stones.
In previous studies, CYP1A1-Ile462Val polymorphism was reported to be associated with an increased risk of GBC in patients from Chile [18], Hungary [19], and Japan [20]. However, in a study by Park et al. from China, CYP1A1-Ile462Val was negatively associated with gallbladder cancer, whereas CYP1A1 IVS1+606G>T (rs2606345) conferred an increased risk of GBC [21]. CYP1A1 polymorphisms have also been reported to be conferring risk of several cancers such as cervical [22], gastric [23], breast [24, 25], and ovarian [26] cancers. These conflicting reports might be due to environmental factors and ethnicity differences. It is also possible that CYP1A1 polymorphisms are in linkage disequilibrium with other functional variations which can influence the results in different populations.
There is only one study on CYP1B1 Leu432Val in gallbladder cancer till now with null association [21]. We also found no association of this polymorphism with GBC risk. Recent meta-analysis studies showed inconsistent association of the CYP1B1 Leu432Val polymorphism with endometrial [27], colorectal [28], lung [29], and breast [30] cancers.
Gallbladder cancer is often associated with cholelithiasis and cholecystitis [31, 32], and 53 % of patients in the present study had associated gallstones and the rest 47 % patients were without gallstones. On stratification of GBC patients on the basis of presence/absence of stones, variant genotypes of CYP1A1-MspI and CYP1A1-Ile462Val polymorphisms were associated with an increased risk of GBC in the patient group without stones. Haplotype analysis also showed an increased risk to GBC in carriers of the risk haplotype [C-val] and in the patient group without stones on subgroup analysis. Previous results of our lab also showed association of CYP1A1-MspI polymorphism with GBC patients without stones. Similarly, Park et al. [21] also found no association of CYP1A1 polymorphisms with gallstone disease in the Chinese population. This might be due to the fact that only a small fraction of patients with cholelithiasis (1–3 %) develop GBC [33], and approximately 53.0 % of patients with GBC in our study were free of gallstones. It is believed that gallbladder cancer involves two separate pathways, one involving gallstones and the other which is gallstone independent. Gallstone-independent effect may be due to alteration in xenobiotic metabolism by CYP1A1 genetic variants. Ours as well as the results of Park et al. [21] suggest that the CYP1A1-related risk of GBC may not involve gallstones as an intermediary step. There are some studies which indicate that people with porcelain gallbladder have a high risk of developing gallbladder cancer [34]. However, in our study, none of our patients were found to have associated porcelain gallbladder; therefore, modulation of GBC risk due to porcelain gallbladder was not studied.
CYP1A1 has been shown to be involved in the activation of tobacco-related N-nitrosamines, [35] which provoke cancer in experimental animals. The procarcinogens require activation by hydroxylation at α-position carbon of the N-nitroso group, a reaction catalyzed by CYPs [36]. The CYP1A1-Ile462Val (rs1048943) and CYP1A1-MspI (rs4646903) are associated with the elevated enzyme activity [11, 37]. The CYP1A1-Ile462Val occurs in the heme-binding motif of the protein, and benzo[a]pyrene (constituent of cigarette smoke and tobacco) metabolism studies have reported an almost twofold increase in variant protein (valine) enzyme activity than that of the wild-type (isoleucine) protein [38]. Ile/Val variant encoding the DNA of CYP1A1 when transfected into a yeast cell expression system, benzo[a]pyrene mutagenic potency, and aromatic hydrocarbon hydroxylase activity were increased [38]. In the present study, CYP1A1-MspI (rs4646903) polymorphism is associated with an increased risk of GBC in tobacco users in case-only analysis. Previous results of our lab in a smaller sample size also showed an increased risk of tobacco-related risk of gallbladder cancer in the North Indian population [15]. Hence, genetic differences in activation/detoxification of carcinogens may be playing an essential role in individual susceptibility towards GBC due to increased carcinogenic intermediates. CYP1A1-MspI polymorphism has also been shown to be associated with head and neck cancer susceptibility in North Indians [39]. A recent study by Lourenco et al. reported association of CYP1A1-MspI polymorphism with tobacco as well as alcohol intake in conferring risk of head and neck squamous cell carcinoma [40].
In addition to the effect of CYP1A1 on xenobiotic metabolism, the encoded enzyme is involved in estrogen metabolism. Results from functional studies suggested that both the val variant of CYP1A1-Ile462Val and the C variant of CYP1A1 may be associated with increased catalytic activity towards estrogens [11, 41], i.e., the presence of these variants may well result in increased production of catechol estrogens and estrogen quinones. Estrogen quinones can react directly with DNA and form both stable adducts and depurinating adducts that can generate mutations [42–44]. These estrogen-derived DNA adducts have been identified in human breast tissue [45]. The elevated conversion of estradiol to 2-OH-E2 has been detected in several tissues, including biliary epithelium [46], and has been linked with biliary tract cancers by causing decreased gallbladder motility, resulting in increased inflammation and infection in the biliary tract [47, 48]. GBC is predominately detected in females and associated with obesity and multiple pregnancies, conditions associated to higher levels of estrogens. It suggests that endogenous estrogens may be involved in disease pathogenesis by altering bile acid composition and gallbladder motility. This study found very significant associations of CYP1A1-MspI polymorphism in female cancer patients.
It is well known that the total risk of gallbladder disease increases with increasing body mass index probably because circulating levels of endogenous estradiol are higher in overweight and obese women. Our results of multivariate analysis showed significant associations of CYP1A1-Ile462Val in GBC cases with obesity (BMI >25 kg/m2). Park et al. also found that CYP1A1 IVS1+606-associated risk of GBC was more pronounced among subjects having BMI >23 kg/m2 [21]. A possible reason may be that obese subjects tend to have lower levels of sex hormone-binding globulin (SHBG) and hence increased levels of bioavailable estradiol [49, 50]. We did not find additional associations between multiparity, earlier age at menarche, higher age at menopause, etc. with GBC risk. Therefore, genes related to estrogen metabolism may also play an important role in gallbladder carcinogenesis. The association of GBC risk in nonvegetarian diets may also be related to CYP1A1-MspI polymorphism. However, this observation requires validation in a larger sample size.
This study has several strengths and limitations. All controls were under Hardy-Weinberg equilibrium; all patients were histopathologically confirmed, age-matched, and gender ethnicity-matched subjects; and strict quality control for genotyping was followed. The sample size in the present study is sufficient to yield 80 % power. Bonferroni correction was applied in subgroup analysis. However, like the majority of association studies, there is a need to replicate this study in other populations for definitive associations with gallbladder carcinogenesis.
Conclusion
Our study showed that CYP1A1 polymorphisms may confer increased risk of gallbladder cancer, through impaired xenobiotic or hormone metabolism in a gallstone-independent pathway.
References
Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118:1591–602.
Lazcano-Ponce EC, Miquel JF, Munoz N, Herrero R, Ferrecio C, Wistuba II, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51:349–64.
Smith G, Stanley LA, Sim E, Strange RC, Wolf CR. Metabolic polymorphisms and cancer susceptibility. Cancer Surv. 1995;25:27–65.
Guengerich FP, Shimada T. Activation of procarcinogens by human cytochrome P450 enzymes. Mutat Res. 1998;400:201–13.
Spink DC, Eugster HP, Lincoln 2nd DW, Schuetz JD, Schuetz EG, Johnson JA, et al. 17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1A1: a comparison of the activities induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in MCF-7 cells with those from heterologous expression of the cDNA. Arch Biochem Biophys. 1992;293:342–8.
Corradini SG, Elisei W, Giovannelli L, Ripani C, Della Guardia P, Corsi A, et al. Impaired human gallbladder lipid absorption in cholesterol gallstone disease and its effect on cholesterol solubility in bile. Gastroenterology. 2000;118:912–20.
Hunter DJ, Riboli E, Haiman CA, Albanes D, Altshuler D, Chanock SJ, et al. A candidate gene approach to searching for low-penetrance breast and prostate cancer genes. Nat Rev Cancer. 2005;5:977–85.
Pharoah PD, Dunning AM, Ponder BA, Easton DF. Association studies for finding cancer-susceptibility genetic variants. Nat Rev Cancer. 2004;4:850–60.
Hayashi SI, Watanabe J, Nakachi K, Kawajiri K. PCR detection of an A/G polymorphism within exon 7 of the CYP1A1 gene. Nucleic Acids Res. 1991;19:4797.
Spurr NK, Gough AC, Stevenson K, Wolf CR. Msp-1 polymorphism detected with a cDNA probe for the P-450 I family on chromosome 15. Nucleic Acids Res. 1987;15:5901.
Kisselev P, Schunck WH, Roots I, Schwarz D. Association of CYP1A1 polymorphisms with differential metabolic activation of 17beta-estradiol and estrone. Cancer Res. 2005;65:2972–8.
Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR. 17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad Sci U S A. 1996;93:9776–81.
Li DN, Seidel A, Pritchard MP, Wolf CR, Friedberg T. Polymorphisms in P450 CYP1B1 affect the conversion of estradiol to the potentially carcinogenic metabolite 4-hydroxyestradiol. Pharmacogenetics. 2000;10:343–53.
Shen Y, Li DK, Wu J, Zhang Z, Gao E. Joint effects of the CYP1A1 MspI, ERalpha PvuII, and ERalpha XbaI polymorphisms on the risk of breast cancer: results from a population-based case-control study in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2006;15:342–7.
Pandey SN, Choudhuri G, Mittal B. Association of CYP1A1 Msp1 polymorphism with tobacco-related risk of gallbladder cancer in a north Indian population. Eur J Cancer Prev. 2008;17:77–81.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4.
Yuan X, Zhou G, Zhai Y, Xie W, Cui Y, Cao J, et al. Lack of association between the functional polymorphisms in the estrogen-metabolizing genes and risk for hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:3621–7.
Tsuchiya Y, Baez S, Calvo A, Pruyas M, Nakamura K, Kiyohara C, et al. Evidence that genetic variants of metabolic detoxication and cell cycle control are not related to gallbladder cancer risk in Chilean women. Int J Biol Markers. 2010;25:75–8.
Kimura A, Tsuchiya Y, Lang I, Zoltan S, Nakadaira H, Ajioka Y, et al. Effect of genetic predisposition on the risk of gallbladder cancer in Hungary. Asian Pac J Cancer Prev. 2008;9:391–6.
Tsuchiya Y, Kiyohara C, Sato T, Nakamura K, Kimura A, Yamamoto M. Polymorphisms of cytochrome P450 1A1, glutathione S-transferase class mu, and tumour protein p53 genes and the risk of developing gallbladder cancer in Japanese. Clin Biochem. 2007;40:881–6.
Park SK, Andreotti G, Sakoda LC, Gao YT, Rashid A, Chen J, et al. Variants in hormone-related genes and the risk of biliary tract cancers and stones: a population-based study in China. Carcinogenesis. 2009;30:606–14.
Sergentanis TN, Economopoulos KP, Choussein S, Vlahos NF. Cytochrome P450 1A1 (CYP1A1) gene polymorphisms and cervical cancer risk: a meta-analysis. Mol Biol Rep. 2012;39:6647–54.
Guo R, Guo X. Quantitative assessment of the associations between CYP1A1 polymorphisms and gastric cancer risk. Tumour Biol. 2012;33(4):1125–32.
Kiruthiga PV, Kannan MR, Saraswathi C, Pandian SK, Devi KP. CYP1A1 gene polymorphisms: lack of association with breast cancer susceptibility in the southern region (Madurai) of India. Asian Pac J Cancer Prev. 2011;12:2133–8.
Shin A, Kang D, Choi JY, Lee KM, Park SK, Noh DY, et al. Cytochrome P450 1A1 (CYP1A1) polymorphisms and breast cancer risk in Korean women. Exp Mol Med. 2007;39:361–6.
Huang M, Chen Q, Xiao J, Zhao X, Liu C. CYP1A1 Ile462Val is a risk factor for ovarian cancer development. Cytokine. 2012;58:73–8.
Wang F, Zou YF, Sun GP, Su H, Huang F. Association of CYP1B1 gene polymorphisms with susceptibility to endometrial cancer: a meta-analysis. Eur J Cancer Prev. 2011;20:112–20.
Xie Y, Liu GQ, Miao XY, Liu Y, Zhou W, Zhong DW. CYP1B1 Leu432Val polymorphism and colorectal cancer risk among Caucasians: a meta-analysis. Tumour Biol. 2012;33:809–16.
Xu W, Zhou Y, Hang X, Shen D. Current evidence on the relationship between CYP1B1 polymorphisms and lung cancer risk: a meta-analysis. Mol Biol Rep. 2012;39:2821–9.
Sillanpaa P, Heikinheimo L, Kataja V, Eskelinen M, Kosma VM, Uusitupa M, et al. CYP1A1 and CYP1B1 genetic polymorphisms, smoking and breast cancer risk in a Finnish Caucasian population. Breast Cancer Res Treat. 2007;104:287–97.
Mumford JL, Li X, Hu F, Lu XB, Chuang JC. Human exposure and dosimetry of polycyclic aromatic hydrocarbons in urine from Xuan Wei, China with high lung cancer mortality associated with exposure to unvented coal smoke. Carcinogenesis. 1995;16:3031–6.
Lowenfels AB, Maisonneuve P, Boyle P, Zatonski WA. Epidemiology of gallbladder cancer. Hepatogastroenterology. 1999;46:1529–32.
Misra S, Chaturvedi A, Misra NC, Sharma ID. Carcinoma of the gallbladder. Lancet Oncol. 2003;4:167–76.
Stephen AE, Berger DL. Carcinoma in the porcelain gallbladder: a relationship revisited. Surgery. 2001;129:699–703.
Fujita K, Kamataki T. Predicting the mutagenicity of tobacco-related N-nitrosamines in humans using 11 strains of Salmonella typhimurium YG7108, each coexpressing a form of human cytochrome P450 along with NADPH-cytochrome P450 reductase. Environ Mol Mutagen. 2001;38:339–46.
Fujita K, Kamataki T. Role of human cytochrome P450 (CYP) in the metabolic activation of N-alkylnitrosamines: application of genetically engineered Salmonella typhimurium YG7108 expressing each form of CYP together with human NADPH-cytochrome P450 reductase. Mutat Res. 2001;483:35–41.
Crofts F, Taioli E, Trachman J, Cosma GN, Currie D, Toniolo P, et al. Functional significance of different human CYP1A1 genotypes. Carcinogenesis. 1994;15:2961–3.
Kawajiri K, Nakachi K, Imai K, Watanabe J, Hayashi S. The CYP1A1 gene and cancer susceptibility. Crit Rev Oncol Hematol. 1993;14:77–87.
Sharma R, Ahuja M, Panda NK, Khullar M. Interactions among genetic variants in tobacco metabolizing genes and smoking are associated with head and neck cancer susceptibility in North Indians. DNA Cell Biol. 2011;30:611–6.
Lourenco GJ, Silva EF, Rinck-Junior JA, Chone CT, Lima CS. CYP1A1, GSTM1 and GSTT1 polymorphisms, tobacco and alcohol status and risk of head and neck squamous cell carcinoma. Tumour Biol. 2011;32:1209–15.
Landi MT, Bertazzi PA, Shields PG, Clark G, Lucier GW, Garte SJ, et al. Association between CYP1A1 genotype, mRNA expression and enzymatic activity in humans. Pharmacogenetics. 1994;4:242–6.
Zhang Y, Gaikwad NW, Olson K, Zahid M, Cavalieri EL, Rogan EG. Cytochrome P450 isoforms catalyze formation of catechol estrogen quinones that react with DNA. Metabolism. 2007;56:887–94.
Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19:1–27.
Cavalieri E, Rogan E, Chakravarti D. The role of endogenous catechol quinones in the initiation of cancer and neurodegenerative diseases. Methods Enzymol. 2004;382:293–319.
Markushin Y, Zhong W, Cavalieri EL, Rogan EG, Small GJ, Yeung ES, et al. Spectral characterization of catechol estrogen quinone (CEQ)-derived DNA adducts and their identification in human breast tissue extract. Chem Res Toxicol. 2003;16:1107–17.
Cosma G, Crofts F, Taioli E, Toniolo P, Garte S. Relationship between genotype and function of the human CYP1A1 gene. J Toxicol Environ Health. 1993;40:309–16.
Kritz-Silverstein D, Barrett-Connor E, Wingard DL. The relationship between reproductive history and cholecystectomy in older women. J Clin Epidemiol. 1990;43:687–92.
Everson GT, McKinley C, Kern Jr F. Mechanisms of gallstone formation in women. Effects of exogenous estrogen (Premarin) and dietary cholesterol on hepatic lipid metabolism. J Clin Invest. 1991;87:237–46.
Pasquali R, Vicennati V, Gambineri A. Adrenal and gonadal function in obesity. J Endocrinol Invest. 2002;25:893–8.
Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril. 2006;85:1319–40.
Acknowledgments
The study was supported by financial support from ICMR, DBT, and DST, Government of India. Funders had no role in the study design, data collection, analysis of data, and writing of the manuscript.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 75 kb)
Rights and permissions
About this article
Cite this article
Sharma, K.L., Agarwal, A., Misra, S. et al. Association of genetic variants of xenobiotic and estrogen metabolism pathway (CYP1A1 and CYP1B1) with gallbladder cancer susceptibility. Tumor Biol. 35, 5431–5439 (2014). https://doi.org/10.1007/s13277-014-1708-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-1708-4