Abstract
Studies investigating the association between cytochrome P450 1B1 (CYP1B1) Leu432Val (432 C/G, rs1056836) polymorphism and colorectal cancer (CRC) risk report conflicting results. The aim of this study was to quantitatively summarize the evidence for such a relationship. Two investigators independently searched the Medline, Embase, China National Knowledge Infrastructure, and Chinese Biomedicine Databases. Summary odds ratios (ORs) and 95% confidence intervals (95% CIs) for CYP1B1 polymorphism and CRC were calculated in a fixed-effects model and a random-effects model when appropriate. The pooled ORs were performed for co-dominant model (GG vs. CC, GC vs. CC), dominant model (GG + GC vs. CC), and recessive model (GG vs. GC + CC). This meta-analysis included ten case–control studies, which included 8,466 CRC cases and 9,301 controls. Overall, the variant genotypes (GG and GC) of the 432 C/G were not associated with CRC risk when compared with the wild-type CC homozygote (GG vs. CC, OR = 1.01, 95% CI = 0.93–1.10; GC vs. CC, OR = 0.97, 95% CI = 0.90–1.04), without any between-study heterogeneity. Similarly, no associations were found in the dominant and recessive models (dominant model, OR = 0.98, 95% CI = 0.92–1.05; recessive model, OR = 1.03, 95% CI = 0.96–1.11). Limiting the analysis to the studies within Hardy–Weinberg equilibrium, the results were persistent and robust. When stratifying for country, matched control and source of controls, no evidence of significant association was observed in any subgroup. No publication bias was found in the present study. No association is found between the CYP1B1 Leu432Val polymorphism and risk of CRC among Caucasians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is one of the most common forms of cancer and is the third leading cause of cancer-related death worldwide [1]. In 2010, an estimated 142,570 new cases will be diagnosed and 51,370 deaths will occur [1]. CRC is a serious problem for public health in many countries. However, the mechanism of colorectal carcinogenesis is still not fully understood. As with other complex diseases, CRC is caused by both genetic and environmental factors [2]. Twin study indicates that about 35% of all colorectal cancer can be ascribed to inherited genetic susceptibility [2]. Because well-recognized genetic predisposition syndromes account for less than 3% of colorectal cancer, low-penetrance genetic factors alone or in combination with environmental factors probably contribute to colorectal cancer development [3].
Cytochromes P450 (CYPs) are the most important enzymes involved in the phase I of biotransformation. CYPs catalyze a large number of reactions modifying dietary and smoking-derived pre-carcinogens and participate in the metabolism of endogenous compounds including hormones and bile acids [4]. Cytochrome P450 1B1 (CYP1B1) is a key P450 enzyme implicated in the metabolism of exogenous and endogenous substrates [5]. A variety of studies have demonstrated that the metabolism of polycyclic aromatic hydrocarbons and other procarcinogens through CYP1B1 may well lead to the activation of the carcinogenic compounds [6, 7].
CYP1B1 gene is located on chr2p22–p21, and there are at least 179 different polymorphism sites in the gene (http:// ncbi.nlm.nih.gov/dbSNP). Of the most studied single-nucleotide polymorphisms, four are reported to result in amino acid substitutions, and they are rs10012 (Arg48Gly), rs1056827 (Ala119Ser), rs1056836 (Leu432Val), and rs1800440 (Asn453Ser). Importantly, these polymorphic variants have been associated with enhanced catalytic activity when compared to the wild-type allele [8, 9]; it has been postulated that this functional finding may confer susceptibility toward cancer at a certain extent [9].
To date, no prospective studies have been done to evaluate the relationship between the CYP1B1 polymorphisms and the risk of CRC. A number of case–control studies were conducted to investigate the association between CYP1B1 Leu432Val (432 C/G, rs1056836) polymorphism and CRC risk in humans. However, the results of these studies are confusing rather than conclusive. No quantitative summary of the evidence has ever been performed. The purpose of this meta-analysis was to quantitatively summarize the evidence for such a relationship.
Materials and methods
Publication search
We searched the PubMed, Embase, China National Knowledge Infrastructure, and Chinese Biomedicine databases for all articles on the association between CYP1B1 polymorphisms and CRC risk (last search update 1 October 2011). The following keywords were used: “colorectal” or “colo*,” “cancer” or “tumor” or “carcinoma,” “CYP1B1” or “cytochrome P450 1B1,” and “polymorphism” or “variant.” The search was without restriction on language, conducted on human subject. The reference lists of reviews and retrieved articles were hand-searched at the same time. We did not consider abstracts or unpublished reports. If more than one article was published by the same author using the same case series, we selected the study where the most individuals were investigated.
Inclusion and exclusion criteria
We reviewed abstracts of all citations and retrieved studies. The following criteria were used to include published studies: (a) case–control studies were conducted to evaluate the association between CYP1B1 Leu432Val polymorphism and CRC risk, (b) sufficient genotype data were presented to calculate the odds ratios (ORs) and 95% confidence intervals (CIs), and (c) the paper should clearly describe CRC diagnoses and the sources of cases and controls. Major reasons for exclusion of studies were (a) no control, (b) duplicate, and (c) no sufficient data were reported.
Data extraction
Two investigators (Yong X and Guo-Qing L) extracted information from all eligible publications independently according to the inclusion criteria listed above. Disagreements were resolved by discussion between the two investigators. The following characteristics were collected from each study: first author, year of publication, country of the first or corresponding author, ethnicity, number of cases and controls, study design, genotyping methods, matching variables, minor allele frequency in controls, and evidence of Hardy–Weinberg equilibrium (HWE) (Table 1).
Statistical analysis
We first assessed HWE in the controls for each study using goodness-of-fit test (chi-square or Fisher’s exact test) and a P < 0.05 was considered as significant disequilibrium. The strength of the association between CRC and the CYP1B1 Leu432Val polymorphism was estimated using ORs, with the corresponding 95% CIs. The pooled ORs were performed for co-dominant model (GG vs. CC, GC vs. CC), dominant model (GG + GC vs. CC), and recessive model (GG vs. GC + CC). We also carried out the stratified analyses by country, matched control (yes/no), HWE in controls (yes/no), and source of controls.
Both the Cochran’s Q statistic [10] to test for heterogeneity and the I 2 statistic to quantify the proportion of the total variation due to heterogeneity [11] were calculated. A P value of more than the nominal level of 0.10 for the Q statistic indicated a lack of heterogeneity across studies, allowing for the use of a fixed-effects model (the Mantel–Haenszel method) [12]; otherwise, the random-effects model (the DerSimonian and Laird method) was used [13]. Sensitivity analysis was performed to assess the stability of results.
Several methods were used to assess the potential publication bias. Visual inspection of funnel plot asymmetry was conducted. The Begg’s rank correlation method [14] and the Egger’s weighted regression method [15] were used to statistically assess publication bias (P < 0.05 was considered statistically significant). All analyses were done using STATA software, version 11.0 (STATA Corp., College Station, TX, USA). All the P values were two-sided.
Results
Characteristics of studies
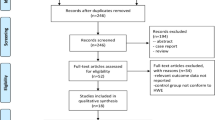
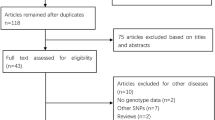
We identified 65 relevant studies when searched the databases. Fifteen publications described the association between CYP1B1 polymorphism and CRC, and their full text were retrieved and carefully studied. Finally, a total of ten eligible studies involving 8,466 cases and 9,301 controls were included in the pooled analyses [3, 16–24]. The characteristics of selected studies are summarized in Table 1. There were ten studies of Caucasian patients, no study of African patients and Asian patients. Studies had been carried out in UK, Spain, Canada, Czech, Polish, German, and France. The cases definition used in the individual studies were pathologically or histologically diagnosed with CRC. Controls were mainly healthy populations and matched for age and/or sex, of which seven were population-based and three were hospital-based. The distribution of genotypes in the controls of all studies was in agreement with HWE except for two studies [19, 22].
Quantitative synthesis
Table 2 listed the main results of this meta-analysis, and Fig. 1 showed the association of CRC risk with CYP1B1 Leu432Val polymorphism. Overall, the variant genotypes (GG and GC) of the 432 C/G were not associated with CRC risk when compared with the wild-type CC homozygote (GG vs. CC, OR = 1.01, 95% CI = 0.93–1.10; GC vs. CC, OR = 0.97, 95% CI = 0.90–1.04), without any between-study heterogeneity. Similarly, no associations were observed in the dominant and recessive models (dominant model, OR = 0.98, 95% CI = 0.92–1.05; recessive model, OR = 1.03, 95% CI = 0.96–1.11).
Forest plots of ORs with 95% CIs for CYP1B1 Leu432Val polymorphism and risk for CRC. The center of each square represents the OR, the area of the square is the number of sample and thus the weight used in the meta-analysis, and the horizontal line indicates the 95% CI a GG vs. CC, b GC vs. CC, c GG + GC vs. CC, and d GG vs. GC + CC
On the basis of the potential underestimation of the true effect of the polymorphism on the CRC risk, we stratified these studies according to country, matched control, source of controls, and HWE in controls. Different countries were categorized as UK, Canada, and others. Different source of controls were defined as hospital-based case–control (HCC) and population-based case–control (PCC). In stratified analyses, the variant genotypes (GG and GC) had no significant relationship with CRC in all of the subgroups, compared with wild-type. The similar results were observed in the recessive model and the dominant model (Table 2).
Heterogeneity and sensitivity analyses
Significant heterogeneity between studies was not observed in overall comparisons and main subgroup analyses. In the sensitivity analysis, the influence of each study on the pooled OR was examined by repeating the meta-analysis while omitting each study, one at a time. This procedure confirmed the stability of our overall results. In addition, when excluding the studies that were not in HWE, the results were persistent and robust (Table 2).
Publication bias
Funnel plot, Begg’s and Egger’s tests were performed to evaluate publication bias of the literature on CRC. Figure 2 displayed a funnel plot that examined the CYP1B1 Leu432Val polymorphism and overall CRC risk included in the meta-analysis in the heterozygous comparison. The shape of funnel plots did not reveal any evidence of funnel plot asymmetry. The statistical results still did not show publication bias (GG vs. CC: Begg’s test P = 0.28, Egger’s test P = 0.58; GC vs. CC: Begg’s test P = 1.00, Egger’s test P = 0.91; dominant model: Begg’s test P = 0.59, Egger’s test P = 0.61; recessive model: Begg’s test P = 0.47, Egger’s test P = 0.68).
Discussion
Xenobiotic clearance is important for the removal of carcinogens and is primarily accomplished by hydroxyl conjugation, involving enzymes in the cytochrome P450 pathway [25]. Cancers of the colon, lung, larynx, kidney, and pancreas have been shown to be associated with environmental exposures to various carcinogens [26, 27], and polymorphisms in several key enzymes involved in xenobiotic clearance have been linked to the risks of various cancers. One enzyme of particular importance is CYP1B1, which is primarily involved in the hydroxylation of 17 β-estradiol at the 2-OH and 4-OH positions [25]. CYP1B1 is encoded by a polymorphic gene [27], and a number of polymorphisms in this gene have been shown to affect the activity of the encoded protein [9, 28]. In recent years, a number of molecular epidemiologic studies have been conducted to evaluate the role of Leu432Val polymorphisms in the CYP1B1 gene on CRC risk; however, the results remain conflicting rather than conclusive [3, 16–24]. Therefore, a systematic review and meta-analysis of association between CYP1B1 Leu432Val polymorphism and CRC risk was of great value.
The present meta-analysis, including 8,466 cases and 9,301 controls from ten case–control studies, explored the association between the Leu432Val polymorphism of the CYP1B1 gene and CRC risk. To the best of our knowledge, this is the first meta-analysis of the comprehensive assessment for the relationship between CYP1B1 Leu432Val polymorphism and the risk of CRC. Overall, we did not find any significant association between CYP1B1 Leu432Val polymorphism and CRC susceptibility among Caucasians (all populations were Caucasians). In the stratified analysis by country, matched control, HWE in controls, and sources of controls, significant associations were still not observed in all genetic models. Our finding is in accordance with one previously published meta-analysis on breast cancer by Yao et al. [29]. They suggest that their meta-analysis provides strong evidence that CYP1B1 Val432Leu polymorphism is not associated with breast cancer risk. However, Wang et al. [30] found that CYP1B1 gene L432V polymorphism was associated with a significantly increased risk of endometrial cancer; Xu et al. [31] found that CYP1B1 432 C/G polymorphism was associated with risk of lung cancer. Meanwhile, they indicated that individuals with 432GG genotype had a 39.7% higher risk of having lung cancer than those with the 432CC genotype and individuals with the 432 G allele had a 26.3% increased risk as well. Although the reasons for this apparent difference in risk with different tumors are as yet unknown, some possibilities should be considered. First, those gene–variant associations vary in different kinds of cancer and may result from the different mechanisms of carcinogenesis among different kinds of cancer. Second, different ethnic composition may contribute to the discrepancy. Different meta-analyses included different original studies which were performed in different races, and the ethnic composition in different meta-analyses may be diversity. For example, original studies included in this meta-analysis were all performed in Caucasian populations.
Results of meta-analyses often depend on control selection procedures [32]. Different controls source may be a confounding factor which may impact on the conclusion of our study because of case–control studies. For instance, some studies used a healthy population as the reference group, whereas others selected inpatients without CRC as the reference group. In order to eliminate interference from the confounding factor, we performed subgroup analysis by source of controls. Our results showed that there was no significant association between CYP1B1 Leu432Val polymorphism and CRC risk in different controls (HCC and PCC), which confirmed the reliability of our overall results.
One of the major concerns in a sound meta-analysis is the degree of heterogeneity that exists between the component studies because non-homogeneous data are liable to result in misleading results. In the present study, the Q test and I 2 statistics were carried out to test the significance of heterogeneity. Obvious heterogeneity between studies was not observed in overall comparisons and main subgroup analyses. Another important issue for any meta-analysis is publication bias due to selective publication of reports. In the current study, funnel plot, Begg’s and Egger’s tests were performed to evaluate this problem. Both the shape of funnel plots and statistical results did not show publication bias.
However, there are still some limitations in this meta-analysis. First, in this meta-analysis, ten studies were all conducted in Caucasian population, and the results of the meta-analysis suggest that there is no association between the CYP1B1 432 G variant and CRC susceptibility, mainly in Caucasian population. Second, another limitation of this analysis is that we did not have original data and we therefore were not able to take into account other factors, like obesity, inflammation, aspirin/NSAID use, vitamin D, and vitamin E intake that may modify the risk estimates, as reported in previous publications. Thus, assessment of the association between CYP1B1 polymorphism and these covariates and CRC is needed in order to determine clearly the impact of CYP1B1 polymorphism on the etiology of CRC. Third, the genotype distribution in control group showed deviation from HWE in two studies.
In conclusion, this meta-analysis reveals that CYP1B1 Leu432Val polymorphism is not associated with altered susceptibility to CRC among Caucasians. Since no study was from non-Caucasian population, it is critical that larger and well-designed multicentric studies based on Asian and African-American patients should be performed to re-evaluate the association.
Abbreviations
- CRC:
-
colorectal cancer
- OR:
-
odds ratio
- CI:
-
confidence interval
- CYP1B1:
-
cytochrome P450 1B1
- SNP:
-
single nucleotide polymorphisms
- HWE:
-
Hardy–Weinberg equilibrium
References
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300.
Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85.
Cleary SP, Cotterchio M, Shi E, Gallinger S, Harper P. Cigarette smoking, genetic variants in carcinogen-metabolizing enzymes, and colorectal cancer risk. Am J Epidemiol. 2010;172:1000–14.
Boursi B, Arber N. Current and future clinical strategies in colon cancer prevention and the emerging role of chemo-prevention. Curr Pharm Des. 2007;13:2274–82.
Paracchini V, Raimondi S, Gram IT, Kang D, Kocabas NA, Kristensen VN, et al. Meta- and pooled analyses of the cytochrome P-450 1B1 Val432Leu polymorphism and breast cancer: a HuGE-GSEC review. Am J Epidemiol. 2007;165:115–25.
Shimada T, Oda Y, Gillam EM, Guengerich FP, Inoue K. Metabolic activation of polycyclic aromatic hydrocarbons and other procarcinogens by cytochromes P450 1A1 and P450 1B1 allelic variants and other human cytochromes P450 in Salmonella typhimurium NM2009. Drug Metab Dispos. 2001;29:1176–82.
Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 2004;95:1–6.
Hanna IH, Dawling S, Roodi N, Guengerich FP, Parl FF. Cytochrome P450 1B1 (CYP1B1) pharmacogenetics: association of polymorphisms with functional differences in estrogen hydroxylation activity. Cancer Res. 2000;60:3440–4.
Shimada T, Watanabe J, Kawajiri K, Sutter TR, Guengerich FP, Gillam EM, et al. Catalytic properties of polymorphic human cytochrome P450 1B1 variants. Carcinogenesis. 1999;20:1607–13.
Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Egger M, DaveySmith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Rudolph A, Sainz J, Hein R, Hoffmeister M, Frank B, Försti A, et al. Modification of menopausal hormone therapy-associated colorectal cancer risk by polymorphisms in sex steroid signaling, metabolism and transport related genes. Endocr Relat Cancer. 2011;18:371–84.
Hlavata I, Vrana D, Smerhovsky Z, Pardini B, Naccarati A, Vodicka P, et al. Association between exposure-relevant polymorphisms in CYP1B1, EPHX1, NQO1, GSTM1, GSTP1 and GSTT1 and risk of colorectal cancer in a Czech population. Oncol Rep. 2010;24:1347–53.
Trubicka J, Grabowska-Kłujszo E, Suchy J, Masojć B, Serrano-Fernandez P, Kurzawski G, et al. Variant alleles of the CYP1B1 gene are associated with colorectal cancer susceptibility. BMC Cancer. 2010;10:420.
Northwood EL, Elliott F, Forman D, Barrett JH, Wilkie MJ, Carey FA, et al. Polymorphisms in xenobiotic metabolizing enzymes and diet influence colorectal adenoma risk. Pharmacogenet Genomics. 2010;20:315–26.
Cotterchio M, Boucher BA, Manno M, Gallinger S, Okey AB, Harper PA. Red meat intake, doneness, polymorphisms in genes that encode carcinogen-metabolizing enzymes, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:3098–107.
Küry S, Buecher B, Robiou-du-Pont S, Scoul C, Sébille V, Colman H, et al. Combinations of cytochrome P450 gene polymorphisms enhancing the risk for sporadic colorectal cancer related to red meat consumption. Cancer Epidemiol Biomarkers Prev. 2007;16:1460–7.
Bethke L, Webb E, Sellick G, Rudd M, Penegar S, Withey L, et al. Polymorphisms in the cytochrome P450 genes CYP1A2, CYP1B1, CYP3A4, CYP3A5, CYP11A1, CYP17A1, CYP19A1 and colorectal cancer risk. BMC Cancer. 2007;7:123.
Landi S, Gemignani F, Moreno V, Gioia-Patricola L, Chabrier A, Guino E, Navarro M, de Oca J, Capellà G, Canzian F, Bellvitge Colorectal Cancer Study Group. A comprehensive analysis of phase I and phase II metabolism gene polymorphisms and risk of colorectal cancer. Pharmacogenet Genomics. 2005;15:535–46.
Sachse C, Smith G, Wilkie MJ, Barrett JH, Waxman R, Sullivan F, Forman D, Bishop DT, Wolf CR, Colorectal Cancer Study Group. A pharmacogenetic study to investigate the role of dietary carcinogens in the etiology of colorectal cancer. Carcinogenesis. 2002;23:1839–49.
Spink DC, Hayes CL, Young NR, et al. The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on estrogen metabolism in MCF-7 breast cancer cells: evidence for induction of a novel 17 beta-estradiol 4-hydroxylase. J Steroid Biochem Mol Biol. 1994;51:251–8.
Landi MT, Bergen AW, Baccarelli A, et al. CYP1A1 and CYP1B1 genotypes, haplotypes, and TCDD-induced gene expression in subjects from Seveso, Italy. Toxicology. 2005;207:191–202.
Agundez JA. Cytochrome P450 gene polymorphism and cancer. Curr Drug Metab. 2004;5:211–24.
Bailey LR, Roodi N, Dupont WD, et al. Association of cytochrome P450 1B1 (CYP1B1) polymorphism with steroid receptor status in breast cancer. Cancer Res. 1998;58:5038–41.
Yao L, Fang F, Wu Q, Zhong Y, Yu L. No association between CYP1B1 Val432Leu polymorphism and breast cancer risk: a meta-analysis involving 40,303 subjects. Breast Cancer Res Treat. 2010;122:237–42.
Wang F, Zou YF, Sun GP, Su H, Huang F. Association of CYP1B1 gene polymorphisms with susceptibility to endometrial cancer: a meta-analysis. Eur J Cancer Prev. 2011;20:112–20.
Xu W, Zhou Y, Hang X, Shen D. Current evidence on the relationship between CYP1B1 polymorphisms and lung cancer risk: a meta-analysis. Mol Biol Rep. 2011.
Benhamou S, Lee WJ, Alexandrie AK, et al. Meta- and pooled analyses of the effects of glutathione S-transferase M1 polymorphisms and smoking on lung cancer risk. Carcinogenesis. 2002;23:1343–50.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, Y., Liu, GQ., Miao, XY. et al. CYP1B1 Leu432Val polymorphism and colorectal cancer risk among Caucasians: a meta-analysis. Tumor Biol. 33, 809–816 (2012). https://doi.org/10.1007/s13277-011-0298-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-011-0298-7