Abstract
Patients with hormone receptor positive breast cancer who are treated with endocrine therapy generally have a good prognosis. However, resistance to hormonal therapy and progression occurs, and the reasons for this are manifold. It has been proposed that the local estrogenic environment has a role in the process of local invasion and progression. We have determined the expression pattern of estrogen receptor α, estrogen receptor β, and the epithelial and stromal expression of the estrogen-metabolizing enzymes aromatase and sulfotransferase by immunohistochemistry in tissue arrays, containing 50 paraffin-embedded sets of tissues obtained from breast cancer and from corresponding metastatic axillary lymph nodes of the same patients. We have found statistically significant higher estrogen receptors α and β expression in primary tumors than in corresponding lymph node metastases (p = 0.0004 and p = 0.003, respectively). Aromatase was also expressed more frequently in epithelial as well as in stromal cells of the malignant tumor when compared to according lymph node metastases (p = 0.08 and p = 0.12, respectively). While in lymph node metastases only estrogen receptor α and stromal aromatase expression were correlated (p = 0.01), significant associations were seen between the estrogen receptor β and stromal aromatase, and epithelial sulfotransferase (p = 0.0006 and p = 0.03, respectively) in the primary tumor. We hypothesize that the decreased expression of local estrogens by aromatase, in combination with a decreased expression of estrogen receptors α and β in lymphatic metastases, renders these metastases hormone insensitive and could contribute to the poor response to endocrine therapy that is often seen in nodal-positive tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The estrogenic receptor (ER) and progesterone receptor (PR) statuses currently represent the best predictive marker for determining a potential response to endocrine therapy strategies. ER and PR expression analyses have therefore emerged as routine parameters that are used to identify those patients who will most likely benefit from antihormonal treatment. The predictive value of ER and PR staining, however, is far from being ideal, since not all patients with receptor-positive tumors will equally benefit, and many will experience tumor progression despite endocrine treatment. To date, the precise mechanisms responsible for resistance to endocrine therapy are not completely understood. Several lines of evidence suggest that cross-talk between ER and a complex system of growth factor receptor pathways, which activate the ER, may have a key role [1, 2].
Other hypotheses that serve to explain why tumor progression occurs under endocrine therapy include a differential local expression of ERs and sex steroid metabolizing enzymes. Three key enzymes regulate the estrogen biosynthesis: aromatase, steroid sulfatase (STS), and estrogen sulfotransferase (EST) [3]. The aromatase enzyme converts androgens to estradiol (E2) and plays a pivotal role in the synthesis of estrogen [4, 5]; STS is responsible for the hydrolysis of steroid sulfates to their unconjugated, biologically active forms [6]. Seventeen-β-hydroxysteroid dehydrogenase type 2 (17β-HSD-2), in turn, reverses this step and inactivates E2 by conversion to estrone (E1). EST catalyzes the transfer of a sulfuryl group to estrogens. The conversion of E1 and E2 to their sulfates (E1S and E2S) prevents them from binding to and thereby activating the ER [7]. Sulfatation of estrogens therefore leads to a further reduction of biologically active estrogen concentrations [8, 9]. Taken together, the activity of intratumoral aromatase and sulfatase enzymes results in an increase in local estrogen concentrations, while sulfotransferase counteracts the effects of both enzymes by reducing the amount of bio-available estradiol in a breast cancer. It is the net balance of the three enzymes, together with the local expression of ERs, that determines the estrogenic response. Since nodal positivity characterizes tumors that are particularly aggressive and that often progress despite endocrine treatment, we have investigated whether a differential expression of ERs and estrogen-converting enzymes in lymph node metastases could be responsible for the resistance to endocrine treatment strategies. We have therefore analyzed the expression of ERs α and β, aromatase, and sulfotransferase in primary breast cancer tissue and the according lymph node metastases.
Materials and methods
Patients and samples
Commercially available tissue arrays (US Biomax BR1001) contain 50 paraffin-embedded sets of tissues obtained from the malignant tumor of the breast and 50 paraffin-embedded sets of tissues from metastatic lymph nodes of the same patients.
As shown in the patients’ characteristics (Table 1), two breast cancer cases and two metastatic lymph node samples have been excluded because of the absence of breast cancer cells (fibro-fatty tissue). Twenty-seven patients (54%) were younger than 50 years of age. The majority of breast cancer cases (84%) were invasive ductal (IDC). The tissue array contained two cases (4%) of invasive lobular carcinoma, two samples (4%) of neuroendocrine cancer, one (2%) micropapillary carcinoma, and one (2%) ductal carcinoma in situ.
Seventeen tumors (34%) were defined as grade 1, 22 cases (44%) as grade 2, and one (2%) sample as grade 3.
Immunohistochemistry
Tissue sections of paraffin-embedded formalin-fixed tissue blocks were dewaxed in xylene for 30 s each, followed by two washes in 100% ethanol for 30 s each, and washed twice in dH2O for 5 min. Slides were then rinsed in tap water followed by antigen retrieval using 10 mM citrate buffer at pH 6.0 for 15 min. Slides were allowed to cool to room temperature (RT) then washed with phosphate-buffered saline (PBS) and distilled water for 30 s. The sections were then blocked with Ultra-V Block (Lab Vision, Westinghouse Drive, Fremont, CA, USA) for 7 min. After a consecutive PBS wash, slides were incubated with the primary ER α antibody (NeoMarkers 1D5, diluted 1:100 in PBS, 60 min at RT, NeoMarkers, Fremont, CA, USA) and ER β antibody (Santa Cruz H-150, diluted 1:50 in PBS, and 80 min at RT). The anti-aromatase antibody (dilution 1:1,000 in PBS/Tween) was generated from a rabbit immunoglobulin fraction of an antiserum prepared against human placental aromatase A (provided by Dr. N. Harada, Fujita Health University Hospital, Toyaoake, Japan [10, 11]), and the monoclonal mouse EST Ab1 antibody (MS-233) was purchased from NeoMarkers (Fremont, CA, USA) and was used at a concentration of 2 μg/ml [10, 11]. Negative controls were performed on all tissue sections by replacing primary antibodies with diluted isotype immunoglobulin (ImmunoCruz™ Staining System, Santa Cruz Biotechnology). The slides were then incubated with goat anti-polyvalent (Lab Vision Polymer Detection System) and streptavidin-HRP (Lab Vision) for 60 min. This was followed by incubation with 3-amino-9-ethylcarbazole (AEC, a widely used chromogen). Slides were then washed in PBS, counterstained with hematoxylin for 5 s, and cover-slipped.
Immunostaining quantification
Two pathologists of our department quantified expression of aromatase and sulfotransferase enzymes using a semiquantitative scoring system described by Remmele et al. [12] independently. The immunoreactive score (IRS) is the product of staining intensity (from 0 = no staining to 4 = strong staining) and the percentage of positive cells (0 = 0% cells stained, 1 = less than 10% of cells stained, 2 = 10–50% of cells stained, 3 = 51–80% stained, and 4 = more than 80% of cells stained). The IRS values ranges from 0 to 12, and an IRS of more than 2 is considered positive. We calculated the ER α scores as the percentage of positively stained nuclei, and tumors were defined “positive” when more than 10% of the nuclei stained positive. Cytoplasmic staining was scored for ER β.
Statistical analysis
The chi-square test was used to identify differences in ER α and β, aromatase, and sulfotransferase expression levels in epithelial and stromal tissue components of the primary tumor and the according lymph node metastases. Bonferroni’s correction was used to correct for multiple comparisons. Correlations between ER α and β, aromatase, and sulfatase and clinical parameters were analyzed by Spearman’s test (two-sided). For all analyses, p value <0.05 was considered statistically significant. Win-SAS V 8 (SAS Institute GmbH, Heidelberg, Germany) statistical software system was used for all calculations.
Results
Expression of ER α and ER β in the primary breast tumor and the according lymph node metastasis
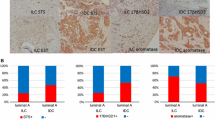
Table 2 shows the protein expression of ER α, ER β, epithelial (Ae) and stromal aromatase (As), and epithelial (ESTe) and stromal sulfotransferase (ESTs) in primary breast cancer tissue and matched lymph node metastases as measured by immunohistochemical analysis. Strong nuclear staining of ER α was found in eight of 48 cases (17%) of primary breast tumors (Fig. 1a). Moderate staining of ER α was observed in six of 48 (13%) breast cancer tissues and weak staining in five samples (11%). ER α was absent in 27 of 48 cases (59%) of primary breast tumors. Cytoplasmic staining of ER β was absent in 19 of 48 cases (41%) of primary breast cancer, weakly expressed in 16 of 48 cases (35%), and moderately expressed in 11 of 48 samples (24%; Fig. 1c). We did not find any sample of primary breast tumor with a strong staining of ER β.
Immunohistochemical analysis of the differential expression of estrogen receptor alpha, estrogen receptor beta, aromatase, and sulfotransferase in primary tumor tissue and according lymph node metastases. a High expression (+++) of estrogen receptor alpha in primary breast tumor. b No expression (−) of estrogen receptor alpha in metastatic lymph node. c Moderate expression (++) of estrogen receptor beta in breast cancer tissue. d No expression (−) of estrogen receptor beta in lymph node metastasis. e High expression (+++) of aromatase in primary breast tumor. f No expression (−) of aromatase in lymphatic metastasis. g High expression (+++) of sulfotransferase in primary breast cancer. h No expression (−) of sulfotransferase in lymph node metastasis (×10 magnification)
In the according lymph node metastases, strong ER α expression was observed in four of 48 cases (9%), while moderate expression was present in nine of 48 (20%) and weak expression in six of 48 samples (13%). ER α staining was absent in 27 of 48 cases (59%; Fig. 1b). ER β protein was absent in 28 of 48 cases (61%) of matched lymph node metastases (Fig. 1d), weakly expressed in 11 cases (24%), and moderately expressed in seven cases (15%), while none of the samples (0%) showed a strong expression of ER β.
When we compared the expression of ER α and ER β between breast cancer tissue and the according lymphatic metastases, we found a statistically significant higher expression of both receptors in the primary tumor (chi-square test; ER α, p = 0.0004; ER β, p = 0.003, respectively).
Expression of aromatase and sulfotransferase in breast cancer and corresponding lymph node metastasis
The expression of aromatase and sulfotransferase in breast cancer and corresponding lymph node metastases is shown in Fig. 1e–h and Table 2. We found strong epithelial aromatase staining in the primary breast cancer in zero of 48 cases (0%), a moderate staining in 21 of 48 cases (45%; Fig. 1e), and a weak staining in 14 of 48 cases (30%). The aromatase expression in the epithelium of the primary tumor samples was absent in 12 of 48 cases (26%). The stromal aromatase expression in primary breast cancer tissue lacked in 12 of 48 cases (26%) was weakly expressed in ten cases (21%) and moderately expressed in 25 cases (53%). Strong staining of the aromatase was not found in any of the 48 samples (0%) of primary breast tumor. When the staining intensity of aromatase in the epithelium of the according lymph node metastases was analyzed, a strong staining was found in one of 48 cases (2%), a moderate staining in two cases (4%), and a weak staining in 11 cases (23%). The epithelial aromatase expression was absent in 34 of 48 cases (71%) of matched lymph node metastases (Fig. 1f). The aromatase in the stroma of lymph node metastases was highly expressed in zero of 48 cases (0%), moderately expressed in 17 cases (35%), and weakly expressed in nine cases (19%). No aromatase staining was found in the stroma of 22 cases (46%) of according lymph node metastases.
When aromatase staining in primary tumor tissue was compared to the according lymph node metastases, a trend towards significantly higher levels of aromatase was observed in the epithelium (Ae, p = 0.08; chi-square test) and the stroma (As, p = 0.12; chi-square test) of the primary tumor.
When we analyzed the EST expression in the epithelium of primary tumor tissue, we found strong staining in zero of 48 cases (0%), moderate staining in four cases (8%; Fig. 1g), and weak staining in five of 48 cases (10%). Epithelial EST expression failed in 39 of 48 samples (81%) of primary breast cancer. The stromal EST expression was absent in 47 of 48 cases (98%) and showed a weak staining in only one sample (2%) of primary tumor tissue. In the lymph node metastases, stromal EST was absent in even 48 of 48 cases (100%), whereas the epithelial expression of EST was observed with a strong staining in one of 48 cases (2%) and a moderate staining in six cases (13%). In 41 of 48 (85%) samples of matched nodal metastases, epithelial EST expression was absent (Fig. 1h).
When we compared the sulfotransferase expression in the primary tumor with the lymph node metastases, we observed a statistically nonsignificant slightly higher expression in the epithelium of primary breast cancer cells (ESTe, p = 0.12; chi-square test). We found no difference in the stromal expression of sulfotransferase, which failed in 98% of breast cancer tissue and 100% of lymph node metastases.
Overall correlations between ER α, ER β, aromatase, and sulfotransferase protein expression
We then investigated whether ER, aromatase, and sulfotransferase expression in lymph node metastases was comparable to the respective expression levels in the primary tumor. Table 3 shows associations between the ER, aromatase, and sulfotransferase protein expression in primary tumor and corresponding lymph node metastases. We found a positive correlation between the expression of aromatase in the epithelium of the primary breast tumor and the according lymph node metastases (r = 0.34, p = 0.02; Spearman’s test). Furthermore, we observed a statistically significant positive correlation (r = 0.44, p = 0.0015; Spearman’s test) between the expression of stromal aromatase in primary tumor tissue and lymphatic metastases. The stromal aromatase of the breast cancer tissue was also positively correlated to the ER β expression of matched lymph node metastases (r = 0.48, p = 0.0006; Spearman’s test).
The expression of ER β in primary breast cancer tissue was significantly and positively correlated with the ER β expression (r = 0.55, p ≤ 0.0001; Spearman’s test) and the epithelial aromatase expression in matched nodal metastases (r = 0.38, p = 0.0095; Spearman’s test). We also found a highly significant correlation between the expression of ER α in primary tumor tissue and according lymph node metastases (r = 0.75, p ≤ 0.0001; Spearman’s test).
Associations between the epithelial and stromal ER α, ER β, aromatase, and sulfotransferase protein expression within the breast and the lymph node subgroup
When we looked at correlations between different estrogen-regulating enzymes and ER status within the breast, we found that the ER β is statistically significant and positively correlated to the expression of aromatase in the stroma (r = 0.48, p = 0.0006; Spearman’s test) and the sulfotransferase expression in the epithelium (r = 0.31, p = 0.03; Spearman’s test; Table 4). None of the other parameters was correlated with each other. We then went on to analyze possible correlations between the epithelial and stromal expression of ER, aromatase, and sulfotransferase within involved axillary lymph nodes. As shown in Table 5, we found a significantly negative correlation between the expression of ER α and the aromatase expression in the stroma of the nodal metastases (r = −0.36, p = 0.01; Spearman’s test). None of the other parameters was correlated.
Discussion
The local estrogenic environment is a consequence of both the expression of ERs on tumor cells and the spatial distribution of estrogen-converting enzymes. While the presence of such enzymes in the primary tumor has been demonstrated previously, considerably less is known about such a network in corresponding lymph nodes [13]. This is somewhat surprising since tumor cells have been shown to lose estrogen responsiveness as they progress and develop distant metastases. To our knowledge, no study exists examining the expression of ER β, aromatase, and sulfotransferase in primary tumor tissue and lymph node metastases, which would help to explain why endocrine therapies are less effective with disease progression. We therefore looked at the expression pattern of ERs α and β, aromatase, and sulfotransferase in breast cancer tissue and the according lymphatic metastases. Our finding of a significantly higher expression of ER α and β and aromatase in the primary breast cancer tissue somewhat contrasts findings by Kristek et al. who detected no difference in the expression of ER α and PR between the primary tumor and the metastatic axillary lymph node [14]. However, in the mouse model, Harrel et al. also observed a downregulation of the ER in corresponding lymphatic metastases. The authors suggested that the lymph node microenvironment alters estradiol signaling and may contribute to local anti-estrogen resistance [15]. We have also investigated ER β protein expression in the corresponding samples because the receptor has a different transcriptional activity than ER α and because it has been implicated in tamoxifen resistance [16, 17]. In addition, ER β modulates ER α activity. Vinayagam et al. showed that high levels of ER β in primary breast cancer are associated with ER α expression and better outcome of adjuvant treatment [18]. Esslimani-Sahla et al. found that a low ER β level is an independent marker to predict tamoxifen resistance, better suited than ER α [19]. Other groups, however, have questioned this hypothesis, since in elderly patients, ER β mRNA expression was neither predictive of response to preoperative toremifene nor did expression levels provide additional information for the likelihood of response [20]. In our hands, aromatase was found in both epithelium (75%) and stroma (74%) of primary tumors. This percentage has been shown in several previous studies of aromatase expression in breast cancer [21–25]. The localization of aromatase activity in malignant breast tissue, however, differs from one study to the other: Santner et al. [26] and Purohit et al. [27] found a higher activity in the stromal than in the epithelial component of breast tumors. In contrast, Esteban et al. [28], Lu et al. [29], and Brodie et al. [30] described an aromatase expression in parenchymal cells. Furthermore, Miki et al. [31] found aromatase protein in both stromal and epithelial components and no difference between the stromal and parenchymal expression of aromatase in breast cancer cells. Until now, however, aromatase expression in lymph node metastases has never been described.
In contrast to aromatase, relatively little is known about sulfotransferase. Data suggest that expression of EST is a potent prognostic factor in human breast carcinoma. Suzuki et al. [32] found a significant inverse correlation of EST with tumor size and lymph node status, and a positive correlation with a decreased risk of recurrence. Hudelist et al. [13] investigated the differential expression of EST in preinvasive and invasive breast cancer and found a significant downregulation in high-grade DCIS compared to non-high-grade cases. To date, sulfotransferase expression in lymphatic metastases has never been investigated. In our analysis, EST was expressed in only 18% of primary breast cancer tissues and in 15% of corresponding metastases. This low expression status suits to the conclusion of previously mentioned studies that more aggressive tumors downregulate EST expression.
Our pilot study is limited by the small sample size and the fact that we analyzed commercially available tissue arrays without the possibility to correlate our findings with clinical parameters. We therefore plan to investigate the expression of ERs and estrogen-modulating enzymes of breast cancer patients before and after neoadjuvant endocrine therapy.
Our results indicate that tumor progression is not only associated with a decrease in estrogen sensitivity but also with a decreased production of local estrogens. Since the estrogen-inactivating sulfotransferase is essentially unchanged in comparison to the primary tumor, this would result in a net decrease of local estrogens. It is not clear, whether the ensuing decrease in estrogen sensitivity is a result of increasing endocrine independence of lymph node metastases. Alternatively, it is possible that tumor cells that have the potential to metastasize into lymph vessels represent a particularly aggressive subgroup that is characterized by de novo estrogen independence.
References
Schiff R, Massarweh S, Shou J, et al. Breast cancer endocrine resistance: how growth factor signaling and estrogen receptor coregulators modulate response. Clin Cancer Res. 2003;9:447–54.
Bedard PL, Freedman OC, Howell A, et al. Overcoming endocrine resistance in breast cancer: are signal transduction inhibitors the answer? Breast Cancer Res Treat. 2008;108:307–17.
Hudelist G, Czerwenka K, Keckstein J, et al. Expression of aromatase and estrogen sulfotransferase in eutopic and ectopic endometrium: evidence for unbalanced estradiol production in endometriosis. Reprod Sci. 2007;14:798–805.
Abul-Hajj YJ, Iverson R, Kiang DT. Aromatization of androgens by human breast cancer. Steroids. 1979;33:205–22.
Lipton A, Santner SJ, Santen RJ, et al. Aromatase activity in primary and metastatic human breast cancer. Cancer. 1987;59:779–82.
Suzuki T, Miki Y, Nakamura Y, et al. Sex steroid-producing enzymes in human breast cancer. Endocr Relat Cancer. 2005;12:701–20.
Zhang H, Varlamova O, Vargas FM, Falany CN, Leyh TS. Sulfuryl transfer: the catalytic mechanism of human estrogen sulfotransferase. J Biol Chem. 1998;273:10888–92.
Geisler J. Breast cancer tissue estrogens and their manipulation with aromatase inhibitors and inactivators. J Steroid Biochem Mol Biol. 2003;86:245–53.
Geisler J, Lønning PE. Aromatase inhibition: translation into a successful therapeutic approach. Clin Cancer Res. 2005;11:2809–21.
Sasano H, Nagura H, Harada N, Goukon Y, Kimura M. Immunolocalization of aromatase and other steroidogenic enzymes in human breast disorders. Hum Pathol. 1994;25:530–5.
Sasano H, Frost AR, Saitoh R, et al. Aromatase and 17 beta-hydroxysteroid dehydrogenase type 1 in human breast carcinoma. J Clin Endocrinol Metabol. 1996;81:4042–6.
Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–40.
Hudelist G, Wülfing P, Kersting C. Expression of aromatase and estrogen sulfotransferase in preinvasive and invasive breast cancer. J Cancer Res Clin Oncol. 2008;134:67–73.
Kristek J, Dmitrović B, Kurbel S, et al. Tumor growth fraction, expression of estrogen and progesterone receptors, p53, bcl-2 and cathepsin D activity in primary ductal invasive breast carcinoma and their axillary lymph node metastases. Coll Antropol. 2007;31:1043–7.
Harrell JC, Dye WW, Harvell DM, et al. Estrogen insensitivity in a model of estrogen receptor positive breast cancer lymph node metastasis. Cancer Res. 2007;67:10582–91.
Speirs V, Parkes AT, Kerin MJ, et al. Coexpression of estrogen receptor alpha and beta: poor prognostic factors in human breast cancer? Cancer Res. 1999;59:525–8.
Rosa FE, Caldeira JR, Felipes J, et al. Evaluation of estrogen receptor alpha and beta and progesterone receptor expression and correlation with clinicopathologic factors and proliferative marker Ki-67 in breast cancers. Hum Pathol. 2008;39:720–30.
Vinayagam R, Sibson DR, Holcombe C, Aachi V, Davies MP. Association of oestrogen receptor beta 2 (ER beta 2/ER beta cx) with outcome of adjuvant endocrine treatment for primary breast cancer- a retrospective study. BMC Cancer. 2007;7:131.
Esslimani-Sahla M, Simony-Lafontaine J, Kramar A, et al. Estrogen receptor beta (ER beta) level but not its ER beta cx variant helps to predict tamoxifen resistance in breast cancer. Clin Cancer Res. 2004;10:5769–76.
Cappelletti V, Celio L, Bajetta E, et al. Prospective evaluation of estrogen receptor-beta in predicting response to neoadjuvant antiestrogen therapy in elderly breast cancer patients. Endocr-Relat Cancer. 2004;11:761–70.
Silva MC, Rowlands MG, Dowsett M, et al. Intratumoral aromatase as a prognostic factor in human breast carcinoma. Cancer Res. 1989;49:2588–91.
Miller WR, Anderson TJ, Jack WJ. Relationship between tumor aromatase activity, tumor characteristics and response to therapy. J Steroid Biochem Mol Biol. 1990;37:1055–9.
Miller WR. Aromatase activity in breast tissue. J Steroid Biochem Mol Biol. 1991;39:783–90.
Bolufer P, Ricart E, Lluch A, et al. Aromatase activity and estradiol in human breast cancer: its relationship to estradiol and epidermal growth factor receptors and to tumor-node-metastasis staging. J Clin Oncol. 1992;10:438–46.
Lipton A, Santen RJ, Santner SJ, Harvey HA, Sanders SI, Matthews YL. Prognostic value of breast cancer aromatase. Cancer. 1992;70:1951–5.
Santner SJ, Feil PD, Santen RJ. In situ estrogen production via the estrone sulfatase pathway in breast tumors: relative importance versus the aromatase pathway. J Clin Endocrinol Metab. 1984;59:29–33.
Purohit A, Newman SP, Reed MJ. The role of cytokines in regulating estrogen synthesis: implications for the etiology of breast cancer. Breast Cancer Res. 2002;4:65–9.
Esteban JM, Warsi Z, Haniu M, Hall P, Shively JE, Chen S. Detection of intratumoral aromatase in breast carcinomas. An immunohistochemical study with clinicopathologic correlation. Am J Pathol. 1992;140:337–43.
Lu Q, Nakmura J, Savinov A, et al. Expression of aromatase protein and messenger ribonucleic acid in tumor epithelial cells and evidence of functional significance of locally produced estrogen in human breast cancers. Endocrinology. 1996;137:3061–8.
Brodie AM, Lu Q, Long BJ, et al. Aromatase and COX-2 expression in human breast cancers. J Steroid Biochem Mol Biol. 2001;79:41–7.
Miki Y, Suzuki T, Tazawa C, et al. Aromatase localization in human breast cancer tissues: possible interactions between intratumoral stromal and parenchymal cells. Cancer Res. 2007;67:3945–54.
Suzuki T, Nakata T, Miki Y, et al. Estrogen sulfotransferase and steroid sulfatase in human breast carcinoma. Cancer Res. 2003;63:2762–70.
Conflicts of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this scientific work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gschwantler-Kaulich, D., Fink-Retter, A., Czerwenka, K. et al. Differential expression pattern of estrogen receptors, aromatase, and sulfotransferase in breast cancer tissue and corresponding lymph node metastases. Tumor Biol. 32, 501–508 (2011). https://doi.org/10.1007/s13277-010-0144-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-010-0144-3