Abstract
Evaluation of expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor 2 (HER2/neu) receptor was carried out on 80 breast cancer patients before and after neoadjuvant chemotherapy (NAC). No differences in expression were noted in 89% with reference to ER, 95% with reference to PR, and 91% with reference to HER2/neu status. A change in receptor status from positive to negative was noted in 12% for ER, 5% for PR, and 21% for HER2/neu after NAC. A negative to positive shift was noted in 11% for ER, 4% for PR, and 4% for HER2/neu after NAC. The possible reasons ascribed for change in receptor status after NAC are as follows: (1) Selection of chemoresistant clones with different receptor expression after NAC. (2) Tumor heterogeneity with variable receptor expression in different areas. (3) Ovarian suppression during NAC leading to alteration in receptor expression. (4) Technical considerations such as staining techniques and intra-observer and inter-observer differences in IHC slide interpretation before and after NAC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor 2 receptor (HER 2/neu) status in breast cancer patients are very important prognostic and predictive indicators. They are used to plan the adjuvant therapy after the patient has undergone surgery for her breast cancer.
A few studies have evaluated the expression of these three receptors before and after neoadjuvant therapy in locally advanced breast cancer with variable outcomes. While some authors have demonstrated alteration in the expression on the receptor as well as the strength of the expression in a few patients after chemotherapy, others have not demonstrated a significant change.
The aim of the present study was to evaluate in Indian breast cancer patients the expression of ER, PR, and HER-2/neu status before and after neoadjuvant chemotherapy.
Patients and Methods
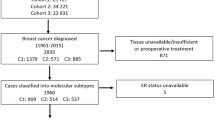
Over a 4-year period beginning in May 2015, a total of 80 histologically proven patients of locally advanced breast cancer scheduled for neoadjuvant chemotherapy were recruited. The patients underwent a thorough clinical evaluation and clinical staging of the disease. A trucut/core needle biopsy specimen was obtained from the breast lump which was subjected to detailed histological examination as well as immunohistochemistry (IHC) for ER, PR, and Her2neu status. Patients received 3–4 cycles of chemotherapy consisting of Adriamycin, cyclophosphamide, and 5-fluorouracil. Following this, patients deemed to have become operable underwent modified radical mastectomy. The mastectomy specimen was subjected to a detailed histopathological examination and a repeat ER, PR, and Her2 neu assessment. Excluded from the study were patients who underwent upfront modified radical mastectomy or those who did not undergo surgery following their chemotherapy. The histological and IHC reports of the core biopsy specimen were correlated with the reports on the mastectomy specimen.
The histopathological grading of the tissue was done using the Nottingham grading system which takes into account the tubule formation, mitotic counts, and nuclear pleomorphism. The ER and PR scoring was done on the basis of the ALLRED system which takes into account the percentage of cells stained and intensity of color produced during IHC examination. The HER 2 neu status was scored on the basis of intensity of membranous staining and percentage of tumor cells stained [1]. A score of 0 or 1+ was considered as negative, 2+ as equivocal, and 3+ as positive for HER2 /neu.
Observation
A total of 80 histologically proven breast cancer patients were included. As per the requirement of the study, only patients who according to their clinical staging required neoadjuvant chemotherapy followed by modified radical mastectomy were included. Hence, only patients with stage III breast cancer were included (Table 1).
Out of the 80 patients recruited in the study, 24 (30%) were labeled as triple negative breast cancer. ER negative status was found in 28 patients (35%) while 52 (65%) were ER positive. PR negative status was noted in 24 patients (30%) while 56 patients (70%) were PR positive. Her 2 neu overexpression was noticed in 24 patients (30%) in their core needle biopsy specimen (Table 2).
Following neoadjuvant chemotherapy, no change in ER, PR, and Her 2 status was noted in 71 (89%), 76 (95%), and 73 (96%) patients. There was a positive to negative shift in 6 (7.5%) regarding ER status, 3 (3.5%) regarding the PR status, and 5 (7.5%) regarding the her 2 neu status. A negative to positive shift in status was noticed in 3 (3.5%) for ER, 1( 1.5%) for PR, and 2 (2.5%) for the HER 2 status (Table 3).
Discussion
Estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER-2) status have important prognostic and predictive value and are used to guide the adjuvant treatment given to breast cancer patients. Information about ER, PR, and HER-2 is obtained by immunohistochemistry (IHC) as a quantitative measure of the receptor expression [2]. The ER, PR, and HER-2 status is characterized as positive or negative based on a cutoff point in the percentage of tumor cells stained and the intensity of the staining on IHC.
In spite of the importance of defining the receptor status and the widespread use of IHC for their assessment, standardization has not been achieved and a wide variety of scoring systems and cutoff points are in use. For evaluation of HER-2 status, although most centers use IHC methods, the current gold standard is the fluorescent in situ hybridization (FISH) which is not routinely available in most centers across India.
There are several studies which have evaluated the effect of NAC on the status of ER, PR, and HER-2 expression (Table4). Although there is no uniformity in results obtained, changes in receptor expression from positive to negative as well as from negative to positive both have been described. Ramteke P et al. [3] demonstrated a 17% discordance in ER status following NACT. Among these 15% of patients who were ER positive on pre-NACT, core needle biopsy was found to be ER negative after resection while 2% who were negative became positive after NAC. Discordance in pre- and post-NAC receptor status was found to be 13% with reference to PR and 11% with reference to HER2. Even among patients who did not undergo NAC, they reported discordant result 8% with reference to ER and PR status and 4% in HER2/neu. The discordance in patients who did not receive NAC was ascribed to intra-tumoral heterogeneity with possible difference in the site of tumor sampling in the core needle biopsy and the mastectomy specimens.
In a study by Taucher et al. [7], out of 214 patients who received pre-operative chemotherapy, 14% of patients who were ER positive on initial biopsy were found to be ER negative on review of the resection specimen. Similarly 51.7% of patients who were PR positive on initial biopsy were found later to be PR negative. Induction of menopause as a result of therapy was postulated as a possible mechanism for decreased ER expression although it was noted that such an occurrence would not account for the change in PR status [8].
In our own study, in 80 patients of breast cancer, we observed that no alteration in the receptor status was observed in 89% (71/80) patients with reference to ER, 95% (76/80) with reference PR, and 91% (73/80) with reference to HER2 status. A positive to negative shift was noted in 12% (6/52) with reference to ER, 5% (3/56) with reference to PR, and 21% (5/24) with reference to HER2 status. A negative to positive was noted in 11% (3/28) with reference to ER, 4% (1/24) with reference to PR, and 4% (2/56) with reference to HER2 status.
All studies have not supported the concept that hormone receptor status changes with administration of pre-operative chemotherapy. Arens et al. [9] compared a group of 25 patients who received neoadjuvant treatment with a control group of 30 patients who did not receive any pre-operative therapy. No significant differences were noted between the core biopsy and the resected specimens with regard to hormone receptor expression. Rare patients in both the control and neoadjuvant therapy group showed either an increase or decrease in receptor expression on the resected specimen as compared with the core biopsy, but overall, these changes did not reach statistical significance.
Similarly with reference to HER2/neu status, some authors have documented a change in expression of this oncoprotein while others have not shown a difference between pre- and post-NAC samples. Varga et al. [10] reported a change in HER-2/neu status on immunohistochemistry in 8 out of 23 patients although only 3 patients showed a change in status of FISH.
Regarding the discrepancies on HER2 status between different published reports, authors feel that the small biopsy sample obtained by core needle biopsy before the NAC may not be representative of the entire tumor. Immunohistochemical methods test for the presence of gene product rather than the gene itself. Minor variations in expression of the gene product in different regions of the tumor may appear more pronounced when interpreted in the context of a small biopsy as obtained by a core needle rather than when viewed in a much larger tissue sample as obtained after tumor resection. Overall good concordance was seen with reference to hormone receptor as well as HER2 status between core biopsy and resected specimens when there was no intervening therapy.
Another theory put forward by Adams et al. [8] to explain the difference in HER2 status pre- and post-therapy is the selection of tumor clones because of chemoresistance. In their study population, there was an increase in the number of tumors with HER2 over expression post-therapy. It seems plausible to speculate that individual tumor clones who over express HER2 may be present within a tumor which is otherwise negative for HER2 over expression. If their clones are chemoresistance, they may become more prevalent in the resected specimen as they continue to grow while the chemosensitive clones succumb during NAC.
Writing on this issue, Ven S van de et al. [2] said that without NAC, little discordance in ER (concordance 98%), PR (concordance 85%), and HER2 (concordance 98.%) status between core needle biopsy and resection specimen has been observed. This they attributed to breast cancer being a heterogenous disease with intra-tumoral heterogeneity, especially PR being concentrated more diversely in the tumor. Secondly, immunohistochemical procedures can be modulated by variations in tissue processing and fixation. A tendency for upgrading score of ER expression in core needle biopsy has been observed. Thirdly, intra- and inter-observer variability can result in differences in receptor status before and after NAC. Overall PR receptor status exhibited most discordance between pre- and post-NAC expression and HER2 was the most stable.
Considering our own work and that of authors from across the world, the possible mechanisms for change in receptor status after chemotherapy could be:
-
1.
Chemotherapy targets chemosensitive tumor cells leaving behind chemoresistant clones with possibly different receptor expression [8].
-
2.
Lower circulatory levels of estrogen due to ovarian insufficiency during or after chemotherapy in pre-menopausal women may cause downregulation of the ER and/or PR receptor [2].
-
3.
Breast cancer demonstrates intra-tumoral heterogeneity which could lead to a selection bias when obtaining a core needle biopsy specimen [2].
-
4.
The IHC techniques test for the presence of gene product rather that the gene itself and minor variations in expression of gene for the receptor could occur after NAC.
-
5.
Differences in receptor status could be the result of variations in staining techniques of tissue specimen as well as inter-observer and intra-observer variations in interpretation of stained slides.
Conclusion
Variations in expression of ER, PR, and HER2/neu receptor before and after NAC have been reported. The same has been noted to a lesser extent in patients who have not received NAC but had their receptor status evaluated on a core needle biopsy before surgery and then again on the resected specimen after surgery. Several possible explanations have been put forward such as selection of chemoresistant clones with different receptor expression, tumor heterogeneity, ovarian suppression during NAC, alteration in gene expression after NAC, and technical consideration such as staining techniques and IHC interpretation. Although the alteration in receptor status affects only a small percentage of patients, it could explain why some patients do not respond to endocrine therapy or trastuzumab therapy as expected.
References
Allred DC, Clark GM, Tandon AK, Molina R, Tormey DC, Osborne CK, Gilchrist KW, Mansour EG, Abeloff M, Eudey L (1992) HER-2/neu in node-negative breast cancer: prognostic significance of overexpression influenced by the presence of in situ carcinoma. J Clin Oncol 10:599–605
van de Ven S, Smit VT, Dekker TJ, Nortier JW, Kroep JR (2011) Discordances in ER, PR and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat Rev 37(6):422–430
Ramteke P, Seenu V, Prashad R, Gupta S, Iyer V, Deo S, Gogia A, Mathur S (2016) Alteration in steroid hormone and Her-2/neu receptor status following neoadjuvant chemotherapy in locally advanced breast cancer: experience at a tertiary care centre in India. Indian J Cancer 53:366–371
Neubauer H, Gall C, Vogel U, Hornung R, Wallwiener D, Solomayer E, Fehm T (2008) Changes in tumour biological markers during primary systemic chemotherapy (PST). Anticancer Res 28:1797–1804
Jin G, Han Y, Liu C, Chen L, Ding B, Xuan S, Liu X, Ma G, Gao J, Tian X (2015) Evaluation of biomarker changes after administration of various neoadjuvant chemotherapies in breast cancer. Int J Clin Exp Pathol 8:914–921
Kasami M, Uematsu T, Honda M, Yabuzaki T, Sanuki J, Uchida Y, Sugimura H (2008) Comparison of estrogen receptor, progesterone receptor and Her-2 status in breast cancer pre- and post-neoadjuvant chemotherapy. Breast 17:523–527
Taucher S, Rudas M, Gnant M, Thomanek K, Dubsky P, Roka S, Bachleitner T, Kandioler D, Wenzel C, Steger G, Mittlböck M, Jakesz R (2003) Sequential steroid hormone receptor measurements in primary breast cancer with and without intervening primary chemotherapy. Endocr Relat Cancer 10:91–98
Adams AL, Eltoum I, Krontiras H, Wang W, Chhieng DC (2008) The effect of neoadjuvant chemotherapy on histologic grade, hormone receptor status, and HER2/neu status in breast carcinoma. Breast J 14(2):141–146
Arens N, Bleyl U, Hildenbrand R (2005) HER2/neu, p53, Ki67, and hormone receptors do not change during neoadjuvant chemotherapy in breast cancer. Virchows Arch 446:489–496
Varga Z, Caduff R, Pestalozzi B (2005) Stability of the HER2 gene after primary chemotherapy in advanced breast cancer. Virchows Arch 446:136–141
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Verma, A., Kar, A.G., Meena, R.N. et al. Evaluation of Estrogen Receptor, Progesterone Receptor, and Human Epidermal Growth Factor Receptor 2 Status Before and After Neoadjuvant Chemotherapy in Breast Cancer Patients. Indian J Surg 83 (Suppl 2), 399–403 (2021). https://doi.org/10.1007/s12262-020-02380-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-020-02380-y