Abstract
The vitamin D receptor (VDR) is a crucial mediator for the cellular effects of vitamin D. The polymorphisms in the VDR gene have been hypothesized to alter the risk of prostate cancer. However, studies investigating the association between VDR polymorphisms (BsmI and FokI) and prostate cancer (PCa) risk report conflicting results , therefore, we conducted a meta-analysis to re-examine the controversy. Published literatures from PubMed, Embase, Google Scholar, and China National Knowledge Infrastructure (CNKI) were searched (updated to March 9, 2013). According to our inclusion criteria, studies that observed the association between VDR BsmI and FokI polymorphisms and PCa risk were included. The principal outcome measure was the odds ratio (OR) with 95 % confidence interval (CI) for PCa risk associated with VDR BsmI and FokI polymorphisms. Thirty-four studies involving 10,267 cases and 11,489 controls were recruited. Overall, we did not find evidence to support an association between any of the VDR polymorphisms and PCa risk. For BsmI, the pooled OR was 0.894 (95 % CI 0.773 to 1.034) for the Bb vs. bb genotypes, 1.002 (95 % CI 0.869 to 1.157) for the BB vs. bb genotypes, 0.922 (95 % CI 0.798 to 1.065) for the dominant model (BB/Bb vs. bb), and 1.018 (95 % CI 0.936 to 1.107) for the recessive model (BB vs. Bb/bb). ORs for the FokI polymorphisms were similar. The results suggest that the VDR BsmI and FokI polymorphisms are not related to PCa risk. Further large and well-designed studies are required to confirm this conclusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is now thought to be one of the most important medical problems in the male population [1]. In European countries, it is recognized as the most common solid neoplasm, with an incidence rate of 214 cases per 1,000 men, outnumbering lung and colorectal cancer [2]. However, the etiology of PCa remains unclear. Biological and epidemiological data suggest that the development of PCa is a multiphase process. So far, a series environmental and lifestyle factors, including pollutants, smoking habit, and diet, as well as geographical and racial factors, have been pointed out as possible contributors to the risk of PCa [3]. In addition, the various risk, incidence, and mortality rates of PCa worldwide suggest that genetic factors also play an important role in PCa initiation and progression [4]. Therefore, the occurrence and development of PCa most likely involve a complex interplay between genetic and environmental factors.
Low levels of vitamin D are hypothesized to be a risk factor for PCa [5]. Experiments have shown that 1,25(OH)2D3, which is the active form of vitamin D, inhibits the proliferation of epithelial cells derived from normal and malignant prostatic tissues [6], and retards the growth of human PCa cell lines [7]. The antiproliferative effects of 1,25(OH)2D3 are thought to be mediated through a pathway involving vitamin D receptor (VDR) [8]. Normal and malignant prostatic epithelial cells have VDRs that bind 1,25(OH)2D3. The VDR gene is located on chromosome 12q12–q14 and several single-nucleotide polymorphisms (SNPs) have been identified that may influence cancer risk [9]. The most frequently studied SNPs are the restriction fragment length polymorphisms FokI (rs2228570) and BsmI (rs1544410), as defined by the endonucleases FokI and BsmI, respectively. The FokI located in the coding region of the VDR gene, results in the production of a VDR protein that is three amino acids longer, which display lower potency than the shorter one [10]. It has been hypothesized that a less active VDR could be associated with either an increased susceptibility to cancer risk or to a more aggressive disease. The BsmI is intronic and located at the 3′ end of the gene. BsmI is strongly linked with a poly (A) microsatellite repeat in the 3′ untranslated region, which may influence VDR messenger RNA stability [11].
In the past years, these two polymorphisms have attracted widespread attention. A number of case–control studies were conducted to investigate the association of variants in the VDR gene and the risk of PCa. However, these studies reported conflicting results. Moreover, three meta-analyses have reported conflicting results. In 2003, Ntais et al. [12] found no statistically significant association between the FokI and BsmI polymorphisms and PCa risk, and in a meta-analysis performed by Berndt et al. [13] that included only 17 studies; the VDR FokI and BsmI polymorphisms were found to not correlate with PCa risk. However, Raimondi et al. [9] noted a decreased risk of PCa for VDR BsmI polymorphism carriers in a meta-analysis.
A single study may not be sufficient to delete a small effect of the polymorphisms on PCa. This is particularly the case when relatively small sample sizes are used. Various types of study populations and study designs may also have contributed to these disparate findings. Hence, an updated meta-analysis based on a total of 34 studies was performed, which may provide the most comprehensive evidence for association of VDR FokI and BsmI polymorphisms with PCa risk.
Materials and methods
Publication search
We searched for studies in the PubMed, Embase, Web of Science, and CNKI (China National Knowledge Infrastructure) electronic databases to include in this meta-analysis, using the terms ‘VDR’, ‘Vitamin D’, ‘polymorphism’, ‘allele’, ‘genetics’ and ‘PCa’. An upper date limit of March 9, 2013 was applied and no lower date limit was used. The search was performed without any restrictions on language and focused on studies conducted in humans. We also reviewed the Cochrane Library for relevant articles. Concurrently, the reference lists of reviews and retrieved articles were searched manually. When the same patient populations appeared in several publications, only the most recent or complete study was included in the meta-analysis.
Inclusion criteria
For inclusion, the studies must have met the following criteria: they (1) evaluated the VDR gene polymorphisms and PCa risk; (2) were case–control studies or nested case–control study; (3) supplied the number of individual genotypes for the VDR FokI and BsmI polymorphisms in PCa cases and controls, respectively. Case-only studies and studies with incomplete data for the control groups were excluded. Studies using men with benign prostatic hyperplasia (BPH) as controls were included because FokI and BsmI polymorphisms in the VDR gene do not appear to be associated with BPH. Pedigree and family-based studies were excluded because these studies are generally linkage studies or family-based transmission disequilibrium studies.
Data extraction
Information was extracted carefully from all eligible publications independently by two authors, based on the inclusion criteria above. Disagreements were resolved through a discussion between the two authors.
The following data were collected from each study: first author’s surname, year of publication, study location, ethnicity, source of controls, laboratory methods to detect VDR polymorphisms, number of cases and controls and P value for Hardy–Weinberg Equilibrium (HWE). If data from any category were not reported in the primary study, the items were designated “not applicable”. We did not contact the author of the primary study to request the information. Ethnic groups were mainly defined as Caucasian, Asian, and African-American. Study designs were stratified into three groups: population-based studies, hospital-based studies, and BPH-based studies. We did not require a minimum number of patients for a study to be included in our meta-analysis.
Statistical analysis
Odds ratios (ORs) with 95 % confidence interval (CIs) were used to determine the strength of the association between the VDR FokI and BsmI polymorphisms and the risk of PCa. For each polymorphism, we estimated the association with PCa risk under certain genotypic models, namely codominant (or robust), additive, recessive, and dominant. Since the reference group for each polymorphism varied among the studies, we made the most common allele for each polymorphism (b for BsmI and F for FokI) the reference allele for our analyses.
The pooled ORs for the risk were calculated. Heterogeneity assumptions were assessed by the chi-square-based Q test [14]. In our study, the I 2 test was used to assess the heterogeneity between studies (I 2 < 25 % no heterogeneity; I 2 = 25–50 % moderate heterogeneity; I 2 > 50 % large or extreme heterogeneity). The heterogeneity was considered statistically significant with I 2 > 50 % or P < 0.10. A P value greater than 0.10 for the Q test indicated a lack of heterogeneity among the studies. Thus, the pooled OR estimate of each study was calculated using the fixed-effects model (the Mantel–Haenszel method) [15]; otherwise, the random-effects model (the DerSimonian and Laird method) was used [16]. In addition, subgroup analysis stratified by ethnicity, study design, source of controls, deviation from HWE, study location, and genotyping method was also performed.
One-way sensitivity analyses were performed to determine the stability of the results; each individual study in the meta-analysis was omitted to reflect the influence of the individual dataset on the pooled OR [17].
Potential publication biases were estimated by the funnel plot, in which the standard error of the log(OR) of each study was plotted against its log(OR). An asymmetrical plot indicates a publication bias. Funnel plot asymmetry was assessed using the Egger’s test. The significance of the intercept was determined by the t test, as suggested by Egger (P < 0.05 was considered a statistically significant publication bias) [18]. If there was some evidence of publication bias, the trim and fill method which estimates the number and results of potential missing studies resulting from publication bias was applied.
All calculations were performed using STATA version 11.0 (Stata Corporation, College Station, TX).
Results
Characteristics of eligible studies
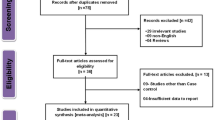
Three hundred and thirty-five potentially relevant citations were reviewed, and 29 articles met the inclusion criteria and were used in our meta-analysis [19–47]. The study search process is shown in Fig. 1. Table 1 presents the principal characteristics of these studies. Four articles contained separate data on different ethnic groups [28 1/2; 30 1/2; 32 1/2; 44 1/2/3], and we treated them as separate studies. In total, 34 studies including 10,267 PCa cases and 11,489 controls were analyzed. The distribution of allele frequency in control groups with different ethnicity was different. For VDR FokI, the variant f-allele frequency was higher in Asian population (47.7 %) than those in Caucasian population (37.2 %). But for VDR BsmI, the variant B-allele frequency was higher in Caucasian population (44.1 %) than those in Asian population (14.5 %).
Of the 34 studies, 31 were published in English and 3 were written in Chinese. The sample sizes ranged from 59 to 2600. All cases were histologically confirmed. Most of the researches contained in this meta-analysis were case–control studies, except eight nested case–control studies [19, 28, 30, 33, 34, 37, 42, 46]. Among the studies, 24 discussed the association between the FokI polymorphism and PCa risk, 24 were about the BsmI. In all eligible studies, there were 14 studies on FokI genotype of Caucasians, 6 studies of Asians, 2 studies of African-Americans, 1 study of Hispanics, and 1 of mixed populations. Accordingly, 11 studies on BsmI genotype were of Caucasians, 8 studies of Asians, 4 studies of African-Americans and 1 of mixed populations. According to the control source, 17 were population-based researches, 10 were hospital-based researches, 3 studies used BPH patients as controls, and two used both hospital-based and BPH patients as controls. In addition, the other two studies were not clarified. All polymorphisms in the control subjects were in HWE, except seven studies for BsmI polymorphism [34, 38–41, 44 2/3] and one for FokI polymorphism [35].
Meta-analysis results
The summary of meta-analysis for VDR gene FokI and BsmI polymorphisms with PCa is shown in Table 2.
Analysis for VDR gene FokI polymorphism
The association between FokI polymorphism and PCa was investigated in 24 independent studies with 8,339 cases and 9,042 controls. The Q test of heterogeneity was almost always not significant and we conducted analyses using fixed-effect models in overall population. We did not detect the association between FokI polymorphism and PCa in overall population when examining the contrast of f-allele vs. F-allele, ff vs. FF, Ff vs. FF, ff vs. Ff + FF and ff + Ff vs. FF genotypes (OR = 0.996, 95 % CI = 0.938–1.057, P heterogeneity = 0.033; OR = 1.022, 95 % CI = 0.933–1.12, P heterogeneity = 0.157; OR = 1.032, 95 % CI = 0.966–1.102, P heterogeneity = 0.429; OR = 1.002, 95 % CI = 0.922–1.088, P heterogeneity = 0.322; OR = 1.029, 95 % CI = 0.967–1.094, P heterogeneity = 0.134, respectively) (Fig. 2). Among the 24 studies, there was one research deviated from HWE [35], so we excluded it and then obtained another result. Nevertheless, this result was similar with the previous one (data not shown).
Fourteen independent studies (6,611 cases and 6,661 controls) were included in sub-analysis of FokI polymorphism in Caucasian population. The Q test of heterogeneity was almost always not significant and we conducted analyses using fixed-effect models. The FokI polymorphism showed no association with PCa in Caucasian population (f vs. F: OR = 1.038, 95 % CI = 0.969–1.112, P heterogeneity = 0.068; ff vs. FF: OR = 1.09, 95 % CI = 0.981–1.212, P heterogeneity = 0.291; Ff vs. FF: OR = 1.053, 95 % CI = 0.977–1.135, P heterogeneity = 0.394; ff vs. Ff + FF: OR = 1.063, 95 % CI = 0.964–1.173, P heterogeneity = 0.479; ff + Ff vs. FF: OR = 1.061, 95 % CI = 0.988–1.138, P heterogeneity = 0.147, respectively). Six independent studies (920 cases and 1,439 controls) were included in sub-analysis of FokI polymorphism in Asian population. As the dramatic heterogeneity, the fixed-effect model was used. The FokI polymorphism showed no association with PCa in Asian population (f vs. F: OR = 0.896, 95 % CI = 0.802–1.001, P heterogeneity = 0.377; ff vs. FF: OR = 0.801, 95 % CI = 0.641–1.0, P heterogeneity = 0.3; Ff vs. FF: OR = 0.938, 95 % CI = 0.781–1.126, P heterogeneity = 0.714; ff vs. Ff + FF: OR = 0.832, 95 % CI = 0.688–1.007, P heterogeneity = 0.373; ff + Ff vs. FF: OR = 0.892, 95 % CI = 0.751–1.06, P heterogeneity = 0.578, respectively). Also it seems that there was no association between PCa risk and the FokI genotype in African-Americans (data not shown).
We also performed subgroup analysis stratified by control source. One study [20] was eliminated as not mentioned the source of controls. Also, low risks were found between PCa and FokI genotypes only in hospital-based controls (f vs. F: OR = 0.724, 95 % CI = 0.537–0.978, P heterogeneity = 0.038; ff vs. FF: OR = 0.676, 95 % CI = 0.501–0.912, P heterogeneity = 0.115; ff vs. Ff + FF: OR = 0.733, 95 % CI = 0.565–0.951, P heterogeneity = 0.233, respectively), but not in population-based or BPH-based controls (data not shown).
Given that in the USA there is a higher use of vitamin D supplementation [9], we also performed stratified analysis by comparing studies conducted in USA and in other countries. We did not observe a significant association between PCa risk and FokI genotype among the studies conducted in USA (data not shown).
Analysis for VDR gene BsmI polymorphism
The meta-analysis for association of BsmI polymorphism with PCa in overall population included 24 independent studies with a total of 7,648 cases and 8,556 controls. With significant between-study heterogeneity by Q test, the analysis was conducted using random effect model. We did not detect the association between BsmI polymorphism and PCa in overall population when examining the contrast of B-allele vs. b-allele, BB vs. bb, Bb vs. bb, BB vs. Bb + bb and BB + Bb vs. bb genotypes (OR = 0.965, 95 % CI = 0.865–1.054, P heterogeneity = 0.000; OR = 1.002, 95 % CI = 0.869–1.157, P heterogeneity = 0.038; OR = 0.894, 95 % CI = 0.773–1.034, P heterogeneity = 0.000; OR = 1.018, 95 % CI = 0.936–1.107, P heterogeneity = 0.649; OR = 0.922, 95 % CI = 0.798–1.065, P heterogeneity = 0.000, respectively) (Fig. 2). Among the 24 studies, there were 7 researches that deviated from HWE [34, 38–41, 44 2/3], so we excluded them and then obtained another result. Nevertheless, this result was similar with the previous one (data not shown).
Eleven independent studies (5,842 cases and 6,266 controls) were included in sub-analysis of FokI polymorphism in Caucasian population. The Q test of heterogeneity was almost always significant and we conducted analyses using random effect models. No association was observed between BsmI polymorphism and PCa risk in Caucasian population (B vs. b: OR = 1.004, 95 % CI = 0.914–1.104, P heterogeneity = 0.001; BB vs. bb: OR = 1.107, 95 % CI = 0.865–1.195, P heterogeneity = 0.022; Bb vs. bb: OR = 0.974, 95 % CI = 0.846–1.121, P heterogeneity = 0.005; BB vs. Bb + bb: OR = 1.023, 95 % CI = 0.934–1.12, P heterogeneity = 0.324; BB + Bb vs. bb: OR = 1.004, 95 % CI = 0.871–1.158, P heterogeneity = 0.001, respectively). Seven independent studies (942 cases and 1,402 controls) were included in sub-analysis of BsmI polymorphism in Asian population. As the dramatic heterogeneity, the random effect model was used. The BsmI polymorphism showed no association with PCa in Asian population (B vs. b: OR = 0.722, 95 % CI = 0.489–1.067, P heterogeneity = 0.001; BB vs. bb: OR = 0.862, 95 % CI = 0.514–1.445, P heterogeneity = 0.5; Bb vs. bb: OR = 0.642, 95 % CI = 0.406–1.015, P heterogeneity = 0.003; BB vs. Bb + bb: OR = 0.93, 95 % CI = 0.569–1.521, P heterogeneity = 0.606; BB + Bb vs. bb: OR = 0.666, 95 % CI = 0.421–1.054, P heterogeneity = 0.001, respectively). Also, it seems that there was no association between PCa risk and the BsmI genotype in African-Americans (data not shown).
The stratified analysis was also performed by source of controls. One study [47] was eliminated as it did mention the source of controls. However, we did not find decreased PCa risk for population-based, hospital-based, or BPH-based controls with the BsmI polymorphism (data not shown). Moreover, we also performed stratified analysis by study location, study design and genotype methods. The available data revealed a result that there was no association between PCa risk and the BsmI genotype among the studies conducted in USA (data not shown). The same results appeared among studies with different study design and genotype methods (data not shown).
Sensitivity analysis
Sensitivity analyses were performed by sequential omission of individual studies for all subjects and subgroups. The corresponding pooled ORs were not materially altered in all subjects and subgroups of FokI and BsmI genotypes (data not shown). The results of sensitivity analyses indicated the stability of the results of this meta-analysis.
Evaluation of publication bias
Begg’s funnel plot and Egger’s test were performed to assess the publication bias of the literatures. No evidence of publication bias was found for comparisons of VDR BsmI B-allele and b-allele (P = 0.709), BB and bb (P = 0.626), Bb and bb (P = 0.970), BB and Bb/bb (P = 0.802), and BB/Bb and bb (P = 0.996) (Fig. 3). However, the shape of the funnel plots seemed asymmetrical for the comparison of different alleles of the VDR FokI polymorphism, suggesting the presence of publication bias. Therefore, Egger’s test was performed to assess funnel plot symmetry statistically. Publication bias was found for comparison of VDR FokI f-allele and F-allele (P = 0.001), ff and FF (P = 0.000), ff and FF/Ff (P = 0.000) and ff/Ff and FF (P = 0.01) (Fig. 3). The association remained non-significant after adjustment for publication bias using the trim and fill method (data not shown).
Discussion
Genetic susceptibility to cancer has been a research focus in scientific community. Development and progression of PCa are influenced by vitamin D synthesis. Therefore, polymorphisms of genes encoding key proteins involved in vitamin D synthesis and metabolism have been primarily chosen as candidate genes for PCa susceptibility. Nowadays, growing number of studies have revealed polymorphic variants of the VDR gene were associated with etiology of PCa. In order to provide the most comprehensive and reliable conclusion, we performed the present meta-analysis of 34 independent case–control studies, including 10,267 cases and 11,489 controls. We explored the association between two common polymorphisms (FokI and BsmI) in the VDR gene region and PCa risk. The results of our meta-analysis do not provide evidence for an association between the VDR FokI and BsmI polymorphisms, and the risk of PCa. It is consistent with the result of former meta-analysis, which was conducted by Berndt et al. in 2006 [13]. However, we included 10,267 cases and 11,489 controls from 34 studies in the present meta-analysis, which is much more than the previous one including only 17 studies. Hence, a more stringent and comprehensive result has been obtained.
It is known that the allele frequencies of metabolic genes are not equally distributed throughout the human population but follow diverse ethnic patterns, therefore, the subgroups according to ethnicity were performed. The strength of linkage disequilibrium between variants in the VDR gene is known to differ among ethnic populations [48]. If an unobserved disease-causing allele is in strong linkage disequilibrium with one of the VDR polymorphisms in one population but not in another population, the observed association between the VDR polymorphism and PCa risk may be substantially different between populations. However, our results indicated that no significant association was found between FokI or BsmI genotypes and PCa risk in the overall population, as well as in Caucasians, Asians, and Africans. The possible reason could be the limited sample size that may have not enough statistical power to detect a real effect or generate a fluctuated estimation.
Furthermore, we also showed that FokI genotypes including allele-contrast, homozygote comparison, and recessive model have strikingly decreased the risk of PCa susceptibility when stratified by control source. However, we obtained the lower risk of PCa when only considered the hospital-based controls. The possible reason may be that FokI genotypes could influence the susceptibility to non-cancer diseases, such as cardiovascular diseases [49], Parkinson's disease [50], and diabetes mellitus [51], so its genotypes frequency possibly differed between the hospital-based and population-based controls. Moreover, several studies used controls obtained from individuals with BPH [21, 26, 27, 31, 43]. Although it has been demonstrated that vitamin D inhibits the growth of cells obtained from BPH tissue [6], few investigators have found that VDR polymorphisms are related to the risk of BPH [52]. If polymorphisms in the VDR gene increase the risk of BPH, the use of men with BPH as controls could attenuate the risk of PCa observed for these polymorphisms. In our meta-analysis, genotype frequencies of the VDR FokI and BsmI polymorphisms in controls appeared similar between studies that did and did not exclude men with BPH. However, since few studies excluded patients with BPH, comparisons were limited and this potential bias cannot be ruled out.
In addition, we also performed stratified analysis by study location. We did not find any evidence of different risk estimates for studies conducted in USA compared with that carried out in other countries. National vitamin D fortification and supplementation practices are generally very different between countries. Fortification of staple foods, such as milk and margarine and spreads, plus other optional fortifications (orange juice, ready-to-eat breakfast cereals, sliced American cheese and yogurt) are mandatory in the USA, while there is no required fortification of foods in other countries.
Our study represents an updated and comprehensive review of the literature on the two most studied VDR polymorphisms and PCa risk. A previous meta-analysis in 2009 found that a significant 17 % reduction in PCa risk for carriers of BsmI Bb compared with bb genotype [9], which is inconsistent with our results. However, because our meta-analysis included eight new studies and three updates of previous publications on PCa compared with that published in 2009 [9], we were able to provide a complete picture of the role of VDR polymorphisms in PCa risk.
There are some limitations in this meta-analysis. First of all, even though we performed subgroup analyses stratified by ethnicity and control source, the heterogeneity for BsmI polymorphism among the studies was extreme. It suggested that there were other potential confounding factors in the included studies, such as the genotyping error, selection bias, or population-specific gene–gene or gene–environment interaction, allelic heterogeneity, or chance [53, 54]. Although evidence of heterogeneity exists, it was found through sensitivity analysis that studies contribute to the heterogeneity do not significantly alter the estimate of overall odds ratio. Secondly, only published studies were included, therefore the publication bias may have occurred. The Egger’s test provided statistical evidence of that. We observed the publication bias when only considered studies about the association between FokI polymorphism and PCa risk, but did not find it in the studies about the PCa risks with BsmI polymorphisms. It is known that positive results usually have a greater probability of being published, and such bias may occur in studies with null or unexpected results. Thirdly, the overall outcomes were based on unadjusted effect estimates. Although the cases and controls were matched on age, sex, and residence in all studies, these confounding factors might slightly modify the effective estimates and a more precise evaluation needed to be adjusted by the potentially suspected factors. Finally, as the meta-analysis remains a retrospective research which is subject to the methodological deficiencies of the included studies, we tried to develop a detailed protocol before initiating the study, and then performed an explicit method for study researching, selection, data extraction and data analysis to minimize the likelihood of bias.
In conclusion, this study is, to the best our knowledge, the largest meta-analysis of associations between VDR gene FokI and BsmI polymorphisms and PCa risk. Although FokI and BsmI polymorphisms were not associated with PCa risk, the possibility of an association in specific subpopulations could not be ruled out and other variants in the VDR gene may affect risk. In the future, well-designed epidemiologic studies would help illuminate the complex interactions of VDR gene polymorphisms, environmental factors, and PCa.
References
Heidenreich A, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71.
Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005;16:481–8.
Fleshner N, Zlotta AR. Prostate cancer prevention: past, present, and future. Cancer. 2007;110:1889–99.
American Cancer Society: Cancer Facts & Figures 2009. 2009.
Schwartz GG, Hulka BS. Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis). Anticancer Res. 1990;10:1307–11.
Peehl DM, Skowronski RJ, Leung GK, Wong ST, Stamey TA, Feldman D. Antiproliferative effects of 1,25-dihydroxyvitamin D3 on primary cultures of human prostatic cells. Cancer Res. 1994;54:805–10.
Miller GJ, Stapleton GE, Hedlund TE, Moffat KA. Vitamin D receptor expression, 24-hydroxylase activity, and inhibition of growth by 1alpha,25-dihydroxyvitamin D3 in seven human prostatic carcinoma cell lines. Clin Cancer Res. 1995;1:997–1003.
Hedlund TE, Moffatt KA, Miller GJ. Vitamin D receptor expression is required for growth modulation by 1 alpha,25-dihydroxyvitamin D3 in the human prostatic carcinoma cell line ALVA-31. J Steroid Biochem Mol Biol. 1996;58:277–88.
Raimondi S, Johansson H, Maisonneuve P, Gandini S. Review and meta-analysis on vitamin D receptor polymorphisms and cancer risk. Carcinogenesis. 2009;30:1170–80.
Whitfield GK, Remus LS, Jurutka PW, et al. Functionally relevant polymorphisms in the human nuclear vitamin D receptor gene. Mol Cell Endocrinol. 2001;177:145–59.
Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143–56.
Ntais C, Polycarpou A, Ioannidis JP. Vitamin D receptor gene polymorphisms and risk of prostate cancer: a meta-analysis. Cancer Epidemiol BiomarkPrev. 2003;12:1395–402.
Berndt SI, Dodson JL, Huang WY, Nicodemus KK. A systematic review of vitamin D receptor gene polymorphisms and prostate cancer risk. J Urol. 2006;175:1613–23.
Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29.
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull. 1999;8:15–7.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in metaanalysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Li H, Stampfer MJ, Hollis JB, et al. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med. 2007;4:e103.
Yang Y, Wang S, Ye Z, Yang W. Association of single nucleotide polymorphism of vitamin D receptor gene start codon and the susceptibility to prostate cancer in the Han nationality in Hubei area. Zhonghua Nan Ke Xue. 2004;10:411–4 [Article in Chinese].
Huang SP, Huang CY, Wu WJ, et al. Association of vitamin D receptor FokI polymorphism with prostate cancer risk, clinicopathological features and recurrence of prostate specific antigen after radical prostatectomy. Int J Cancer. 2006;119:1902–7.
Mishra DK, Bid HK, Srivastava DS, Mandhani A, Mittal RD. Association of vitamin D receptor gene polymorphism and risk of prostate cancer in India. Urol Int. 2005;74:315–8.
Bai Y, Yu Y, Yu B, et al. Association of vitamin D receptor polymorphisms with the risk of prostate cancer in the Han population of Southern China. BMC Med Genet. 2009;10:125.
Holick CN, Stanford JL, Kwon EM, Ostrander EA, Nejentsev S, Peters U. Comprehensive association analysis of the vitamin D pathway genes, VDR, CYP27B1, and CYP24A1, in prostate cancer. Cancer Epidemiol Biomark Prev. 2007;16:1990–9.
Hayes VM, Severi G, Padilla EJ, et al. Genetic variants in the vitamin D receptor gene and prostate cancer risk. Cancer Epidemiol Biomark Prev. 2005;14:997–9.
Bodiwala D, Luscombe CJ, French ME, et al. Polymorphisms in the vitamin D receptor gene, ultraviolet radiation, and susceptibility to prostate cancer. Environ Mol Mutagen. 2004;43:121–7.
Rukin NJ, Luscombe C, Moon S, et al. Prostate cancer susceptibility is mediated by interactions between exposure to ultraviolet radiation and polymorphisms in the 5' haplotype block of the vitamin D receptor gene. Cancer Lett. 2007;247:328–35.
Oakley-Girvan I, Feldman D, Eccleshall TR, et al. Risk of early-onset prostate cancer in relation to germ line polymorphisms of the vitamin D receptor. Cancer Epidemiol Biomark Prev. 2004;13:1325–30. 1/2.
John EM, Schwartz GG, Koo J, Van Den Berg D, Ingles SA. Sun exposure, vitamin D receptor gene polymorphisms, and risk of advanced prostate cancer. Cancer Res. 2005;65:5470–9.
Torkko KC, van Bokhoven A, Mai P, et al. VDR and SRD5A2 polymorphisms combine to increase risk for prostate cancer in both non-Hispanic White and Hispanic White men. Clin Cancer Res. 2008;14:3223–9. 1/2.
Ruan L, Li Z, Li G. Relationship between snp of vitamin d receptor start codon and prostate cancer. IMHGN. 2009;15:12–4. Article in Chinese.
Holt SK, Kwon EM, Peters U, Ostrander EA, Stanford JL. Vitamin D pathway gene variants and prostate cancer risk. Cancer Epidemiol Biomark Prev. 2009;18:1929–33. 1/2.
Mikhak B, Hunter DJ, Spiegelman D, Platz EA, Hollis BW, Giovannucci E. Vitamin D receptor (VDR) gene polymorphisms and haplotypes, interactions with plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D, and prostate cancer risk. Prostate. 2007;67:911–23.
Chokkalingam AP, McGlynn KA, Gao YT, et al. Vitamin D receptor gene polymorphisms, insulin-like growth factors, and prostate cancer risk: a population-based case–control study in China. Cancer Res. 2001;61:4333–6.
Cicek MS, Liu X, Schumacher FR, Casey G, Witte JS. Vitamin D receptor genotypes/haplotypes and prostate cancer risk. Cancer Epidemiol Biomark Prev. 2006;15:2549–52.
Correa-Cerro L, Berthon P, Häussler J, et al. Vitamin D receptor polymorphisms as markers in prostate cancer. Hum Genet. 1999;105:281–7.
Tayeb MT, Clark C, Haites NE, Sharp L, Murray GI, McLeod HL. Vitamin D receptor, HER-2 polymorphisms and risk of prostate cancer in men with benign prostate hyperplasia. Saudi Med J. 2004;25:447–51.
Cheteri MB, Stanford JL, Friedrichsen DM, et al. Vitamin D receptor gene polymorphisms and prostate cancer risk. Prostate. 2004;59:409–18.
Szendroi A, Speer G, Tabak A, et al. The role of vitamin D, estrogen, calcium sensing receptor genotypes and serum calcium in the pathogenesis of prostate cancer. Can J Urol. 2011;18:5710–6.
Huang SP, Chou YH, Wayne Chang WS, et al. Association between vitamin D receptor polymorphisms and prostate cancer risk in a Taiwanese population. Cancer Lett. 2004;207:69–77.
Onen IH, Ekmekci A, Eroglu M, Konac E, Yesil S, Biri H. Association of genetic polymorphisms in vitamin D receptor gene and susceptibility to sporadic prostate cancer. Exp Biol Med (Maywood). 2008;233:1608–14.
Ingles SA, Coetzee GA, Ross RK, et al. Association of prostate cancer with vitamin D receptor haplotypes in African-Americans. Cancer Res. 1998;58:1620–3.
Habuchi T, Suzuki T, Sasaki R, et al. Association of vitamin D receptor gene polymorphism with prostate cancer and benign prostatic hyperplasia in a Japanese population. Cancer Res. 2000;60:305–8.
Nam RK, Zhang WW, Trachtenberg J, et al. Comprehensive assessment of candidate genes and serological markers for the detection of prostate cancer. Cancer Epidemiol Biomark Prev. 2003;12:1429–37. 1/2/3.
Suzuki K, Matsui H, Ohtake N, et al. Vitamin D receptor gene polymorphism in familial prostate cancer in a Japanese population. Int J Urol. 2003;10:261–6.
Ma J, Stampfer MJ, Gann PH, et al. Vitamin D receptor polymorphisms, circulating vitamin D metabolites, and risk of prostate cancer in United States physicians. Cancer Epidemiol Biomark Prev. 1998;7:385–90.
Liu JH, Li HW, Wang JQ, et al. Vitamin D receptor gene Bsm I polymorphism and the susceptibility to prostate cancer in northern Chinese Han population. Zhonghua Nan Ke Xue. 2003;9:413–6. Article in Chinese.
Nejentsev S, Godfrey L, Snook H, et al. Comparative high-resolution analysis of linkage disequilibrium and tag single nucleotide polymorphisms between populations in the vitamin D receptor gene. Hum Mol Genet. 2004;13:1633–9.
Vélayoudom-Céphise FL, Larifla L, Donnet JP, et al. Vitamin D deficiency, vitamin D receptor gene polymorphisms and cardiovascular risk factors in Caribbean patients with type 2 diabetes. Diabetes Metab. 2011;37:540–5.
Han X, Xue L, Li Y, Chen B, Xie A. Vitamin D receptor gene polymorphism and its association with Parkinson's disease in Chinese Han population. Neurosci Lett. 2012;525:29–33.
Wang Q, Xi B, Reilly KH, Liu M, Fu M. Quantitative assessment of the associations between four polymorphisms (FokI, ApaI, BsmI, TaqI) of vitamin D receptor gene and risk of diabetes mellitus. Mol Biol Rep. 2012;39:9405–14.
Bousema JT, Bussemakers MJ, van Houwelingen KP, et al. Polymorphisms in the vitamin D receptor gene and the androgen receptor gene and the risk of benign prostatic hyperplasia. Eur Urol. 2000;37:234–8.
Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28:123–37.
Lin PI, Vance JM, Pericak-Vance MA, Martin ER. No gene is an island: the flip-flop phenomenon. Am J Hum Genet. 2007;80:531–8.
Acknowledgments
This study was supported by “The Youth Innovation Fund of the First Affiliated Hospital of Zhengzhou University” and “Foundation of Henan Educational Committee (No. 13A320688)”.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhan Guo and Jianguo Wen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Guo, Z., Wen, J., Kan, Q. et al. Lack of association between vitamin D receptor gene FokI and BsmI polymorphisms and prostate cancer risk: an updated meta-analysis involving 21,756 subjects. Tumor Biol. 34, 3189–3200 (2013). https://doi.org/10.1007/s13277-013-0889-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-0889-6