Abstract
Members of the Botryosphaeriaceae commonly cause stem cankers and dieback of tropical and subtropical woody plants. A survey was conducted to isolate and identify the fungal causal agents of dieback and sooty canker of trees in some areas of Khuzestan province, southwestern Iran, 2015–2022. Following visual inspection, 63 trees belonging to 22 species were detected with typical signs of the disease in eight townships, and symptomatic material including branches and stems were sampled from those trees. Fungal isolation was performed on Potato Dextrose Agar (PDA), and the isolates obtained were induced into sporulation using pine needles in Water Agar (WA). All isolates were identified as Neoscytalidium novaehollandiae, based on multi-gene phylogenetic analyses of combined ITS, LSU, and tef-1α sequence data, in combination with morphological comparisons. In a pathogenicity trial, five representative strains of N. novaehollandiae were inoculated onto healthy stem fragments from their respective original host tree species, resulting in severe necrotic lesions in all cases. Field assessment of sooty canker and dieback of trees in areas under investigation showed that at least 5–30 percent of ornamental and fruit trees were affected by N. novaehollandiae, with disease severity of 11.1 to 28.2 percent. Ficus benghalensis, Morus nigra, Juglans regia and Eucalyptus camaldulensis trees were more frequently and severely affected, as compared to Cassia fistula and Syzygium cumini. This study reports 22 new hosts for N. novaehollandiae in association with serious disease symptoms in Iran.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Canker and dieback diseases are common, widespread, and destructive on a broad range of woody plants, including ornamental and fruit trees (Shurtleff 1997; Horst 2013). The damage of these diseases is aggravated by wounding and weakening of trees caused by frost, mechanical injuries, mineral deficiencies and other abiotic factors because the canker pathogens mostly infect trees, which are low in vigor (Calavan and Wallace 1954; Whiteside 1988; Elshafie and Ba-Omar 2002). Among fungi, most members of the family Botryosphaeriaceae are well known as pathogens causing branch and stem canker, dieback, and gummosis of a wide range of fruit, ornamental, and forest trees (Mayorquin et al. 2016; Berraf-Tebbal et al. 2020). The Botryosphaeriaceae are widely distributed in most geographical and climatic areas throughout the world.
The genus Neoscytalidium was erected within the Botryosphaeriaceae to accommodate strains of Hendersonula toruloidea Nattrass (≡ Dothiorella mangiferae Syd. & P. Syd., ≡ Nattrassia mangiferae (Syd. & P.Syd.) B.Sutton & Dyko, ≡ Fusicoccum dimidiatum (Penz.) D.F.Farr) that formed Scytalidium-like synanamorphs (Crous et al. 2006; Madrid et al. 2009; Phillips et al. 2013). These fungi are also known to produce multilocular or pycnidial conidiomata having holoblastic, thin-walled, cylindrical to fusiform, 1–3-euseptate conidia and the arthric conidia produced in dry powdery chains on aerial mycelia (Phillips et al. 2013). The number of authentic species currently recognized in Neocytalidium might be a subject of debate. The global fungal nomenclator registry, Index Fungorum, still lists five species, including the type N. dimidiatum (Penz.) Crous & Slippers (crous et al. 2006), N. novaehollandiae Pavlic, T.I.Burgess & M.J.Wingf. (Pavlic et al. 2008), N. orchidacearum S.K. Huang, Tangthir., J.C. Kang & K.D. Hyde (Huang et al. 2016), N. oculi R.P. Calvillo-Medina, J. Mena, Raymundo & V. Bautista-de-Lucio (Calvillo-Medina et al. 2018), and N. hylocereum Kheawleng, Intaraa-nun & Rodkaew (Wonglom et al. 2023). However, Zhang et al. (2021) proposed to retain only N. dimidiatum and N. orchidacearum, and to reduce all other species to synonyms to N. dimidiatum. However, N. novaehollandiae is distinguished from other species in producing muriform and dichomera-like conidia (Zhu and Liu 2012).
Neoscytalidium species are capable of causing diseases in plants, humans, and animals (Ruíz-Cendoya et al. 2010; Bakhshizadeh et al. 2014; Dionne et al. 2015; Shokoohi et al. 2019). This genus is known as an opportunistic pathogen with the potential to cause severe invasive disease on a wide range of plants in tropical and sub-tropical regions including California, China, Egypt, Europe, India, Iran, Iraq, Jamaica, Niger, South Tunisia, Southern Thailand, Turkey, and West Africa (Nattrass 1933; Calavan and Wallace 1954; Sommer 1955; Meredith 1963; Natour and El-Haideri 1967; Giha 1975; Reckhans and Adamou 1987; Cao and Wang 1989; Granata and Sidoti 1991; Harsh and Tiwari 1992; Matheron and Sigler 1993; Elliott et al. 1997; Msikita et al. 1997; Tsahouridou and Thanassoulopoulos 2000; Elshafie and Ba-Omar 2002; Heidarian and Alizadeh 1995; Namsi et al. 2010, Oksal and Özer 2021; Ören et al. 2020a, b; Wonglom et al. 2023). Neoscytalidium species can have a detrimental effect on various woody plants by causing cankers, dieback, gummosis, necrosis, wood rot and wilt (Phillips et al. 2013; Goudarzi and Moslehi 2020; Brito et al. 2020).
Neoscytalidium novaehollandiae was initially described as a new species for four isolates singularly producing Dichomera-like synanamorphs, but otherwise morphologically similar to N. dimidiatum (Pavlic et al. 2008). These isolates were obtained from asymptomatic branches of Acacia synchronica Maslin, Adansonia gibbose F.Muell., Crotalaria medicaginea Lam. and Grevillia agrifolia A.Cunn. ex R.Br. in north-western Australia (Pavlic et al. 2008). N. novaehollandiae was subsequently reported as a plant pathogen in Australia, in association with dieback of mango and common fig (Ray et al. 2010). Since then, accumulating reports have depicted this fungus as a destructive pathogen causing canker, decline, dieback, stem blight and shoot blighton various shrubs and trees in Australia, China, Iran and Turkey, including the species Mangifera indica (Ray et al. 2010; Sakalidis et al. 2011), Ulmus densa (Zhu and Liu 2012), Pistacia vera (Kurt et al. 2019), Vitis vinifera (Akgül et al. 2019), Prunus dulcis and Diospyros kaki (Ören et al. 2020a, b), Solanum lycopersicum L. (Derviş et al. 2020), Pyrus communis L. (Oksal and Özer 2021) and Salvia officinalis L. (Derviş et al. 2021). This species was also reported to cause wood canker and dieback symptoms on Quercus brantii (Sabernasab et al. 2019) and Pinus eldarica in Iran (Alizadeh et al. 2022).
Various fruit and ornamental trees are common in Khuzestan province in the southwest of Iran. These trees are affected by numerous plant diseases including leaf spot and blight (Ascochyta sp., Pleospora sp. and Stemphylium sp.), anthracnose (Colletotrichum spp.), stem canker and decay (Inonotus rickii (Pat.) D.A. Reid) and dieback and sooty canker (Fusicoccum dimidiatum) (Ershad 2009). In recent years, dieback and sooty canker diseases have become highly prevalent on a wide range of the ornamental and fruit trees in this province. Therefore, the purpose of this study was to identify the probable fungal pathogens responsible for canker and dieback on fruit and ornamental trees in Khuzestan province. To that end, fungi were isolated from symptomatic materials and characterized using morphological features and phylogenetic analyses of DNA sequence data. The pathogenic potential of representative isolates was also evaluated via artificial inoculation on healthy plant material. Furthermore, a field survey was undertaken to assess the incidence of the disease throughout the province.
Materials and methods
Sampling and fungal isolation
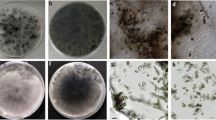
Samples were collected between 2015 and 2022 from eight townships, including Ahvaz, Andimeshk, Baghmalek, Behbahan, Dezful, Izeh, Masjedsoleyman and Molasani, in the Khuzestan province, southwestern Iran. The parks, boulevards, and gardens were evaluated, and in each place, the trees were visually inspected for the symptoms of dieback and sooty canker (Fig. 1). The diseased branches and trunks were collected from 63 trees belonging to 22 tree species (Table 1).
Disease symptoms on specimens collected. (a–f). Lamination of the trunk bark and canker on, Conocarpus erectus, Eucalyptus camaldulensis, Bauhinina purpurea and Syzygium cumini respectively. (e–j). Sooty canker on Morus nigra, Hibiscus rosa-sinensis, Albizia lebbeck, Prunus persica, Juglans regia and Nerium oleander respectively. (k). Trunk cracking of Ficus benghalensis. (l-m). Dieback on Ficus benghalensis and Ficus religiosa respectively
Small pieces (0.5–1 cm in size) were excised from healthy and discolored margins of symptomatic branches and surface-sterilised by dipping them in 2% NaOCl for 1–3 min. Pieces were then rinsed three times with sterile distilled water for 30 s and left to dried on sterile filter paper. Then, the fragments were plated on potato dextrose agar (PDA; Difco, USA) medium amended with streptomycin sulphate (30 mg/L). The plates were incubated in the dark at 28 °C for 5 to 7 days and individual colonies were transferred to fresh PDA. Purified cultures were obtained using a dilution method on quarter-strength PDA (Mehrabi-Koushki et al. 2018). A serial dilution of spore suspension was prepared for each isolate and 100 μl of each dilution was plated on quarter-strength PDA. The plates were incubated in the dark at 28 °C for 24–48 h and individual small colonies were transferred on new PDA plates as purified isolates. The representative cultures from the isolates tested in this study were deposited in the Iranian Fungal Culture Collection (Iranian Research Institute of Plant Protection, Tehran, Iran) and Collection of Fungal Cultures, Department of Plant Protection, Shahid Chamran University of Ahvaz, Iran (SCUA).
Morphological characterization
Microscopic features, colony shape, and growth speed, were evaluated on PDA, at 28 °C, under photoperiod of 12 h. The color rate was determined according to the Methuen handbook of color (Kornerup and Wanscher 1967) and the diameter of colonies was measured at 24-h intervals up to 7 days of incubation. Fungal structures were mounted in a drop of lactophenol or lactophenol cotton blue on microscopic slides. In order to induce sporulation and formation of the fruiting bodies (pycnidia), two ways were performed: i) double–autoclaved pine needles were placed into 2% water agar (WA, 2% agar; Merck) plates, and a mycelial disk from the pure culture of each isolate was placed in the center of each petri plate. The plates were incubated at 25 °C, with 12–hour light–dark regime for six weeks. ii) the isolates were grown on oatmeal agar (OA; oatmeal 30–60 g L−1, agar 12 g L−1) at 25 °C under 12 h photoperiod for 7–14 days. Photomicrographs were taken with an OLYMPUS DP12 digital camera fixed on OLYMPUS BX51 microscope. At least, 50 measurements of each fungal structure were made using a Leitz Wetzlar (SM-LUX) Basic Biological Light Microscope at 400 × and 1000 × magnification. A maximum and minimum range, 95% confidence intervals, means and standard deviations were calculated for the size of each structure.
DNA extraction and amplification
The isolates were grown on PDA at 25 °C in darkness for 1–3 weeks. Mycelial biomass was scraped off from the surface of each culture using a sterile glass slide. Total genomic DNA was extracted using the method described by Raeder and Broda (1985), with some modifications (Ahmadpour et al. 2017). Briefly, the ground mycelia were lysed with a SDS-based lysis buffer and then extracted three times by Phenol: chloroform: isoamyl alcohol (25:24:1). The first extract was treated with RNase a and then subjected to two following extractions. The genomic DNA was recovered through an ethanol-precipitation method. DNA extracts were qualified and quantified using a spectrophotometer (Eppendorf BioPhotometer plus). Nuclear genomic regions, including the first and second internal transcribed spacers and intervening 5.8S subunit (ITS) and the D1/D2 domain of the 28S subunit (LSU) of the nuclear ribosomal RNA, as well as partial translation elongation factor 1- alpha (tef-1α) were amplified in polymerase chain reactions (PCR). The primer combinations utilized were ITS1/NL4 (White et al. 1990; O’Donnell 1993) and EF1- 688F/ EF2-R (Alves et al. 2008; O’Donnell et al. 1998) for the ITS-LSU and tef-1α, respectively. Each 50 μL reaction mixture contained 5 μL of 10 × prime Taq Reaction Buffer (GenBio, South Korea), 6 μL of MgCl2 (25 mM), 0.6 μL of Prime Taq DNA Polymerase (5U/μL), 2 μL of each primer (10 mM), 2 μL of dNTP (10 mM mix), 100–500 ng of DNA and miliqure water up to final volume. PCR amplification was performed in a thermocycler (MJ MiniTM Gradient Thermal Cycler) using the following parameters: initial melting at 94 °C for 5 min, followed by 35 cycles of 30 s at 94 °C, 30 s at 55 °C (ITS-LSU) or 56 °C (tef1-α) and 90 s at °72 C, followed a final extension at 72 °C for 10 min. PCR products were evaluated by agarose gel electrophoresis in 1.0 × Tris–acetic acid-EDTA (TAE) buffer for amplicon size. The amplicons of the expected size were purified with the GF-1 AmbiClean Kit (Vivantis, Malaysia) and sequenced in the forward and reverse directions (Codon Genetics, Tehran, Iran). The nucleotide sequences were edited in BioEdit v. 7.0.9.0 (Hall 1999) and assembled using DNA Baser Sequence Assembeler v4 programs (2013, Heracle BioSoft, www.DnaBaser.com). All DNA sequences generated in this study are deposited in GenBank (Table 2).
Phylogenetic analyses
To identify taxa most closely related to our isolates based on sequence similarity, our newly generated sequences were queried against the nucleotide sequence database of the NCBI's GenBank using the BlASTn algorithm. This enabled us to select sequences of authentic strains of Neoscytalidium and other genera of Botryospheariaceae that were included in phylogenetic analyses (Table 2). The phylogenetic analyses were performed with a combined sequence data of ITS, LSU, and tef1-α regions. The alignments of ITS, LSU, and tef1-α were generated generated separately using Clustal W in BioEdit v. 7.0.9.0 (Hall 1999) and manually adjusted where necessary. The combined ITS-LSU-tef1-α dataset was created by concatenation of all individual alignments.
Phylogenetic analyses were conducted using maximum likelihood (ML) in the raxmlGUI 2.0 beta (Edler et al. 2019), maximum parsimony (MP) in MEGA7 (Tamura et al. 2013) and Bayesian analysis (BI) in MrBayes v.3.2.6 (Ronquist et al. 2012). The phylogenetic tree was rooted with Lasiodiplodia theobromae (Pat.) Griffon & Maubl. strain CBS 164.96. ML analysis was done using general time-reversible model with gamma distributed and invariant sites (GTR + G + I). Bootstrap values in MP (MPBS) and ML (MLBS) analyses were evaluated from 1000 replicates. The best fit evolutionary models for each single gene partition were estimated based on the lowest BIC score (Bayesian information criterion) in jModelTest 2 (Darriba et al. 2012) for the Bayesian phylogenetic analysis. As a result, GTR + G was used for ITS, GTR + I was used for LSU and HKY + G was used for tef1-α. The Bayesian inference posterior probabilities (PP) distribution was estimated by Markov chain Monte Carlo (MCMC) algorithm with the following options: four MCMC chains were sampled over 10,000,000 generations, sampling every 1000 generations, the standard deviation below 0.01 and removing the first 25% of trees for calculating posterior probability values (BPP). Data matrixes used for analysis were deposited in TreeBase under the accession number of S30423 (http://purl.org/phylo/treebase/phylows/study/TB2:S30423).
To investigate the genetic diversity and detect a possible structure in the global population of N. novaehollandiae, we used the unweighted pair group method with arithmetic average (UPGMA), as implemented in MEGA7 (Tamura et al. 2013), to build a dendrogram of N. novaehollandiae isolates from various locations and hosts based tef1-α sequence data. All stains represented in the GenBank in that respect were included. We also included three sequences from Khuzestan newly generated in this study. The tef1-α gene locus was selected for this analysis because of its greater variability as detected in sequences alignments.
Field disease assessment
During 2021–2022, the health status of trees in the sampling areas as indicated earlier was evaluated. To assess disease incidence (DI) and severity (DS), 50 trees were randomly selected for each tree in each area (township). The disease incidence was calculated using the following formula (1):
where: A = Number of trees showing dieback and sooty canker and B = Number of trees examined.
Disease severity was also evaluated using an infection category scoring from 0 to 9, based on the sooty canker area and percentage of dryness of the branches (Table 3). Then, disease severity was calculated with the following formula (2):
where: n = The number of trees in each severity category, v = Score for disease severity, N = Number of trees examined, Z = The score for the highest category.
Pathogenicity test
Pathogenicity tests of five representative isolates were performed on the same hosts from which the isolates were obtained, including Bauhinia purpurea, Conocarpus erectus, Eucalyptus camaldulensis, Ficus religiosa and Morus nigra. Pathogenicity trials were performed on detached stems of the host trees (8–10 years old) with similar height (~ 15–20 cm), diameter (~ 2 cm) and healthy under a controlled condition. The fragments were disinfected with 2% sodium hypochlorite (2–5 min), then washed three times with sterile distilled water. After drying, two wounds with a diameter of 5 mm were created on the stem fragments by a metal cork borer at a distance of 50 mm from each end. A mycelial PDA plug from the actively growing margin of each 3-day-old colony was inoculated into each wound. For each isolate, four stem fragments were used and an equal number was also inoculated with sterile PDA plugs as negative controls. Inoculated and control stems of each isolate were separately placed on a plastic mesh into water-containing desiccators, sterilised as a moist chamber. Then, the desiccators were kept at 28 °C under a 12-h photoperiod. The severity of the lesions induced by isolates were estimated in terms of percentage of wood discoloration (PWD) 10 days after inoculation when discoloration reached about 85% in at least one of the stem fragments. The PWD values obtained on each host was summarized as mean ± standard deviation. Stem fragments from each treatment and control were randomly selected for re-isolation to fulfill Koch's postulates. All pathogenicity tests were repeated once.
Results
Two hundred symptomatic branches and stems from 22 tree species, showing dieback and sooty canker, were collected. In total, 80 fungal isolates with identical culture characteristics were isolated from sample materials (Table 1). of those, 13 isolates were characterized based on PCR amplification and sequencing of the ITS, LSU and tef1-α gene loci, generating 1200 bp and 500 bp DNA sequence data, respectively. Aligning the newly generated sequences for comparison revealed that all the isolates share 100% sequence similarity with one another at all three loci, respectively. Evaluation of our sequences against the GenBank using BLASTn revealed that our isolates were 100% identical to the type strain of N. novohollandiae (CBS 122071) at the ITS and LSU. They showed a 0.7% difference with CBS 122071 at the tef1-α. This difference rested on 2/293 bp variation detected when the two sequences were aligned.
Phylogenetic analyses
Combined sequence data (ITS, LSU, and tef1-α) used in phylogeny consisted of 88 sequences from 32 ingroup taxa (Table 2), with Lasiodiplodia theobromae strain CBS 164.96 as the outgroup. The resulting concatenated aligned dataset contained a total of 1198 bp (410 bp for ITS, 505 bp for LSU and 283 bp for tef1-α) including alignment gaps. Among those sites, 953 bp were constant and 233 bp were variable (180 bp were parsimony informative and 53 bp were parsimony-uninformative). Single-locus trees did not show any conflicts in their topologies for the 70% reciprocal trees, which allowed to combine the three loci for a multi-locus phylogenetic analysis. The ML tree showed a similar topology to those obtained in BI and MP analyses in major clades (Fig. 2). In the three loci phylogenetic tree (Fig. 2), the 13 surveyed strains were grouped in a moderately-supported clade (MLBS 75%, MPBS 77%, BPP 0.91) with an internal subclade containing three reference strains of Neoscytalidium novaehollandiae, including CBS 122071, CBS 122610 and WAC 13286 (MLBS 85%, MPBS 90%, BPP 0.99). Hence, we identified our strains as N. novaehollandiae.
Phylogenetic tree constructed from a maximum likelihood analysis based on the combined ITS, LSU and tef1-α sequences. Bootstrap values obtained in maximum likelihood (MLBS) and maximum parsimony (MPBS) analyses ≥ 50% and Bayesian posterior probability values (BYPP) ≥ 0.5 are shown at the nodes, respectively. The scale bar shows the expected number of changes per site. The tree is rooted in Lasiodiplodia theobromae strain CBS 164.96. Letter T indicates the ex-type strains. Taxa under study are shown with black-color filled circles
In the UPGMA dendrogram (Fig. 3), the strains of N. novaehollandiae clustered in three low-supported groups including most strains of Turkey, China, the present study (Khuzestan Province, Iran) and one other strain from Iran (Tehran Province) in group 1, two other strains from Turkey in group 2, and strains of Australia and two other strains from Iran (Kermanshah Province) and Turkey in group 3.
UPGMA method phylogenetic tree based on tef1-α sequences of Neoscytalidium novaehollandiae strains obtained in this study and all existing strains in GenBank. The percentage of replicate in which the associated taxa clustered together in the bootstrap test (1000) is shown next to the branches. Taxa under study are shown with black-color filled circles
Morphological characteristics
Neoscytalidium novaehollandiae Pavlic, T.I. Burgess & M.J. Wingf., 2008 Fig. 4.
Conidiomata pycnidial or multilocular stromata, semi-immersed or superficial, black, globose. Conidia of the coelomycetous state formed in two distinct types: i) ellipsoid to ovoid, with rounded apex, at first hyaline, aseptate, becoming cinnamon to sepia, 0–1-septate or 2-septate with darker central cell, (4.2–)5–9.8(–10.3) × (2–)2.5–6.1(–7.9) µm, ii) variable in shape and size, globose, subglobose to obpyriform with muriform septa, at first hyaline with age becoming cinnamon to sepia, (5–)5.2–11(–15) × (3–)3.5–8.8(–10) µm. Aerial mycelium produced chains of one-celled spores (arthroconidia). Arthroconidia dry powdery to the touch, occurring in disarticulating aerial mycelium, cylindrical, oblong–obtuse to doliiform, thick-walled, initially hyaline, with age becoming cinnamon to sepia, 0–1-septate, (5–)6–10(–11.2) × 2.5–3.8(–4) μm.
Culture characteristics: Colonies on PDA, 90 mm diameter after three days of incubation at 28 ± 0.5 C, white to olivaceous, buff with regular margins at early growth stage, becoming blackish green in the central and olivaceous green at the edge within seven days, and black with age; reverse blackish green to black.
Pathogenicity test
Five representative isolates of Neoscytalidium novaehollandiae were examined on the trees from where they were originally isolated, including the strain SCUA-Ahv-Bau on Bauhinia purpurea, IRAN 4172C on Conocarpus erectus, IRAN 4181C on Eucalyptus camaldulensis, SCUA-Y29 on Ficus religiosa, and IRAN 4179C on Morus nigra. These strains were able to grow and sporulate in the bark surrounding the inoculation point on stem fragments of the mentioned plants. Results of the pathogenicity tests showed that all isolates were pathogenic on inoculated detached stems (Table 4). No lesions were produced on control shoots. The colonies of N. novaehollandiae developed on the inoculation points on the stem fragments two weeks after inoculation which was associated with wood necrosis and discoloration in the xylem (Fig. 5). The severity of induced lesions in inoculated material was in the following order: Eucalyptus camaldulensis and Morus nigra > Conocarpus erectus > Ficus religiosa > Bauhinia purpure. Accordingly, the rate of wood necrosis in E. camaldulensis was higher than others and B. purpure was the lowest. Masses of black fungal spores appeared under the bark and the canker surface on the stem fragments of C. erectus, E. camaldulensis, and M. nigra after three weeks of inoculation (Fig. 5). This pathogenic fungus was re-isolated from the necrosis-like areas formed on stem fragments and re-identified as N. novaehollandiae species based on morphological characterization. None of the fungal isolates were re-isolated from the control shoots.
Pathogenicity tests. (a–e) Necrosis on a stem fragment of Bauhinina purpurea, Ficus religiosa, Conocarpus erectus, Eucalyptus camaldulensis, and Morus nigra respectively. (f) compared to a control fragment. (g–h) Pycnidia and masses of black fungal spores on Eucalyptus camaldulensis and Conocarpus erectus respectively
Field disease incidence
In recent years, widespread presence and high incidence of dieback and sooty canker on trunks and branches associated with tree decline were observed in some areas of Khuzestan province. Field disease symptoms of the different hosts are shown in Fig. 1. In detail, all investigated hosts showed various external disease symptoms including shoot and twig dieback, sooty canker, and cracking of the bark. Internal observation of symptomatic tissues revealed a brown wood discoloration. Isolations were made from the discolored edge of cankers on branches and stems, dried shoots and branches, and also from the inner bark and woody tissues of the hosts. In our study, 29 isolates of Neoscytalidium novaehollandiae were recovered from various ornamental and fruit trees including, Citrus sinensis, Cassia fistula, C. floribunda, Conocarpus erectus, Cordia myxa, Cupressus sempervirens, Eucalyptus camaldulensis, Ficus religiosa, F. benghalensis, Juglans regia, Hibiscus rosa-sinensis, Morus nigra, Prunus persica, Punica granatum, Nerium oleander, Syzygium cumini and Ziziphus spina-christi. Visual inspection of the trees showed that in some cases, young shoots were affected initially, and as the disease progressed, branches and stems were affected as well. In most cases, necrotic girdling of stem tissues resulted in death of shoots and branch. This survey included about 5850 trees in all studied regions. According to the disease estimation (Table 5), disease incidence and severity varies between sampling areas (ranging from 5 to 30 percent) and trees (ranging from 11.1 to 28.2 percent). Results show that Ahvaz, Masjed-soleyman and Molasani had the most disease incidence and severity (Table 5).
Discussion
The present study is the first to investigate the incidence and etiology of dieback and sooty canker of various ornamental and fruit trees in the Khuzestan province of Iran. All fungal isolates obtained from symptomatic materials were identified to represent the same botryosphaeriacious species, Neocytalidium novaehollandiaea, based on the production of typical muriform, Dichomera-like conidia (Pavlic et al. 2008), as well as their phylogenetic placement in a monophyletic clade, alongside the type specimens of the species. However, the newly collected isolates, which appear to represent the same strain based on available sequence data, formed a discrete subclade resulting from two point-mutations detected in the tef-1α gene sequence. The UPGMA dendogram based on tef1-a of a global population of N. novaehollandiae reveals that there are at least three genetic groups associated with this fungus. All three groups occur in Turkey and two have been detected in Iran. But more generally the dendrogram shows that a greater diversity of N. novaehollandiae exists in Asia where all three groups are present, as compared to Australia, for instance, where only one group has been detected so far. Therefore, one can arguably assume that the diversification center of this fungus would be located in Asia, even-though a more representative sampling throughout the world will be needed to reach robust conclusions.
Artificially inoculating the fungus back onto various original host materials resulted in a pathogenic interaction in all cases, but it also highlighted a variation in susceptibility among hosts. For instance, wood discolorations and black powdery appearance in Conocarpus erectus, Eucalyptus camaldulensis and Morus nigra stems were significantly greater than those in Ficus religiosa and Bauhinia purpurea. This conformed with the results of disease assessment in the field that showed a variation in disease incidence and severity among investigated tree species.
Visual assessment of sooty canker and dieback of trees in areas under investigation showed that the disease index and tree mortality positively correlate with environmental stress. At least 5–30 percent of ornamental and fruit trees were affected by Neoscytalidium novaehollandiae in Khuzestan Province (Table 5). Since drought and extremely hot summers became more common in Khuzestan during the last decade, higher than usual incidence of die back and sooty canker diseases may be due to drought stresses and higher annual temperatures that made trees more susceptible to the disease. During this period, some members of the family Botryosphaeriaceae, including Botryosphaeria, Lasiodiplodia, Neofussicoccum and Neoscytalidium species, have emerged as destructive pathogens of many tree species, mainly causing canker and dieback (Manawasinghe et al. 2016; Alizadeh et al. 2022). Observational assessment showed that there was a clear increase in decline symptoms in the zones with high light intensity, low fertility soils, drought, water-logging, prolonged exposure to extremely high temperatures, summer sunscald, nutrient deficiency, soil compaction, changes in the soil grade and mechanical injuries. Previous studies showed that weakening by abiotic stress factors, such as high temperatures and drought periods could play a role in increasing the virulence and expansion of the Botryosphaeriaceae and other decline pathogens (Smith et al. 1996; Kim et al. 2009; Arnold and Herre 2003; Desprez-Loustau et al. 2007; Slippers and Wingfield 2007; Botella et al. 2010; Dissanayake et al. 2015; Fan et al. 2016; Anonym 2017; Delgado-Cerrone et al. 2016). Results of this study show that the canker and dieback of fruit and ornamental trees caused by N. novaehollandiae is a serious epidemic, that affects a broad range of tree species in Iran. In recent years, there are many scattered reports of detection of this fungus associated with emerging diseases prevalence on various plants from many regions of Asia and Australia. In view of the fact that this fungus is typically widespread in tropical and subtropical regions (Crous et al. 2006), it can also be assumed that it is well adapted to the environmental conditions of southwestern and southern Iran (Goudarzi and Moslehi 2020).
Most members of the Botryosphaeriaceae have a cosmopolitan distribution and cause disease on a broad host range of woody plants (Slippers and Wingfield 2007). N. novaehollandiae has been reported from a wide range of plants from Australia, China and Turkey and a few plants from Iran. The present study reports a wide range of new hosts (22 tree species) for N. novaehollandiae in Iran, in association with dieback and sooty canker. Previously, this fungus has been reported as causal agent of various diseases on Acacia synchronica, Adansonia gibbosa, Carpinus betulus, Crataegus pentagyna, Crotalaria medicaginea, Diospyros kaki, Fagus orientalis, Grevillia agrifolia, Mangifera indica, Morus alba, Pinus eldarica, Pistacia vera, Prunus dulcis, Pyrus communis, Quercus brantii, Salvia ofcinalis, Solanum lycopersicum, Ulmus densa, and Vitis vinifera (Rahmani 2018; Sabernasab et al. 2019; Kazemzadeh Chakusary et al. 2019; Pavlic et al. 2008; Ray et al. 2010; Sakalidis et al. 2011; Zhu and Liu 2012; Kurt et al. 2019; Ören et al. 2020a, b; Derviş et al. 2021; Oksal and Özer 2021; Alizadeh et al. 2022). Furthermore, Alizadeh et al. (2022) presented the host range of this pathogen, and showed that these potential hosts are prone to this pathogen under high temperature and low humidity.
In conclusion, Neoscytalidium species cause canker and dieback diseases on a large number of shrubs and trees with agricultural, industrial, forestry and ornamental importance. This study establishes N. novaehollandiae as the causal agent of dieback and sooty canker on many fruit and ornamental trees in Khuzestan province, Iran. The great number of affected tree species, together with the high disease indices estimated suggest that we are dealing with a problem that is growing in importance, probably in connection with global environmental change. This also means that numerous woody plants are at risk of severe disease due to N. novaehollandiae in various regions of the world. This study lays a foundation for further investigation into the economic impact of this pathogen, factors affecting disease development and dissemination, strategies to prevent and manage diseases and minimizing diseases losses in different climates.
Data availability
The data that support the findings of this study are openly available in GenBank at https://www.ncbi.nlm.nih.gov.
References
Kim KW, Lee IJ, Thoungchaleun V, Kim CS, Lee DK, Park EW (2009) Visualization of wound periderm and hyphal profiles in pine stems inoculated with the pitch canker fungus Fusarium circinatum. Microscopy Research and Technique 72:965–973
Ahmadpour SA, Mehrabi-Koushki M, Farokhinejad R (2017) Neodidymelliopsis farokhinejadii, a new fungal species from dead branches of trees in Iran. Sydowia 69:171–182
Akgül DS, Savaş NG, Özarslandan M (2019) First Report of Wood Canker Caused by Lasiodiplodia exigua and Neoscytalidium novaehollandiae on Grapevine in Turkey. Plant Disease 103:1036
Alizadeh M, Safaie N, Shams-Bakhsh M, Mehrabadi M (2022) Neoscytalidium novaehollandiae causes dieback on Pinus eldarica and its potential for infection of urban forest trees. Scientific Reports 12:9337
Alves A, Crous PW, Correia A, Phillips AJ (2008) Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Diversity 28:1–13 http://www.fungaldiversity.org/fdp/sfdp/28-1.pdf
Anonym (2017) Thousand cankers disease survey guidelines for 2017. United States Department of Agriculture, Forest Service (FS) and Plant Protection and Quarantine (PPQ), USA
Arnold AE, Herre EA (2003) Canopy cover and leaf age affect colonization by tropical fungal endophytes: ecological pattern and process in Theobroma cacao (Malvaceae). Mycologia 95:388–398
Bakhshizadeh M, Hashemian HR, Najafzadeh MJ, Dolatabadi S, Zarrinfar H (2014) First report of rhinosinusitis caused by Neoscytalidium dimidiatum in Iran. Journal of Medical Microbiology 63:1017–1019
Berraf-Tebbal A, Mahamedi AE, Aigoun-Mouhous W, Špetík M, Čechová J, Pokluda R, Baranek M, Eichmeier A, Alves A (2020) Lasiodiplodia mitidjana sp. nov. and other Botryosphaeriaceae species causing branch canker and dieback of Citrus sinensis in Algeria. PLoS ONE 15:e0232448
Botella L, Santamaría O, Diez J (2010) Fungi associated with the decline of Pinus halepensis in Spain. Fungal Diversity 40:1–11
Brito AC, De Mello JF, Câmara MP, Vieira JC, Michereff SJ, Souza-Motta CM, Machado AR (2020) Diversity and pathogenicity of Botryosphaeriaceae species associated with black root rot and stem cutting dry rot in Manihot esculenta in Brazil. European Journal of Plant Pathology 157:583–598
Calavan EC, Wallace JM (1954) Hendersonula toruloidea Natrass on citrus in California. Phytopathology 44:635–639
Cao ZM, Wang X (1989) A survey of stem diseases of Zanthoxylun. Forest Pest Disease 1:47
Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WF, Philips AJ, Alves A, Burgess T, Barber P, Groenewald JZ (2006) Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55:235–253
Calvillo-Medina RP, Martínez-Neria M, Mena-Portales J, Barba-Escoto L, Raymundo T, Campos-Guillén J, Jones GH, Reyes-Grajeda JP, González-y-Merchand JA, Bautista-de Lucio VM (2018) Identification and biofilm development by a new fungal keratitis aetiologic agent. Mycoses 62:62–72
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9:772–772
Delgado-Cerrone L, Mondino-Hintz P, Alaniz-Ferro S (2016) Botryosphaeriaceae species associated with stem canker, die-back and fruit rot on apple in Uruguay. European Journal of Plant Pathology 146:637–655
Derviş S, Güney İG, Koşar İ, Bozoğlu T, Özer G (2021) First report of Neoscytalidium novaehollandiae on common sage (Salvia ofcinalis). Australasian Plant Disease Notes 16:1–4
Derviş S, Özer G, Türkölmez Ş (2020) First report of Neoscytalidium novaehollandiae causing stem blight on tomato in Turkey. Journal of Plant Pathology 102:1339–1340
Desprez-Loustau ML, Robin C, Reynaud G, Déqué M, Badeau V, Piou D, Husson C, Marçais B (2007) Simulating the effects of a climate-change scenario on the geographical range and activity of forest-pathogenic fungi. Canadian Journal of Plant Pathology 29:101–120
Dionne B, Neff L, Lee SA, Sutton DA, Wiederhold NP, Lindner J, Fan H, Jakeman B (2015) Pulmonary fungal infection caused by Neoscytalidium dimidiatum. Journal of Clinical Microbiology 53:2381–2384
Dissanayake AJ, Zhang W, Li X, Zhou Y, Chethana T, Chukeatirote E, Hyde KD, Yan J, Zhang G, Zhao W (2015) First report of Neofusicoccum mangiferae associated with grapevine dieback in China. Phytopathologia Mediterranea 54:414–419
Edler D, Klein J, Antonelli A, Silvestro D (2019) raxmlGUI 2.0 beta: a graphical interface and toolkit for phylogenetic analyses using RAxMl. bioRxiv, https://doi.org/10.1101/800912
Elliott M, Edmonds RL, Suff LJ (1997) Role of stress in development of Arbutus canker (Nattrassia mangiferae). Phytopathology 87:S27
Elshafie AE, Ba-Omar T (2002) First report of Albizia lebbeck dieback caused by Scytalidium dimidiatum in Oman. Mycopathologia 154(1):37–40
Ershad J (2009) Fungi of Iran. Iranian Research, Educations Institute of plant protection, Iran, Tehran, p 531
Fan XL, Du Z, Hyde KD, Liang YM, PAN YP, Tian CM (2016) Cryptosporella platyphylla, a new species associated with Betula platyphylla in China Phytotaxa 253:285–292
Giha OH (1975) Hendersonula toruloidea associated with a serious wilt disease of shade trees [Fiscus benghalensis] in the Sudan [Fungus diseases]. Plant Disease Reporter 59:899–902
Goudarzi A, Moslehi M (2020) Distribution of a devastating fungal pathogen in mangrove forests of southern Iran. Crop Protection 128:104987
Granata G, Sidoti A (1991) Grapevine death caused by Nattrassia toruloidea. Vitis 30:219–222
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/ NT. Nucleic Acids Symposium Series 41:95–98
Harsh NSK, Tiwari CK (1992) Top dying and mortality in provenence trial of Gemelina arborea. Journal of Tropical Forest 8(1):55–61
Heidarian A, Alizadeh A (1995) Citrus dieback and decline caused by Nattrassia mangiferae and its other hosts in Khouzestan province. In Proceedings of the 12th Iranian Plant Protection Congress 2–7 September 1995, Karaj, Iran, pp 231
Horst RK (2013) Field manual of diseases on trees and shrubs (p. 196). Springer, Dordrecht
Huang SK, Tangthirasunun N, Phillips AJ, Dai DQ, Wanasinghe DN, Wen TC, Bahkali AH, Hyde KD, Kang JC (2016) Morphology and Phylogeny of Neoscytalidium orchidacearum sp. nov. (Botryosphaeriaceae). Mycobiology 44:79–84
Kazemzadeh Chakusary M, Mohammadi H, Khodaparast SA (2019) Diversity and pathogenicity of Botryosphaeriaceae species on forest trees in the north of Iran. European Journal of Forest Research 15:685–704
Kornerup A, Wanscher JH (1967) Methuen handbook of colour. England, London
Kurt Ş, Uysal A, Soylu EM, Kara M, Soylu S (2019) First record of Neoscytalidium novaehollandiae associated with pistachio dieback in the Southeastern Anatolia region of Turkey. Mycologia Iranica 6:55–57
Madrid H, Ruíz-Cendoya M, Cano J, Stchigel A, Orofino R, Guarro J (2009) Genotyping and in vitro antifungal susceptibility of Neoscytalidium dimidiatum isolates from different origins. International Journal of Antimicrobial Agents 34:351–354
Manawasinghe IS, Phillips AJ, Hyde KD, Chethana KW, Zhang W, Zhao WS, Yan JY, Li XH (2016) Mycosphere Essays 14: Assessing the aggressiveness of plant pathogenic Botryosphaeriaceae. Mycosphere 7:883–892
Matheron ME, Sigler L (1993) First report of Eucalyptus die-back cause by Nattrassia mangiferae in North America. Plant Disease 78:432
Mayorquin JS, Wang DH, Twizeyimana M, Eskalen A (2016) Identification, distribution, and pathogenicity of Diatrypaceae and Botryosphaeriaceae associated with citrus branch canker in the southern California desert. Plant Disease 100:2402–2413
Mehrabi-Koushki M, Khodadadi-Pourarpanahi S, Jounbozorgi S (2018) Fungal endophytes associated with some thermotolerant plants in salt-stress ecosystem. Mikologiya i Fitopatologiya 52:187–195
Meredith DS (1963) Tip rot of banana fruits in Jamaica. I. Hendersonula toruloidea on Dwarf Cavendish bananas. Transactions of the British Mycological Society 46:473–481
Msikita W, Yaninek JS, Ahounou M, Baimey H, Fagbemissi R (1997) First report of Nattrassia mangiferae root and stem rot of cassava in West Africa. Plant Disease 81:332
Namsi A, Zouba A, Triki MA, Mahmoud MO, Takrouni ML (2010) Study on Nattrassia mangiferae, the causal agent of apricot tree decline disease in the Oases of South Tunisia: biology and in vitro evaluation of some fungicides. The African Journal of Plant Science and Biotechnology 4:88–90
Natour RM, El-Haideri HAIDER (1967) Occurrence of branch wilt disease caused by Hendersonula toruloidea in Iraq. Plant Disease Reporter 51:371–373
Nattrass RM (1933) A new species of Hendersonula toruloideae on deciduous trees in Egypt. Transactions of the British Mycological Society 18:189–198
O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC (1998) Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences 95:2044–2049
O’Donnell K (1993) Fusarium and its near relatives. In: Reynolds DR, Taylor JW (eds) The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CAB International, Wallingford, pp 225–233
Oksal E, Özer G (2021) First report of shoot blight and branch canker of Pyrus communis by Neoscytalidium novaehollandiae in Turkey. Journal of Plant Pathology 103:673–674
Ören E, Koca G, Bayraktar H (2020a) First report of Neoscytalidium novaehollandiae associated with branch dieback on Japanese persimmon in Turkey. Journal of Plant Pathology 102:1311–1312
Ören E, Koca G, Gencer R, Bayraktar H (2020b) First report of Neoscytalidium novaehollandiae associated with stem canker and branch dieback of almond trees Australasian Plant Disease. Notes 15:1–3
Pavlic D, Wingfield MJ, Barber P, Slippers B, Hardy GE, Burgess TI (2008) Seven new species of the Botryosphaeriaceae from baobab and other native trees in Western Australia. Mycologia 100:851–866
Phillips AJ, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, Groenewald JZ, Crous PW (2013) The Botryosphaeriaceae: genera and species known from culture. Studies in Mycology 55:53–63
Raeder U, Broda P (1985) Rapid preparation of DNA from filamentous fungi. Letters in Applied Microbiology 1:17–20
Rahmani S (2018) Morphological and molecular identifcation of Neoscytalidium dimidiatum isolates and other endophyte fungi dieback in Sistan and Kerman Province. Doctoral dissertation, University of Zabol
Ray JD, Burgess T, Lanoiselet V (2010) First record of Neoscytalidium dimidiatum and N. novaehollandiae on Mangifera indica and N. dimidiatum on Ficus carica in Australia. Australasian Plant Disease Notes 5:48–50
Reckhans P, Adamou I (1987) Hendersonula dieback of Mango in Niger. Plant Disease 71:1045
Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61:539–542
Ruíz-Cendoya M, Madrid H, Pastor FJ, Mayayo E, Mariné M, Guarro J (2010) Development of murine models of disseminated infection by Neoscytalidium dimidiatum. Medical Mycology 48:681–686
Sabernasab M, Jamali S, Marefat A, Abbasi S (2019) Morphological and molecular characterization of Neoscytalidium novaehollandiae, the cause of Quercus brantii dieback in Iran. Phytopathologia Mediterranea 58:347–357
Sakalidis ML, Ray JD, Lanoiselet V, Hardy GE, Burgess TI (2011) Pathogenic Botryosphaeriaceae associated with Mangifera indica in the Kimberley region of Western Australia. European Journal of Plant Pathology 130:379–391
Shokoohi GR, Ansari S, Abolghazi A, Gramishoar M, Nouripour-Sisakht S, Mirhendi H, Makimura K (2019) The First Case of Fingernail Onychomycosis Due to Neoscytalidium novaehollandiae, Molecular Identification and Antifungal Susceptibility. Journal De Mycologie Medicale 30:100920
Shurtleff MC (1997) Deter canker and dieback diseases. Grounds Maintenance 32:49–56
Slippers B, Wingfield MJ (2007) Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biology Reviews 21:90–106
Smith H, Wingfield MJ, Petrini O (1996) Botryosphaeria dothidea endophytic in Eucalyptus grandis and Eucalyptus nitens in South Africa. Forest Ecology and Management 89:189–195
Sommer NF (1955) Sunburn predisposes walnut trees to branch wilt. Phytopathology 45:607–613
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30:2725–2729
Tsahouridou PC, Thanassoulopoulos CC (2000) First report of Hendersonula toruloidea as a foliar pathogen of strawberry-tree (Arbutus unedo) in Europe. Plant Disease 84:487
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322
Whiteside JO (1988) Miscellaneous fungi and associated diseases. In: Whiteside JO, Garnsey SM, Timmer LW (eds) Compendium of Citrus Diseases. The American Phytopathological Society, Minnesota, USA, p 29
Wonglom P, Pornsuriya C, Sunpapao A (2023) A New Species of Neoscytalidium hylocereum sp. nov. Causing Canker on Red-Fleshed Dragon Fruit (Hylocereus polyrhizus) in Southern Thailand. Journal of Fungi 3:197
Zhang W, Groenewald JZ, Lombard L, Schumacher RK, Phillips AJ, Crous PW (2021) Evaluating species in Botryosphaeriales. Persoonia-Molecular Phylogeny and Evolution of Fungi 46:63–115
Zhu XM, Liu XF (2012) A new species and genus distribution record from China: Neoscytalidium novaehollandiae. Indian Journal of Microbiology 52:565–568aaa
Acknowledgements
Authors would like to appreciate the administration of plant protection Department, Faculty of Agriculture, Shahid Chamran University of Ahvaz for their support in accomplishment of this study. The authors are thankful to anonymous reviewers for beneficial comments and suggestions on the manuscript.
Funding
This work was financially supported by grant (SCU.AP1401.294) from Research Council of Shahid Chamran University of Ahvaz.
Author information
Authors and Affiliations
Contributions
Seyedeh Akram Ahmadpour carried out sample preparation, fungal isolation and purification, morphometric and morphological determination, DNA isolation and amplification, DNA and phylogenetic analysis and the writing of the manuscript. Mehdi Mehrabi-Koushki carried out the design and implementation of the research and revising of the manuscript. Reza Farokhinejad contributed to the implementation of the research and revising of the manuscript. Zahra Mirsoleymani, provided some fungal isolates and revising of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmadpour, S.A., Mehrabi-Koushki, M., Farokhinejad, R. et al. Characterization and pathogenicity of Neoscytalidium novaehollandiae causing dieback and sooty canker in Iran. Trop. plant pathol. 48, 493–507 (2023). https://doi.org/10.1007/s40858-023-00591-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-023-00591-8