Abstract
The vitamin D receptor (VDR) gene polymorphisms have been suggested to be involved in the development of diabetes mellitus, including type 1 diabetes (T1DM) and type 2 diabetes (T2DM). However, the results have been inconsistent. In this study, we performed a meta-analysis to investigate the associations. Literature was retrieved from PubMed, ISI Web of Science and Chinese databases. Pooled odds ratios (ORs) with 95 % confidence intervals (CIs) were calculated using a random or fixed effect model. 79 studies (FokI: 22 studies; BsmI: 25 studies; ApaI: 17 studies; TaqI: 15 studies) on T1DM and 44 studies (FokI: 10 studies; BsmI: 10 studies; ApaI: 14 studies; TaqI: 10 studies) on T2DM were included. The results indicated that BsmI polymorphism was associated with an increased risk of T1DM (B vs. b: OR 1.31, 95 % CI 1.10–1.55, P = 0.002), especially in East Asians (B vs. b: OR 2.57, 95 % CI: 1.55–4.24, P < 0.001); FokI polymorphism was associated with an increased risk of T2DM (f vs. F: OR 1.30, 95 % CI: 1.17–1.45, P < 0.001), especially in East Asians (f vs. F: OR 1.36, 95 % CI: 1.21–1.54, P < 0.001). However, no significant association was observed between ApaI or TaqI polymorphism and diabetes risk with the exception of significant association between ApaI polymorphism and T1DM risk in East Asians. Thus, the authors found BsmI polymorphism in the VDR gene may increase the risk of T1DM in East Asians and the FokI polymorphism may increase the risk of T2DM in East Asians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a common chronic disease with high rates of disability and mortality caused by its vascular complications. Type 1 diabetes mellitus (T1DM), accounting for only 5–10 % of diabetes cases worldwide [1], is a T cell mediated autoimmune disease [2] and results from autoimmune destruction of β-cells of the pancreas [1]. Type 2 diabetes mellitus (T2DM), accounting for 90–95 % of those with diabetes, results from a combination of resistance to insulin action and an inadequate compensatory insulin secretory response [1]. Although the two forms of DM can be attributed to environmental factors, such as geography [3], obesity [1], diet and exercise [4], genetic predispositions also play important roles in both developments of DM [1, 3]. The genome-wide association studies (GWAS) have identified many potential loci associated with DM, including SH2B3, ERBB3, PTPN22, IL27, KCNQ1, GLIL3, PEPD, KCNK16 [3, 5, 6]. Candidate gene studies have also revealed some DM loci, including MHC, INS, PTPN2, TCF7L2, PPARGC1A [3, 6–8]. Despite both environmental and genetic factors mentioned above, the complicated mechanisms behind DM remain unclear.
Evidence has suggested that taking vitamin D supplements in early childhood and high vitamin D intake may be inversely associated with risk of incident T1DM and T2DM, respectively [9, 10]. Vitamin D possesses the actions of immunity regulation, especially T cell mediated immunity and affects the immune cells with relevance to autoimmunity [11]. High levels of vitamin D can enhance pancreatic β-cell secretion functions and improve insulin resistance [12, 13]. The active form of Vitamin D, 1,25-dihydroxyvitamin D, exerts its bioactivities through the vitamin D receptor (VDR), which is an intracellular hormone receptor belonging to the steroid hormone receptor superfamily. Hence, the VDR gene may be involved in the pathogenesis and progression of DM including T1DM and T2DM.
At present, FokI (rs10735810), BsmI (rs1544410), ApaI (rs7975232) and TaqI (rs731236) are the four common single nucleotide polymorphisms (SNPs) in the VDR gene that have been most frequently studied [14, 15]. A previous meta-analysis by Guo et al. [16] regarding the associations between the four VDR gene polymorphisms and T1DM risk did not identify any genetic variant associated with T1DM. However, since this article was published, sixteen additional papers investigating these associations have been published. In addition, a great number of studies have also investigated the associations between the four VDR gene polymorphisms and T2DM risk. However, the results of these studies have been inconsistent.
In this study, a meta-analysis was performed to clarify the associations between the four common polymorphisms (FokI, ApaI, BsmI, TaqI) in the VDR gene and T1DM and T2DM susceptibilities.
Materials and methods
Literature and search strategy
The databases PubMed, ISI Web of Science, Chinese Biomedical Literature Database, Chinese National Knowledge Infrastructure and Chinese Wanfang Data were searched. The search strategy to identify all possible articles in English or Chinese language involved the use of combination of the following key words: (vitamin D receptor or VDR) AND (polymorphism or variation or variant) AND (type 2 diabetes mellitus or NIDDM or type 1 diabetes mellitus or IDDM or diabetes mellitus). If more than one article was published using the same study data, only the study with the largest sample size was included. The literature search was updated on November 11th, 2011.
Inclusion criteria and data extraction
Studies were included if they met the following inclusion criteria: (1) an original article; (2) case–control or cohort study; (3) evaluating the associations of the VDR gene polymorphisms (FokI, ApaI, BsmI, TaqI) with T1DM or T2DM risk; and (4) providing sufficient data for calculation of an odds ratio (OR) with 95 % confidence interval (CI). Studies were excluded if: (1) they were case-only reports or review papers; (2) the cases with other diseases combined such as osteoporosis or coronary heart disease; or (3) the study was based on pedigree data. The following information was extracted from each study: (1) name of first author; (2) year of publication; (3) country of origin; (4) ethnicity of the study population; (5) gender distribution and mean age of subjects in cases and controls; (6) mean age of onset in cases; (7) genotype distributions in cases and controls; and (8) P value for the test of Hardy–Weinberg equilibrium (HWE) in controls. Two authors independently assessed the articles for compliance with the inclusion criteria, and disagreement was followed by discussion until consensus was reached.
Quality assessment
The quality of included studies was also independently assessed by two authors using the procedure known as “extended quality score”, which was used in the paper by Xu et al. [17] and based on the recommendations of the MOOSE guidelines and other related meta-analytic papers. The procedure with eleven items stems from epidemiological and genetic considerations and the full score is 14 points [17]. Studies were categorized as “high” quality if the score was equal to or greater than 11 points, “medium” if the score was equal to or greater than 7 points and less than 11 points and “poor” if the score was less than 7 points.
Statistical analysis
The associations between VDR gene polymorphisms (FokI, ApaI, BsmI and TaqI) and T1DM or T2DM risk were estimated by calculating pooled ORs with 95 % CIs assuming a multiplicative genetic model (FokI: f vs. F; ApaI: a vs. A; BsmI: B vs. b; TaqI: t vs. T). The significance of the pooled ORs was determined using Z tests (P < 0.05 was considered statistically significant). The heterogeneity among studies was evaluated by the Q-statistic test and I 2-statistic test [18]. A random- (DerSimonian–Laird method) [19] or fixed- (Mantel–Haenszel method) [20] effects model was used to calculate the pooled ORs in the presence (P ≤ 0.10) or absence (P > 0.10) of heterogeneity. Meta-regression with maximum likelihood estimation was performed to explore the potentially important sources of heterogeneity among studies. Subgroup analysis was also performed based on ethnicity. Sensitivity analysis, removing one study at a time, was performed to evaluate the stability of the results. Begg’s funnel plot, a scatter plot of effect against study size, was generated as a visual aid to detect bias or systematic heterogeneity [21]. Publication bias was assessed by Egger’s test [22] (P < 0.05 was considered statistically significant). Data analysis was performed using STATA version 11 (StataCorp LP, College Station, Texas, USA).
Results
Characteristics of the studies
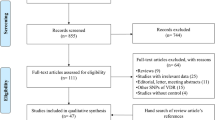
The detailed steps of our literature search are shown in Fig. 1. Based on the search strategy and inclusion criteria, 29 papers were included in the meta-analysis of the associations between polymorphisms in the VDR gene and T1DM risk [23–51]. There were 22 studies (2,940 cases and 4,942 controls) for FokI polymorphism, 25 studies (3,854 cases and 6,498 controls) for BsmI polymorphism, 17 studies (2,136 cases and 3,877 controls) for ApaI polymorphism, and 15 studies (1,719 cases and 1,843 controls) for TaqI polymorphism, respectively. Regarding to the associations between polymorphisms in the VDR gene and T2DM risk, 24 papers were included in the meta-analysis [29, 35, 38, 42, 52–71]. For FokI polymorphism, there were 10 studies involving 1,562 cases and 1,461 controls; for BsmI, 10 studies, 1,778 cases and 2,800 controls; for ApaI, 14 studies, 1,430 cases and 2,441 controls; for TaqI, 10 studies, 1,388 cases and 2,438 controls.
All the included studies were case–control designs. All studies was categorized as “medium” or “high” quality, with the exception that the studies from 3 papers on T1DM [23, 26, 34] and 2 papers on T2DM [53, 67] were considered as “pool” quality. The characteristics of the studies are listed in Supplementary Tables 1 and 2.
Quantitative synthesis
Type 1 diabetes mellitus
After excluding the studies deviating from HWE in controls, the overall results showed that there was a statistically significant association between BsmI polymorphism and an increased risk of T1DM (B vs. b: OR 1.31, 95 % CI 1.10–1.55, P = 0.002), with evidence of heterogeneity among studies (I 2 = 74.2 %, P < 0.001) (Table 1; Fig. 2). For the other three polymorphisms, no significant association was found in the overall population after the studies deviating from HWE in controls were excluded. For FokI polymorphism, the pooled summary OR was 0.97 (95 % CI: 0.82–1.16, P = 0.766), with evidence of heterogeneity among studies (I 2 = 74.7 %, P < 0.001); for ApaI polymorphism, the pooled summary OR was 0.91 (95 % CI 0.82–1.02, P = 0.095), with moderate heterogeneity between studies (I 2 = 35.5 %, P = 0.106); for TaqI polymorphism, the pooled summary OR was 1.12 (95 % CI: 0.96–1.32, P = 0.148), and there was no evidence of heterogeneity between studies (I 2 = 0.0 %, P = 0.445) (Table 1; Supplementary Figs. 1–3). Further subgroup analyses by ethnicity showed that the effect sizes were statistically significant in East Asians for BsmI (B vs. b: OR 2.57, 95 % CI: 1.55–4.24, P < 0.001) and ApaI polymorphisms (a vs. A: OR 0.78, 95 % CI: 0.63–0.96, P = 0.017) (Table 1).
Type 2 diabetes mellitus
After excluding the study deviating from HWE in controls, the overall result showed that there was a statistically significant association between FokI polymorphism and an increased risk of T2DM (f vs. F: OR 1.30, 95 % CI: 1.17–1.45, P < 0.001), with moderate heterogeneity between studies (I 2 = 36.8 %, P = 0.124) (Table 2; Fig. 3). For BsmI polymorphism, a marginally significant association with T2DM risk was found after the study deviating from HWE in controls was excluded (B vs. b: OR 1.49, 95 % CI: 1.03–2.15, P = 0.033), with evidence of heterogeneity between studies (I 2 = 84.8 %, P < 0.001). For ApaI polymorphism, the pooled summary OR was 1.00 (95 % CI: 0.88–1.13, P = 0.978), with no sign of heterogeneity between studies (I 2 = 0 %, P = 0.476). For TaqI polymorphism, the pooled summary OR was 0.92 (95 % CI: 0.72–1.18, P = 0.526), with evidence of heterogeneity between studies (I 2 = 60.8 %, P = 0.018) (Table 2; Supplementary Figs. 4–6). Further subgroup analyses by ethnicity showed that the effect sizes were statistically significant in East Asians for FokI (f vs. F: OR 1.36, 95 % CI: 1.21–1.54, P < 0.001) and BsmI polymorphisms (B vs. b: OR 2.60, 95 % CI: 1.82–3.72, P < 0.001) (Table 2).
Sensitivity analysis
A sensitivity analysis was performed by excluding one study at a time. Results for the association between BsmI polymorphism and T1DM risk remained statistically significant, with the ORs with 95 % CIs ranging from 1.22 (1.06–1.41) to 1.35 (1.14–1.60). In addition, the positive association between FokI polymorphism and T2DM risk was also confirmed by the sensitivity analysis, with the ORs with 95 % CIs ranging from 1.26 (1.13–1.41) to 1.36 (1.21–1.53). However, the sensitivity analysis indicated that the significant association between BsmI polymorphism and T2DM risk was not robust (data not shown).
Sources of heterogeneity
The meta-regression was conducted with the covariates publication year, ethnicity, latitude of the city or region, sex frequency of cases and controls, mean age of cases and controls, age of onset in cases and BMI of cases and controls. However, no covariate was identified as a potential source of heterogeneity among studies for any comparison.
Potential publication bias
Using Egger’s test, no publication bias could be detected for studies published on the associations of the above-mentioned polymorphisms in the VDR gene with DM risk (T1DM: FokI, P = 0.653; BsmI, P = 0.052; ApaI, P = 0.719; TaqI, P = 0.367; T2DM: FokI, P = 0.460; BsmI, P = 0.421; ApaI, P = 0.721; TaqI, P = 0.269).
Discussion
The vitamin D has important biological functions, such as modulating immunity system, influencing insulin secretion and improving insulin resistance [11, 13], which are involved in the etiology of DM and more likely to be influenced by VDR gene polymorphisms. Given the controversial results from published individual studies with small sample sizes, we performed a meta-analysis to clarify the association between the VDR gene polymorphisms and susceptibility to diabetes mellitus.
To our knowledge, the present study is the first meta-analysis of the association between VDR gene polymorphisms (FokI, ApaI, BsmI, TaqI) and risk of T2DM. In addition, the included sample size in our meta-analysis is more than double that of the meta-analysis on T1DM risk by Guo et al. [16]. Our meta-analysis suggests that FokI polymorphism in the VDR gene is significantly associated with T2DM risk, especially in East Asians, but not with T1DM risk; BsmI polymorphism in the VDR gene was associated with T1DM risk, especially in East Asians, but not with T2DM risk in the overall population, except in East Asians. However, no significant association was found between ApaI or TaqI polymorphism and any type of DM with the exception of significant association between ApaI polymorphism and T1DM risk in East Asians.
The human VDR gene, with a resolution of >100 kb, is located on chromosome 12q12–q14. So far, a number of polymorphisms have been found in and around exons 1f–1c in the 5′ promoter area, in and around the eight protein-coding exons 2–9, and in the 3′ untranslated region (UTR) of the gene [15]. However, among these loci, the FokI polymorphism (ATG–ACG) located in the exon 2 of the gene is the only known locus affecting the structure of the VDR protein produced. The f allele encodes a 427 amino acid protein while the F allele encodes a 424 amino acid protein [15, 72, 73]. The shorter VDR protein variant seem to function more effectively and further increase its capacity of binding 1,25-dihydroxyvitamin D [74], and the relatively higher level of vitamin D, in turn, can reduce the risk of T2DM by enhancing pancreatic β-cell secretion function and improve insulin resistance [12, 13]. This biological mechanism could explain the association between the f allele of FokI polymorphism and susceptibility to T2DM.
It has been suggested that the TaqI polymorphism is a silent mutation despite being located in exon 9, and both BsmI and ApaI are located in the intron between extons 8 and 9 and do not alter the amount of the VDR protein, structure or function [15]. However, in our meta-analysis, a significant association was suggested between BsmI polymorphism and T1DM risk in the overall population especially in East Asians while the association between BsmI polymorphism and T2DM risk was only found in East Asians. So far, the potential mechanism underlying the association of BsmI polymorphism with DM risk remains unclear. The polyA variable number of tandem repeat (VNTR) in the 3′ UTR may be the real effect variation predisposing to the disease, which is in strong linkage disequilibrium (LD) with BsmI polymorphism and related to VDR messenger RNA stability [15, 75]. In addition, it is possible that the BsmI polymorphism in the VDR gene contributes to T-helper 1 response involved in the development of T1DM because of higher levels of interferon-gamma (IFN-γ) produced by peripheral blood mononuclear cells of T1DM patients with BB genotype [45, 46]. Some evidence suggests that the BsmI polymorphism may influence gene transcription, thereby increasing the level of parathormone and osteocalcin and reducing the level of intestinal calcium absorption. These factors, in turn, increase intracellular calcium in adipocytes, enhance lipogenesis and increase demand for insulin, leading to a higher incidence of insulin resistance and T2DM [76]. Further research is required to probe into the actual action of the BsmI polymorphism.
Several limitations in the present meta-analysis should be noted. First, the pooled results were based on unadjusted estimates and therefore potential covariates were not controlled for, including age, gender, vitamin D status varying with ultraviolet level and dietary intake, as well as factors such as physical activity and BMI. Second, although there was heterogeneity among studies in some comparisons in our meta-analysis, no covariate was identified as a potential source of heterogeneity between studies by meta-regression. Therefore, other unknown confounding factors may help explain the between-study heterogeneity. Third, despite gene–gene and gene-environment interactions involved in the pathogenesis of DM, these effects were not assessed in our meta-analysis due to insufficient data from the included studies. Finally, the results of our meta-analysis should be interpreted with caution because the sample size was reduced after the subgroup analysis was performed. However, as a useful statistical tool, meta-analysis allows pooling data from individual studies, thereby undoubtedly increasing the statistical power and the precision of effect estimates.
In conclusion, our meta-analysis suggests that the BsmI polymorphism in the VDR gene is significantly associated with T1DM risk in East Asians and the FokI polymorphism is significantly associated with T2DM risk in East Asians. However, future studies considering gene–gene and gene-environment interactions are needed to investigate these associations. Furthermore, more in depth research is also required to clarify the mechanisms behind the associations of the VDR polymorphisms and DM.
References
American Diabetes Association (2005) Diagnosis and classification of diabetes mellitus. Diabetes Care 28:S37–S42
Herzog BA, Ott PA, Dittrich MT, Quast S, Karulin AY, Kalbacher H et al (2004) Increased in vivo frequency of IA-2 peptide-reactive IFNgamma +/IL-4- T cells in type 1 diabetic subjects. J Autoimmun 23:45–54
Polychronakos C, Li Q (2011) Understanding type 1 diabetes through genetics: advances and prospects. Nat Rev Genet 12:781–792
Zisser H, Gong P, Kelley CM, Seidman JS, Riddell MC (2011) Exercise and diabetes. Int J Clin Pract Suppl 71–75
Cho YS, Chen CH, Hu C, Long J, Hee Ong RT, Sim X et al. (2011) Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in East Asians. Nat Genet. doi: 10.1038/ng.1019
Prokopenko I, McCarthy MI, Lindgren CM (2008) Type 2 diabetes: new genes, new understanding. Trends Genet 24:613–621
Lukacs K, Hosszufalusi N, Dinya E, Bakacs M, Madacsy L, Panczel P (2011) The type 2 diabetes-associated variant in TCF7L2 is associated with latent autoimmune diabetes in adult Europeans and the gene effect is modified by obesity: a meta-analysis and an individual study. Diabetologia. doi: 10.1007/s00125-011-2378-z
Yang Y, Mo X, Chen S, Lu X, Gu D (2011) Association of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PPARGC1A) gene polymorphisms and type 2 diabetes mellitus: a meta-analysis. Diabetes Metab Res Rev 27:177–184
Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM (2001) Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 358:1500–1503
Mitri J, Muraru MD, Pittas AG (2011) Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr 65:1005–1015
Lemire J (2000) 1,25-Dihydroxyvitamin D3-a hormone with immunomodulatory properties. Z Rheumatol 59(S1):24–27
Pittas AG, Lau J, Hu FB, Dawson-Hughes B (2007) The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 92:2017–2029
Ozfirat Z, Chowdhury TA (2010) Vitamin D deficiency and type 2 diabetes. Postgrad Med J 86:18–25
Zmuda JM, Cauley JA, Ferrell RE (2000) Molecular epidemiology of vitamin D receptor gene variants. Epidemiol Rev 22:203–217
Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP (2004) Genetics and biology of vitamin D receptor polymorphisms. Gene 338:143–156
Guo SW, Magnuson VL, Schiller JJ, Wang X, Wu Y, Ghosh S (2006) Meta-analysis of vitamin D receptor polymorphisms and type 1 diabetes: a HuGE review of genetic association studies. Am J Epidemiol 164:711–724
Xu M, Sham P, Ye Z, Lindpaintner K, He L (2010) A1166C genetic variation of the angiotensin II type I receptor gene and susceptibility to coronary heart disease: collaborative of 53 studies with 20,435 cases and 23,674 controls. Atherosclerosis 213:191–199
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22:719–748
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Hauache OM, Lazaretti-Castro M, Andreoni S, Gimeno SG, Brandão C, Ramalho AC et al (1998) Vitamin D receptor gene polymorphism: correlation with bone mineral density in a Brazilian population with insulin-dependent diabetes mellitus. Osteoporos Int 8:204–210
Chang TJ, Lei HH, Yeh JI, Chiu KC, Lee KC, Chen MC et al (2000) Vitamin D receptor gene polymorphisms influence susceptibility to type 1 diabetes mellitus in the Taiwanese population. Clin Endocrinol 52:575–580
Ban Y, Taniyama M, Yanagawa T, Yamada S, Maruyama T, Kasuga A et al (2001) Vitamin D receptor initiation codon polymorphism influences genetic susceptibility to type 1 diabetes mellitus in the Japanese population. BMC Med Genet 2:7
Yokota I, Satomura S, Kitamura S, Taki Y, Naito E, Ito M et al (2002) Association between vitamin D receptor genotype and age of onset in juvenile Japanese patients with type 1 diabetes. Diabetes Care 25:1244
Fassbender WJ, Goertz B, Weismüller K, Steinhauer B, Stracke H, Auch D et al (2002) VDR gene polymorphisms are overrepresented in german patients with type 1 diabetes compared to healthy controls without effect on biochemical parameters of bone metabolism. Horm Metab Res 34:330–337
Györffy B, Vásárhelyi B, Krikovszky D, Madácsy L, Tordai A, Tulassay T et al (2002) Gender-specific association of vitamin D receptor polymorphism combinations with type 1 diabetes mellitus. Eur J Endocrinol 147:803–808
Dong YH, Zhai MX, Li CG, Wang HY, Liu L, Qu N et al (2002) Association of vitamin D receptor gene polymorphism with bone mineral density in the patients with diabetes mellitus. Zhong Hua Nei Fen Mi Dai Xie Za Zhi 18:111–115
Skrabić V, Zemunik T, Situm M, Terzić J (2003) Vitamin D receptor polymorphism and susceptibility to type 1 diabetes in the Dalmatian population. Diabetes Res Clin Pract 59:31–35
Motohashi Y, Yamada S, Yanagawa T, Maruyama T, Suzuki R, Niino M et al (2003) Vitamin D receptor gene polymorphism affects onset pattern of type 1 diabetes. J Clin Endocrinol Metab 88:3137–3140
Turpeinen H, Hermann R, Vaara S, Laine AP, Simell O, Knip M et al (2003) Vitamin D receptor polymorphisms: no association with type 1 diabetes in the Finnish population. Eur J Endocrinol 149:591–596
Martí G, Audí L, Esteban C, Oyarzábal M, Chueca M, Gussinyé M et al (2004) Association of vitamin D receptor gene polymorphism with type 1 diabetes mellitus in two Spanish populations. Med Clin (Barc) 123:286–290
Bianco MG, Minicucci L, Calevo MG, Lorini R (2004) Vitamin D receptor polymorphisms: are they really associated with type 1 diabetes? Eur J Endocrinol 151:641–642
Shen BS (2004) The association of vitamin D receptor gene polymorphism with diabetes mellitus in the Han nationality of Tianjin area. Master’s Thesis of Tianjin Medical University, pp 1–49
San-Pedro JI, Bilbao JR, Perez de Nanclares G, Vitoria JC, Martul P, Castaño L (2005) Heterogeneity of vitamin D receptor gene association with celiac disease and type 1 diabetes mellitus. Autoimmunity 38:439–444
Zemunik T, Skrabic V, Boraska V, Diklic D, Terzic IM, Capkun V et al (2005) FokI polymorphism, vitamin D receptor, and interleukin-1 receptor haplotypes are associated with type 1 diabetes in the Dalmatian population. J Mol Diagn 7:600–604
Liao L (2005) The association of vitamin D status with latent autoimmune diabetes in adults. Doctor’s Thesis of Central South University, pp 1–128
Capoluongo E, Pitocco D, Concolino P, Santonocito C, Di Stasio E, d’Onofrio G et al (2006) Slight association between type 1 diabetes and “ff” VDR FokI genotype in patients from the Italian Lazio Region. Lack of association with diabetescomplications. Clin Biochem 39:888–892
Xiao XH, Liu ZL, Wang H, Sun Q, Li WH, Yang GH et al (2006) Effects of vitamin D receptor gene polymorphisms on susceptibility to type 1 diabetes mellitus. Chin Med Sci J 21:95–98
García D, Angel B, Carrasco E, Albala C, Santos JL, Pérez-Bravo F (2007) VDR polymorphisms influence the immune response in type 1 diabetic children from Santiago, Chile. Diabetes Res Clin Pract 77:134–140
Shi YJ, Shen Y, Cai LQ, Hu F, Yang YY (2007) Relationship between vitamin D receptor gene polymorphism and diabetes mellitus. Zhong Guo Tang Niao Bing Za Zhi 15:219–221
López T, García D, Angel B, Carrasco E, Codner E, Ugarte F et al (2008) Association between Fok I vitamin D receptor (VDR) gene polymorphism and plasmatic concentrations of transforming growth factor-beta1 and interferon gamma in type 1 diabetes mellitus. Med Clin (Barc) 130:81–84
Lemos MC, Fagulha A, Coutinho E, Gomes L, Bastos M, Barros L et al (2008) Lack of association of vitamin D receptor gene polymorphisms with susceptibility to type 1 diabetes mellitus in the Portuguese population. Hum Immunol 69:134–138
Shimada A, Kanazawa Y, Motohashi Y, Yamada S, Maruyama T, Ikegami H et al (2008) Evidence for association between vitamin D receptor BsmI polymorphism and type 1 diabetes in Japanese. J Autoimmun 30:207–211
Panierakis C, Goulielmos G, Mamoulakis D, Petraki E, Papavasiliou E, Galanakis E (2009) Vitamin D receptor gene polymorphisms and susceptibility to type 1 diabetes in Crete, Greece. Clin Immunol 133:276–281
Mory DB, Rocco ER, Miranda WL, Kasamatsu T, Crispim F, Dib SA (2009) Prevalence of vitamin D receptor gene polymorphisms FokI and BsmI in Brazilian individuals with type 1 diabetes and their relation to beta-cell autoimmunity and to remaining beta-cell function. Hum Immunol 70:447–451
Israni N, Goswami R, Kumar A, Rani R (2009) Interaction of vitamin D receptor with HLA DRB1 0301 in type 1 diabetes patients from North India. PLoS One 4:e8023
Sheng ZY, Zhang WW, You L, Cheng JY, Wang YF, Wang YF et al (2009) The association of vitamin D receptor gene polymorphism with type 1 diabetes mellitus in Han nationality of Shanghai area. Zhong Guo Tang Niao Bing Za Zhi 17:666–668
Kocabaş A, Karagüzel G, Imir N, Yavuzer U, Akçurin S (2010) Effects of vitamin D receptor gene polymorphisms on susceptibility to disease and bone mineral density in Turkish patients with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 23:1289–1297
Mohammadnejad Z, Ghanbari M, Ganjali R, Afshari JT, Heydarpour M, Taghavi SM et al. (2011) Association between vitamin D receptor gene polymorphisms and type 1 diabetes mellitus in Iranian population. Mol Biol Rep (doi: 10.1007/s11033-011-0805-3)
Boullu-Sanchis S, Leprêtre F, Hedelin G, Donnet JP, Schaffer P, Froguel P et al (1999) Type 2 diabetes mellitus: association study of five candidate genes in an Indian population of Guadeloupe, genetic contribution of FABP2 polymorphism. Diabetes Metab 25:150–156
Speer G, Cseh K, Winkler G, Vargha P, Braun E, Takács I et al (2001) Vitamin D and estrogen receptor gene polymorphisms in type 2 diabetes mellitus and in android type obesity. Eur J Endocrinol 144:385–389
Ye WZ, Reis AF, Dubois-Laforgue D, Bellanné-Chantelot C, Timsit J, Velho G (2001) Vitamin D receptor gene polymorphisms are associated with obesity in type 2 diabetic subjects with early age of onset. Eur J Endocrinol 145:181–186
Oh JY, Barrett-Connor E (2002) Association between vitamin D receptor polymorphism and type 2 diabetes or metabolic syndrome in community-dwelling older adults: the Rancho Bernardo Study. Metabolism 51:356–359
Malecki MT, Frey J, Moczulski D, Klupa T, Kozek E, Sieradzki J (2003) Vitamin D receptor gene polymorphisms and association with type 2 diabetes mellitus in a Polish population. Exp Clin Endocrinol Diabetes 111:505–509
Li HM, Miao H, Lu YB, Geng HF, Jiang XQ (2005) Association between DNA polymorphism of human vitamin D receptor gene and type 2 diabetes mellitus. Zhong Guo Xian Dai Yi Xue Za Zhi 15:989–992
Li HM, Miao H, Lu YB, Cheng JL, Geng HF, Jiang XQ (2005) Association between the polymorphism of human vitamin D receptor gene and the susceptibility of diabetic nephropathy in Chinese Han population. Zhong Guo Lin Chuang Kang Fu 15:1–4
Xu JR, Lu YB, Geng HF, Wu J, Miao H (2007) Association between the polymorphism of huaman vitamin D receptor gene and type 2 diabetes. Zhong Guo Zu Zhi Gong Cheng Yan Jiu Yu Lin Chuang Kang Fu 11(5881–5883):6018
Du T, Zhou ZG, Liao L (2008) Association between the VDR gene FokI polymorphism and type 2 diabetes. Nan Jing Da Xue Xue Bao (Li Ke Ban) 32:489–492
Zhang P, Su WL, Shen BS, Li DQ, Qiu MC (2008) Association between vitamin D receptor gene polymorphism and type 2 diabetes mellitus of Han nationality in Tianjin. Tian Jin Yi Yao 36:255–257
Zhai MX, Liu F, Chen XP, Lv ZY, Pan L (2008) Association of vitamin D receptor gene polymorphism with Igt and type 2 diabetes mellitus. Wei Fang Yi Xue Yuan Xue Bao 30:47–49
Wang CX (2009) Investigations on the gene polymorphisms of vitamin D receptor and IL-10 as the risk factors for chronic periodontitis and type 2 diabetes mellitus. Doctor’s Thesis of Southern Medical University, pp 1–108
Bai R, Liu M, Du JL, Xing Q, Liu Y, Sun LP, Li CC (2009) Relationship of vitamin D receptor (VDR) FokI gene polymorphism with type 2 diabetes and the combined type 2 diabetes and atherosclerosis. Zhong Guo Tang Niao Bing Za Zhi 17:892–894
Bid HK, Konwar R, Aggarwal CG, Gautam S, Saxena M, Nayak VL et al (2009) Vitamin D receptor (FokI, BsmI and TaqI) gene polymorphisms and type 2 diabetes mellitus: a North Indian study. Indian J Med Sci 63:187–194
Lan XC, Huo XJ (2009) Relationship of vitamin D receptor (VDR) BsmI gene polymorphism with type 2 diabetes mellitus. Hai Xia Yi Xue 21:141–142
Ding HG, Liu PY, Liu JY, Li Y, Ye H, Wu L et al (2009) The association of vitamin D receptor gene polymorphism with latent autoimmune diabetes in adults. Shen Zhen Zhong Xi Yi Jie He Za Zhi 19(336–338):355
Mukhopadhyaya PN, Acharya A, Chavan Y, Purohit SS, Mutha A (2010) Metagenomic study of single-nucleotide polymorphism within candidate genes associated with type 2 diabetes in an Indian population. Genet Mol Res 9:2060–2068
Nosratabadi R, Arababadi MK, Salehabad VA, Shamsizadeh A, Mahmoodi M, Sayadi AR et al (2010) Polymorphisms within exon 9 but not intron 8 of the vitamin D receptor are associated with the nephropathic complication of type-2 diabetes. Int J Immunogenet 37:493–497
Dilmec F, Uzer E, Akkafa F, Kose E, van Kuilenburg AB (2010) Detection of VDR gene ApaI and TaqI polymorphisms in patients with type 2 diabetes mellitus using PCR-RFLP method in a Turkish population. J Diabetes Complications 24:186–191
Zhao T, Yi B, Zhang H (2011) Vitamin D receptor gene polymorphism and the susceptibility of type 2 diabetes mellitus. Yi Xue Lin Chuang Yan Jiu 28:668–670
Arai H, Miyamoto K, Taketani Y, Yamamoto H, Iemori Y, Morita K et al (1997) A vitamin D receptor gene polymorphism in the translation initiation codon: effect on protein activity and relation to bone mineral density in Japanese women. J Bone Miner Res 12:915–921
Nejentsev S, Godfrey L, Snook H, Rance H, Nutland S, Walker NM (2004) Comparative high-resolution analysis of linkage disequilibrium and tag single nucleotide polymorphisms between populations in thevitamin D receptor gene. Hum Mol Genet 13:1633–1639
Reis AF, Hauache OM, Velho G (2005) Vitamin D endocrine system and the genetic susceptibility to diabetes, obesity and vascular disease. A review of evidence. Diabetes Metab 31:318–325
Obi-Tabot ET, Tian XQ, Chen TC, Holick MF (2000) A human skin equivalent model that mimics the photoproduction of vitamin D3 in human skin. In Vitro Cell Dev Biol Anim 36:201–204
Ortlepp JR, Lauscher J, Hoffmann R, Hanrath P, Joost HG (2001) The vitamin D receptor gene variant is associated with the prevalence of type 2 diabetes mellitus and coronary artery disease. Diabet Med 18:842–845
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11033_2012_1805_MOESM2_ESM.tif
Supplementary Fig. 1. Meta-analysis of the association between FokI polymorphism in the VDR gene and type 1 diabetes (f vs. F) (TIFF 1579 kb)
11033_2012_1805_MOESM3_ESM.tif
Supplementary Fig. 2. Meta-analysis of the association between ApaI polymorphism in the VDR gene and type 1 diabetes (a vs. A) (TIFF 1703 kb)
11033_2012_1805_MOESM4_ESM.tif
Supplementary Fig. 3. Meta-analysis of the association between TaqI polymorphism in the VDR gene and type 1 diabetes (t vs. T) (TIFF 1794 kb)
11033_2012_1805_MOESM5_ESM.tif
Supplementary Fig. 4. Meta-analysis of the association between BsmI polymorphism in the VDR gene and type 2 diabetes (B vs. b) (TIFF 1672 kb)
11033_2012_1805_MOESM6_ESM.tif
Supplementary Fig. 5. Meta-analysis of the association between ApaI polymorphism in the VDR gene and type 2 diabetes (a vs. A) (TIFF 1767 kb)
11033_2012_1805_MOESM7_ESM.tif
Supplementary Fig. 6. Meta-analysis of the association between TaqI polymorphism in the VDR gene and type 2 diabetes (t vs. T) (TIFF 1839 kb)
Rights and permissions
About this article
Cite this article
Wang, Q., Xi, B., Reilly, K.H. et al. Quantitative assessment of the associations between four polymorphisms (FokI, ApaI, BsmI, TaqI) of vitamin D receptor gene and risk of diabetes mellitus. Mol Biol Rep 39, 9405–9414 (2012). https://doi.org/10.1007/s11033-012-1805-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1805-7