Abstract
Members of the Botryosphaeriaceae are well known fungi associated with dieback, canker and fruit rot on various hosts worldwide, including mango. The aim of this study was identify a large collection of Botryosphaeriaceae species associated with dieback and stem-end rot of mango in the semi-arid region of Northeastern Brazil, and compare the species in relation to mycelial growth, pathogenicity and virulence. A total of 115 isolates were sampled and based on morphology and DNA sequence data (ITS and EF1-α) seven taxa were identified, namely, Botryosphaeria dothidea, B. mamane, Fusicoccum fabicercianum, Neofusicoccum parvum, N. brasiliense sp. nov, Neoscytalidium dimidiatum and Pseudofusicoccum stromaticum. B. dothidea and P. stromaticum were the most commonly isolated species, which represented 37 % and 33 % of all isolates respectively. B. mamane is reported for the first time in association with mango diseases worldwide. There were significant differences among the species obtained in this study in relation to optimum temperature for mycelial growth and mycelial growth rates. All species were pathogenic on mango fruit. There were significant differences in virulence among the species, with Ne. dimidiatum and N. parvum being the most virulent species, while P. stromaticum was the least virulent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brazil is one of the biggest producer and exporter of tropical fruits in the world. Mango (Mangifera indica L.) is one of the most exported products. Most of the mangoes grown for the international market comes from the São Francisco Valley region located in the Northeast part of the country. In 2010 the national production was 1 197 million t and the planted area reached 75 416 ha, generating about US$ 334 million. This ranks Brazil as third largest world producer after China and India (Agrianual 2012).
Mangoes are affected by various pests and pathogens. Among the wide range of destructive fungal pathogens that impact on mango production in Brazil are members of the Botryosphaeriaceae (Costa et al. 2010). The first report of species associated with mango in Brazil was in 1947 (Batista 1947) and they have become increasingly important (Tavares et al. 1991; Tavares 2002).
The species within Botryosphaeriaceae have a worldwide distribution and occur on a large variety of plant hosts including monocotyledons, dicotyledons, gymnosperms and angiosperms, on which they are found as saprophytes, parasites, and endophytes (von Arx 1987; Slippers and Wingfield 2007). These fungi are associated with different symptoms such as fruit rots, shoot blights, stem cankers, dieback and gummosis (von Arx 1987). In Brazil, stem-end rot is the main disease induced by Botryosphaeriaceae on mango, reducing fruit shelf-life and causing serious post-harvest losses (Junqueira and Junqueira 2007).
The taxonomy of species in the Botryosphaeriaceae is commonly based on the morphology of the anamorph states, which are most frequently encountered in nature. However, overlapping morphological characteristics has emphasized the utility of applying DNA sequence comparisons to resolve species (De Wet et al. 2008). Considerable changes have taken place recently in the taxonomy of the Botryosphaeriaceae (Liu et al. 2012). Historically, more than 18 anamorph genera have been associated with Botryosphaeria Ces. & De Not. In a phylogenetic study based on part of the 28S ribosomal DNA gene together with morphological characters revealed that Botryosphaeria is composed of several distinct lineages that correspond to individual genera (Crous et al. 2006). Only B. dothidea (Moug. : Fr.) Ces. & De Not. and B. corticis (Demaree & M.S. Wilcox) Arx & E. Müll. were retained in Botryosphaeria, while other species with Fusicoccum Corda like anamorphs were transferred to Neofusicoccum Crous, Slippers & A.J.L. Phillips. Pseudofusicoccum Mohali, Slippers & M.J. Wingf., or Neoscytalidium Crous & Slippers, which were introduced in that study.
The use of molecular tools has made a significant contribution towards the recognition of species in the Botryosphaeriaceae and numerous species have been described in recent years, both in native vegetation, and in diverse crops of economic importance (Phillips et al. 2002; Slippers et al. 2004a, b; Luque et al. 2005; Phillips et al. 2005; Liu et al. 2012). Pavlic et al. (2008) identified seven new species in Australian native vegetation. Based on DNA sequence data for five nuclear loci Pavlic et al. (2009a, b) identified three new species of Neofusicoccum within the N. parvum/N. ribis species complex in South Africa. In 2010, two new species, N. batangarum Begoude, Jol. Roux, Slippers and Lasiodiplodia mahajangana Begoude, Jol. Roux, Slippers were described from Terminalia catappa L. (Begoude et al. 2010). More recently Fusicoccum ramosum Pavlic, Burgess, M.J. Wingfield (Pavlic et al. 2008), F. atrovirens J.W.M. Mehl & B. Slippers (Mehl et al. 2011), F. fabicercianum S.F. Chen, D. Pavlic, M.J. Wingf. & X.D. Zhou (Chen et al. 2011), B. fusispora Boonmee, J.K. Liu & K.D. Hyde (Liu et al. 2012), and B. schariffi Abdollahzadeh, Zare, A.J.L. Phillips (Abdollahzadeh et al. 2013) were described in the Botryosphaeria. Regarding the genus Lasiodiplodia, 16 new species have been reported since 2004. (Pavlic et al. 2004, 2008; Burgess et al. 2006; Damm et al. 2007; Alves et al. 2008; Begoude et al. 2010; Abdollahzadeh et al. 2010; Ismail et al. 2012; Úrbez-Torres et al. 2012). The increase in the number of new species introduced is largely a result of the widespread use of DNA sequence data, but is also due to the exploration of new geographic regions and habitats.
Several species of Botryosphaeriaceae have been found associated with diseased mango trees and fruits worldwide: N. parvum (Pennycook & Samuels) Crous, Slippers & A.J.L. Phillips, N. mangiferae (Syd. & P. Syd.) Crous, Slippers & A.J.L. Phillips, B. dothidea and L. theobromae (Pat.) Griffon & Maubl are some of the more common pathogens (Slippers et al. 2005; Javier-Alva et al. 2009; Costa et al. 2010). Recently, four new species of Lasiodiplodia, B. schariffi and N. mediterraneum Crous, M.J. Wingf. & A.J.L. Phillips were associated with this host in Iran (Abdollahzadeh et al. 2010, 2013). In Australia, species of Botryosphaeriaceae: Neoscytalidium novaehollandiae Pavlic, Burgess, M.J. Wingfield, Ne. dimidiatum (Penz.) Crous & Slippers, Pseudofusicoccum adansoniae Pavlic, Burgess, M.J. Wingfield, P. ardesiacum Pavlic, Burgess, M.J. Wingfield, P. kimberleyense Pavlic, Burgess, M.J. Wingfield, L. iraniensis Abdollahzadeh, Zare & A.J.L. Phillips and L. pseudotheobromae A.J.L. Phillips, A. Alves & Crous were isolated from cankers and tip dieback of mango (Ray et al. 2010; Sakalidis et al. 2011), and in 2012, L. egyptiacae A.M. Ismail, L. Lombard & Crous was recorded in Egypt (Ismail et al. 2012).
In Brazil, dieback and stem end rot was reported in the early 1900s. Recently, diseases caused by members of the Botryosphaeriaceae have gained importance mainly because of changes in the cultivation management. Historically, this disease was attributed exclusively to L. theobromae. Recent studies using molecular methods revealed the presence of more species namely B. dothidea, N. parvum and P. stromaticum (Mohali, Slippers & M.J. Wingf.) Mohali, Slippers & M.J. Wingf. and six species of Lasiodiplodia causing diseases in mango in Northeast Brazil (Costa et al. 2010; Marques et al. 2012; Marques et al. 2013).
Considering the number of species associated with mango elsewhere it seems likely that more species would be associated with this host in Brazil. Therefore, the objective of this study was to combine morphological characters with ITS, BT and EF1-α sequence data to characterize a large number of isolates and identify the species of Botryosphaeriaceae associated with M. indica in Brazil and to compare the species in relation to mycelial growth, pathogenicity and virulence.
Materials and methods
Sampling and fungal isolation
From April to June 2010, isolates were collected from plant tissue exhibiting dieback and stem end rot in commercial plantations of mango located in the São Francisco Valley, Northeastern Brazil. Plant tissues were surface disinfested with 70 % ethanol for 30 s and 1.5 % NaOCl for 1 min. Samples were then rinsed in sterile distilled water for 30 s and dried before small pieces (4–5 mm) of tissue were taken from the margin between necrotic and apparently healthy tissue and plated onto potato dextrose agar (PDA, Acumedia, Lansing, USA) amended with 0.5 g l−1 streptomycin sulfate (PDAS). Plates were incubated at 25 °C in the dark for 3 to 4 days. Fungal colonies emerging from plant tissue pieces that were morphologically similar to species of Botryosphaeriaceae (Sutton 1980; Phillips 2006) were transferred to fresh PDA plates and incubated at 25 °C in the dark, with observation at 3, 5 and 15 day. To obtain single-spore isolates, pycnidia were produced on 2 % water agar (WA) with autoclaved pine needles as a substrate after incubation for 3-week at 25 °C under a 12 h daily photoperiod with near-ultraviolet light (Slippers et al. 2004a,b). A single pycnidium was cut from each isolate under a stereo microscope (Zeiss Stemi DV4; Carl Zeiss, Berlin, Germany) and placed in 250 μl of sterile water to produce a conidial suspension. A 20 μl aliquot was spread on PDAS and incubated at 28 °C in the dark for 24 h. A single germinating conidium was transferred to a new PDA plate. Stock cultures were stored on PDA slants at 5 °C in the dark.
DNA isolation, PCR amplification and sequencing
A portion of the translation elongation factor 1α (EF1-α) gene was sequenced for all 115 isolates collected from mango orchards. A total of 28 isolates were selected as representatives of the taxa found in our survey. For these isolates, the entire ITS rDNA cluster was sequenced and for six selected isolates of Neofusicoccum part of the β-tubulin genes was sequenced, in order to clarify the relationships among isolates obtained in this study (Table 1). Using a sterile 10 μl pipette tip, a small amount of aerial mycelium was scraped from the surface of a culture grown for 5 days on PDA at 25 °C and genomic DNA was extracted using the AxyPrep™ Multisource Genomic DNA Miniprep Kit (Axygen Scientific Inc., Union City, USA) following the manufacturer’s instructions. The ITS region was amplified using the primers ITS1 and ITS4 (White et al. 1990) as described by Slippers et al. (2004a), part of the β-tubulin gene was amplified using the primers BT2a and BT2b (Glass and Donaldson 1995) and part of the EF1-α gene was amplified using the primers EF1-688F and EF1-1251R (Alves et al. 2008) as described by Phillips et al. (2005). Each 50-μl polymerase chain reaction (PCR) mixture included 21 μl of PCR-grade water, 1 μl of DNA template, 1.5 μM of each primer, and 1 μl of PCR Master Mix (2X) (0.05 u μl-1 de Taq DNA polimerase, reaction buffer, 4 mM MgCl2, 0.4 mM of each dNTP; Thermo Scientific, Waltham, USA). PCR reactions were carried out in a thermal cycler (Biocycler MJ 96; Applied Biosystems, Foster City, USA). The PCR amplification products were separated by electrophoresis in 1.5 % agarose gels in 1.0× Tris-acetate acid EDTA (TAE) buffer and were photographed under UV light after staining with ethidium bromide (0.5 μg ml−1) for 1 min. PCR products were purified using the AxyPrep™ PCR Cleanup Kit (Axygen) following the manufacturer’s instructions. ITS and EF1-α regions were sequenced in both directions using an ABI PRISM® 3100-Avant Genetic Analyzer (Applied Biosystems) at the Sequencing Platform LABCEN/CCB in the Universidade Federal de Pernambuco (Recife, Brazil).
Phylogenetic analyses
Sequences were edited with Chromas v. 2.32 (Technelysium Pty Lda, Brisbane, Australia). Sequences of both DNA regions of additional isolates were retrieved from GenBank. Sequences were aligned with ClustalX v. 1.83 (Thompson et al. 1997) and manually adjusted when necessary. Phylogenetic information contained in indels (gaps) was incorporated into the phylogenetic analyses using simple indel coding as implemented by GapCoder (Young and Healey 2003). A partition homogeneity test was done to determine the possibility of combining the ITS and EF1-α datasets (Farris et al. 1995; Huelsenbeck et al. 1996). Sequences of other Botryosphaeriaceae species obtained from GenBank were included in the analyses (Table 1). P. stromaticum (CMW 13434 and CMW 13435) were used as outgroup in the phylogenetic analyses of Botryosphaeria and Neoscytalidium species and B. dothidea (CMW8000 and CBS110302) were used as outgroup to Neofusicoccum species and Pseudofusicoccum species.
Phylogenetic analyses were performed using PAUP v. 4.0b10 (Swofford 2003) for Maximum-parsimony and MrBayes v. 3.0b4 (Ronquist and Huelsenbeck 2003) for Bayesian analyses. Maximum-parsimony analyses were performed using the heuristic search option with 1,000 random taxa addition and tree bisection and reconnection (TBR) as the branch-swapping algorithm. All characters were unordered and of equal weight and gaps were treated as missing data. Branches of zero length were collapsed and all multiple, equally parsimonious trees were saved. The robustness of the most parsimonious trees was evaluated from 1,000 bootstrap replications (Hillis and Bull 1993). Other measures used were consistency index (CI), retention index (RI) and homoplasy index (HI).
Bayesian analyses employing a Markov Chain Monte Carlo method (MCMC) were performed. The general time-reversible model of evolution (Rodriguez et al. 1990), including estimation of invariable sites and assuming a discrete gamma distribution with six rate categories (GTR+Γ+G) was used. Four MCMC chains were run simultaneously, starting from random trees for 1,000,000 generations. Trees were sampled every 100th generation for a total of 10,000 trees. The first 1,000 trees were discarded as the burn-in phase of each analysis. Posterior probabilities (Rannala and Yang 1996) were determined from a majority-rule consensus tree generated with the remaining 9,000 trees. This analysis was repeated three times starting from different random trees to ensure trees from the same tree space were sampled during each analysis. Phylogenetic trees were visualized using Treeview (Page 1996). Sequences derived in this study were deposited in GenBank. Representative isolates obtained in this study were deposited in the Culture Collection of Phytopathogenic Fungi “Prof. Maria Menezes” (CMM) at the Universidade Federal Rural de Pernambuco (Recife, Brazil). Phylogenetic trees were deposited in TreeBASE (S14389).
Morphological characterization
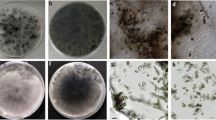
Representatives of the different groups identified in the phylogenetic analysis were used to study colony morphology and conidial characteristics. The color and aerial hyphal growth from isolates were recorded during 15 days of growth on 2 % PDA at 25 °C in the dark. Conidial characteristics were determined from cultures grown on 2 % WA containing autoclaved pine needles and incubated under near-ultraviolet light, as described above. Conidia and other structures were mounted in 100 % lactic acid and digital images recorded with a Leica DFC320 camera on a Leica DMR HC microscope fitted with Nomarski differential interference contrast optics (Leica Microsystems Imaging Solutions Ltd., Cambridge, UK). The length and width of 50 conidia per isolate were measured with the Leica IM500 measurement module. Mean and standard errors of the conidial measurements, including mean length to width (L/W) of the ratio conidial measurements were calculated.
These isolates were also used to determine the effect of temperature on colony growth of different species. A 3-mm-diameter mycelial plug from the growing margin of a 3-day-old colony was placed in the center of a 90-mm-diameter 2 % PDA plate, and four replicates of each isolate were incubated at temperatures ranging from 5 °C to 35 °C in 5 °C intervals in the dark. After a 2-day incubation period, the colony diameter (mm) was measured in two perpendicular directions. The experiment was done twice. Colony diameters were plotted against temperature and a curve was fitted by a cubic polynomial regression (y = a + bx + cx2 + dx3). Optimal temperature was estimated from the regression equation and numeric summary with TableCurve™ 2D v. 5.01 (SYSTAT Software Inc., Chicago, USA). Optimum temperature was defined as the temperature that produced the maximum mycelial growth rate. The colony diameter data at 30 °C were used to calculate the mycelial growth rate (mm/day). One-way analyses of variance (ANOVA) were conducted with data obtained from optimum temperature and mycelial growth rate experiments, and means were compared by Fisher’s least significant difference (LSD) test at the 5 % significance level using STATISTIX v. 9.0 (Analytical Software, Tallahassee, USA).
Pathogenicity and virulence in fruits
The isolates used in the morphological characterization were selected for this test. Mango fruits (cv. Tommy Atkins) at stage three of maturation (Assis 2004), which had not been treated with fungicides, were washed in running water, surface disinfested in 70 % ethanol for 1 min and 1 % NaOCl for 5 min, then rinsed in sterile distilled water. After drying, the fruits were placed on plastic trays, on the base of each were four layers of paper towels wetted with distilled water to increase humidity. Each fruit was put on a sterilized Petri plate to avoid direct contact with water and was wounded at the median region by pushing the tip of four sterile pins through the surface of the skin to a depth of 3 mm. A mycelial plug (5 mm in diameter) removed from the margin of a 5-day-old PDA culture grown at 28 °C in the dark of each isolate was immediately placed on the wound. A non-colonized agar plug was used for the control. The trays were enclosed in plastic bags and incubated at 25 °C in the dark. The plastic bags and paper towels were removed after 48 h, and the fruits were kept at the same temperature. Isolates were considered pathogenic when the lesioned area advanced beyond the 5-mm diameter inoculum point. The virulence of the isolates was evaluated from measurement of the lesion length at 5 days after inoculation in two perpendicular directions on each fruit. The experiment was arranged in a completely randomized design with six replicates per treatment (isolate) and one fruit per replicate. The experiment was conducted twice. Differences in virulence caused by Botryosphaeriaceae species were determined by one-way ANOVA and means were compared by LSD test at the 5 % significance level using STATISTIX.
Results
DNA sequencing and phylogenetic analyses
A total of 115 isolates were obtained from mango stems and fruits collected from Northeast regions of Brazil. From this total, 7 species of Botryosphaeriaceae were identified based on phylogenetic analysis of the partial translation elongation factor 1α (EF1-α) gene: Botryosphaeria dothidea, B. mamane, N. parvum, Fusicoccum fabicercianum, Neofusioccum sp., Ne. dimidiatum and Pseudofusicoccum stromaticum. To confirm the identity of the isolates, the internal transcribed spacer (ITS) sequence was obtained for 28 isolates representing each putative species and part of the β-tubulin gene was sequenced for six isolates of Neofusicoccum.
PCR products for the ITS were approximately 580 bp in size, while those for β-tubulin and EF1-α were approximately 450 bp. The ITS and EF1-α sequences were combined in three different data sets corresponding to 1) Botryosphaeria and Neoscytalidium species, 2) Neofusicoccum species, 3) Pseudofusicoccum species. Each data set was analyzed separately to produce three phylogenetic trees, one for each genus.
The combined ITS and EF dataset of the Botryosphaeria and Neoscytalidium consisted of 29 ingroup and 2 outgroup sequences. The alignment contained 827 characters including coded alignment gaps. Of these characters 543 were constant, 11 were variable and parsimony uninformative and 273 were parsimony informative. A heuristic search of the 273 parsimony informative characters generated 16 most parsimonious trees (TL = 387; CI = 0.902; RI = 0.965; HI = 0.098) each with similar clade topologies, and one is presented in Fig. 1. The phylogenetic analyses of Maximum-parsimony (MP) and Bayesian methods (BM) produced nearly identical topologies (Bayesian tree not shown). Isolates from mango clustered in three different clades corresponding to known species. The isolates CMM3928, CMM3925, CMM3923, CMM3899 and CMM3905 clustered together with F. fabicercianum (CMW27094=CBS127193, culture ex-type) in a strongly supported clade (MP bootstrap = 95 %, BM probability = 0.83). Isolates CMM3937, CMM3938, CMM3940 clustered within B. dothidea. Isolates CMM3941 and CMM 1390 clustered within B. mamane D.E. Gardner in a well-supported clade (MP/BM: 100/1.0), while CMM3979 and CMM3980 resided in a clade together with Ne. dimidiatum.
One of 16 most parsimonious trees (TL = 387; CI = 0.902; RI = 0.965; HI = 0.098) obtained from combined ITS and EF1-α sequence data. Maximum parsimony bootstrap support values from 1,000 replications and Bayesian posterior probability scores are shown at the nodes. The tree was rooted to P. stromaticum (CMW 13434 and CMW 13435). The bar represents 10 changes
The Neofusicoccum combined ITS and EF dataset (9 isolates from this study and 40 sequences retrieved from GenBank) was composed of 783 characters including gaps, of which 557 were constant, 14 were variable and parsimony uninformative and 212 were parsimony informative. A heuristic search generated 70 most parsimonious trees (TL = 361; CI = 0.770; RI = 0.919; HI = 0.230) with similar clade topologies, and one is presented in Fig. 2a. In this dataset, the isolates from Brazil clustered in two clades. The majority of isolates (CMM3945, CMM3944, CMM1271, CMM3943, CMM1291, CMM1465) clustered together with N. parvum supported by an MP bootstrap of 96 % and BM probability of 1.00. While the isolates CMM1285, CMM1338 and CMM1269 were not clearly resolved and bootstrap support for the branches was generally low, they grouped next to N. cordaticola and N. kwambonambiense. The Neofusicoccum combined ITS, β-tubulin and EF1-α dataset included 20 ingroup and 2 outgroup taxa and was composed of 1,183 characters including gaps, of which 1,090 were constant, 22 were variable and parsimony uninformative and 71 were parsimony informative. Maximum parsimony analysis of the remaining 71 parsimony informative characters produced a single most parsimonious tree with TL = 104; CI = 0.913; RI = 0.940; HI = 0.087. Isolates CMM1285, CMM1338 and CMM1269 formed a well-supported clade (MP/BM = 99/1.00) representing a new phylogenetic species. And the other isolates clustered together with N. parvum (MP/BM = 99/1.0) (Fig. 2b).
a. One of the most 70 parsimonious trees (TL = 361; CI = 0.770; RI = 0.919; HI = 0.230) obtained from combined ITS and EF1-α. The bar represents 1 change. b. One of the two most parsimonious trees (TL = 104 CI = 0.913; RI = 0.940; HI = 0.087) resulting from maximum parsimony analysis of combined ITS, EF1-α and BT sequence data for Neofusicoccum species. Maximum parsimony bootstrap support values from 1,000 replications and Bayesian posterior probability scores are shown at the nodes. The bar represents 10 changes
The Neofusicoccum spp. combined ITS and EF dataset included 17 taxa including the outgroup comprised 864 characters including gaps, of which 521 were constant, 308 were variable and parsimony uninformative. Maximum parsimony analysis of the remaining 35 parsimony informative characters resulted in a single tree with TL = 364 steps CI = 0.975; RI = 0.920; HI = 0.025 (Fig. 3). In this dataset, the mango isolates grouped together with P. stromaticum with MP bootstrap = 51 % and BM probability of 0.94.
Single most parsimonious tree (TL = 364; CI = 0.975; RI = 0.920; HI = 0.025) obtained from combined ITS and EF1-α sequence data for Pseudofusicoccum species. Maximum parsimony bootstrap support values from 1,000 replications and Bayesian posterior probability scores are shown at the nodes. The tree was rooted to B. dothidea (CAP 288 and CMW 8000). The bar represents 10 changes
Results of this study showed that B. dothidea (37 %) and P. stromaticum (33 %) were the most prevalent fungi isolated from Mangifera indica in São Francisco Valley, followed by N. parvum (9 %), F. fabicercianum (7 %), Ne. brasiliense (10 %), and Ne. dimidiatum (2 %) (Fig. 4).
Taxonomy
Neofusicoccum brasiliense M.W. Marques, A.J.L. Phillips & M.P.S. Câmara sp. nov. MycoBank MB804730.
Etymology: The name refers to Brazil, the country where this fungus was first found.
Cultures sterile. Neofusicoccum brasiliense differs from its closest phylogenetic neighbor, N. kwambonambiense and N. umdonicola, by unique fixed alleles in three loci based on alignments of the separate loci deposited in TreeBase as study S14389: ITS positions 132 (C), 137 (T), 154 (C), 164 (A), 363 (A), 407 (T); EF-1α positions 56(T) and 206 (GAP); BT positions 32(C), 61 (T), 96 (C), 115(A), 175 (C), 235 (A), 251 (A), 301 (C) 316(C), 391(T);
Culture characteristics: aerial mycelia white, becoming dark greenish-grey or greyish with the reverse side of the colonies greenish black after 4–5 days at 25 °C. Optimum temperature for mycelial growth: 27.7 ± 0.6. Mycelial growth rate: 29.5 ± 1.89 mm/day.
Holotype: Brazil, Pernambuco, Petrolina, Lote 1195 - DISNC (40° 31′ 00″, 09° 20′ 14.8″), on Mangifera indica stems, 2010, coll. M.W. Marques, holotype living culture CMM 1338; isotype living culture in URM 7005.
Specimen examined: Brazil, Pernambuco, Petrolina, Farm Boa Esperança (40°27′30.8″, 09°20′03.2″), on Mangifera indica stem, 2006, coll. V.S.O. Costa (paratype living culture CMM 1269, ex-paratype living culture URM 7006). Brazil, Bahia, Casa Nova, Farm Fortaleza (40°52′ 46.2″, 09°17′07.2″), on Mangifera indica stem, 2006, coll. V.S.O. Costa (paratype living culture CMM 1285, ex-paratype living culture URM 7004).
Notes: A light yellowish pigment was observed in the media of three isolates examined. Isolates could not be induced to sporulate on any of the media defined in this study, nor on sterilized plant host tissue placed on WA.
Morphology and cultural characteristics
The isolates that were identified in the phylogenetic analysis using the combined data set were used to study colony morphology and conidial characteristics. Isolates of N. brasiliense did not sporulate and for this reason the description was based on molecular data. The conidia of Ne. dimidiatum were ellipsoid to ovoid, hyaline, with an acutely rounded apex, truncate base, initially aseptate, becoming brown and 2-septate at maturity, with the central cell darker than the end cells. The mycelium was composed of branched, septate, brown hyphae which disarticulated into 0-1-septate phragmospores. All other isolates produced anamorph structures on the pine needles on WA within 2–4 week. No sexual (teleomorph) structures were observed during this study and the species produced hyaline, elongate and thin-walled, fusoid conidia. All isolates on PDA grew rapidly, covering the entire surface of the Petri dishes within 4 days. The aerial mycelium was initially white, turning dark greenish-grey or greyish after 4–5 days at 25 °C under near UV-light. Conidial dimensions of the species obtained in this study were similar to those previously described in the literature (Table 2).
There were significant differences (P ≤ 0.05) among the species obtained in this study in relation to optimum temperature for mycelial growth and mycelial growth rates. The optimum temperature varied from 25.4 °C to 30.8 °C. Optimum temperature for growth differed significantly between the species. Ne. dimidiatum (30.8 °C) and P. stromaticum (30.4 °C) had the highest optimum temperature for growth, while N. parvum had the lowest. The mycelial growth rate of Ne. dimidiatum (41.2 mm/day) was significantly higher than all other species. Growth rates differed significantly among the other species, which varied from 19.7 to 29.5 mm/day. B. dothidea and F. fabicercianum had the lowest mycelial growth rates with 21.9 and 19.7 respectively (Table 3).
Pathogenicity and virulence on fruits
All species of Botryosphaeria, Neofusicoccum, Neoscytalidium and Pseudofusicoccum collected in this study were pathogenic in fruit. Inoculations resulted in irregularly shaped, roughly circular, black to brown lesions on the surface of the fruit. Analysis of variance showed that there were significant differences (P ≤ 0.05) in the virulence among species. Ne. dimidiatum, N. parvum and B. dothidea were the most virulent species. P. stromaticum produced the smallest lesions on mango fruits. The lesion length induced by B. mamane, F. fabicercianum and N. brasiliense had intermediate levels of virulence (Fig. 5).
Mean lesion lengths (mm) caused by species associated with dieback and stem-end rot of mango in Northeastern Brazil, 72 h after inoculation with mycelium colonized agar plugs onto wounded fruits of Tommy Atkins cultivar. Bars above columns are the standard error of the mean. Columns with same letter do not differ significantly according to Fisher’s LSD test (P ≤ 0.05)
Discussion
In this study, seven different species of Botryosphaeriaceae distributed in four genera were identified and characterized from M. indica in Northeastern Brazil. These identifications were supported by morphology and DNA sequence data (ITS and EF1-α).
In Northeastern Brazil, the mango cultivation has increased considerably lately and the use of new cultivation technology, such as floral induction by application of potassium nitrate and paclobutrazol together with long periods of exposure of plants to water stress (Junqueira and Junqueira 2007), has likely contributed to the resurgence of diseases such as stem-end rot and dieback. These diseases were associated exclusively with L. theobromae and, later N. parvum, B. dothidea and P. stromaticum were reported (Costa et al. 2010; Marques et al. 2012). In this study, four more species were found to be associated with these diseases. These include: B. mamane, Fusicoccum fabicercianum, N. brasiliense and Ne. dimidiatum.
The species B. dothidea and N. parvum are commonly associated with mango diseases worldwide (Slippers et al. 2005) and are generally found in temperate regions (Burgess et al. 2006; Pavlic et al. 2007; Sakalidis et al. 2011). Recent work reported B. dothidea had a broad distribution on M. indica in Iran, and was found in a variety of climates ranging from temperate and humid to the semi-arid regions and the humid tropical regions (Abdollahzadeh et al. 2013). In the present study, B. dothidea was the most prevalent species found in the semi-arid regions of Brazil and represented 37 % of all the isolates examined. B. dothidea is common on both cultivated and indigenous hosts in the Northern Hemisphere (Zhou and Stanoxz 2001; Slippers et al. 2004a; Piskur et al. 2011). This suggests a Northern Hemisphere origin for this fungus and implies that it was introduced into the Southern Hemisphere together with plant material. Worldwide, this species is associated with a wide variety of hosts. In Brazil it has been reported on pear, apple, Vitis sp., Eucalyptus and mango (Mendes et al. 1998; Becker and Ieki 2002; Costa et al. 2010).
Neofusicoccum parvum is one of the most common pathogens of mango causing fruit stem-end rot, dieback and blossom blight (Slippers et al. 2005). It was reported in Brazil for the first time in 2009 (Costa et al. 2010). This species is closely related to N. ribis, often showing overlapping morphological characters, which has led to confusion and misidentification, requiring, therefore the use of molecular methods. However, the use of only ITS may underestimate the real diversity, mainly among closely related species or cryptic species (Taylor et al. 2000). Therefore, the sequence data of ITS is normally combined with the other genes such as EF1-α, β -tubulin, which have been applied successfully to discriminate cryptic species and elucidate phylogenetic relations (Mohali et al. 2007; De Wet et al. 2008; Phillips et al. 2008; Sakalidis et al. 2011). Recently, four species, N. batangarum, N. cordaticola Pavlic, Slippers, M.J. Wingfield, N. kwambonambiense Pavlic, Slippers, M.J. Wingfield and N. umdonicola Pavlic, Slippers, M.J. Wingfield were identified in this complex based on congruence between genealogies of multiple genes (Pavlic et al. 2009a, b; Begoude et al. 2010).
Neofusicoccum brasiliense is recognized as another species in the complex N. parvum/N. ribis, closely related to N. kwambonambiense and N. cordaticola. Since it failed to sporulation in culture, our description was based in molecular data. In this study, this species was the third most prevalent species found on mango.
Fusicoccum fabicercianum was recently described from Eucalyptus sp. in southern China (Chen et al. 2011). Phylogenetically, F. fabicercianum is closely related to B. corticis, B. dothidea, B schariffi and F. ramosum. This species is a new record for Brazil and is reported here for the first time on mango.
An interesting finding in this study was the identification of B. mamane associated with mango. This species has previously only been reported in Hawaiian (Gardner 1997) and Venezuelan native vegetation (Mohali et al. 2006). Only the ITS sequence is available for the ex-type isolate (isolate 97-59). For this reason sequences of regions other than ITS come from isolates that are not associated with the type, its host or locality. Unfortunately, these isolates might not be true B. mamane since there are 4 bp differences in ITS from the ex-type.
In this study P. stromaticum was the second most abundant species comprising 38 % of the isolates, indicating this genus is more widely distributed than it believed earlier. This species has been reported only on non-native Eucalyptus and Acacia spp. in Venezuela (Mohali et al. 2006, 2007). Later, it was reported causing a disease of mango in Brazil (Marques et al. 2012). According to Pavlic et al. (2008), the fact that all Pseudofusicoccum spp. occurred on native hosts in a relatively undisturbed area of Australia or in the case of P. stromaticum on Australian plants suggests that the species are most likely native to that country. Based in our survey this is clearly not the case, since this species was found in non-native plant species (mango) and intensively cultivated areas.
The species Ne. dimidiatum is characterized by conidia formed in arthric chains in the aerial mycelium, powdery to the touch. In addition to arthroconidia the cultures produce Fusicoccum-like conidia in pycnidia. Ne. dimidiatum has been isolated from different substrates including plant tissues, soil, human skin and nails, and is known as a plant pathogen (Punithalingam and Waterston 1970; Crous et al. 2006). In Australia this species was collected as an endophyte in tissues of plants (Pavlic et al. 2008). Later, these fungus were isolated from mango showing dieback and canker symptoms and produced lesions during pathogenicity trials on mango fruit and excised stems (Ray et al. 2010; Sakalidis et al. 2011). The same was found in the present study, this species was isolated only from mango branches with dieback or canker. However, it was able to cause lesions on mango fruit, and was the most virulent species in the pathogenicity test. In Brazil, this species was reported for the first time causing collar and root rot in the biofuel plant Jatropha curcas L. (Machado et al. 2012). To our knowledge this is the first report related to mango trees in Brazil. Studies about these species resurging in the country and their roles in the disease epidemiology must be undertaken.
In Brazil, B. dothidea, N. parvum and L. theobromae were found in the main mango production regions, but their relative prevalence differed in each region. In the São Francisco Valley, B. dothidea and N. parvum were more prevalent than L. theobromae, whereas L. theobromae was the predominant species in the Assú Valley (Costa et al. 2010). In this study, all species were reported only in São Francisco Valley. In Western Australia, eight taxa of Botryosphaeriaceae were identified in mango, the most commonly encountered species included Ne. novaehollandiae, Ne. dimidiatum and P. adansoniae (Sakalidis et al. 2011). In eastern Australia the species identified were N. parvum, N. mangiferum, B. dothidea and P. kimberleyense (Slippers et al. 2005). The species distribution may be due to a number of factors including the predominant cultivars, differences in climate and soil type and cultivation practices (Lazzizera et al. 2008).
Inoculation of mango fruits was made and all the species showed the potential to cause damage. Ne. dimidiatum and N. parvum caused the largest lesions on the fruit. Similar data were found by Sakalidis et al. (2011), when pathogenicity of Botryosphaeriaceae species was tested in fruits and branches of mango. Studies about its epidemiology and its impact on mango culture should be done. In this study, the origins of the Botryosphaeriaceae species collected from M. indica are unknown. Maybe, the semi-arid climatic conditions and the use of practices that lead the plant to longer periods of stress (floral induction and water restriction) have led to these diseases acquiring more importance lately. The incidence and pathogenicity indicate that all the species are economically important for mango cultivation, because they have the potential to reduce fruit quality especially in postharvest.
References
Abdollahzadeh J, Javadi A, Mohammadi-Goltapeh E, Zare R, Phillips AJL (2010) Phylogeny and morphology of four new species of Lasiodiplodia from Iran. Persoonia 25:1–10
Abdollahzadeh J, Javadi A, Zare R, Phillips AJL (2013) Phylogeny and taxonomy of Botryosphaeria and Neofusicoccum species in Iran, with description of Botryosphaeria scharifii sp. nov. Mycologia 105:210–220
Agrianual (2012) Anuário da agricultura brasileira. Informa Economics/FNP South America, São Paulo, 482 p
Alves A, Crous PW, Correia A, Phillps AJL (2008) Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Divers 28:1–13
Assis JS (2004) Cultivo da mangueira: colheita e pós-colheita. Embrapa Semi-Árido, Petrolina. http://sistemasdeproducao.cnptia.embrapa.br/FontesHTML/Manga/CultivodaMangueira/colheita.htm Accessed 23 June 2012
Batista AC (1947) Mal do Recife (grave doença da mangueira). Tese de concurso para a Cadeira de Fitopatologia e Microbiologia Agrícola, Escola Superior de Agricultura, Recife, 109 p
Becker WF, Ieki H (2002) Ocorrência e patogenicidade de Botryosphaeria dothidea como agente causal da seca de ramos em pereira japonesa no Estado de Santa Catarina, Brasil. Summa Phytopathol 28:201–203
Begoude BAD, Slippers B, Wingfield MJ, Roux J (2010) Botryosphaeriaceae associated with Terminalia catappa in Cameroon, South Africa and Madagascar. Mycol Prog 9:101–123
Burgess TI, Barber PA, Mohali S, Pegg G, de Beer W, Wingfield MJ (2006) Three new Lasiodiplodia spp. from the tropics, recognized based on DNA comparisons and morphology. Mycologia 98:423–435
Chen SF, Pavlic D, Roux J, Slippers B, Xie YJ, Wingfield MJ, Zhou XD (2011) Characterization of Botryosphaeriaceae from plantation grown Eucalyptus species in South China. Plant Pathol 60:739–751
Costa VSO, Michereff SJ, Martins RB, Gava CAT, Mizubuti ESG, Camara MPS (2010) Species of Botryosphaeriaceae associated on mango in Brazil. Eur J Plant Pathol 127:509–519
Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Philips AJL, Alves A, Burgess T, Barber P, Groenewald JZ (2006) Phylogenetic lineages in the Botryosphaeriaceae. Stud Mycol 55:235–253
Damm U, Crous PW, Fourie PH (2007) Botryosphaeriaceae as potential pathogens of Prunus in South Africa, with descriptions of Diplodia Africana and Lasiodiplodia plurivora sp. nov. Mycologia 99:664–680
De Wet J, Slippers B, Preisig O, Wingfield DB, Wingfield MJ (2008) Phylogeny of the Botryosphaeriaceae reveals patterns of host association. Mol Phylogenet Evol 46:116–126
Farris JS, Kallersjo M, Kluge AG, Bult C (1995) Testing significance of incongruence. Cladistics 10:315–319
Gardner DE (1997) Botryosphaeria mamane sp. nov. associated with witches’-brooms on the endemic forest tree Sophora chrysophylla in Hawaii. Mycologia 89:298–303
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330
Hillis DM, Bull JJ (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst Biol 42:182–192
Huelsenbeck JP, Bull JJ, Cunningham CV (1996) Combining data in phylogenetic analysis. Trends Ecol Evol 11:152–158
Ismail AM, Cirvilleri G, Polizzi G, Crous PW, Groenewald JZ, Lombard L (2012) Lasiodiplodia species associated with dieback disease of mango (Mangifera indica) in Egypt. Australas Plant Pathol 41:649–660
Javier-Alva J, Gramaje D, Alvarez LA, Armengol J (2009) First report of Neofusicoccum parvum associated with dieback of mango trees in Peru. Plant Dis 93:426
Junqueira NTV, Junqueira LP (2007) Manejo das principais doenças da mangueira. In: Núcleo de Estudos em Fitopatologia/Universidade Federal de Lavras (ed) Manejo integrado de doenças de fruteiras. Sociedade Brasileira de Fitopatologia, Brasília, pp 129–150
Lazzizera C, Frisullo S, Alves A, Phillips AJL (2008) Morphology, phylogeny and pathogenicity of Botryosphaeria and Neofusicoccum species associated with drupe rot of olives in Southern Italy. Plant Pathol 57:948–956
Liu JK, Phookamsak R, Doilom M, Wikee S, Li YM, Ariyawansha H, Boonmee S, Chomnunti P, Dai DQ, Bhat JD, Romero AI, Zhuang WY, Monkai J, Jones EBG, Chukeatirote E, Ko Ko TW, Zhao YC, Wang Y, Hyde KD (2012) Towards a natural classification of Botryosphaeriales. Fungal Divers 57:149–210
Luque J, Martos S, Phillips AJL (2005) Botryosphaeria viticola sp. nov. on grapevines: a new species with a Dothiorella anamorph. Mycologia 97:1111–1121
Machado AR, Pinho DB, Dutra DC, Pereira OL (2012) First report of collar and root rot of physic nut (Jatropha curcas) caused by Neoscytalidium dimidiatum in Brazil. Plant Dis 96:1697
Marques MW, Lima NB, Michereff SJ, Câmara MPS, Souza CRB (2012) First report of mango dieback caused by Pseudofusicoccum stromaticum in Brazil. Plant Dis 96:144–145
Marques MW, Lima NB, Morais MA Jr, Barbosa MAG, Souza BO, Michereff SJ, Phillips AJL, Câmara MPS (2013) Species of Lasiodiplodia associated with mango in Brazil. Fungal Divers. doi:10.1007/s13225-013-0231-z
Mehl JW, Slippers B, Roux J, Wingfield MJ (2011) Botryosphaeriaceae associated with Pterocarpus angolensis (kiaat) in South Africa. Mycologia 103:534–553
Mendes MAS, da Silva VL, Dianese JC, Ferreira MASV, Santos CEN, Gomes Neto E, Urben AF, Castro C (1998) Fungos em Plantas no Brasil. Embrapa-SPI/Embrapa-Cenargen, Brasilia, 555 p
Mohali S, Slippers B, Wingfield MJ (2006) Two new Fusicoccum species from Acacia and Eucalyptus in Venezuela, based on morphology and DNA sequence data. Mycol Res 110:405–413
Mohali S, Slippers B, Wingfield MJ (2007) Identification of Botryosphaeriaceae from Eucalyptus, Acacia and Pinus in Venezuela. Fungal Divers 25:103–125
Page RD (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Pavlic D, Slippers B, Coutinho TA, Gryzenhout M, Wingfield MJ (2004) Lasiodiplodia gonubiensis sp. nov., a new Botryosphaeria anamorph from native Syzygium cordatum in South Africa. Stud Mycol 50:313–322
Pavlic D, Slippers B, Coutinho TA, Wingfield MJ (2007) Botryosphaeriaceae occurring on native Syzygium cordatum in South Africa and their potential threat to Eucalyptus. Plant Pathol 56:624–636
Pavlic D, Wingfield MJ, Barber P, Slippers B, Harder GES, Burgess TI (2008) Seven new species of the Botryosphaeriaceae from baobab and other native trees in Western Australia. Mycologia 100:851–866
Pavlic D, Slippers B, Coutinho TA, Wingfield MJ (2009a) Multiple gene genealogies and phenotypic data reveal cryptic species of the Botryosphaeriaceae: a case study on the Neofusicoccum parvum ⁄ N. ribis complex. Mol Phylogenet Evol 51:259–268
Pavlic D, Slippers B, Coutinho TA, Wingfield MJ (2009b) Molecular and phenotypic characterization of three phylogenetic species discovered within the Neofusicoccum parvum ⁄ N. ribis complex. Mycologia 5:636–647
Phillips AJL (2006) The Botryosphaeria site. http://www.crem.fct.unl.pt/botryosphaeria_site. Accessed 10 June 2010
Phillips AJL, Fonseca F, Povoa V, Castilho R, Nolasco G (2002) A reassessment of the anamorphic fungus Fusicoccum luteum and description of its teleomorph Botryosphaeria lutea sp. nov. Sydowia 54:59–77
Phillips AJL, Alves A, Correia A, Luque J (2005) Two new species of Botryosphaeria with brown, 1-septate ascospores and Dothiorella anamorphs. Mycologia 97:513–529
Phillips AJL, Alves A, Pennycook SR, Johnston PR, Ramaley A, Akulov A, Crous PW (2008) Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia 21:29–55
Piskur B, Pavlic D, Slippers B, Ogris N, Maresi G, Wingfield MJ, Jurc D (2011) Diversity and pathogenicity of Botryosphaeriaceae on declining Ostrya carpinifolia in Slovenia and Italy following extreme weather conditions. Eur J Forest Res 130:235–249
Punithalingam E, Waterston JM (1970) Hendersonula toruloidea. CMI descriptions of pathogenic fungi and bacteria, No. 274. Commonwealth Mycological Institute, Kew, 2 p
Rannala B, Yang Z (1996) Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. J Mol Evol 43:304–311
Ray JD, Burgess TI, Lanoiselet VM (2010) First record of Neoscytalidium dimidiatum and N. novaehollandiae on Mangifera indica and N. dimidiatum on Ficus carica in Australia. Plant Dis Notes 5:48–50
Rodriguez F, Oliver JF, Marin A, Medina JR (1990) The general stochastic model of nucleotide substitutions. J Mol Evol 142:485–501
Ronquist F, Huelsenbeck JP (2003) MrBayes3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Sakalidis ML, Ray JD, Lanoiselet V, Hardy GESJ, Burgess TI (2011) Pathogenic Botryosphaeriaceae associated with Mangifera indica in the Kimberley Region of Western Australia. Eur J Plant Pathol 130:379–391
Slippers B, Wingfield MJ (2007) Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biol Rev 21:90–106
Slippers B, Crous PW, Denman S, Coutinho TA, Wingfield BD, Wingfield MJ (2004a) Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea. Mycologia 96:83–101
Slippers B, Fourie G, Crous PW, Coutinho TA, Wingfield BD, Wingfield MJ (2004b) Multiple gene sequences delimit Botryosphaeria australis sp. nov. from B. lutea. Mycologia 96:1028–1039
Slippers B, Johnson GI, Crous PW, Coutinho TA, Wingfield B, Wingfield MJ (2005) Phylogenetic and morphological re-evaluation of the Botryosphaeria species causing diseases of Mangifera indica. Mycologia 97:99–110
Sutton BC (1980) The Coelomycetes: fungi imperfecti with pycnidia, acervuli and stromata. Commonwealth Mycological Institute, Kew, 696 p
Swofford DL (2003) PAUP: phylogenetic analysis using parsimony (*and other methods) Version 4. Sinauer Associates, Sunderland
Tavares SCCH (2002) Epidemiologia e manejo integrado de Botryodiplodia theobromae - situação atual no Brasil e no mundo. Fitopatol Bras 27:46–52
Tavares SCCH, Menezes M, Choudhury MM (1991) Infecção da mangueira por Botryodiplodia theobromae Pat. na região semi-árida de Pernambuco. Rev Bras Frutic 13:163–166
Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, Hibbett DS, Fisher MC (2000) Phylogenetic species recognition and species concepts in fungi. Fungal Genet Biol 31:21–32
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Úrbez-Torres JR, Peduto F, Striegler RK, Urrea-Romero KE, Rupe JC, Cartwright RD, Gubler WD (2012) Characterization of fungal pathogens associated with grapevine trunk diseases in Arkansas and Missouri. Fungal Divers 52:169–189
von Arx JA (1987) Plant-pathogenic fungi. Cramer, Berlin
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols, a guide to methods and applications. Academic, San Diego, pp 315–322
Young ND, Healey J (2003) GapCoder automates the use of indel characters in phylogenetic analysis. BMC Bioinformatics 4:6. http://www.biomedcentral.com/content/pdf/1471-2105-4-6.pdf
Zhou S, Stanoxz GR (2001) Relationship among Botryosphaeria species and associated anamorphic fungi inferred from the analyses of ITS and 5.8S rDNA sequences. Mycologia 95:516–527
Acknowledgments
We are grateful to Sequencing Platform LABCEN/CCB in the Universidade Federal de Pernambuco for use of its facilities. This work was financed by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 141275/2009-0) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/BEX 0245/12-7). M. P. S. Câmara, Marcos A. de Morais Junior and S. J. Michereff also acknowledge the CNPq research fellowship. A.J.L. Phillips thanks Fundação para a Ciência e a Tecnologia (Portugal) for financial support through grant PEst-OE/BIA/UI0457/2011
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marques, M.W., Lima, N.B., de Morais, M.A. et al. Botryosphaeria, Neofusicoccum, Neoscytalidium and Pseudofusicoccum species associated with mango in Brazil. Fungal Diversity 61, 195–208 (2013). https://doi.org/10.1007/s13225-013-0258-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-013-0258-1