Abstract
The genus Cochliobolus (anamorphs Bipolaris, Curvularia) comprises many destructive plant pathogens that cause severe crop losses worldwide. The taxonomy of Cochliobolus is confused as frequent nomenclatural changes have occurred in the sexual and asexual states of species over the past 50 years. We provide an overview of these nomenclatural changes together with a morphological circumscription of the genus. Taxonomic notes and information about the life history of 55 species epithets of Cochliobolus listed in Index Fungorum are also given. Further information is given concerning the location of type cultures; availability of DNA sequence data derived from type cultures; mode of life; novel metabolite production; and use of Cochliobolus species in biocontrol. We provide a multilocus phylogenetic tree based on DNA sequence data derived from 25 ex-type and authentic cultures that shows the group as monophyletic. This paper represents the first comprehensive overview of Cochliobolus since 1987, including a summary of applications of species and molecular phylogenetic research. The 55 species of Cochliobolus are listed alphabetically, with synonyms, hosts and diseases, brief notes concerning taxonomic and phylogenetic research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species of Cochliobolus Drechsler (1934) and its anamorphs Bipolaris Shoemaker (1959) and Curvularia Boedijn (1933) are worldwide pathogens of mostly grasses (Poaceae). Some species have caused devastating disease epidemics of important food crops such as rice, wheat and maize (Sivanesan 1987; Scheffer 1997; Berbee et al. 1999). In 1943, Cochliobolus miyabeanus (S. Ito & Kurib.) Drechsler ex Dastur caused famine in Bengal by reducing rice yields by 40–90% and as a result two million people died of starvation (Scheffer 1997). In 1970 in the USA, about 19 million metric tons of Zea mays were devastated by Southern corn leaf blight caused by a specific race of Cochliobolus heterostrophus (Drechsler) Drechsler (Tatum 1971). Cochliobolus species are also reported as plant pathogens on hosts in several other plant families including Alliaceae, Anacardiaceae, Araceae, Euphorbiaceae, Fabaceae, Malvaceae, Rutaceae and Zingiberaceae.
The estimated number of species of Cochliobolus ranges from 22 in The Dictionary of the Fungi (Kirk et al. 2008) to 35 species associated with grasses (Sivanesan 1987). There are 26 species listed in the USDA database (http://nt.ars-grin.gov/fungaldatabases accessed on 31 Aug. 2011), and 55 species epithets listed in Index Fungorum (www.indexfungorum.org; accessed 31 Aug. 2011). Frequent name changes, together with refinements in taxonomy, have caused some confusion for mycologists and plant pathologists (Sivanesan 1987). A natural classification of this pathogenic system and correct identification of species are important for disease control, plant breeding and establishment of phytosanitary measures (Cai et al. 2009; Hyde et al. 2010). This paper provides a comprehensive account of the genus Cochliobolus. We have used Sivanesan (1987) as the starting point and include additional reports of Cochliobolus available. Host location and additional notes are given for each accepted species. Taxa needing further research are discussed. As name changes have been frequent all synonyms are listed. Available ex-type cultures and derived sequences are also listed. The objectives of this paper are to (i) review the taxonomic history and nomenclature of the group, (ii) review the knowledge on life cycles, metabolite production, and biocontrol (iii) provide a multilocus phylogeny based on type derived sequences (ITS, GPDH, LSU, EF), and (iv) list the teleomorphs annotated with synonyms, hosts and diseases, notes on each taxa considered.
Taxonomic review
Graminicolous Helminthosporium
The graminicolous Helminthosporium species can be segregated into Bipolaris, Curvularia, Drechslera and Exserohilum. Cochliobolus sexual states are only found associated with Bipolaris and Curvularia anamorphs (Sivanesan 1987). The basic morphology of Helminthosporium sensu stricto is based on the type species H. velutinum Link. (1809). this includes solitary, cylindrical, unbranched, brown conidiophores that produce obclavate, distoseptate conidia through small pores in walls of apical and intercalary cells of the conidiophores (Goh et al. 1998). The graminicolous Helminthosporium species (Drechsler 1923; Misra 1973) are, however, fundamentally different from Helminthosporium sensu stricto in producing solitary conidia at the apex of brown geniculate conidiophores, and resumption of growth by sympodial extension from the subepidermal region of the conidiophores with distinct conidial scars (Goh et al. 1998). Graminicolous Helminthosporium was, therefore, divided into two groups by Nisikado (1928), who introduced the subgenera Cylindro-Helminthosporium and Eu-Helminthosporium. The subgenus Cylindro-Helminthosporium was raised to generic rank as Drechslera by Ito (1930), who characterized the members of the new genus as having cylindrical, not curved conidia and the sexual states often associated with Pyrenophora.
The genus Bipolaris with the type species Bipolaris maydis (Y. Nisik. & C. Miyake) Shoem. was introduced by Shoemaker (1959) to accommodate members of the subgenus Eu-Helminthosporium. Subramanian and Jain (1966), however, amended the description of Drechslera to include all Helminthosporium species from grasses including all species disposed as Bipolaris (Alcorn 1983b).
Bipolaris as circumscribed by Shoemaker (1959) was heterogeneous (Alcorn 1983b). In contrast to other Bipolaris species, which have Cochliobolus sexual states, Bipolaris turcium (Pass.) Shoem. and B. rostrata (Drechsler.) Shoem. have Trichometasphaeria sexual states. These species also have conidia with a protuberant hilum in contrast to other species. Leonard and Suggs (1974) removed this heterogeneity by introducing a new genus Exserohilum, typified by Exserohilum turcicum (Pass.) K.J. Leonard & Suggs, to accommodate these Bipolaris species. They also introduced a new genus Setosphaeria K.J. Leonard and Suggs (1974) to accommodate the sexual states of Exserohilum (Sivanesan 1987). The anamorphic genera Bipolaris, Drechslera and Exserohilum have distinctly different sexual states, respectively in Cochliobolus, Pyrenophora and Setosphaeria (Sivanesan 1987). Some species such as Helminthosporium geniculatum Tracy & Earle of Helminthosporium sensu stricto were segregated into the genus Curvularia (Boedijn 1933), typified by Curvularia lunata (Wakker) Boedijn.
Thus, the graminicolous Helminthosporium species can be segregated into four genera, Bipolaris, Curvularia, Drechslera and Exserohilum (Shoemaker 1959; Luttrell 1963, 1964; Ellis 1971; Alcorn 1983b, 1988; Sivanesan 1987; Goh et al. 1998). Cochliobolus sexual states are found with Bipolaris and Curvularia anamorphs (Sivanesan 1987).
Cochliobolus: sexual state
Cochliobolus was introduced and typified by C. heterostrophus Drechsler (1934). Previous to this many species of Cochliobolus were placed in Ophiobolus (Riess 1854), a genus with bitunicate asci and scolecospores (Von Arx and Oliviera 1952; Drechsler 1934). Drechsler (1934) found that graminicolous forms of Helminthosporium produced teleomorphs with greater ascus and ascospore width and strongly helicoid ascospores with bipolar germination which deviated from typical Ophiobolus species. Drechsler (1934) introduced Cochliobolus to accommodate these species. Ophiobolus kusanoi Y. Nisik., O. miyabeanus S. Ito & Kurib., O. sativus Ito & Kurib. and O. setariae S. Ito & Kurib were subsequently transferred to Cochliobolus by Dastur (1942), who also added a novel species C. tritici Daustar (Alcorn 1983a). Cochliobolus is placed in the family Pleosporaceae, in the order Pleosporales, and bears a unilocular ascostromata, also known as a pseudothecium (Alexopoulos et al. 1996; Zhang et al. 2012).
Tsuda and Uyama (1977) distinguished the genus Pseudocochliobolus from Cochliobolus by two main characters. One was the presence of stromatic tissue below the ascomata body in Pseudocochliobolus and the other was the degree of ascospore coiling, which is slight or absent in Pseudocochliobolus (Alcorn 1983a). Alcorn (1983a) doubted the value of these characters and considered Pseudocochliobolus a synonym of Cochliobolus.
Berbee et al. (1999) used a molecular phylogenetic approach based on analysis of ITS and GPDH regions to examine the phylogenetic distribution of 32 Cochliobolus species (including asexual species). Their analysis recognized two distinct groups of Cochliobolus. Group 1 comprised highly virulent pathogens with Bipolaris asexual states. Group 2 comprised mild pathogens with both Bipolaris and Curvularia asexual states. The host ranges of Cochliobolus overlapped in Group 1 and 2. The Pseudocochliobolus species clustered in Group 2, which was shown to be monophyletic. Phylogenetic analysis of Bipolaris species based on the Brn1 sequence region support the division of Bipolaris into two clusters corresponding to Cochliobolus and Pseudocochliobolus (Shimizu et al. 1998). By introducing Pseudocochliobolus as a separate genus, Tsuda et al. (1977) had diagnosed a deep divergence in Cochliobolus (Berbee et al. 1999). Berbee et al. (1999), however, recommended further phylogenetic analysis as the results based on phylogeny needed to be clarified for C. homomorphus Luttr. & Rogerson, which did not cluster in either Group 1 or 2.
Bipolaris and Curvularia: asexual states
Because of their economic impact, Cochliobolus species and their asexual states have been the focus of careful taxonomical attention (Berbee et al. 1999). Shoemaker (1959) raised Eu-Helminthosporium to the generic rank of Bipolaris and connected it with the Cochliobolus teleomorph. Since then further Cochliobolus teleomorphs have been described in association with Bipolaris anamorphs (Alcorn 1983a: Sivanesan 1987). Curvularia was first connected to Cochliobolus by Nelson (1960a) who linked Curvularia intermedia with Cochliobolus intermedius. There have since been many associations between Cochliobolus and Curvularia (Nelson 1964b; Sivanesan 1987).
Bipolaris and Curvularia share some morphological similarities. For example, they may or may not form stromata, conidia in both genera germinate from one or both polar cells and form a basal germ tube originating close to the hilum or apex that grows semi-axially with a conidial hilum that is distinctly protuberant, conidiogenous cells are either smooth or verrucose on both genera (Sivanesan 1987). These morphological similarities prompted von Arx and Luttrell (1979) to suggest that Bipolaris and Curvularia were synonymous. The two genera are differentiated only by conidial morphology and such a division could be artificial as many species in Bipolaris and Curvularia have conidia that are intermediate between the two genera (Goh et al. 1998). Goh et al. (1998) demonstrated that species in Bipolaris and Curvularia were heterogeneous when considering restriction phenotypes, providing further evidence for the synonymy of Bipolaris and Curvularia. The phylogenetic analysis of Berbee et al. (1999) also showed neither Curvularia nor Bipolaris is monophyletic. The separation of Bipolaris and Curvularia is controversial (Sivanesan 1987; Alcorn 1988). Clearly new approaches are needed to resolve the taxonomy of Bipolaris and Curvularia (Nakada et al. 1994). DNA fingerprinting with intrageneric probes is a potentially useful tool for species separation and identification in Bipolaris and Curvularia when coupled with another characteristic such as conidial morphology (Nakada et al. 1994). A molecular phylogenetic approach is needed to establish a robust taxonomic frame of Cochliobolus and its anamorphs.
Morphological identification of Cochliobolus and its anamorphs
Cochliobolus ascomata (Figs. 1c and 2a) are dark brown to black, unilocular with a globose body and a long or short cylindrical ostiolate neck. Hyaline to brown sterile hyphae and conidiophores (Figs. 1d, e, f and 2b) often occur on the ascomata and less so on the neck (Sivanesan 1987). The wall comprises cells which are more or less equal in thickness. Asci are bitunicate, 2-8-spored, cylindrical to obclavate or obclavate cylindrical (Sivanesan 1987) (Figs. 1a and 2c). Ascospores are filiform and more or less coiled in a helix in the ascus (Sivanesan 1987) (Fig. 1b). Most species of Cochliobolus form protothecia (sclerotia) which are sterile without any ascogenous hyphae (Shoemaker 1955).
When the generic descriptions of Bipolaris and Curvularia are compared, the two genera are morphologically very similar and cannot easily be distinguished by any distinct taxonomic criteria (Sivanesan 1987). However, these two genera have a few morphological differences, such as septal structure; conidia of Curvularia species are euseptate (Figs. 1h and 2e, f) (Ellis 1971) and those of Bipolaris are distoseptate (Figs. 1g and 2d, g) (Luttrell 1963).
Application of names: anamorph vs. teleomorph
The International Code of Botanical Nomenclature permits pleomorphic fungi to be given two names; however, there has been a worldwide intention to provide all fungi with a single name (Hawksworth et al. 2011). Many Cochliobolus species have their asexual states, and consequently synonyms, in either Bipolaris or Curvularia. For example, Cochliobolus heterostrophus is the same biological species as Bipolaris maydis (Y. Nisik. & C. Miyake) Shoemaker, and Cochliobolus geniculatus R.R. Nelson is the same biological species as Curvularia geniculata (Tracy & Earle) Boedijn. There are, however, a few exceptions; Cochliobolus boutelouae R. Sprague, C. miakei I. Hino & Katum., C. palmivora P.N. Rao & Chaudhury, C. sasae I. Hino & Katum., and C. sitharamii S.M. Reddy are not linked to any asexual state (Alcorn 1983a). Further, we could not find any references for the asexual state of C. buteae S.D. Patil & C. Ramesh. The asexual states of C. tritici Dastur and C. sporoboli E. Castell. were recorded in Helminthosporium sensu stricto (Alcorn 1983a) but further details could not be found in the available literature.
Numerous fungi have lost their capacity to form a teleomorph (Gehlot et al. 2010). Thus, many Bipolaris and Curvularia species are not linked to a Cochliobolus name because of their inability to produce the sexual state. Sivanesan (1987) listed 35 names in Cochliobolus, 33 in Curvularia and 46 in Bipolaris. Of the 35 Cochliobolus spp., 33 were linked to asexual states in Bipolaris (23 names) and to Curvularia (10 names). There were 22 Curvularia names and 26 Bipolaris names that were not linked to Cochliobolus. Bipolaris micropus (Drechsler) Shoemaker was recorded as having a Cochliobolus teleomorph but it was not named and described. In Index Fungorum, 56 Cochliobolus names, 116 Bipolaris names and 113 Curvularia names are listed. Of these only 43 Cochliobolus teleomorphs are linked to Bipolaris (31 names) and Curvularia (12 names) asexual states.
Berbee et al. (1999) studied nine Curvularia and Bipolaris species that did not have known sexual states and found that all to have a similar ancestor as sexual species of Cochliobolus. Molecular work carried out on species of Bipolaris and Curvularia showed that none was monophyletic (Berbee et al. 1999). There are various views as to which name should be adopted when choosing a unique name for a fungal species, i.e., the oldest name, the teleomorphic name, or the most important name (Hyde et al. 2011). To avoid confusion, we use the name of the sexual state Cochliobolus in this review. The name Curvularia (Boedijn 1933) is older than Cochliobolus (Drechsler 1934) and Bipolaris (Shoemaker 1959) and once changes are made to the Botanical Code in 2013 where Article 59 is no longer applicable it may be necessary to use Curvularia for all species of these genera.
As a starting point for the study of Cochliobolus and its anamorphs we have chosen to give a taxonomic review for all 55 Cochliobolus names listed in Index Fungorum, which also includes all Cochliobolus names listed by Sivanesan (1987). Bipolaris and Curvularia spp. which are not linked to a Cochliobolus name will be discussed in future studies.
Importance of Cochliobolus and its anamorphs
Some species of Cochliobolus are economically important pathogens worldwide and some examples of diseases they cause are given in this section. In the conference on ‘Wheat for the National Warm Areas’ held in Brazil in 1990, Cochliobolus sativus (Ito & Kurib.) Drechsler ex Dastur (asexual state: Bipolaris sorokiana) was confirmed to be the most economically important wheat foliar pathogen in all warm regions (Duveiller and Gilchrist 1994). C. sativus is the causal agent of southern leaf botch, seedling blights, crown rot, node infections, head blights and black point on kernels of wheat (Duveiller and Gilchrist 1994). As wheat is newly grown in warm areas it has become a serious pathogen in various parts of the world including eastern Bangladesh, Brazil, India and Nepal (van Ginkel and Rajaram 1998). According to Joshi et al. (2004), total yield loss due to C. sativus could be between 20% and 80% and losses of up to 100% can occur under severe conditions.
Southern leaf blight of maize (Zea mays) caused by Cochliobolus heterostrophus (Drechsler) Drechsler (asexual state: Bipolaris maydis) is a serious disease worldwide in warm humid conditions (Carson 1998). Typical symptoms of the disease on leaves include numerous eye spots (Lev et al. 1999), While decay of ears, infection of kernels and rotting of stalks has also been observed (Ullstrup 1972). The disease has devastating effects on maize with T cytoplasm, with those having sensitive mitochondria to the host specific polyketide toxin produced by race T pathogens being most sensitive (Lev et al. 1999). The disease spread rapidly throughout the USA and UK in 1970 (Ullstrup 1972). In some regions in the Corn Belt of mid western USA, yield per acre (0.4 ha) was reduced by 50% (Ullstrup 1972). The standard test weight for marketing grain reduced significantly (Ullstrup 1972). The serious southern leaf blight epidemic resulted in a remarkable increase in the maize price in 1970, and as a consequence British farmers shifted their livestock feed from maize to barley (Ullstrup 1972).
The most serious epidemic caused by a Cochliobolus was the famine of Bengal, in India (1943–1944), caused by C. miyabeanus (S. Ito & Kurib.) Drechsler ex Dastur. According to the data, the disease reduced rice yield in India by 40%–90% (Scheffer 1997). The price of rice increased significantly and about 2 million people starved to death. The most significant symptoms caused by C. miyabeanus are brown spots on leaves and glumes. Symptoms may also appear on coleoptiles, leaf sheath panicles and branches, but rarely in roots, young sheaths or stems. The leaf spots are uniform and fairly evenly distributed over the leaf surface. The disease may weaken seedlings and older plants, but when it infects the seeds it affects the grain quality and reduces yield (Ou 1985).
Other economically important species include Cochliobolus melinidis Alcorn, which causes spots on leaves, inflorescences and commercially important seed samples of Melinis minutiflora (Sivanesan 1987). Cochliobolus luttrellii Alcorn is found on Dactyloctenium aegyptium, while C. peregianensis Alcorn mainly causes leaf spots on grass species such as Cynodon dactylon, and Zea mays. C. victoriae Nelson is the causal agent of Victoria blight on oat. C. intermedius R.R. Nelson and C. perotidis Alcorn cause diseases on some grasses. Some species such as C. australiensis (Tsuda & Ueyama) Alcorn, C. hawaiiensis Alcorn and C. lunatus Nelson & Haasis are opportunistic human pathogens.
Bipolaris and Curvularia asexual states of Cochliobolus reported to cause several human diseases such as phaeohyphomycosis (Costa et al. 1991), Nasal phaeohyphomycosis (Koshi et al. 1987) and Corneal ulcer (Anandi et al. 1988). An unidentified Cochliobolus sp. has been recorded, inhabiting marine sponges although the ecological role of the fungus is poorly understood (Paz et al. 2010).
Life cycle of a Cochliobolus plant pathogen
Cochliobolus sativus (S. Ito & Kurib.) Drechsler ex Dastur (asexual state: Bipolaris sorokiniana (Sacc.) Shoemaker) is selected to illustrate the disease life cycle (Fig. 3). This fungus is recorded as a necrotrophic fungal pathogen causing leaf blotch, root rot, seed rot, germination failure, crown rot, seedling blight, head blight and black point on wheat grains (Zillinsky 1983; Mishra et al. 2001). Intensive symptomless biotrophic development of C. sativus occasionally results in enhanced senescence of all leaves (Dehne and Oerke 1985). Asexual propagation of the taxon is common in nature, whereas the sexual state is rarely reported, except in one Zambia sample, where two opposite mating types were recorded (Raemaekers 1988). Conidia are the main dispersal and survival propagules (Reis and Wunschr 1984). As with many other fungal leaf pathogens, this fungus is seed borne and the primary infection can be through infected seeds, infected crop residues, collateral hosts and free dormant conidia in soil (Reis 1991; Lanoiselet et al. 2005). Survival of C. sativus in seeds is probably the major source of primary inocula (Mehta 1993). If the seeds were infected when they sown it provide inocula for the newly grown crop (Reis 1991). Seed infection reduces seedling emergence by up to 38% (Clark and Wallen 1969).
The fungus also overwinters in the soil and crop residues and infects young seedlings in the spring. The trends of survival duration in wheat crop residues and infected soil is more or less similar and it was found that the population of C. sativus increased initially for 2 months in both soils and crop residues and declined thereafter (Malaker et al. 2007). However, conidia may survive in soil for more than 2 years (Ledingham et al. 1960). It has been observed that the parasitic conidia on wheat straw can form conidial aggregates. These clumps of conidia are morphologically similar to sclerotia (Chand et al. 2002). Secondary infection may be caused by airborne conidia (Duveiller et al. 2005) and this increases the severity of the disease. Nutrients available in the conidia of C. sativus are sufficient for germination and formation of appressoria, however, conidia need an exogenous supply of nutrients to enable successful penetration (Yadav 1981). Nutrients which leach out from the healthy wheat leaf tissue are absorbed by the conidia and utilized for penetration (Yadav 1981). In the tropics inocula are generally found in the soil year round (Ou 1985).
Mode of life
Fungi interact differently with their host and exhibit different life modes depending on the type of interaction. Species belonging to Cochliobolus and its anamorphs are found in association with various hosts as epiphytes, pathogens, endophytes or saprobes and examples are discussed here. Switching between these life modes is also possible.
Epiphytic Cochliobolus
The surfaces of aerial plant parts provide a habitat for epiphytic microorganisms, many of which are capable of influencing the growth of pathogens (Blakeman and Fokkema 1982). Relationships between epiphytes and endophytes have important implications for fungal biodiversity and plant health (Santamaría and Bayman 2005). For example, Curvularia pallescens isolated as an epiphytic from the surface of banana fruit shows in vitro antagonism towards Lasiodiplodia theobromae, which is a common fungal pathogen (Alvindia and Natsuaki 2008).
Endophytic Cochliobolus
Endophytes are commonly referred to a group of fungi that reside asymptomatically inside living plant tissues (Huang et al. 2009). These endophytic fungi can be mutualists, commensals, or latent pathogens (Stone et al. 2000; Hyde and Soytong 2008). Much attention is now being paid to endophytic biodiversity, to the chemistry and bioactivity of endophytic metabolites, and to the relationship between endophytes and their host plants (Huang et al. 2009; Aly et al. 2010; Xu et al. 2010). Some Cochliobolus species have been isolated as endophytes associated with different plant species (Table 1), but their percentage occurrence is usually relatively low (less than 10%) when compared to other species (Suryanarayanan et al. 2002: Thongkantha et al. 2008). Bipolaris sorokiniana (sexual state: Cochliobolus sativus) had a high frequency of occurrence in wheat glumes (Larran et al. 2007). Endophytes might be a latent pathogen on wheat leaves and glumes (Larran et al. 2007). Some Curvularia endophytes are latent pathogens and cause severe disease of Musa spp. when plants are stressed (Photita et al. 2004). Change of host plant susceptibility, excessive humidity, or poor nutrient supply may induce the transition of life mode from endophyte to pathogen (Fisher and Petrini 1992).
Bipolaris and Curvularia asexual states are most commonly recorded as endophytes; records are lacking for the occurrence of the sexual state as endophytes. Curvularia protuberata is an endophyte of Dichantelium lanuginosum (Marquez et al. 2007: Morsy et al. 2010). This fungus carries a dsRNA mycovirus (Curvularia thermal tolerance virus) and develops a three-way symbiotic relationship with host plants enabling their survival in extreme soil temperatures (Morsy et al. 2010). Bipolaris asexual states are ubiquitous in marine sponges and also found in association with sea grasses (Sakayaroj et al. 2010). A crude extract of endophytic Cochliobolus sp. isolated from Piptadenia adiantoides killed 90% of the amastigote-like forms of Leishmania amazonensis, which is a protozoan parasite and are etiological agents of severe neglected tropical diseases (NTDs) (Campos et al. 2008). A Bipolaris species was associated with the marine sponge Gelliodes carnosa and showed a close relationship to B. sorokiniana (sexual state: Cochliobolus sativus) (Liu et al. 2010), a well known plant pathogen.
Saprobic Cochliobolus
Cochliobolus species and its asexual states have been also isolated as saprobes (Ellis 1966; Kodsueb et al. 2008) and from dead wood in wounded stems of Aquilaria malaccensis (Mohamed et al. 2010). Anamorphic states of Cochliobolus can be found in association with many species of Poaceae such as bamboo and also other host plants. For example, Curvularia lunata is a frequently recorded saprobe of bamboo clumps (Hyde et al. 2002).
Although saprobic asexual states of Bipolaris and Curvularia have been recorded from stored grains such as wheat, the Cochliobolus state is less common as a saprobe. Fourteen percent of grain mould development in stored rice in southern USA was due to Curvularia spp. (Jurjevic et al. 2006) and such infestations may cause significant losses. Biosecurity regulations have been established in USA to specify that shipment of corn should be free from Cochliobolus heterostrophus (= Bipolaris maydis) (Christensen and Meronuck 1986). Some examples of saprobic Cochliobolus taxa are listed in Table 2.
Cochliobolus in biological control
Using fungi as biological control agents is a rapidly growing area of research with implication for plant productivity, animal and human health, and food production (Butt et al. 2001). Weeds are an economic constraint to agricultural production (Evans et al. 2001). Biological control of weeds by using plant pathogens has gained acceptance as a practical, safe, environmentally beneficial weed management method applicable to agroecosystems. The use of mycoherbicides is important in the move towards organic farming and the reduction in the use of chemical herbicides.
Many Cochliobolus and its anamorphic species are pathogens of weeds and can successfully be applied as weed herbicides because the weeds and pathogens have co-evolved over a long period. Some examples of research that shows Bipolaris and Curvularia can be used as potential mycoherbicides are listed in Table 3. The pathogens may have evolved biochemical mechanisms to kill the weed host or have significance influence on the host gross physiology (Strobel et al. 1991). Bipolaris spp. were tested as a potential herbicide agent against serrated tussock in Australia (Casonato et al. 2005). It was found that 16% of the underground seed bank was infected and the fungus was causing seed destruction. The virulence of Bipolaris spp. was higher than three other proven or potential pathogenic fungi (Arthrinium, Dinemasporium, Fusarium) found on serrated tussock
Alisma plantago-aquatica, Sagittaria spp. and Echinochloa spp. are weeds of rice cultivation and cause significant yield losses if not controlled (Podkin et al. 1981). Cochliobolus lunatus was shown to be a good biocontrol agent against some of these weeds (Motlag 2011). Alisma plantago-aquatica was most affected by C. lunatus, while Sagitaria trifolia was most tolerant. There was no significant difference in the susceptibility of Echinochloacrus-galli and E. oryzicola to C. lunatus (Motlag 2011). Cochliobolus eragrostidis is a biological herbicide used against large crab grasses (Digitaria sanguinalis) in cotton, soybean, watermelon and peanut cultivation (Zhu and Qiang 2004). The fungus produces the mycotoxin α,β-dehydrocurvularin, which inhibits photosynthetic electron transport within Digitaria sanguinalis (Shu-jun and Sheng 2005).
The biological control of weeds has generally been limited by the slow development of effective, broader-spectrum biological control agents (Zhang et al. 2007), and more effective biocontrol agents need to be developed. An effective biocontrol agent was produced by protoplast fusion between Cochliobolus lunatus and Helminthosporium gramineum subsp. echinochloae (Zhang et al. 2007) and such improvements should be developed to produce cost effective, broad spectrum biological control agents.
Novel metabolites and toxins produced by Cochliobolus species
The fungal kingdom includes many species with unique and unusual biochemical pathways (Keller et al. 2005). The final products and by-products of these pathways include important pharmaceuticals, potent toxins, and some metabolites that are both toxic and pharmaceutically useful (Keller et al. 2005). These natural products, along with many other low molecular weight fungal metabolites, are classified as secondary metabolites (Keller et al. 2005). Secondary metabolite production can be used in fungal systematics as demonstrated in chemotaxonomic studies carried out in Eurotiales and Xylariales (Guarro et al. 1999) and Xylariaceae (Fournier et al. 2010; Stadler 2011). New chromatographic techniques have resulted in a huge amount of secondary metabolite data which are now being incorporated into a database (Guarro et al. 1999).
Curvularia and Bipolaris species produce metabolites and toxins but their production cannot be used to differentiate the two genera (Sivanesan 1987). For example, curvulin is formed by a Curvularia sp. and also by Bipolaris papendorfii (Sivanesan 1987).
Curvularides, cochlioquinones, anthroquinones and some novel proteins involved in cyclic peptide regulation and cell wall degradation have been reported from Cochliobolus strains. These compounds may have important medicinal values, such as anti-fungal properties, and thus have the potential to be used in medical science (Chomcheon et al. 2010). For example, curvularide A-E is produced by Curvularia geniculata and has anti-fungal properties with curvularide B showing anti-fungal activity against Candida albicans, which is an opportunistic pathogen, especially in HIV patients. Curvularide B also exhibits synergistic activity with a fluconazole drug (Chomcheon et al. 2010). Some examples of novel metabolites and toxins from Cochliobolus strains are listed in Tables 4 and 5.
Mycotoxin production by Cochliobolus
Mycotoxins are toxic metabolites produced by fungi (Yassin et al. 2010). In many tropical countries mycotoxin contamination of food supplies is a major threat (Pitt and Hocking 1995). Mycotoxins can be used as a taxonomic tool to distinguish some taxa. For example, the differentiation of Group I and Group II of Penicillium viridicatum was based on mycotoxins xanthomegnin and viomellein, respectively (Ciegler et al. 1981). Bipolaris species produce more important mycotoxins than species of Curvularia. Mycotoxins produced by Bipolaris species may or may not be host-specific (Sivanesan 1987). HS toxin (a peptide and a secondary amine) produced by Cochliobolus victoriae, HC toxin (a polypeptide) produced by Cochliobolus carbornum race 1, and T toxin produced by C. heterostrophus are examples of host-specific toxins (Sivanesan 1987). Ophiobolins (terpinoid) produced by C. miyabeanus and carbotoxin produced by C. carbornum are examples of non host-specific toxins (Sivanesan 1987). Curvularin is a common mycotoxin produced by Curvularia species. For example, it is produced by Curvularia lunata and has been recoded as a toxin on sorghum and rice grains (Anthony et al. 2009). Curvularia is an important contaminant of stored rice in Nigeria (Makun et al. 2007).
Some mycotoxins have medicinal properties and these could be used in therapy against various diseases. For example, ophiobolins possess a wide range of inhibitory effects against nematodes, fungi and bacteria while cochlioquinones have been reported as anti-angiogenic agents and antagonists of human chemokine receptor CCR5, a crucial target for current anti-HIV-1-therapy (Phuwapraisirisan et al. 2007). These findings indicate a potential for using ophiobolin and cochlioquinon as pharmaceuticals. Some important mycotoxins produced by the genus Cochliobolus and its anamorphs are listed in Table 5.
Type cultures of Cochliobolus
Gene sequence data is commonly used in resolving taxonomic problems at the species or higher taxonomic levels (Shenoy et al. 2007, 2010; Cheewangkoon et al. 2010). Such studies are often flawed in that ex-type cultures of a species or genus being researched have not been included in the study (Than et al. 2008; Hyde et al. 2009). In order to stabilize the application of species names it is necessary to sequence ex-holotype, ex-isotype or ex-epitype cultures if they exist (Cai et al. 2009; Hyde et al. 2009). We summarize the genes sequenced from ex-type cultures of species of the genus Cochliobolus (Table 6). When the type derived sequences are lacking or type cultures could not be located, sequences of cultures obtained from the original author or authenticated in reliable ways were included. These type derived sequences (Table 6) can be used for analysis and identification of fresh collections in future work in this genus.
Multi locus phylogeny based on type derived cultures
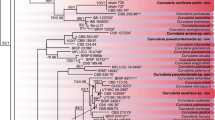
DNA was extracted from 15 ex-type cultures, 4 authentic strains (as stated in the CBS database), five cultures collected by the original authors and one culture collected from the same host plant as in the protologue. These authentic strains will be epitypified in our future publications. rDNA ITS, GPDH and EF1α sequences were generated (Manamgoda et al. 2011) and sequences were aligned using BioEdit and the alignment was optimized manually. All gaps were treated as missing data. Phylogenetic analysis was carried out for the dataset using PAUP* 4.0b10 (Swofford 2002). Ambiguously aligned regions were excluded from all analyses. Trees were figured in Treeview (Page 1996). One of the most parsimonious trees generated from phylogenetic analysis based on the above sequences for 25 strains is provided (Fig. 4). Phylogram derived from combined gene analysis of rDNAITS, LSU (28S ribosomal DNA), GPDH (glycerldehyde-3-phoshate dehydrogenase) (Berbee et al. 1999), and TEF1 (translation elongation factor-1 alpha) (Schoch et al. 2009) is presented here as a phylogenetic treatment for the genus Cochliobolus (Fig. 4). The tree shows the robust lineages for most of the well identified species analyzed using type, extype or authentic cultures.
Phylogram generated from the parsimony analysis based on combined genes of ITS,GPDH,LSU, Ef 1α sequence data derived from type, epitype and other selected specimens. Bootstrap support values >50% are shown below or above the branch and strict consensus branches are thickened. The tree is rooted with Alternaria sp. (Berbee et al. 1999)
Cochliobolus names in current use
Fifty-five Cochliobolus names were found in the literature and available databases. As a starting point, Cochliobolus names are first considered, while Bipolaris and Curvularia anamorphs that do not have a Cochliobolus sexual state are not included. The Cochliobolus names are listed alphabetically below with notes as follows:
-
Species name with authorities and publication details
-
Synonyms
-
Putative diseases and their symptoms are listed for the pathogenic species
-
A list of hosts and distribution of the fungus based on available literature
-
Essential notes emphasizing research needs
Cochliobolus aberrans Alcorn, Mycotaxon 39: 362 (1990)
= Bipolaris aberrans Alcorn, Mycotaxon 39: 364 (1990)
Holotype: BRIP 16364; Isotype: IMI 335209.
Hosts and diseases: Leaf spots of Eragrostis parviflora (Poaceae).
Distribution: Australia.
References: Alcorn 1990.
Notes: The Bipolaris anamorph, isolated from Eragrostis parviflora, formed a Cochliobolus teleomorph after mating. Sivanesan (1987) listed 16 Bipolaris and Curvularia species from the same Eragrostis parviflora host and most of them are distinct from the anamorph of C. aberrans. Only Cochliobolus kusanoi (Y. Nisik.) Drechsler ex Dastur and C. nodulosus Luttr. share some similar morphological characters. Alcorn (1990) confirmed the anamorph-teleomorph connection between C. aberrans and Bipolaris aberrans. There is little published information about C. aberrans. This species is differentiated from other species by morphology and phylogenetic analysis is needed to infer its relationship with other species.
Cochliobolus akaii (Tsuda & Ueyama) Sivan., Mycol. Pap. 158: 106 (1987)
≡ Pseudocochliobolus akaii Tsuda & Ueyama, Trans. Mycol. Soc. Japan 26: 324 (1985)
= Curvularia akaii Tsuda & Ueyama, Trans. Mycol. Soc. Japan 26: 325 (1985)
Holotype: TNS-F.
Hosts and disease: Themeda triandra (Poaceae).
Distribution: Japan.
References: Sivanesan 1987; Lenne 1990.
Notes: The teleomorph is heterothallic and was obtained by pairing compatible monoconidial isolates on Sach’s agar medium incorporating sterilized rice straw (Tsuda and Ueyama 1985). Ascospore length in C. akaii is similar to C. heteropogonis Alcorn (Alcorn 1990). This species is differentiated from other species by morphology and phylogenetic analysis is needed to infer its relationship with other species.
Cochliobolus akaiiensis Sivan., Mycol. Pap. 158: 110 (1987)
= Curvularia akaiiensis Sivan., Mycol. Pap. 158: 110 (1987)
Holotype: IMI 172167.
Hosts and diseases: Unknown.
References: Sivanesan 1987.
Notes: This species was recorded as homothallic. There are no reports of this species since Sivanesan (1987). The sexual state was only observed on TWA + wheat straw medium and has not been observed in the nature. The conidia are similar in shape to that of C. akaii but smaller and wider (Sivanesan 1987). This species is differentiated from other species based on morphology and phylogenetic analysis is needed to infer its relationship with other species.
Cochliobolus australiensis (Tsuda & Ueyama) Alcorn, Mycotaxon 16: 373 (1983)
≡ Pseudocochliobolus australiensis Tsuda & Ueyama, Mycologia 73: 92 (1981)
= Bipolaris australiensis (M.B. Ellis) Tsuda & Ueyama, Mycologia 73: 90 (1981)
≡ Drechslera Bugnic. ex M.B. Ellis, Dematiaceous Hyphomycetes (Kew): 413 (1971)
≡ Drechslera australiensis Bugnic. ex Subram. & B.L. Jain [as ‘australiense’], Curr. Sci. 35: 354 (1966)
≡ Helminthosporium australiense Bugnic., Rev. gén. Bot. 62: 242 (1956)
Holotype: TNS-F-195405; Isotypes: BPI, CUP, IMI, K, L.
Hosts and diseases: Cosmos bipinnatus, Helianthus annuus, Senecio mesogrammoides, Zinnia elegans (Asteraceae), Ananas comosus (Bromeliaceae), Acacia koa, Arachis hypogaea, anamorph is recorded as an endophyte on Medicago laciniata (Fabaceae), on leaves of Quercus xalapensis (Fagaceae), Passiflora edulis (Passifloraceae), Chloris gayana, Cymbopogon winterianus, Cynodon transvaalensis, Hordeum vulgare, Lolium multiflorum, Pennisetum americanum, P. typhoides, Sorghum, Zea mays (Poaceae), seeds of Lycopersicon esculentum (Solanaceae).
The species causes leaf blights and brown leaf spot especially of grasses. The anamorph Bipolaris australiensis has been recorded as an opportunistic human pathogen.
Distribution: Australia, China, Hawaii, India, Japan, Kenya, Libya, Mexico, New Zealand, Zimbabwe.
References: Sarbhoy et al. 1971; Baker et al. 1979; Kumar and Dwivedi 1981; Sharma et al. 1981; Srivastava and Gupta 1981a; b; Chalet et al. 1986; Sivanesan 1987; Pennycook 1989; Sobti and Bansal 1989; Lenne 1990; Portales et al. 1995; Caretta et al. 1999; Kobayashi 2007; Dyer et al. 2008.
Notes: This heterothallic species is widespred with a large host range. The disease is transmitted by infected seeds and airborne conidia. In Ellis (1971) list the species under the name Drechslera. However, Tsuda and Ueyama (1981) found that Drechslera australiensis had a sexual state in Pseudocochliobolus (later transferred to Cochliobolus) and thus they transferred Drechslera australiensis to Bipolaris australiensis. This species is an important human pathogen and has been used in medical research (Newell et al. 2006; McGinnis et al. 1986).
Cochliobolus bicolor A.R. Paul & Parbery, Trans. Br. mycol. Soc. 49: 386 (1966)
= Bipolaris bicolor (Mitra) Shoemaker, Can. J. Bot. 37: 884 (1959)
≡ Helminthosporium bicolor Mitra, Trans. Br. mycol. Soc. 15: 286 (1931) [1930]
≡ Drechslera bicolor (Mitra) Subram. & B.L. Jain, Curr. Sci. 35: 354 (1966)
≡ Drechslera bhawanii O. Prakash & A.P. Misra [as ‘bhawani’], Sydowia 33: 242 (1980)
≡ Helminthosporium bhawanii A.P Misra, Helminthosporium species occurring on cereals and other Graminae: 214 (1976), non rite publ. (Art.36)
Holotype: MELU F2220.
Hosts and diseases: Bactris gasipaes (Arecaceae), Quercus xalapensis (Fagaceae), Andropogon aciculatus, Apluda aristata, Brachiaria ruziziensis, Eleusine coracana, Eragrostis japonica, Pennisetum clandestinum, P. glaucum, P. typhoides, Melanocenchris abyssinica, Oryza sativa, Panicum maximum, Setaria sp., Sorghum vulgares, Triticum aestivum, Urochloa panicoides, Zea mays, Zizania aquatica (Poaceae).
Generally causes leaf spots.
Distribution: Africa, Australia, Brazil, Canada, Cote d’Ivoire, Denmark, India, New Zealand, Nigeria, Swaziland.
References: Paul and Parbery 1966; Sivanesan 1987; Lenne 1990; Portales et al. 1995; Mendes et al. 1998; Morejon et al. 1998.
Notes: This species was first reported as Helminthosporium bicolor Mitra (1930) in Poona, India associated with foot rot of wheat. Resemblance conidia dimensions of H. carbonum Ullstrup, H. setariae Saw. and H. cynodontis Marig. The typical bicoloration of conidia separates this species from others (Paul and Parbery 1966). The sexual stage was produced on Sach’s agar. High humidity stimulated mycelia and conidial growth, but in these conditions ascomata development failed (Paul and Parbery 1966).
Cochliobolus boutelouae R. Sprague, Mycologia 43: 550 (1951)
Holotype: A.S. 20726 WSP.
Hosts and diseases: Boutealoua gracilis (Poaceae)
The taxon causes dark brown, almost black charred, linear leaf spots.
Distribution: USA.
References: Sprague 1951; Rogerson 1958.
Notes: No conidial state for this fungus has been recorded. We cannot found any report of the occurrence of the species after its introduction in 1958. Epityfication is required.
Cochliobolus buteae S.D. Patil & C. Ramesh, Indian Botanical Reporter 5: 28 (1986)
Hosts: Butea monosperma (Fabaceae).
Distribution: India.
References: Patil and Ramesh 1986.
Notes: There are no records of this species after its introduction in 1986 and the species should be recollected and subjected to molecular analysis to prove its distinctness. The asexual state of the species is unknown.
Cochliobolus carbonum R.R. Nelson, Phytopathology 49: 809 (1959)
= Bipolaris zeicola (Stout) Shoemaker, Can. J. Bot. 37: 885 (1959)
≡ Helminthosporium zeicola Stout, Mycologia 22: 273 (1930)
≡ Helminthosporium carbornum Ullstrup, Phytopathology 34: 219 (1944)
≡ Drechslera zeiocola (Stout) Subram. & B.L. Jain, Curr. Sci. 35: 355 (1966)
≡ Drechslera carbonum (Ullstrup) Sivan., Bitunicate Ascomycetes and their Anamorphs (Vaduz): 369 (1984)
Holotype: BPI, K
Hosts and diseases: Sorghum, Zea mays (Poaceae), Malus domestica (Rosaseae). The species is highly virulent on corn causing black ear rot and Northern leaf spot of sweet corn. C. carbornum causes spots on leaves, kernels, ear and stems of maize of and also causes diseases on seeds of Sorghum. The asexual state is the casual agent of Helminthosporium corn leaf spot (HCLS) (Jones and Dunkle 1993).
Distribution: Australia, Brazil, Cambodia, Canada, China, Congo, Denmark, Egypt, India, Kenya, New Zealand, Nigeria, Solomon island, USA
References: Nelson 1959; Lijia et al. 1998. Litzenberger et al. 1962; Turner 1971; Leath and Leonard 1986; Sivanesan 1987; Lenne 1990; Mendes et al. 1998
Notes: Cochliobolus carbornum is a common pathogenic species. HC-toxin is a host selective toxin produced by C. carbornum Race 1 which is essential for its pathogenicity on maize (Panaccione et al. 1992). On the basis of inter-specific fertility C. carbornum and C. victoriae seem closely related. However only 1% of attempted crosses of these two isolates are fertile (Nelson 1960b). A close connection between C. carbornum and C. victoriae was apparent as the two species could not be distinguished on the phylogenetic tree (Berbee et al. 1999). Genetic analysis of the progeny from individual asci of C. carbornum by (Nelson 1960b) showed that asci rarely contain a full complement of eight ascospores and also found a link between the number of asci and segregation ratio.
Cochliobolus chloridis Alcorn, Trans. Br. Mycol. Soc. 70: 61 (1978)
= Bipolaris chloridis (Alcorn) Alcorn, Mycotaxon 16: 373 (1983)
≡ Drechslera chloridis Alcorn, Trans. Br. mycol. Soc. 67: 148 (1976)
Holotype: IMI 213865; Isotype: BRIP 12106.
Hosts and diseases: Chloris gayana (Poaceae). Causes leaf blight
Distribution: Australia, India, Kenya, Malawi, Tanzania, Zambia.
References: Alcorn 1978; Sivanesan 1987.
Notes: The species is heterothallic and morphologically very similar to Cochliobolus heterostrophus, which is the type species of the genus. It differs only in having ascomata with a wider diameter range, and a shorter narrow beak; however its conidial state is quite distinct from that of C. heterostrophus (Alcorn 1978). The species was described using morphological characters and phylogenetic analysis using type derived sequences are essential to infer its relationships with other species.
Cochliobolus cymbopogonis J.A. Hall & Sivan., Trans. Br. mycol. Soc. 59: 315 (1972)
= Curvularia cymbopogonis (C.W. Dodge) J.W. Groves & Skolko [as ‘cymbopogi’], Canadian Journal of Research, Section C 23: 96 (1945)
≡ Helminthosporium cymbopogonis C.W. Dodge [as ‘cymbopogi’], Ann. Mo. bot. Gdn 29: 139 (1942)
Holotype: IMI 130402.
Hosts and diseases: Cannabis sativa (Cannabaceae) Andropogon sambesi, A. tectorum, A. gabonensis, Cymbopogon citrates, C. confertiflorus, C. martini, Cymbopogon winterianus, Hyparrhenia rufa, H. dissoluta, H. glabriuscula, Loudetia superb, Sorghum vulgare, Zea mays (Poaceae).
Causes seed and seedling blights and leaf spots on dicotyledons, monocotyledons worldwide.
Distribution: Colombia, Ghana, India, Jamaica, Malaysia, Nigeria, Sierra Leone, Sudan, USA, Venezuela, Zambia.
References: Litzenberger et al. 1962; Sivanesan 1987; Lenne 1990; Mcpartland and Cubeta 1997
Notes: Homothallic pseudothecia of C. cymbopogonis have been seen only in cultures (Mcpartland and Cubeta 1997). Asci of C. cymbopogonis are partially bitunicate and both layers of the ascus wall are functional in ascospore liberation (El Shafie and Webster 1980). Cochliobolus cymbopogonis shows an unusual organization of MAT gene (Dyer et al. 2003); with the species carry both homothallic and heterothallic idiomorphs in MAT loci (Yun et al. 1999).
Cochliobolus cynodontis R.R. Nelson, Mycologia 56: 67 (1964)
≡ Bipolaris cynodontis (Marignoni) Shoemaker, in Azbukina et al. (eds), Overs. K. danske Vidensk. Selsk. Forh. Medlemmers Arbeider 79: (1959)
= Bipolaris cynodontis (Marignoni) Shoemaker, Can. J. Bot. 37: 883 (1959)
≡ Helminthosporium cynodontis Marignoni, Micromiceti di Schio: 27 (1909)
≡ Drechslera cynodontis (Marignoni) Subram. & B.L. Jain, Curr. Sci. 35: 354 (1966)
Holotype: BPI 85101.
Hosts and diseases: Axonopus affinis, Cynodon dactylon var. dactylon, Digitaria spp., Lolium multiflorum-perenne, Miscanthus spp., Oryza sativa, Panicum maximum, Sorghum arundinaceum var. sudanense (Poaceae), Eucalyptus sp. (Myrtaceae), Rosa spp. (Rosaceae).
Leaf spot of Bermuda grass and other crops, leaf blight and brown patches of turf and lawns.
Distribution: Australia, India, Kenya, New Zealand, Papua New Guinea, South Africa, Taiwan and USA
References: Endo 1961; Nelson 1964a; Matsushima 1980; Shaw 1984; Sivanesan 1987; Pennycook 1989; Lenne 1990; Crous et al. 2000; Roane 2004.
Notes: This species was first isolated from dry leaves of Cynodon dactylon in Italy in 1909 (Nelson 1964a). Although the species is not considered to be a primary pathogen it is very common on Cynodon dactylon. Morphological characters of this species are congeneric with the description of C. heterostrophus which is the type species of the genus. Pigmentation was previously used as a taxonomic character in the genus Bipolaris, but studies on B. cynodontis showed that cultural conditions affected pigmentation and thus pigmentation cannot be used as a taxonomic character (White and Johnson 1971). The species is heterothallic (Nelson 1964a).
Cochliobolus dactyloctenii Alcorn, Mycotaxon 15: 3 (1982)
= Bipolaris dactyloctenii Alcorn, Mycotaxon 15: 3 (1982)
Holotype: BRIP 13498.
Hosts and diseases: Dactyloctenium radulans, Melinis minutiflora, Sorghum, Zea mays (Poaceae).
The species forms lesions on leaves.
Distribution: Australia, India.
References: Alcorn 1982; Sivanesan 1987.
Notes: The anamorph of this fungus was first collected on caryopses of Melinis minutiflora and the heterothallic fungus formed the teleomorph in paired single-ascospore cultures (Alcorn 1982a). Conidia of B. dactyloctenii can be differentiated from the other species with small straight conidia such as B. australiensis, B. hawaiiensis (M.B. Ellis) J.Y. Uchida & Aragak and B. spicifera (Bainier) Subram (Alcorn 1982) by the shape and width of conidia.
Cochliobolus eleusines Alcorn, Mycotaxon 39: 367 (1990)
= Bipolaris eleusines Alcorn & R.G. Shivas, Mycotaxon 39: 369 (1990)
Holotype: BRIP 16334; Isotype: IMI 335211.
Hosts and diseases: Eleusine indica (Poaceae).
Generally causes leaf spots.
Distribution: Australia, Papua New Guinea, Vanuatu.
References: Alcorn 1990; Shivas and Alcorn 1996.
Notes: The sexual state is similar to C. sativus, but differs in its greater size range of ascomata and shorter and narrower ascospores with fewer septa (Kuribayashi 1929; Tinline 1951; Sivanesan 1987). Harding and Tinline (1983) examined this relationship by mating C. eleusines and C. sativus and found that there was no interfertility although protothecia formed in many crosses. Subsequently, Alcorn (1990) named C. eleusines as a different species. Cochliobolus eleusines shows a very close relationship to C. sativus at the phylogenetic level (Berbee et al. 1999). Since 1990 there have been no records of this species.
Cochliobolus ellisii Alcorn, Trans. Br. mycol. Soc. 81: 172 (1983)
= Bipolaris ellisii (Danquah) Alcorn, Trans. Br. mycol. Soc. 81: 174 (1983)
≡ Drechslera ellisii Danquah, Trans. Br. mycol. Soc. 64:545 (1975)
Holotype: BRIP 13633; Isotypes: DAR 41671, IMI 267702.
Hosts and diseases: Crotalaria pseudospartium (Fabaceae), on dead needles of Pinus khasya (Pinaceae), Dactyloctenium aegyptium (Poaceae), isolated from seeds of Capsicum sejuncta (Solanaceae).The species is generally recorded as a saprobes.
Distribution: Australia, Ghana, Kenya, Thailand.
References: Danquah 1975; Alcorn 1983c; Sivanesan 1987; Hyde and Alcorn 1993; Tokumasu et al. 1990; Caretta et al. 1999.
Notes: Drechslera ellisii isolates was found to form the teleomorph in paired cultures. Morphological characters such as shape, manner of germination, septum ontogeny and hilum morphology of asexual state indicated that it would be more appropriate to be placed in Bipolaris (Alcorn 1983c). There are no records of the species since 1999.
Cochliobolus eragrostidis (Tsuda & Ueyama) Sivan., Mycol. Pap. 158: 113 (1987)
= Curvularia eragrostidis (Henn,) J.A. Mey, Publ. Inst. nat. Étude agron. Congo belge, Sér. sci. 75: 183 (1959)
≡ Brachysporium eragrostidis Henn, Annals de Musée du Congo, Botanique Série 5 2: 230 (1908)
≡ Spondylocadium maculans Bancroft, Bull. Dep. Afric. F.M.S. 16: 16 (1913)
≡ Curvularia maculans (Bancroft) Boedijin, Bull. Jard. bot. Buitenz. 3 Sér. 125: (1933)
≡ Pseudocochliobolus eragrostidis Tsuda & Ueyama, Trans. Mycol. Soc. Japan 26: 322 (1985)
Holotype: TNS-F 198560.
Hosts and diseases: The species is distributed worldwide and reported from several plant families. Liquidambar macrophylla (Altingiaceae), Allium cepa, A. sativum (Amaryllidaceae), Cananga odorata, Annona odorata (Annonaceae), Colocasia esculenta (Araceae), Areca catechu, Cocos nucifera, Elaeis guineensis, (Arecaceae), Raphanus sativus (Brassicaceae), Ananas comosus (Bromeliaceae), Rhoeo discolor, R. spathacea (Commelinaceae), Dioscorea alata, D. cayenensis, D. pentaphylla (Dioscoreaceae), Alysicarpus monilifer, Acacia aulacocarpa, Pueraria phaseoloides, Stylosanthes gracilis, S. humilis, Vigna unguiculata (Fabaceae), Ficus spp., (Moraceae), Eucalyptus globulosus, E. tereticornis (Myrtaceae), Phalaenopsis amabilis (Orchidaceae).Pandanus tectorius (Pandanaceae), Pinus patula (Pinaceae), Brachiaria decumbens, Cynodon dactylon, Cymbopogon ambiguous, C. martini, C. winterianus, Dactyloctenium aegyptium, Digitaria exilis, Eragrostis chapilieri, Imperata cylindrica, Oryza sativa, Panicum miliaceum, Pennisetum purpureum, Rottboellia exaltata, Saccharum officinarum, Sorghum vulgare, Sporobolus poiretii, Triticum, Zea mays (Poaceae), Pentas lanceolata (Rubiaceae), Capsicum annuum, Lycopersicon esculentum (Solanaceae), Camellia sinensis (Theaceae)
Cochliobolus eragrostidis and its asexual state Curvularia eragrostidis is responsible for numerous diseases including leaf spots and blights. Important examples include foliage diseases of tea (Camellia sinensis) where it causes extensive disease particularly to cloned cuttings and in young tea plantations (Saha et al. 2001). Curvularia eragrostidis causes economic damage to young tea plantations and research has been carried out to investigate the pathogenicity on tea plants. Pathogenicity to different varieties of tea was related to the level of common antigens present between host and pathogen (Dasgupta et al. 2005). The asexual state Curvularia eragrostidis causes yam blight which is a major foliar disease in yam (Michereff et al. 1994). One strain was isolated from air in Hong Kong (Zhuang 2001).
Distribution: Australia, Brazil, Brunei, China, Congo, Cuba, Darussalam, Fiji, Hong Kong, India, Indonesia, Japan, Malawi, Malaysia, Mexico, Myanmar, New Zealand, Nigeria, Sierra Leone, South Africa, Solomon Islands, Sri Lanka, Thailand, USA.
References: Ostazeski 1959; Johnston 1960; Turner 1971; Miller 1971; Liu 1977; Peregrine and Ahmad 1982; Matsushima 1983; Urtiaga 1986; Sivanesan 1987; Matsushima 1989; Shivas 1989; Lenne 1990; Tokumasu et al. 1990; Hyde and Alcorn 1993; Vittal and Dorai 1994; Portales et al. 1995; Mendes et al. 1998; Crous et al. 2000; Lu et al. 2000; Minter et al. 2001; Zhuang 2001; Michereff et al. 2008; Thaung 2008
Notes: Cochliobolus eragrostidis is a heterothallic species and the sexual state was observed on Sach’s agar medium containing sterilized rice straw (Sivanesan 1987). Goh et al. (1998) included the anamorph in a phylogenetic study based on ITS and 28S rRNA combined analysis and found that it clustered with Bipolaris maydis and Curvularia oryzae. This cluster was distinct from the main cluster of Bipolaris and Curvularia species.
Cochliobolus geniculatus R.R. Nelson, Mycologia 56: 777 (1964)
= Curvularia geniculata (Tracy & Earle) Boedijn, Bull. Jard. bot. Buitenz, 3 Sér. 13: 129 (1933)
≡ Helminthosporium geniculatum Bull. Torrey bot. Club 23: 207 (1896)
≡ Pseudocochliobolus geniculatus (R.R. Nelson) Tsuda, Ueyama & Nishih. Mycologia 69: 1118 (1977)
Holotype: BPI.
Hosts and diseases: The species was found associated with various hosts such as Amaranthus gangeticus (Amaranthaceae), Petroselinum crispum (Apiaceae), Caladium bicolor, Colocasia esculenta (Araceae), Zinnia elegans (Asteraceae), Impatiens balsamina (Balsaminaceae), Basella rubra (Basellaceae), Brassica oleracea (Brassicaceae), Opuntia spp. (Cactaceae), Ipomoea batatas (Convolvulaceae) Arachis hypogaea, rotting pods of Phaseolus vulgaris, Cause leaf spots on Cajans cajan, Centrosema pubescens, Glycine max, Senna alexandrina, Stylosanthes capitata (Fabaceae), Episcia cupreata (Gesneriaceae), Maranta spp. (Marantaceae), Jasminum sambac (Oleaceae) seeds of Eschscholzia californica (Papaveraceae), Axonopus compressus, Brachiaria spp., Cymbopogon sp, Cynodon dactylon, Imperata cylindrical, Ischaemum ciliare, Oryza sativa, Panicum miliaceum, Paspalum sp., Pennisetum sp., Saccharum officinarum, Sporobolus poiretii, S. raveneli, Themeda arguens, Triticum aestivum, T. durum, Zea mays (Poaceae) Capsicum frutescens, Lycopersicon esculentum, Solanum melongena (Solanacae), Vitis vinifera (Vitaceae), Zingiber officinale (Zingiberaceae).
Causes leaf spots, seed and seedling blights also isolated as a saprobe.
Distribution: Cochliobolus geniculatus is recorded most commonly in tropical regions including Australia, Bangladesh, Bhutan, Brunei, Canada, Cuba, India, Fiji, Hong Kong, Jamaica, Malaysia, Myanmar, Nepal, Nigeria, Papua New Guinea, Peru, Sierra Leone, Singapore, Seychelles, Solomon Islands, Tobago, Thailand, Trinidad, Uganda, Venezuela.
References: Nelson 1964b; Firman 1972; Srivastava and Gupta 1981a; Peregrine and Ahmad 1982; Sivanesan and Holliday 1982; Shaw 1984; Urtiaga 1986; Sivanesan 1987; Lenne 1990; Richardson 1990; Boa and Lenné 1994; Thaung 2008.
Notes: Drechsler found that it was different from Helminthosporium sensu lato but he did not separate it from the genus. Later Boedijn (1933) transferred H. geniculatus and other similar species to Curvularia. The species is heterothallic and the sexual stage was formed in paired cultures (Nelson 1964b). Tsuda et al. (1977) introduced the new genus Pseudocochliobolus and transferred the species as P. geniculatus together with P. lunatus, P. nisikadoi, and P. specifer based on the development of columnar to flat ascomata; or flat stroma firmly adhering to the substrate at the base; and also parallel to loosely coiled arrangement of ascomata in ascus. These characters were thought to represent intra-specific variation within Cochliobolus. Alcorn (1983a), however, synonymized Pseudocochliobolus with Cochliobolus. The discharge of ascospores of C. geniculatus is through splitting of ascus (Alcorn 1981a) which is somewhat different from the generic type species C. heterostrophus where ascospores are released though a swelling of the ascus and circumcessile rupture in the epical portion of the ascus wall (Alcorn 1981a).
Cochliobolus graminicola Alcorn, Proc. R. Soc. Qd. 107: 1 (1998)
= Curvularia graminicola Alcorn, Proc. R. Soc. Qd. 107: 2 (1998)
Holotype: BRIP 24308.
Hosts and disease: dead leaves of Triodia and Aristida, and Lolium × multiflorum-perenne (Poaceae).
Distribution: Australia, New Zealand.
References: Pennycook 1989; Alcorn 1998.
Notes: Records on species distribution and phylogenetic analysis are lacking. This species was introduced based on morphology, and a phylogenetic analyses is necessary to establish its distinctiveness.
Cochliobolus hawaiiensis Alcorn, Trans. Br. mycol. Soc. 70: 64 (1978)
= Bipolaris hawaiiensis (M.B. Ellis) J.Y. Uchida & Aragaki, Phytopathology 69: 1115 (1979)
≡ Drechslera hawaiiensis Bugnic. ex M.B. Ellis, Dematiaceous Hyphomycetes : 415 (1971)
≡ Drechslera hawaiiensis Bugnic. ex Subriam. & B.L. Jain, [as ‘hawaiiense’], Curr. Sci. 35: 354 (1966)
≡ Helminthosporium hawaiiense Bugni, Rev. gén. Bot. 62: 238 (1955)
≡ Pseudocochliobolus hawaiiensis (Alcorn) Tsuda & Ueyama, Mycologia 73: 92 (1981)
Holotype: IMI213864; Isotype: BRIP 12105.
Hosts and diseases: Hypoestes forskaolii (Acanthaceae), Cosmos bipinnatus, Dahlia variabilis (Asteraceae), Dianthus sp. (Caryophyllaceae), Cleome spinosa (Cleomaceae), Bauhinia Lupinus, Glycine ussuriensis (Fabaceae), Quercus sp. (Fagaceae), Juncus roemerianus (Juncaceae) Artocarpus integra (Moraceae), Musa sp. (Musaceae), Pandanus sp. (Pandanaceae), Bambusa sp., Cenchrus setigerus, Chloris gayana, C. inflate, Cynodon dactylon, C. transvaalensis, Digitaria decumbens, Hordeum vulgare, Oryza sativa, Pennisetum sp., Saccharum officinarum, Setaria italica, Sorghum sp., Triticum sp., Zea mays (Poaceae) and soil. Causes leaf blights and leaf spots (Subramanyam et al. 1990) and has been recorded as a weak pathogen and a saprobe associated with rotten rice kernels and seeds of Cajanus cajan. It has been recorded as a human pathogen causing phaeohyphomycosis (Costa et al. 1991) such as nasal phaeohyphomycosis (Koshi et al. 1987) and Corneal ulcer (Anandi et al. 1988). Bipolaris hawaiiensis cause sinusitis and is more aggressive than the other hyphomycete pathogens which cause sinusitis (Fothergill 1996).
Distribution: This species is widespread throughout tropical and subtropical regions including Australia, China, Cuba, Denmark, Egypt, India, Hawaii, Kenya, Nepal, New Zealand, Mozambique, Myanmar, Pakistan, Papua New Guinea, South Africa, Tanzania, Thailand, and USA.
References: Alcorn 1978; Fell and Hunter 1979; Mishra et al. 1981; Srivastava and Gupta 1981a; b; Sivanesan 1987; Matsushima 1989; Lenne 1990; Richardson 1990; Boa and Lenné 1994; Bettucci et al. 1997; Caretta et al. 1999; Crous et al. 2000; Liu and Zhang 2004; Thaung 2008; Worapattamasri et al. 2009.
Notes: The morphologies of the ascomatal neck are generally regarded as a generic feature of Cochliobolus (Alcorn 1978; Sivanesan 1987). Studies with C. hawaiiensis showed that this is not a fixed character as considerable variation occurs in the neck of this species (i.e. ascomata neck can vary from long cylindrical to short or in some ascomata neck is absent) even when grown in the same conditions (Alcorn 1978). Cochliobolus geniculatus, C. cymbopogonis and C. lunatus have similar ascomata size to C. hawaiiensis, but their Curvularia conidial states are distinguishable.
Cochliobolus heliconiae Alcorn, Aust. Syst. Bot. 9: 813 (1996)
= Bipolaris heliconiae Alcorn, Aust. Syst. Bot. 9: 814 (1996)
Ex -Holotype: BRIP 17349 a
Hosts and diseases: Cause lesions on Heliconia sp. (Heliconiaceae)
References: Alcorn 1996
Notes: The species is heterothallic and ascomata are produced in culture after mating. The species has not been included in phylogenetic analyses. No additional distribution records can be found in the literature.
Cochliobolus heteropogonis Alcorn, Mycotaxon 39: 371 (1990)
= Curvularia heteropogonis Alcorn, Mycotaxon 39: 372 1990
Holotype: BRIP 16087; Isotype: IMI 335213.
Hosts and diseases: Heteropogon contortus, Cymbopogon citratus (Poaceae). Highly pathogenic causing numerous linear orange-brown lesions, which often coalesce resulting leaf blight (Alcorn 1990).
Distribution: Australia.
References: Alcorn 1990.
Notes: This fungus was first isolated from a leaf of Heteropogon contortus and identified as Curvularia andropogonis (Zimm.) Boedjin (Alcorn 1990). However, conidial dimensions and cell proportions (basal cell to second cell) differed from those of C. andropogonis and the isolate formed a sexual state in mating experiments. The species is most similar to C. cymbopogonis; but differs in having a much longer ostiolar neck, longer asci and larger ascospores. Ascospores are similar in length to those of C. akaii (Alcorn 1990). Records of this species are few. As this species is described only by morphologically this could be easily misidentified. It is necessary to carry out a molecular analysis of C. heteropogonis with similar species using type derived sequences to show its phylogenetic distinctness.
Cochliobolus heterostrophus (Drechsler) Drechsler, Phytopathology 24: 973 (1934)
= Bipolaris maydis (Y. Nisik. & C. Miyake) Shoemaker, Can. J. Bot.: 882 (1959) ≡ Ophiobolus heterostrophus Drechsler, Journal of Agricultural Research 31: 701 (1925)
≡ Helminthosporium maydis Nisikado and Miyake, Journal of Plant Protection, Tokyo 13: 20–27 (1926)
≡ Drechslera maydis (Nisikado and Miyake) Subram and Jain, Curr. Sci. 35: 354 (1966)
Holotype: BPI.
Hosts and disease: The species is recorded on a wide range of hosts including Elaeis guineensis, E. indica (Arecaceae) Dianthus caryophyllus (Caryophyllaceae) Vigna sinensis (Fabaceae) Antirrhinum majus (Plantaginaceae) Brachiria foliosa, Bothriochloa insculpta, Chloris virgata, C. gayana, Coix lacryma-jobi, Cynodon dactylon, C. martini, C. citrates, Dactyloctenium aegyptium, Digitaria ciliaris, D. marginata, Echinochloa colonum, E. crus-galli, Eleusine indica, Eriochloa procera, Euchalaena maxicana, Leersia hexandra, Panicum bisulcatum, P. maximum, P. miliaceum, P. palmifolium, Panicum reptan, Paspalum scrobiculatum, Pennisetum glaucum, P. purpureum, Perotis indica, Rottboellia exaltata, Saccharum officinarum, Sorghum halepenes, S. bicolor, Setaria barbata, S. homonyma, S. italica, S. sphacelata, Triticum sp, Zea mays (Poaceae), Populus deltoides (Salicaceae). Causes southern corn leaf blight.
Distribution: Australia, Bahamas, Bhutan, Bolivia, Brunei, China, Congo, Cuba, Cyprus, Denmark, Egypt, Georgia, Ghana, Guatemala, Guinea, Guyana, Hawaii, Hong Kong, Jamaica, New Zealand, Nigeria, Malaysia, Malawi, Pakistan, Panama, Papua New Guinea, Philippines, Samoa, South Africa, Sudan, Swaziland, Taiwan, Thailand, USA, Vanuatu, Yugoslavia, Zambia, Zimbabwe .
References: Drechsler 1934; Teodoro 1937; Dade 1940; Boughey 1946; Sprague 1950; Mc Guire Jr and Crandall 1967; Misra and Prakash 1972; Williams and Liu 1976; Gorter 1977; Anonymous 1979; Alfieri et al. 1984; Urtiaga 1986; Turner 1971; Hanlin et al. 1978; Sivanesan 1987; Lenne 1990; Richardson 1990; Chauhan and Pandey 1992; Crous et al. 2000; Deng and Zhang 2002.
Notes: Cochliobolus heterostrophus, the maize leaf spot pathogen is the generic type of Cochliobolus. The taxon is heterothallic (Nelson 1957). Two races of C. heterostrophus, Race T and O have been identified (Hooker et al. 1970). Both races are morphologically similar, but differ in alleles at the host specific toxin locus (Tox 1) (Klittich and Bronson 1986). Strains with TOX-1 allele (Race T) produce T- toxin and cause spindle-shaped lesions on maize leaves with T-cms cytoplasm (Texas male sterile cytoplasm); strains with tox-1 do not produce T-toxin (Race O) and cause small, parallel lesions on T-cms maize leaves (Klittich and Bronson 1986).
Cochliobolus heveicola Tsukib. & W.H. Chung, Mycoscience 46: 20 (2005)
= Bipolaris heveae (Petch) Arx, Beih. Nova Hedwigia 87: 288 (1987)
≡ Drechslera heveae (Petch) M.B. Ellis, Dematiaceous Hyphomycetes: 451 (1971)
≡ Helminthosporium heveae Petch, Ann. R. bot. Gdns Peradeniya 3: 8 (1906)
Holotype: NIAES 20555.
Hosts and diseases: Hevea brasiliensis (Euphorbiaceae) Cynodon dactylon, Distichlis spicata var. stricta, Zoysia japonica, Panicum virgatum (Poaceae), Acer truncatum (Sapindaceae).
Causes minute, orbicular purple spots with a brown border on leaves of rubber (Hevea brasiliensis) and brown stripe disease on leaves of Cynodon dactylon and Zoysia japonica (Tsukiboshi et al. 2005). Cochliobolus heveicola (as Bipolaris heveae) was also recorded as causing brown stripe in the salt marsh grass Distichlis spicata var. stricta.
Distribution: Brazil, Cambodia, China, Congo, Ghana, Guinea, Honduras, India, Indonesia, Japan, Malaysia, Myanmar, Nicaragua, Panama, Papua New Guinea, Philippines, Sri Lanka, USA.
References: Dade 1940; Johnston 1960; Anonymous 1960; Litzenberger et al. 1962; Kranz 1963; Mc Guire Jr and Crandall 1967; Shaw 1984; Zhuang 2001; Lang and Tisserat 2005; Tsukiboshi et al. 2005; Piepenbring 2006; Ghimire et al. 2011; Sun et al. 2011
Notes: Even though the anamorph is a common pathogen in rubber (Hevea brasiliensis), the teleomorph was first recorded from leaf lesions of Cynodon dactylon and Zoysia japonica (Tsukiboshi et al. 2005).
Cochliobolus homomorphus Luttr. & Rogerson, Mycologia 51: 195 (1959)
= Bipolaris homomorphus (Luttr. & Rogerson) Subram. ex Alcorn, Mycotaxon 16: 374 (1983)
≡ Helminthosporium homomorphus Luttr. & Rogerson, Mycologia 51: 195 (1959)
≡ Drechslera homomorpha (Luttr. & Rogerson) Sivan, The bitunicate ascomycetes and their anamorphs: 375 (1984)
Holotype: BPI, K.
Hosts and diseases: No disease records are known for this species.
Distribution: USA.
References: Luttrell and Rogerson 1959.
Notes: The type strain of this species was obtained from air with a paddy-rittis sampler located 175 ft high in Kansas State College, USA. The teleomorph was obtained by inoculating the conidial isolates into wheat straw partially embedded into Sach’s agar medium (Luttrell and Rogerson 1959). Cochliobolus homomorphus is one of the four homothallic species in the genus. Cochliobolus homomorphus can be identified by its unique arrangement of MAT locus (Yun et al. 1999).
Cochliobolus intermedius R.R. Nelson, Mycologia 52: 776 (1960)
= Curvularia intermedia Boedjin, Bull. Jard. bot. Buitenz. III, 13:126 (1933)
Holotype: BPI, K.
Hosts and diseases: Worldwide distribution. Recorded from Mangifera indica (Anacardiaceae), Tillandsia caput-medusae (Bromeliaceae), Carica papaya (Caricaceae), Cupressus arizonica (Cupressaceae), Glycine max, Phaseolus vulgaris (Fabaceae), Cynodon dactylon, Dactyloctenium adscendens, Digitaria adscendens, D. ciliaris, Ischaemum ciliare, Miscanthus sp., Oryza sativa, Thaumastochloa rariflora, Triticum aestivum, Pennisetum sp., Saccharum sp., Sorghum vulgare, S. bicolor subsp. drummondii, S. arundinaceum var. sudanense, Zea mays, (Poaceae), Elettaria cardamomum (Zingiberaceae)
Causes disease of grasses resulting in some economical losses when associated with Sudan grasses (Sorghum bicolor subsp. drummondii) (Komoto et al. 1980).
Distribution: Australia, Brazil, Brunei, China, Guinea, Hong Kong, India, Japan, Korea, Malaysia, Malawi, Papua New Guinea, Tanzania, USA, Venezuela, Zimbabwe.
References: Nelson 1960a; Turner 1971; Davis et al. 1972; Peregrine and Siddiqi 1972; Jones 1976; Hanlin et al. 1978; Matsushima 1980; Bhale and Khare 1982; Peregrine and Ahmad 1982; Grand 1985; Urtiaga 1986; Sivanesan 1987; Shivas 1989; Lenne 1990; Miller 1991; Richardson 1990; Hyde and Alcorn 1993; Mendes et al. 1998; Zhuang 2001; Cho and Shin 2004; Liu and Zhang 2004.
Notes: Cochliobolus intermedius is heterothallic and the teleomorph is obtained by pairing compatible isolates on Sach’s agar medium (Nelson 1960a). C. intermedius is a potential herbicide against crab grasses and some other specific grass plants (Zhu et al. 2004) and for susceptible weed grasses in crops such as cotton, soybean, and peanut (Tilley and Walker 2002).
Cochliobolus kusanoi (Y. Nisik.) Drechsler ex Dastur, Indian J Agr Res 12: 733 (1942)
=Bipolaris kusanoi (Y. Nisik.) Shoem., Can. J. Bot. 37: 883 (1959)
≡ Drechslera kusanoi (Y. Nisik.) Subram. & P.C. Jain, Curr. Sci. 35: 354 (1966)
≡ Helminthosporium kusanoi Y. Nisik, J. Jap. Bot. 4: 108 (1929)
≡ Ophiobolus kusanoi Y. Nisik., J. Jap. Bot. 4: 99 (1929)
Hosts and disease: Eragrostis cilianensis, E. multicaulis, E. pilosa (Poaceae). Causes sooty heads on Eragrostis species.
Distribution: Japan, Sudan, USA.
References: Drechsler 1942; Rogerson 1958; Sivanesan 1984, 1987.
Notes: The asexual state has long conidiophores which are nearly nodulose from the base, and resemble the long conidiophores produced on seed heads by B. nodulosa, thus the sexual state of Cochliobolus kusanoi belongs to the nodulosus group of the genus Bipolaris. C. kusanoi is homothallic.
Cochliobolus lunatus R.R. Nelson & F.A. Haasis, Mycologia 56: 316 (1964)
= Curvularia lunata (Wakker) Boedijn, Bull. Jard. bot. Buitenz, 3 Sér. 13: 127 (1933)
≡ Acrothecium lunatum Wakker, Wakker & Went, De Ziektan van het Suikerriet op Java: 196 (1898)
≡ Pseudocochliobolus lunatus (R.R. Nelson & F.A. Haasis) Tsuda, Ueyama & Nishih, Mycologia 69: 1118 (1978) [1977]
≡ Helminthosporium caryopsidum Sacc, Annls mycol. 12: 313 (1914)
≡ Helminthosporium sudanensis Cif. and Frag, Bol. R. Soc. Espana Hist. Nat. 26: 497 (1926)
≡ Curvularia caryopsidum (Sacc.) S.C Teng., Fungi of China: 760 (1963)
Holotype: BPI 626381.
Hosts and diseases: Justicia adhatoda (Acanthaceae), Polianthes tuberosa (Asparagaceae), Amaranthus gangeticus (Amaranthaceae), Allium sativum, A. tuberosum (Amaryllidaceae), Mangifera indica (Anacardiaceae), Cocos nucifera, Trachycarpus fortunei, Elaeis guineensis (Arecaceae), Dracaena sp. (Asparagaceae), Cosmos bipinnatus (Asteraceae), Impatiens balsamina (Balsaminaceae), Annasas comosus, Billbergia sp. (Bromeliaceae), Hylocereus polyrhizus (Cactaceae), Casuarina equisetifolia (Casuarinaceae) Cecropia schreberiana (Cecropiaceae), Gloriosa abyssinica (Colchicaceae), Thuja orientalis (Cupressaceae), Hevea brasiliensis, Manihot esculenta, Ricinus communis (Euphorbiaceae), Arachis villosa, Cajanus cajan, Cassia surattensis, Centrosema pubescens, Crotalaria juncea, C. micans, Lablab purpureus, Lens culinaris, Leucaena leucocephala, Macrotyloma uniflorum, Medicago sativa, Erythrina variegate, Glycine max, Phaseolus vulgari, Tephrosia purpurea, Senna tora, Vigna adenantha, V. unguiculata (Fabaceae), Hydrilla verticillata (Hydrocharitaceae), Gladiolus communis (Iridaceae), Ocimum sp. (Lamiacaeae), Cinnamomum zeylanicum (Lauraceae), Gossypium hirsutum, G. barbadense, Pachira macrocarpa, Urena lobata, U. sinuate (Malvaceae), Musa nana (Musaceae), Syzygium aromaticum (Myrtaceae), Jasminum sambac (Oleaceae), Phalaenopsis amabilis (Orchidaceae), Oxalis sp. (Oxalidaceae), Pinus densiflora (Pinaceae), Piper aduncum, P. nigrum (Piperaceae), Rosa sp. (Rosaceae), Citrus sinensis (Rutaceae), Populus sp. (Salicaceae) Boehmeria nivea (Urticaceae), Andropogon sorghum, Avena sativa, Axonopus compressus, A. affinis, Bambusa vulgaris, Brachiaria mutica, Cenchrus brownie, Cynodon dactylon, Cymbopogon ambiguous, C. citrates, Dactyloctenium aegyptium, Digitaria sanguinalis, D. horizontalis, Echinochloa colona, Eragrostis tenella, Hemarthria altissima, H. fasciculate, Heteropogon contortus, Ischaemum villosum, I. aristatum Oryza sativa, Panicum maximum var. trichoglume, Paspalum sp, P. frumentaceum, P. miliaceum, Pennisetum subangustum, P. typhoides, P. americanum, P. purpureum, Phyllostachys bambusoides, Rottboellia exaltata, Sporobolus pyramidalis, Saccharum officinarum, Setaria italica, Sorghum vulgare, S. plumosum, S. bicolor, S. sudanense, Triticum sp., Typha angustata, Zea mays (Poaeceae), Kaempferia galangal, Curcuma longa (Zingiberaceae). Cochliobolus lunatus causes leaf spots and seedling blights on some of host species listed above. C. lunatus is well known as a facultative pathogen which cause seed germination failure and seedling blights of cereals and other monocotyledonous crops (Sivanesan 1987).
The species also causes disease in humans, such as mycotic keratitis in the eyes, allergic bronchopulmonary disease and allergic fungal sinusitis.
Distribution: Occurrence throughout every continent except Antarctica, Australia, Bolivia, Brazil, Brunei, Cambodia, Cameroon, Canada, China, Costa Rica, Cote d’Ivoire, Cuba, El Salvador, Eritrea, Ethiopia, Ghana, Guinea, India, Jamaica, Japan, Kenya, Korea, Malaysia, Mauritius, Myanmar, Nigeria.
References: Castellani and Ciferri 1937; Dade 1940; Wiehe 1948; Hughes 1953; Resplandy et al. 1954; Johnston 1960; Nattrass 1961; Litzenberger et al. 1962; Farr and Stevenson 1963; Kranz 1963; Nelson and Haasis 1964; Kranz 1965; Mc Guire Jr and Crandall 1967; Orieux and Felix 1968; Sarbhoy et al. 1971; Turner 1971; Ellis and Gibson 1975; Matsushima 1975; Williams and Liu 1976; Liu 1977; Williams and Liu 1976; Wellman 1977; Tai 1979; Sharma et al. 1981; Srivastava and Gupta 1981b; McAleer et al. 1981; Peregrine and Ahmad 1982; Arnold 1986; Urtiaga 1986; Sivanesan 1987; Macmillan et al. 1987; Shivas 1989; Lenne 1990; Richardson 1990; Hyde and Alcorn 1993; Boa and Lenné 1994; Teng 1996; Msikita et al. 1997; Mendes et al. 1998; Caretta et al. 1999; Xi et al. 2000; Zhuang 2001; Delgado-Rodriguez et al. 2002; Taylor and Hyde 2003; Cho and Shin 2004; Liu and Zhang 2004; Kobayashi 2007; Thaung 2008; Hawa et al. 2009.
Notes: Wakker and Went (1898) described the asexual state of Cochliobolus lunatus from dead leaves of sugar cane as Acrothecium lunatum. Later, Boedijn (1933) introduced Curvularia to include some species of Acrothecium, with Acrothecium lunatum chosen as the generic type and renamed Curvularia lunata. The species is similar to C. trifolii in spore size (Parmelee 1956). Ellis (1966) described a new variety C. lunata var. aeria, which differed from the type in forming regular and abundant stromata in culture and having zonate colonies on potato dextrose agar. No sexual state is known for this variety. Tsuda and Uyama (1977) transferred the sexual stage of Curvularia lunatus into Pseudocochliobolus. The characters used to separate there genera were, however, considered doubtful and thus Cochliobolus lunatus was regarded as the appropriate name by Alcorn (1983a). Cochliobolus lunatus has also been recorded as a saprotroph. Saprobic soil inhabiting C. lunatus has caused various infections in humans (Rohwedder et al. 1979). C. lunatus is also used in bio-control of weeds (Motlag 2011).
Cochliobolus luttrellii Alcorn, Mycotaxon 39: 377 (1990)
= Bipolaris luttrellii Alcorn, Mycotaxon 39: 378 (1990)
Holotype: BRIP 14791; Isotype: IMI 335215.
Hosts: Sexual state found on the leaves of Dactyloctenium aegyptium (Poaceae).
Distribution: Australia.
References: Alcorn 1990.
Notes: The species is homothallic. Formation of ascomata is light dependent this is similar to other homothallic species in the genus such as C. hormomophus and C. kusanoi (Alcorn 1990). All self compatible species in the genus including C. luttrellii carry both MAT genes in one nucleus and their order is unique for each species (Yun et al. 1999).
Cochliobolus melinidis Alcorn, Mycotaxon 15: 5 (1982)
= Bipolaris melinidis Alcorn, Mycotaxon 15: 7 (1982)
≡ Drechslera curvispora El Shafie, Trans. Br. Mycol. Soc. 78: 545 (1982)
≡ Bipolaris curvispora (El Shafie) Sivan., Myco. Pap. 158: 47 (1987)
≡ Drechslera pluriseptata Khetarpal, Nath & Lal, Ind. Phytopath. 37: 320 (1984)
Holotype: BRIP 12764.
Hosts and diseases: Melinis minutiflora, Panicum maximum, Setaria anceps, Triticum aestivum, Oryza sativa (Poaceae). This species causes leaf spots on all above hosts. Sometimes can be found on inflorescences and commercial seed samples of Melinis minutiflora (Sivanesan 1987).
Distribution: Australia, Brazil, Costa Rica, India, Paraguay.
References: Alcorn 1982; Sivanesan 1987; Farias et al. 2011.
Notes: The conidiophores of the asexual state of Cochliobolus melinidis are similar to those of C. heterotrophus (Alcorn 1982). But ascocarps of Cochliobolus heterotrophus are smaller than those of C. melinidis, also the later species have shorter asci and the beak is longer and more or less cylindrical in the former species (Alcorn 1982). Mating was carried out to evaluate the possibility that C. melinidis could be a different taxon (Alcorn 1982). Ascospores were formed after pairing C. melinidis with C. heterotrophus, which were black and globose with a short beak. As these ascomata differ from those of C. heterostrophus, the two taxa were suggested to be closely related, but distinct. Thus, C. melinidis was introduced to accommodate the fungus on Melinis (Alcorn 1982). Phylogenetic analysis using type derived sequences of C. melinidis and C. heterostrophus should be used to distinguish two species.
Cochliobolus miakei I. Hino & Katum, J. Jap. Bot. 41: 292 (1966)
Holotype: YAM.
Hosts: Eragrostis sp., Pleioblastus argenteo, P. distichus var. nezasa, Sasa senanensis (Poaceae).
Distribution: Ethiopia, Japan.
References: Hino and Katum 1966; Richardson 1990; Eriksson and Yue 1998; Kobayashi 2007.
Notes: There are very few records of this species even though it was described 45 years ago. There are no type derived sequences and species has not been included in phylogenetic analyses. Molecular analysis using type derived sequences are essential to establish if this is a distinct species.
Cochliobolus microlaenae Alcorn, Mycotaxon 39: 381 (1990)
= Bipolaris microlaenae Alcorn, Mycotaxon 39: 382 (1990)
Holotype: BRIP 16363; Isotype: IMI 335217.
Hosts and diseases: Microlaena stipoides (Poaceae). Causes small dark leaf spots.
Distribution: Australia.
Reference: Alcorn 1990.
Notes: Cochliobolus microlaenae can be distinguished from most other Cochliobolus species on the basis of ascomata, ascus and ascospore dimensions. Only the ascus width is similar to that of C. sativus and C. victoriae. This species has not been recorded since it was introduced. The species was not used in the previous phylogenetic analysis and there are no type derived sequences. Recollection and analysis of epitype derived sequences are needed to establish if this is a distinct species.
Cochliobolus miyabeanus (S. Ito & Kurib.) Drechsler ex Dastur, Indian Journal of Agricultural Research 12: 733 (1942)
= Bipolaris oryzae (Breda de Haan) Shoemaker, Can. J. Bot. 37: 883 (1959)
=Bipolaris oryzae Sawada, Can. J. Bot. 37: 883 (1959)
≡ Drechslera oryzae (Breda de Haan) Subram. & B.L. Jain, Can. J. Bot. 37: 883 (1959)
≡ Helminthosporium macrocarpum Grev, Scott. crypt. fl., 2: 148 (1824) [1825]
≡ Helminthosporium oryzae Breda de Haan, Bulletin Inst. Bot. Buitenzorg 6: 11 (1900)
≡ Luttrellia oryzae (Breda de Haan) Gornostaĭ [as ‘Lutrellia’], in Azbukina et al. (eds), Vodorosli, Griby i Mkhi Dal’nego Vostoka (Vladivostok): 81 (1978)
≡ Ophiobolus miyabeanus S. Ito & Kurib, Proc. Imp. Acad. Hokkaido Imp. Univ 6 (1927)
≡ Spondylocladium macrocarpum (Grev.) G. Arnaud, Bull. trimest. .Soc. mycol. Fr. 69: 288 (1954) [1953]
Hosts and diseases: Cosmos bipinnatus (Asteraceae), Cordia trichotoma (Boraginaceae), Alopecurus aequalis, A. geniculatus, Eleusine indica, Ischaemum rugosum, Leersia hexandra, Oryza australiensis, O. glaberrima, O. latifolia, O. sativa, O. rufipogon, Panicum colonum, P. maximum, P. virgatum, Setaria italica, Triticum aestivum, Zea mays, Zizania palustris, Z. aquatic (Poaceae). Causes brown spots and seedling blights of rice (Oryza sativa). Leaf lesions are ovoid (up to 1 cm long) and brownish or rarely purplish at the beginning. Later turn white or grey and coalescing to form target spots on leaves. Roots, seeds and seedlings can be infected.
Distribution: Australia, Bangladesh, Bhutan, Bolivia, Brazil, Brunei, Cambodia, Cameroon, China, Colombia, Costa Rica, Cote d’Ivoire, Cuba, Dominican Republic, Egypt, Fiji, France, Gambia, Ghana, Greece, Hong Kong, India, Indonesia, Iran, Italy, Jamaica, Japan, Korea, Malawi, Malaysia, Mauritius, Mexico, Myanmar, Nepal, New Zealand, Nicaragua, Nigeria, Pakistan, Papua New Guinea, Philippines, Puerto Rico, Sierra Leone, Solomon Islands, Sri Lanka, South Africa, Sudan, Taiwan, Tanzania, Thailand, Togo, Turkey, Uganda, Venezuela, Yugoslavia, Zambia, Zimbabwe.
References: Chardon and Toro 1930; Deighton 1936; Teodoro 1937; Dade 1940; Larter and Martyn 1943; Boughey 1946; Sprague 1950; Baker and Dale 1951; Stoner 1951; Thompson and Johnston 1953; Resplandy et al. 1954; Green 1958; Parris 1959; Sawada 1959; Anonymous 1960; Riley 1960; Ciferri 1961; Bean and Schwartz 1961; Litzenberger et al. 1962; Kranz 1963; Schroeder 1964; Mc Guire Jr and Crandall 1967; Ahmad 1969; Dennis 1970; Ellis 1971; Sarbhoy et al. 1971; Turner 1971; Firman 1972; Pantidou 1973; Aulakh et al. 1974; Stevenson 1975; Alvarez 1976; Kernkamp et al. 1976; Williams and Liu 1976; Gorter 1977; Ueyama and Tsuda 1977; Ahmad 1978; Tai 1979; Matsushima 1980; Cholil and De Hoog 1982; Percich and Nickleson 1982; Imolehin 1983; Shaw 1984; Arnold 1986; Urtiaga 1986; Sivanesan 1987; French 1989; Shivas 1989; Lenne 1990; Richardson 1990; Dahal et al. 1992; Johansen and Percich 1992; Jones et al. 1993; Nyvall et al. 1995; Teng 1996; Ahmad et al. 1997; Mendes et al. 1998; Kihara et al. 1998; Guo 1999; Crous et al. 2000; Zhuang 2001; Deng and Zhang 2002; Cho and Shin 2004; Krupinsky et al. 2004; Zhuang 2005; Paz et al. 2006; Piepenbring 2006; Thaung 2008; Bobev 2009; Peterson and Balbalian 2010.
Notes: Bipolaris oryzae, the asexual state of Cochliobolus miyabeanus has conidia with either light or dark hila, but in most cases both biotypes are mixed in cultures and there can be considerable variations in the conidial morphology (Sivanesan 1987). Wild type C. miyabeanus form dark green colonies whereas mutants, which are defective in melanin biosynthesis, form grey colonies (Kubo et al. 1989). The phytotoxins Ophiobolin A and Ophiobolin B are produced in cultural media (Xio et al. 1991). The most favorable temperature for ascospore formation is 20–24°C (Tsuda and Ueyama 1975), however the sexual stage is not common in nature (Kulkarni et al. 1980). The species is heterothallic. C. miyabeanus is a soil inhabiting plant pathogen and can survive in dry soil for about 4–6 weeks. Seed-borne pathogens may cause pre-emergence death of seedlings (Kulkarni et al. 1980). Some myxobacteria can cause small perforation and etched hollows on the mycelial and conidial walls and suppress the fungal growth to some extent (Homma 1984). As this is an important pathogen which causes severe economical loss, many fungicides and biocontrol measures have been tested for use against C. miyabeanus (Amadioha 2002; Manandhar et al. 1998).
Cochliobolus neergaardii Alcorn, Mycotaxon 39: 385 (1990)
= Bipolaris neergaardii (Danquah) Alcorn, Mycotaxon 17: 68 (1983)
≡ Drechslera neergaardii Danquah, Trans. Br. mycol. Soc. 64: 545 (1975)
Holotype: BRIP 16385; Isotype: IMI 335219.
Hosts: Hibiscus esculentus (Malvaceae), Oryza sativa (Poaceae).
Distribution: Australia, Ghana, Saudi Arabia.
References: Sivanesan 1987; Alcorn 1990.
Notes: Only the asexual state of C. neergaardii has been found in nature, the teleomorph connection was demonstrated by Alcorn (1990) after mating experiments. There have been no records of C. neergaardii since 1990 and it has not been included in any previous phylogenetic analysis.
Cochliobolus nisikadoi (Tsuda, Ueyama & Nishih.) Alcorn, Mycotaxon 16: 373 (1983)
= Bipolaris coicis (Nisikado) Shoemaker, Can. J. Bot. 37: 883 (1959)
≡ Curvularia coicis Castell, Nuovo G. bot. Ital 62: 554 (1955)
≡ Pseudocochliobolus nisikadoi Tsuda, Ueyama & Nishihara, Mycologia 69: 1117 (1977)
≡ Helminthosporium coicis Nisikado, Sp. Rep. Ohara Inst. Agric. Res. 4: 136 (1928)
≡ Drechslera coicis (Nisikado) Subram. & Jain, Curr. Sci. 35: 354 (1966)
Holotype: TNSF 193037; Isotypes: BPI, CUP, IMI, L; Paratype: TNSF 193038.
Hosts and diseases: Coix lachrymal, Thysanolaena maxima, Zea mays (Poaceae). Causes leaf blight on Coix species. Leaf spots are brown and elongated, sometimes reaching half of the length of the leaf, sporulation occurs on leaves and inflorescences.
Distribution: Brunei, India, Papua New Guinea.
References: Alcorn 1983a; Sivanesan 1987.
Notes: Two isolates of Helminthosporium coicis formed the sexual stage in artificial media and Cochliobolus nisikadoi was originally described as Pseudocochliobolus nisikadoi (Tsuda and Uyama 1977). Later Pseudocochliobolus was placed in synonymy with Cochliobolus (Alcorn 1983a). Occurrence of sexual stage in nature is not common; however, formation of the sexual stage in vitro is possible (Kim and Yang 1998). The species is heterothallic.
Cochliobolus nodulosus Luttr., Phytopathology 47: 547 (1957)
= Bipolaris nodulosa (Berk. & M.A. Curtis) Shoemaker, Can. J. Bot. 37: 883 (1959)
≡ Bipolaris leucostyla (Drechsler) Shoemaker, Can. J. Bot. 37: 883 (1959)
≡ Drechslera nodulosa (Berk. & M.A. Curtis ex Sacc.) Subram. & B.L. Jain, Curr. Sci. 35: 354 (1966)
≡ Helminthosporium leucostylum Drechsler, Journal of Res. 24: 711 (1923)
≡ Helminthosporium nodulosum Berk. & M.A. Curtis, Syll. Fung. 4: 421 (1886)
≡Helminthosporium nodulosum var. tritici Patel, Kamat & Y.A. Padhye, Indian Phytopath. 6: 25 (1954)
≡ Helminthosporium nodosum Berk. & M.A. Curtis in Berk, Grevillea 3: 102 (1875)
Holotype: BPI, K.
Hosts and diseases: Cassia siamea (Fabaceae), Digitaria sp., Eleusine africana, E. compressa, E. coracana, E. indica, Eragrostis pilosa, Paspalum sp., Pennisetum clandestinum (Poaceae). On Eleusine indica cause seedling blight, spotting and stripping on leaves and sooty heads of inflorescences. Young seedlings are killed, shriveled and blackened with conidiophores and conidia of the fungus. In moist weather, lesions are covered with a compact layer of short conidiophores and conidia and closely resemble stripe smut symptoms. Infected inflorescences are stunted and do not produce viable seeds (Luttrell 1957).
Distribution: Australia, Ethiopia, Georgia, India, Kenya, Malaysia, Mauritius, New Zealand, Nigeria, Pakistan, Papua New Guinea, Sierra Leone, South Africa, Tanzania, Uganda, Zambia.
References: Luttrell 1957; Riley 1960; Hanlin 1963; Sarbhoy et al. 1971; Turner 1971; Sivanesan 1987; Richardson 1990; Crous et al. 2000.
Notes: There was confusion as to the asexual state of Cochliobolus nodulosus as it was classified as Helminthosporium nodulosum. An entirely different fungus was referred to as H. nodulosus by Nisikado (1928), Butler (1918) and (Mitra and Meheta 1934). Other species such as H. leucostylum Drechsler (Drechsler 1923), H. kusanoi and H. hadrotrichoides (Ellis & Everh.) (Sprague 1950) appear to be closely related to H. nodulosus (Luttrell 1957). Luttrell (1957) also found that the species described by Mitra and Meheta (1934) was different to H. nodulosum. Drechsler (1923) found that H. leucostylum was similar to H. nodulosum and they were made synonymous. Even though the type material of H. hadrotrichoides was inadequate Sprague (1950) synonymized it with H. leucostylum. Nisikado (1928) reported on the distinctness between H. nodulosus and H. kusanoi based on mating and pathogenicity tests. The sexual state Cochliobolus nodulosa was produced in culture but is uncommon in nature. C. nodulosa and C. kusanoi are very similar in morphology and the former species differs only by shorter conidia, fewer conidial septa and a shorter length to width ratio. The asexual state of C. nodulosa has similar morphology to Bipolaris hadrotrichoides, but the former species differs by consistent 3-septate conidia and prominently swollen tips of its conidiophores (Luttrell 1957). Three closely related species, asexual states of C. nodulosa, C. kusanoi and Bipolaris hadrotrichoides form the nodulosa group of the genus Bipolaris. The three species share many morphological similarities (Luttrell 1969).
Cochliobolus oryzae-sativae Sivan
Holotype: IMI 289758.
Hosts: Oryza sativa (Poaceae).
Distribution: Argentina.
Notes: Index Fungorum refers to Cochliobolus oryzae-sativae Sivan. but in the original description the species is referred to as a Curvularia oryzae-sativae without a Cochliobolus teleomorph. There has been no mention of Curvularia oryzae-sativae in the literature following its introduction in 1987. This species is differentiated from other species by morphology and phylogenetic analysis is needed to infer its relationship with other species.
Cochliobolus ovariicola Alcorn, Mycotaxon 39: 388 (1990)
= Bipolaris ovariicola Alcorn, Mycotaxon 15:45 1982
Holotype: BRIP 15917; Isotype: IMI 335220.
Hosts: Eragrostis bahiensis, E. interrupta (Poaceae).
Distribution: Australia.
References: Alcorn (1990).
Notes: The sexual state was obtained by mating experiments in culture. The species has not been included in previous phylogenetic studies. This species is differentiated by morphology and phylogenetic analysis is needed to infer its relationships.
Cochliobolus pallescens (Tsuda & Ueyama) Sivan, Mycol. Pap. 158: 118 (1987)
= Curvularia pallescens Boedijn, Bull. Jard. bot. Buitenz, 3 Sér. 13: 127 (1933)
≡ Pseudocochliobolus pallescens Tsuda & Ueyama, Mem Col Agr, Kyoto Imperial University 122: 86 (1983)
≡ Curvularia leonensis M.B. Ellis, Mycol. Pap. 106: 28 (1966)
Holotype: TNSF 197849, CUP.
Hosts and diseases: Recorded from many plant families, Such as Liquidambar macrophylla (Altingiaceae), Amaranthus gangeticus (Amaranthaceae), Allium odorum (Amaryllidaceae), Mangifera indica (Anacardiaceae), Colocasia esculenta (Araceae), Elaeis guineensis (Arecaceae), Dahlia variabilis, Gaillardia picta (Asteraceae), Cocos nucifera, Bursera simaruba (Burseraceae), Gloriosa superba (Colchicaceae), Citrullus vulgaris (Cucubitaceae), Cyperus antillanus (Cyperaceae) Hevea brasiliensis, Ricinus communis (Euphorbiaceae), Arachis hypogaea, Bahunia sp., Cajanus cajan, Centrosema plumier, C. pubescens, Glycine max, Lens culinaris, Phaseolus vulgaris, Psophocarpus tetragonolobus Senna occidentalis, S. tora, Stylosanthes guianensis, Vicia faba (Fabaceae), Quercus germane, Q. xalapensis (Fagaceae), Gmelina arborea (Lamiaceae), Punica granatum (Lythraceae), Ceiba pentandra, Urena sinuata (Malvaceae), Stephania abyssinica (Menispermaceae), Eucalyptus globules, E. tereticornis (Myrtaceae), Bougainvillea spectabilis (Nyctaginaceae), Pinus patula, P. caribaea, P. elliottii, P. merkusi, P. taiwanensis (Pinaceae), Axonopus compressus, Bambusa tuldoides, Brachiaria dictyoneura, B. humidicola, B. mutica, Chloris gayana, Cymbopogon Citrates, Cynodon dactylon, Dactyloctenium aegyptium, Digitaria horizontalis, Echinochloa colona, E. mexicana, Imperata cylindrica, Oryza sativa, Panicum laxum, P. maximum, P. miliaceum, Paspalum scrobiculatum, Pennisetum pedicellatum, P. polystachion, P. purpureum, P. typhoides, Rottboellia exaltata, Schizachyrium hirtiflorum, Setaria anceps, S. glauca, S. italica, S. sphacelata, Sorghum arundinaceum, S. bicolor, S. halepense, Sporobolus poireti, Stenotaphrum secundatum, Zea mays (Poaceae), Fagopyrum sp. (Polygonaceae), Citrus sinensis (Rutaceae), Cupania macrophylla (Sapindaceae), Capsicum annuum, Lycopersicon esculentum, Solanum tuberosum (Solanaceae), Alpinia zerumbet (Zingiberaceae). Causes leaf spots, rots on some of above hosts. Also recorded from soil. Sometimes recorded as a human pathogen causing cutaneous phaeohyphomycosis.
Distribution: Australia, Barbados, Brazil, Colombia, Cuba, Denmark, Guinea, Guyana, Hong Kong, India, Indonesia, Jamaica, Pakistan, Malaysia, Malawi, Mexico, Myanmar, Nepal, Nigeria, Pakistan, Samoa, Saudi Arabia, Sierra Leone, South Africa, Sri Lanka, Tanzania, Thailand, Togo, Uruguay, USA, Venezuela, West indies, Zambia, Zimbabwe.
References: Nattrass 1961; Goos 1963; Kranz 1963; Conners 1967; Turner 1971; Sarbhoy et al. 1971; Peregrine and Siddiqi 1972; Norse 1974; Wallbridge and Pinegar 1975; Williams and Liu 1976; Liu 1977; Ebbels and Allen 1979; Gulya et al. 1979; Peregrine and Ahmad 1982; Sierra 1984; Arnold 1986; Manoch et al. 1986; Urtiaga 1986; Sivanesan 1987; Madhukar and Reddy 1988, Shivas, Lenne 1990; Richardson 1990; Khare 1991; Dahal et al. 1992; Bettucci and Saravay 1993; Vittal and Dorai 1994; Berg et al. 1995; Agrawal and Singh 1995; McKenzie 1996; Mendes et al. 1998; Caretta et al. 1999; Crous et al. 2000; Lu et al. 2000; Minter et al. 2001; Zhuang 2001; Delgado-Rodriguez et al. 2002; Thaung 2008; Dadwal and Verma 2009; Yassin et al. 2010.
Notes: The sexual state was obtained on Sach’s agar medium in mating experiments, and is uncommon in nature. The species was not included in previous phylogenetic analysis.
Cochliobolus palmivora P.N. Rao & Chaudhury, Mycopath. 23: 38 (1964)
Holotype: K.
Hosts and disease: Livistona chinensis (Arecaceae). Causes leaf spots
Distribution: India.
References: Rao and Chaudhury 1964.
Notes: The sexual state of Cochliobolus palmivora was observed in nature. We could not find any record of this species after the first publication in 1964. This species is differentiated from other species by morphology and phylogenetic analysis is needed to infer its relationship with other species.
Cochliobolus peregianensis Alcorn, Mycotaxon 15: 9 (1982)
= Bipolaris peregianensis Alcorn, Mycotaxon 15: 9 (1982)
Holotype: BRIP 12790; Isotypes: IMI 264355, DAR 35057.
Hosts and diseases: Cynodon dactylon, Zea mays (Poaceae). Causes leaf lesions (oblong–elliptical dark purplish 2 × 1 mm).
Distribution: Australia.
References: Alcorn 1982; Sivanesan 1987.
Notes: The asexual state of C. peregianensis is similar to that of C. cynodontis, but conidia of the latter species are smaller, paler, more septate and less curved than those of C. peregianensis (Alcorn 1982). Cochliobolus peregianensis is heterothallic and the teleomorph was obtained after mating in cultures. C. peregianensis is closely related to C. miyabeanus, which is a common pathogen of rice.
Cochliobolus perotidis Alcorn, Mycotaxon 15: 11 (1982)
= Bipolaris perotidis Alcorn, Mycotaxon 15: 13 (1982)
Holotype: BRIP 12804.
Hosts and diseases: Aristida behriana, Cenchrus setigerus, Digitaria smutsii, Eragrostidis brownii, Heteropogon triticeus, Perotis rara, Rottboellia exaltata, Triticum aestivum, Zea mays (Poaceae). Recorded as a saprobe and pathogen that causes leaf lesions.
Distribution: Australia.
References: Alcorn 1982; Sivanesan 1987; Lenne 1990
Notes: There are no reports of C. perotidis since its description in 1982. According to Alcorn (1982), the asexual state of C. perotidis is similar to the unnamed anamorph of C. tritici. However an anamorph was not observed on the type material of C. tritici (Alcorn 1982) to compare with the anaorph of C. perotidis .
Cochliobolus ravenelii Alcorn, Mycotaxon 13: 341 (1981)
=Bipolaris ravenelii (M.A. Curtis) Shoemaker, Can. J. Bot. 33: 884 (1959)
≡ Drechslera ravenelii (M.A. Curtis) Subram. & B.L. Jain, Curr. Sci. 35: 354 (1966)
≡ Helminthosporium hoffmannii Berk. [as ‘hoffmanni’], Intr. crypt. bot. : 298 (1857)
≡Helminthosporium ravenelii M.A. Curtis ex Berk, J. Linn. Soc., Bot. 10: 360 (1868) [1869]
≡ Helminthosporium tonkinense P. Karst. & Roum, Revue mycol., Toulouse 12: 78 (1890)
≡ Heterosporium callospermum Speg, Anal. Soc. cient. argent. 22: 213 (1857)
≡ Napicladium ravenelii (M.A. Curtis) Speg, Anal. Soc. cient. argent. 26: 71 (1888)
Holotype : BRIP 13165; Paratype: BRIP 13027, BRIP 13028.
Hosts and diseases: Cirsium arvense (Asteraceae), Axonopus affinis, Bromus ciliates, Eleusine indica, Eragrostis interrupta, E. pilosa, Sporobolus africanus, S. berteroanus, S. capensis, S. diander, S. elongates, S. fertilis, S. indicus, S. poiretii, S. pyramidalis, Sporobolus fimbriatus, S. poiretii, S. pyramidalis, S. tenacissimus, Panicum auritum, P. caesium, Sporobolus cryptandrus, S. diandrus, S. neglectus, S. poiretii (Poaceae). Usually causes false smut disease (black mold like appearance) on above grass species.
Distribution: Australia, Brazil, China, Colombia, Costa Rica, Georgia, Ghana, Hawaii, Hong Kong, India, Jamaica, Kenya, Malawi, Malaysia, Mauritius, Myanmar, New Zealand, Nepal, Papua, New Guinea, Georgia, Ghana, Philippines, Singapore, Sri Lanka, South Africa, Taiwan, Turkey, Uruguay, UK, USA, Venezuela, Zambia.
References: Stevens 1925; Chardon and Toro 1930; Teodoro 1937; Wolf et al. 1938; Dade 1940; Greene 1944; Wiehe 1948; Doidge 1950; Greene 1950; Sprague 1950; Hughes 1952; Thompson and Johnston 1953; Parris 1959; Anonymous 1960; Ciferri 1961; Simmonds 1966; Whiteside 1966; Orieux and Felix 1968; Benjamin and Slot 1969; Cooke 1971; Turner 1971; Peregrine and Siddiqi 1972; Stevenson 1975; Luttrell 1976; Williams and Liu 1976; Anonymous 1979; Alcorn 1981b; Raabe et al. 1981; Gorter 1981; Alfieri et al. 1984; Grand 1985; Urtiaga 1986; Sivanesan 1987; Pennycook 1989; McKenzie 1992; Teng 1996; Mendes et al. 1998; Roane and Roane 1997; Crous et al. 2000; Zhuang 2001; Piepenbring 2006; Thaung 2008; Eschen et al. 2010
Notes: C. ravenelii was first described from Sporobolus and has been found in association with many Sporobolus species. It is morphologically distinguishable from C. sporoboli, which is a common Sporobolus pathogen. The species is heterothallic and the teleomorph has been produced in culture. Cochliobolus ravenelii has been used as a biocontrol agent (Hetherington et al. 1999).
Cochliobolus sasae I. Hino & Katum, Bull. Faculty of Agriculture, Yamaguchi University 11: 26 (1960)
Holotype: YAM.
Hosts and diseases: Recorded as a saprobe on Sasa tambaensis (Poaceae).
Distribution: Japan.
References: Hino and Katumoto 1960; Eriksson and Yue 1998; Kobayashi 2007.
Notes: There has been no mention of C. sasae in the literature following its introduction in 1960. This species is differentiated from other species by morphology and a phylogenetic analysis is needed to infer its relationship with other species.
Cochliobolus sativus (S. Ito & Kurib.) Drechsler ex Dastur, Indian J Agr Res12: 733 (1942)
= Bipolaris sorokiniana (Sacc.) Shoemaker, Can. J. Bot. 37: 884 (1959)
≡ Helminthosporium acrothecioides Lindf, Sevensk bot. Tidskr. 12:562 (1918)
≡ Helminthosporium californicum Mackie & G.E. Paxton, Phytopathology 13: 562 (1923)
≡ Helminthosporium sativum Pammel, C.M. King & Bakke, Bulletin of Iowa State College 116: 180 (1910)
≡ Helminthosporium sorokinianum Sacc.in Sorok, Trans. Soc. Nat. Univ. Kazan 22: 15 (1890)
≡ Ophiobolus sativus S. Ito & Kurib, Trans. Sapporo nat. Hist. Soc. 10: 138 (1929)
≡ Bipolaris californica (Mackie & G.E. Paxton) Gornostaĭ [as ‘californicum’], in Azbukina et al. (eds), Vodorosli, Griby i Mkhi Dal’nego Vostoka (Vladivostok): 80 (1978).
≡ Drechslera sorokiniana (Sacc.) Subram. & Jain, Curr. Sci. 35: 354 (1966)
Hosts and diseases: Distributed throughout tropical and temperate regions on Allium sp. (Amaryllidaceae), Taraxacum kok-saghyz (Asteraceae), Calluna vulgaris (Ericaceae), Cicer arietinum, Lablab purpureus, Phaseolus vulgaris (Fabaceae), Linum usitatissimum (Linaceae), Agropyron cristatum, Agrostis sp., Aira caryophyllea, A. geniculatus, Alopecurus pratensis, Aneurolepidium chinense, Avena sativa, Bromus inermis, B. racemosus,, B. willdenowii, Chloris gayana, C. virgata, Clinelymus dahuricus, C. sibiricus, Cynodon dactylon, C. transvaalensis, Cynosurus echinatus, Dactylis glomerata, Danthonia californica, Deschampsia caespitosa, Echinochloa crus, Eleusine corocana, E. indica, Elymus junceus, Festuca elatior, Festuca myuros, Glyceria borealis, Holcus lanatus, Hordeum brevisubulatum, H. distichon, H. murinum, H. gussoneanum, Hordeum sativum, H. vulgare, Lolium multiflorum, L. perenne, L. temulentum, Microlaena stipoides, Oryza sativa, Panicum lacromanianum, P. typhoides, Phleum pratense, Setaria italica, Trisetum aestivum, T. durum, T. sativum, T. secale, Triticum sphaerococcum, T. turgidum, T. vulgare, Zea mays (Poaceae), Guzmania sp. (Tillandsioideae). The fungus causes spot blotch or foot and root rot of temperate cereals (Sivanesan 1987). Spot blotch of wheat causes severe epidemics in many tropical countries sometimes resulting in yield losses of up to 87% in highly susceptible varieties (Hetzler et al. 1990). Foot rot of wheat causes brown streaks and blotches on the stem bases (Sivanesan 1987).
Distribution: Argentina, Australia, Belgium, Bhutan, Brazil, Bulgaria, Canada, Cameroon, China, Cyprus, Denmark, Egypt, Ethiopia, Finland, Greece, Indonesia, Israel, Germany, Hungary, Italy, Kenya, Korea, Malawi, Myanmar, New Zealand, Nepal, Papua New Guinea, Poland, Sudan, Tanzania, Taiwan, Turkey, Uganda, UK, USA, Zimbabwe.
References: Sprague 1950; Roane and Starling 1958; Riley 1960; Foister 1961; Nattrass 1961; Simmonds 1966; Orieux and Felix 1968; Peregrine and Siddiqi 1972; Pantidou 1973; Hanlin et al. 1978; Anonymous 1979; Tai 1979; Krupinsky and Berdahl 1984; Shaw 1984; Sierra 1984; Ginns 1986; Krupinsky 1986; Sivanesan 1987; Pennycook 1989; Richardson 1990; McKenzie 1992; Mendes et al. 1998; Crous et al. 2000; Hilton 2000; Cho and Shin 2004; Kowalik and Sagan 2005; Bobev 2009, Crepel et al. 2006.
Notes: The sexual state of Cochliobolus sativus is very similar to C. elusinensis but the latter differs in having larger ascomata, somewhat shorter and narrower asci, and much shorter ascospores with fewer septa. Tinline (1951) demonstrated that C. sativus is heterothallic and each thallus is bisexual and self sterile. The sexual state of C. sativus is not common in nature but has been produced under laboratory conditions. Environmental conditions, such as substrate, temperature and pH influence perithecia formation (Tinline and Dickson 1958). Conidia of C. sativus can be white to olivaceous but this is not linked to mating type (Tinline and Dickson 1958). C. sativus is highly variable in pathogenicity and virulence. Dosdall (1923) showed that C. sativus isolates can induce differential infection reactions in barley. Kline and Nelson (1963) identified 13 pathogenicity types from 28 isolates based on their varying capacity to cause disease on selected species of Poaceae. Zhong and Steffenson (2001) identified two pathotypes of C. sativus from the USA.
Cochliobolus setariae (S. Ito & Kurib.) Drechsler ex Dastur, Indian J Agr Res 12: 733 (1942)
= Bipolaris setariae Shoemaker, Can. J. Bot. 37: 884 (1959)
≡ Drechslera setariae (Shoemaker) Subram. & B.L. Jain, Curr. Sci. 35: 354 (1966)
≡ Helminthosporium setariae Sawada, Spec. Bull. Agric. Exp. Station Formosa 19: 656 (1919)
≡ Helminthosporium setariae Lind, Danish Fungi : 527 (1913)
≡ Ophiobolus setariae S. Ito & Kurib., Proc. Imper. Acad. Tokyo 6: 352 (1930)
Hosts and diseases: Most commonly found on Setaria grass species and many other hosts such as Chamaedorea seifrizii, C. elegans, Chrysalidocarpus lutescens, (Arecaceae), Manihot esculenta (Euphorbiaceae), Stylosanthes guianensis (Fabaceae) Persea americana (Lauraceae), Antirrhinum majus (Plantaginaceae), Maranta arundinacea, M. leuconeura var. erythroneura, M. leuconeura var. kerchoviana, M. leuconeura var. leuconeura (Marantaceae), Dendrobium sp. (Orchidaceae), Agrostis tenuis, Avena sativa, Brachiaria mutica, B. reptans, B. eruciformis, B. ramose, Brachiaria reptans, Cynodon sp., Desmostachya bipinnata, Digitaria granularis, Echinochloa indica, Eleusine coracana, Eragrostis sp., Hordeum sp., Ischaemum rugosum, Oryza sativa, Oryzopsis holciformis, Panicum clandestinum, P. fasciculatum, P. maximum, P. miliaceum, Paspalidium flavidum, P. distichum, Pennisetum americanum, P. pedicellatum, Saccharum officinarum, Setaria faberi, S. geniculata, S. glauca, S. italica, S. lutescens, S. tomentosa, S. sphacelata, Triticum, Zea mays (Poaceae), Rosa sp. (Rosaceae), Calathea sp. (Zingiberaceae) Causes spots on leaves and petals, leaf blights, on millet (Balasubramanian 1980). Leaf lesions are elongated and variable in size depending on the host and environment conditions, but are usually white or grey in the centre and becoming darker towards the margin (Sivanesan 1987).
Distribution: Argentina, Australia, Bangladesh, China, Cuba, Egypt, Ethiopia, Georgia, Hawaii, India, Myanmar, New Guinea, New Zealand, Pakistan, Sierra Leone, South Africa, Turkey, Uganda, USA, Venezuela.
References: Dastur 1942; Sprague 1950; Sarbhoy et al. 1971; Luttrell et al. 1974; Misra and Prakash 1972; Singh and Misra 1979; Tai 1979; Singh et al. 1981; Chase 1982; Simone and Brunk 1983; Alfieri et al. 1984; Arnold 1986; Urtiaga 1986; Sivanesan 1987; Lenne 1990; Richardson 1990; Roane and Roane 1997; Crous et al. 2000; Zhuang 2001; Cho and Shin 2004; Roane 2004; Zhuang 2005; Thaung 2008; Shi et al. 2010.
Notes: Cochliobolus setariae is suspected to be heterothallic but the sexual state has not been observed in culture (Sivanesan 1987). The species has not been incorporated in previous phylogenetic analyses and no type derived sequences are available. Cochliobolus setariae has potential for use as a microbial herbicide and further details are discussed under the topic Cochliobolus in biocontrol. Type derived sequences are needed to infer the relationships of this pathogenic species.
Cochliobolus sitharamii S.M. Reddy, Indian Phytopath. 29: 199 (1977) [1976]
Host: The species was recorded as a saprobe on Bambusa sp. (Poaceae).
References: Reddy 1977.
Notes: There has been no mention of C. sitharamii in the literature following its introduction in 1977. This species is differentiated from other species by morphology and phylogenetic analysis is needed to infer its relationship with other species.
Cochliobolus spicifer R.R. Nelson, Mycologia 56: 198 (1964)
= Bipolaris spicifera (Bainier) Subram., Hyphomycetes : 756 (1971)
≡ Bipolaris tetramera (McKinney) Shoemaker, Can. J. Bot. 37: 884 (1959)
≡ Brachycladium spiciferum Bainier, Bull. Soc. mycol. Fr. 24: 81 (1908)
≡ Brachysporium spiciferum (Bainier) Corbetta, Riso 12: 30 (1963)
≡ Curvularia spicifera (Bainier) Boedijn, Bull. Jard. bot. Buitenz, 3 Sér. 13: 127 (1933)
≡ Curvularia tetramera (McKinney) Boedijn ex J.C. Gilman, Manual of Soil Fungi: 303 (1945)
≡ Dendryphion spiciferum (Bainier) Sacc. & Traverso, Syll. fung. (Abellini) 19: 560 (1910)
≡ Drechslera spicifera (Bainier) Arx, Icon. microfung. Matsush. lect. : 65 (1975)
≡ Drechslera tetramera (McKinney) Subram. & B.L. Jain, Curr. Sci. 35: 355 (1966)
≡ Helminthosporium spiciferum (Bainier) Nicot, Öst. bot. Z. 100: 482 (1953)
≡ Helminthosporium tetramera McKinney, Bull. U.S. Department of Agriculture 1347: 33 (1925)
≡ Pseudocochliobolus spicifer (R.R. Nelson) Tsuda, Ueyama & Nishih., Mycologia 69: 1119 (1978) [1977]
Holotype: BPI.
Hosts and diseases: Pistacia vera (Anacardiaceae), Daucus carota (Apiaceae), Elaeis guineensis (Arecaceae), Dahlia variabilis, Zinnia sp. (Asteraceae), Heterophragma adenophylla (Bignoniaceae), Casuarina equisetifolia (Casuarinaceae), Commelina benghalensis (Commelinaceae), Citrullus vulgaris, C. lanatus, Cucurbita maxima (Cucubitaceae), Manihot esculenta (Euphobiaceae), Arachis hypogaea, Phaseolus vulgaris, Cassia fistula, Cyamopsis psoralioides, C. tetragonoloba, Erythrina variegate, Glycine ussuriensis, G. max, Lablab purpureus, Lens culinaris, Lupinus termis, Phaseolus radiates, P. vulgaris, Pisum sativum, Vicia faba (Fabaceae), Quercus xalapensis (Fagaceae), Gossypium sp. (Malvaceae) Pinus sylvestris (Pinaceae), Agropyron cristatum, Agrostis tenuis, Arundo donax, Avena sativa, Bromus inermis, Chloris verticillata, Cynodon dactylon, Cynosurus cristatus, Deschampsia flexuosa, Dactylis glomerata, Desmostachya bipinnata, Echinochloa frumentacea, Eragrostis cilianensis, Festuca idahoensis, F. rubra, Heteropogon contortus, Hordeum vulgare, Lolium multiflorum, Miscanthus sinensis, Oryza sativa, Oryzopsis holciformis, Paspalum sp., Panicum miliaceum, Pennisetum americanum, P. typhoides, Phleum pratense, Poa pratensis, Saccharum officinarum, Secale cereale, Setaria glauca, S. lutescens, Sorghum bicolor, S. halepense, Triticum aestivum, Zea mays (Poaceae), Prunus dulcis (Rosaceae), Citrus sinensis (Rutaceae), Salix caprea (Salicaceae), Capsicum annuum, Lycopersicon esculentum (Solanaceae), Aloe barbadensis (Xanthorrhoeaceae) Zingiber officinale (Zingiberaceae). Cause foliar blights, leaf spots on some of above hosts and soft rot on some fruit species. Asexual state of C. spicifer cause diseases in human such as fungus balls of the sinuses and chronic rhinosinusitis with massive polyposis (Buzina et al. 2003)
Distribution: Australia, Bangladesh, Brazil, Canada, China, Cyprus, Egypt, Georgia, India, Iran, Japan, Kenya, New Zealand, Pakistan, Saudi Arabia, South Africa, Sudan, Taiwan, Tanzania, Turkey, USA.
References: Preston 1945; Luttrell 1952; Georghiou and Papadopoulos 1957; Greene 1960; Anonymous 1960; Nelson 1964c; Ahmad 1969; Turner 1971; Sarbhoy et al. 1971; Srivastava and Gupta 1981a; Pirozynski 1972; Chandra 1974; Jones and Wiggins 1974; Matsushima 1975; Ahmad 1978; Phillips et al. 1979; Mallaiah et al. 1981; Alfieri et al. 1984; Hafez 1984; Sivanesan 1987; Madhukar and Reddy 1988; Cook and Dubé 1989; Pennycook 1989; Lenne 1990; Richardson 1990; Bourbos and Skoudridakis 1992; Boa and Lenné 1994; Portales et al. 1995; Roane and Roane 1996; Ahmad et al. 1997; Pourabdollah and Ershad 1997; Roane and Roane 1997; Mendes et al. 1998; Zhuang 2001; Chen et al. 2002; Liu and Zhang 2004; Roane 2004; Pratt 2006; Majumdar et al. 2007; Mulenko et al. 2008; Roane 2009; Mhadri et al. 2009.
Notes: Cochliobolus spicifer is similar to C. australiensis on PDA. A hyaline area just above the dark hilum is characteristic of the conidia C. spicifer (Ruppel 1974). The species is heterothallic, and the asexual conidial state has bipolar germination. This species is an opportunistic human pathogen and resembles two of its close relatives, C. australiensis and C. hawaiiensis (Emami and Hack 2002).
Cochliobolus sporoboli E. Castell., Mycopathologia 6: 56 (1951)
Holotype: OTT.
Hosts: Sporobolus affinis, S. marginatus, S. ruspolianus (Poaceae).
Distribution: Africa, Ethiopia, Myanmar, Pakistan.
References: Castellani and Ciferri 1950; Castellani 1951; Ahmad 1969, 1978; Patil and Ramesh 1986; Alcorn 1991; Ahmad et al. 1997; Thaung 2008.
Notes: The species has not been included in previous phylogenetic analysis. As a type culture is not available, recollection and epitypification is essential.
Cochliobolus stenospilus T. Matsumoto & W. Yamam., J Plant Protect. Tokyo 23: 14 (1936)
= Bipolaris stenospila (Drechsler) Shoemaker, Can. J. Bot. 37: 884 (1959)
≡ Drechslera stenospila (Drechsler) Subram. & B.L. Jain, Curr. Sci. 35: 354 (1966)
≡ Helminthosporium stenospilum Drechsler, Phytopathology 18: 136 (1928)
Hosts and diseases: Brachiaria platyphylla, Cynodon dactylon, Imperata arundinacea, Pennisetum glaucum, Saccharum officinarum (Poaceae). Causes brown stripe disease of leaves.
Distribution: Africa, Australia, Brazil, China, Cuba, Fiji, Georgia, Guyana, Hawaii, India, Malawi, Malaysia, Puerto Rico, Samoa, Taiwan, USA, Zambia.
References: Matsumoto and Yamamoto 1936; Doidge 1950; Sprague 1950; Luttrell 1954; Freeman 1957; Anonymous 1960, 1979; Simmonds 1966; Firman 1972; Peregrine and Siddiqi 1972; Misra and Prakash 1972; Stevenson 1975; Gorter 1977; Raabe et al. 1981; Alfieri et al. 1984; Arnold 1986; Urtiaga 1986; Sivanesan 1987; Mendes et al. 1998; McKenzie 1996; Tai 1979; Zhuang 2001; Crous et al. 2000; Pratt 2006.
Notes: In Index Fungorum, Cochliobolus stenospilus was maintained in synonymy with B. stenospila which is the asexual state. Alcorn (1983a) recorded C. stenospilus as the teleomorph of B. stenophilus. The Cochliobolus teleomorph was, however, published in Japanese and without a Latin description and is therefore invalid (Sivanesan 1987). The asexual state is difficult to distinguish from B. heveae from their original description (Ellis 1971). Type material could not be located and this species should be recollected and validly published.
Cochliobolus tripogonis Alcorn, Mycotaxon 13: 342 (1981)
= Bipolaris tripogonis (A.S. Patil & V.G. Rao) Alcorn, Mycotaxon 13: 344 (1981)
≡ Drechslera tripogonis A.S. Patil & V.G. Rao, Trans.Br. mycol. Soc. 59:340 (1972)
Holotype: BRIP 12375; Paratype: BRIP 12273.
Hosts and diseases: Tripogon jacquemontii, T. loliiformis (Poaceae). Infection on the inflorescences of Tripogon is characterized by strands and spongy sooty colonies of the fungus turning the entire inflorescence into a dark, thick, rigid, stick-like structure (Patil and Rao 1972).
Distribution: Australia, India.
References: Alcorn 1981b.
Notes: The sexual state of Cochliobolus tripogonis formed in culture by pairing compatible strains (Sivanesan 1987) and differed from many other Cochliobolus species by having a very long ascomata. Cochliobolus cymbopogonis, C. hawaiiensis and C. ravenelii, which also have long ascomata, differ from this species by other morphological characters (Alcorn 1981a, b). Cochliobolus tripogonis is heterothallic.
Cochliobolus tritici Dastur, Indian J. agric. Sci. 12: 735 (1942)
Host: Triticum vulgare (Poaceae).
Distribution: India.
References: Dastur 1942.
Notes: An asexual state of C. tritici was mentioned by Dastur (1942), however it was unnamed and morphology was similar to the asexual state of Cochliobolus perotidis, but the sexual states of the two species are distinct (Alcorn 1982). No records of this species have been made since the original description.
Cochliobolus tuberculatus Sivan., Trans. Br. mycol. Soc. 84: 548 (1985)
= Curvularia tuberculata B.L. Jain, Trans. Br. mycol. Soc. 45: 539 (1962)
Ex-type: IMI 288001
Hosts and diseases: Coccinia indica (Cucurbitaceae), Cyperus malaccensis (Cyperaceae) Vigna aconitifolia (Fabaceae), Juncus roemerianus (Juncaceae), Eucalyptus globulosus, E. tereticornis, Psidium guava (Myrtaceae), Pinus caribaea (Pinaceae), Oryza sativa, Pennisetum sp., Triticum sp., Sorghum vulgare (Poaceae), Citrus limonia (Rutaceae). Causes leaf spots, leaf blights.
Distribution: Denmark, India, Pakistan, Taiwan, USA.
References: Lele et al. 1968; Rao 1969; Sarbhoy et al. 1971; Fell and Hunter 1979; Matsushima 1980, 1983; Mallaiah et al. 1981; Sivanesan 1985, 1987; Lenne 1990; Richardson 1990; Vittal and Dorai 1994; Majumdar 1997
Notes: The species is heterothallic and the sexual state was obtained by pairing mono conidial compatible isolates (Sivanesan 1985). This taxon has not been incorporated in a previous phylogenetic analysis and type derived sequences are not available in GenBank.
Cochliobolus verruculosus (Tsuda & Ueyama) Sivan., Bitunicate Ascomycetes and their Anamorphs: 366 (1984)
≡ Pseudocochliobolus verruculosus Tsuda & Ueyama, Mycologia 74: 565 (1982)
= Curvularia verruculosa Tandon & Bilgrami ex M.B. Ellis, Mycol. Pap. 106: 20 (1966)
= Curvularia verruculosa Tandon & Bilgrami, Curr. Sci. 31: 254 (1962)
Holotype: TNS-F 234525, Isotype: CUP, IMI, L, Lectotype: IMI.
Hosts and diseases: Reported on various hosts such as Zinnia elegans (Asteraceae), Kigelia pinnata (Bignoniaceae), Canna indica (Cannaceae), Dioscorea alata (Dioscoreaceae), Cassia fistula, Lablab purpureus, Vicia faba, Vigna sinensis (Fabaceae), Tinospora cordifolia (Menispermaceae), Musa sp. (Musaceae), Pinus caribaea (Pinaceae), Brachiaria mutica, Chloris radiate, Cynodon dactylon, Echinochloa crus-galli, Oryza sativa, Paspalum scrobiculatum, Pennisetum americanum, P. typhoides, Saccharum sp., Setaria viridis, Sorghum vulgare, Zea mays (Poaceae). Cause leaf spots and blights.
Distribution: China, Egypt, India, Indonesia, Iran, Israel, Jamaica, Samoa, Taiwan, Venezuela, Malaysia, West Indies.
References: Chandra 1974; Singh and Chohan 1974; Khan and Kamal 1974; Wallbridge and Pinegar 1975; Ebbels and Allen 1979; Srivastava and Gupta 1981a; Matsushima 1983; Sivanesan 1984, 1987; Urtiaga 1986; Singh and Singh 1986; Lenne 1990; Richardson 1990; Boa and Lenné 1994; McKenzie 1996; Ahmad et al. 1997; Minter et al. 2001; Sun et al. 2003; Zhuang 2005.
Notes: The asexual state (Curvularia verruculosa) is characterized by 3-septate conidia with a verrucose conidial wall. It is easily distinguished from other species with verrucose cell walls, 3-septate conidia such as C. tuberculatus and by the position of the largest cell in the conidium. The largest cell is second from the base in C. tuberculatus and third from the base in C. verruculosus (Tsuda and Ueyama 1982). Conidial size and cell wall ornamentation are highly variable depending on cultural conditions (Tsuda and Ueyama 1982).
Cochliobolus victoriae R.R. Nelson, Phytopathology 50: 775 (1960)
= Bipolaris victoriae (F. Meehan & H.C. Murphy) Shoemaker, Can. J. Bot. 37: 882 (1959)
≡ Drechslera victoriae (F. Meehan & H.C. Murphy) Subram. & B.L. Jain, Curr. Sci. 35: 355 (1966)
≡ Helminthosporium sativum var. victoriae (F. Meehan & H.C. Murphy) H.R. Rosen, Wiser & J.O. York, Beitr. Bau. Flecht. 533: 22 (1953)
= Helminthosporium victoriae F. Meehan & H.C. Murphy, Science, N.Y. 104: 413 (1946)
Holotype: BPI, K.
Hosts and diseases: Chenopodium sp.(Amaranthaceae), Commelina benghalensis (Commelinaceae), Glycine max (Fabaceae), Agropyron cristatum, Avena sativa, A. byzantine, Cymbopogon flexuosus, Digitaria ciliaris, Dactylis glomerata, Hordeum vulgare, Hordeum vulgare, Oryza sativa, Paspalum scrobiculatum, P. notatum, Phalaris arundinacea, Phleum pretense, Setaria sphacelata, S. verticillata, S. viridis, Triticum, Zea mays (Poaceae). Causal agent of Victoria blight of oat. Also causes leaf spots, culm necrosis, seeds and seedling blights. Seedling leaves become reddened, often giving a striped effect, and leaves and seedlings can be killed (Sivanesan 1987).
Distribution: Australia, Bolivia, Brazil, Canada, Florida, Georgia, India, Kenya, Malaysia, United Kingdom, USA, Zambia, Zimbabwe.
References: Gilman 1949; Earhart 1950, 1952,1955,1957; Moseman and Hebert 1950; Josephson and Reid 1950; Rosen 1950; Lyle 1950; Livingston 1950; Sprague 1950; Miller et al. 1952; Atkins 1951; Tisdale 1952; Summers and Bowman 1953; Futrell and Atkins 1954; Elliott 1955; Luttrell 1955; Miller et al. 1955; Gore et al. 1956; Farrar and Gore 1957; Farrar and Stacy 1958; Ivanoff et al. 1958; Roane and Starling 1958; Futrell et al. 1959; Parris 1959; Anonymous 1960; Nelson 1960c; Simmonds 1966; Conners 1967; Scheffer and Nelson 1967; Mankin 1969; Misra and Prakash 1972; Hanlin et al. 1978; Sivanesan and Holliday 1981; Alfieri et al. 1984; Epsteina and Lockwooda 1984; Cannon et al. 1985; Grand 1985; Sivanesan 1987; Shivas 1989; Lenne 1990; Christiansen et al. 1998; Mendes et al. 1998; Caretta et al. 1999.
Notes: This species is heterothallic. A victroin host selective toxin is produced by this species (Wolpert et al. 1988). Cochliobolus victoriae is a soil inhabiting species but prolonged exposure to soil can reduce the virulence of fungal conidia (Filonow et al. 1983). Some soil inhabiting bacteria can suppress the germination of its conidia (Epsteina and Lockwooda 1984).
Cochliobolus zeae H.S. Chang, Bot. Bull. Acad. sin., Taipei 33: 175 (1992)
Cochliobolus zeae H.S. Chang was first invalidly published (Art. 36.1) in the Annual Report, Institute of Botany, Academia Sinica (Taipei): 5 (1988) [1987]. It was also invalidly published (Art. 36.1) in Abstracts of Papers, 5th International Congress of Plant Pathology Kyoto, Japan, August 20–27, 1988 (Kyoto): 138 (1988).
= Bipolaris zeae Sivan., Trans. Br. mycol. Soc. 84: 418 (1985)
Holotype: ASIB-02; Isotype: IMI 350958
Hosts: Alloteropsis semialata, Brachiaria decumbens, Cenchrus ciliaris, Imperata cylindrica, Panicum virgatum, Pennisetum americanum, P. clandestinum, P. glaucum, P. typhoides, Zea mays (Poaceae), Acer truncatum (Sapindaceae), Eucalyptus sp. (Myrtaceae).
Distribution: Australia, Brazil, China, Colombia, India.
References: Sivanesan 1985, 1987; Shivas 1989; Lenne 1990; Chang 1992; Krupinsky et al. 2004; Sun et al. 2011.
Notes: The teleomorph was obtained after mating single conidial isolations on Sach’s agar medium. (Chang 1992). The species is heterothallic.
Excluded species
Cochliobolus thalassiae Orpurt & Boral
This species is listed in Index Fungorum, but no literature could be found on this species.
References
Abraham WR, Meyer H, Abate D (1995) Curvupallides, a new class of alkaloids from the fungus Curvularia pallescens. Tetrahedron 51:4947–4952
Agrawal A, Singh SM (1995) Two cases of cutaneous phaeohyphomycosis caused by Curvularia pallescens. Mycoses 38:301–303. doi:10.1111/j.1439-0507.1995.tb00412.x
Ahmad S (1969) Fungi of West Pakistan. Biol Soc Pakistan Monogr 5:1–110
Ahmad S (1978) Ascomycetes of Pakistan part II. Biol Soc Pakistan Monogr 8:1–144
Ahmad S, Iqbal SH, Khalid AN (1997) Fungi of Pakistan. Sultan Ahmad mycological society of Pakistan 248
Ahn JH, Walton JD (1998) Regulation of cyclic peptide biosynthesis and pathogenicity in Cochliobolus carbonum by TOXEp, a novel protein with a bZIP basic DNA-binding motif and four ankyrin repeats. Mol Gen Genet 260:462–469. doi:10.1007/PL00008632
Alcorn JL (1978) Two new Cochliobolus species. Trans Br Mycol Soc 70:61–65
Alcorn JL (1981a) Ascus structure and function in Cochliobolus species. Mycotaxon 13:349–360
Alcorn JL (1981b) Cochliobolus ravenelii sp.nov and Cochliobolus tripogonis sp.nov. Mycotaxon 13:341
Alcorn JL (1982) New Cochliobolus and Bipolaris species. Mycotaxon 15:1–19
Alcorn JL (1983a) On the genera Cochliobolus and Pseudocochliobolus. Mycotaxon 16:353–379
Alcorn JL (1983b) Generic concepts of Drechslera, Bipolaris and Exserohilum. Mycotaxon 17:1–86
Alcorn JL (1983c) Cochliibolus ellisii sp.nov. Trans Br Mycol Soc 81:172–174
Alcorn JL (1988) The taxonomy of Helminthosporium species. Annu Rev Phytopathology 26:37–56
Alcorn JL (1990) Additions to Cochliobolus, Bipolaris and Curvularia. Mycotaxon 39:361–392
Alcorn JL (1991) New combinations and synonymy in Bipolaris and Curvularia, and a new species of Exserohilum. Mycotaxon 41:329–343
Alcorn JL (1996) Cochliobolus heliconiae sp.nov. (Ascomycota). Aust Syst Bot 9:813–817
Alcorn JL (1998) A new Cochliobolus species and its Curvularia anamorph. Proc R Soc Qd 107:1
Aldridge DC, Turner WB (1970) 9-Hydroxyprehelminthosporol, a metabolite of Cochliobolus (Helminthosporium)sativus. J Chem Soc C 686–688. doi:10.1039/J39700000686
Alexopoulos CJ, Mims CW, Blackwell M (1996) Introductory mycology. Wiley, New York
Alfieri JR, Langdon SA, Wehlburg KRC, Kimbrough JW (1984) Index of plant diseases in florida (revised). Fla Dep Agr Consum Serv Div Plant Ind Bull 11:1–389
Alvarez MG (1976) Primer catalogo de enfermedades de plantas Mexicanas. Fitofilo 71:1–169
Alvindia DG, Natsuaki KT (2008) Evaluation of fungal epiphytes isolated from banana fruit surfaces for bio-control of banana crown rot disease. Crop Prot 27:1200–1207
Aly AH, Debbab A, Kjer J, Proksch P (2010) Fungal endophytes from higher plants: a prolific source of phytochemicals and other bioactive natural products. Fungal Divers 41:1–16. doi:10.1007/s13225-010-0034-4
Amadioha AC (2002) Fungitoxic effects of extracts of Azadirachta Indica against Cochliobolus miyabeanus causing brown spot disease of rice. Arch Phytopathol Pfl 35:37–42
Anandi V, Suryawanshi NB, Koshi G, Padhye AA, Ajello L (1988) Corneal ulcer caused by Bipolaris hawaiiensis. Med Mycol 26:301–306. doi:10.1080/02681218880000411
Anonymous (1960) Index of plant diseases in the United States. USDA Agr Handbook 165:1–531
Anonymous (1979) List of plant diseases in Taiwan. Pl Protect Soc Republ of China 404 pp.
Anthony MH, Ayinla GT, Helmina AO, Ezekiel SA, Haruna OG (2009) Health implications of toxigenic fungi found in two Nigerian staples: guinea corn and rice. Afr J Food Sci 3:250–256
Arnold GRW (1986) Lista de hongos fitopatogenos de Cuba. Ministerio de Cultura Editorial Cientifico-Tecnica 207 pp
Atkins JG Jr (1951) Helminthosporium victoriae as a leaf-spotting pathogen on oats. Phytopathology 41:300–301
Aulakh KS, Mathur SB, Neergaard P (1974) Seed heath testing of rice and comparison of field incidence with laboratory counts of Drechslera oryzae and Pyricularia oryzae. Seed Sci Tech 2:385–398
Baker RED, Dale WT (1951) Fungi of trinidad and tobago. Mycol Pap 33:1–123
Baker GE, Dunn PH, Sakai WS (1979) Fungus communities associated with leaf surfaces of endemic vascular plants in Hawaii. Mycologia 71:272–292
Balasubramanian KA (1980) Association of Drechslera setariae with downy mildew affected pearl millet. Curr Sci 49:233–234
Bean GA, Schwartz R (1961) A severe epidemic of Helminthosporium brown spot disease on cultivated wild rice in northern Minnesota. Pl Dis Rep 45:901
Benjamin CR, Slot A (1969) Fungi of Haiti. Sydowia 23:125–163
Berbee ML, Pirseyedi M, Hubbard S (1999) Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 91:964–977
Berg D, Garcia JA, Schell WA (1995) Cutaneous infection caused by Curvularia pallescens: a case report and review of the spectrum of disease. J Am Acad Dermatol 32:375–378. doi:10.1016/0190-9622(95)90408-5
Bettucci L, Saravay M (1993) Endophytic fungi of Eucalyptus globulus: a preliminary study. Mycol Res 97:679–682
Bettucci L, Alonso R, Fernandez LM (1997) A comparative study of fungal populations in healthy and symptomatic twigs and seedlings of Eucalyptus globulus in Uruguay. Sydowia 49:109–117
Bhale MS, Khare MN (1982) Seed-borne fungi of Sorghum in Madhya Pradesh and their significance. Indian Phytopathology 35:676–678
Blakeman JP, Fokkema NJ (1982) Potential for biological control of plant diseaseson the phylloplane. Annu Rev Phytopathol 20:167–192
Boa E, Lenné J (1994) Diseases of nitrogen fixing trees in developing countries. An annotated list. Natural Resources Institute, Kent
Bobev S (2009) Reference guide for the diseases of cultivated plants. Unknown journal or publisher 466 pp.
Boedijn (1933) Bull. Jard. bot. Buitenz, 3 Sér. 13:123
Boughey AS (1946) A preliminary list of plant diseases in the Anglo-Egyptian Sudan. Mycol Pap 14:1–16
Bourbos VA, Skoudridakis MT (1992) Soft rot of kiwifruit caused by Bipolaris spicifera, anamorph of Cochliobolus spicifer. FAO Plant Protect Bull 40:110
Butler EJ (1918) Fungi and deceases in plant. Thacker, spink and Co. Culcutta
Butt TM, Jackson CW, Magan N (2001) Fungi as bio-controlling agents Progress, problem and potential. CABI Publishing 1–9
Buzina W, Braun H, Schimpl K, Stammberge H (2003) Bipolaris spicifera causes fungus balls of the sinuses and triggers polypoid chronic rhinosinusitis in an immunocompetent patient. Clin Microbiol 41:4885–4887. doi:10.1128/JCM.41.10.4885-4887.2003
Cai L, Hyde KD, Taylor PWJ, Wei BS, Waller J, Abang MM, Zhang JZ, Yang YL, Phoulivong S, Liu ZY, Prihastuti H, Shivas RG, McKenzie EHC, Johnston PR (2009) A polyphasic approach for studying Colletotrichum. Fungal Divers 39:183–204
Campos FF, Rosa LH, Cota BB, Caligiorne RB, Rabello ALT, Alves TMA, Rosa CA, Zani CL (2008) Leishmanicidal metabolites from Cochliobolus sp., an endophytic fungus isolated from Piptadenia adiantoides (fabaceae). PLoS Negl Trop Dis 2:1–11
Cannon PF, Hawksworth DL, Sherwood-Pike MA (1985) The British ascomycotina. An annotated checklist. Commonwealth Mycological Institute, Kew
Carson ML (1998) Aggressiveness and perennation of isolates of Cochliobolus heterostrophus from North Carolina. Plant Dis 82:1043–1047
Carruthers JR, Cerrini S, Fedeli W, Casinovi CG, Galeffi C, Vaccaro AMT, Scala A (1971) Structures of cochlioquinones A and B, new metabolites of Cochliobolus miyabeanus: chemical and X-ray crystallographic determination. J Chem Soc D Issue 3:164–166. doi:10.1039/C29710000164
Caretta G, Piontelli E, Picco AM, Del Frate G (1999) Some filamentous fungi on grassland vegetation from Kenya. Mycopathologia 145:155–169
Casonato S, Lawrie A, McLaren D (2005) Prospects for biological control of serrated tussock Tussock Terminators Research Forum: 32–35
Castellani E, Ciferri R (1937) Prodromus mycoflorae africae orientalis italicae. Istituto agricolo coloniale italiano
Castellani E, Ciferri R (1950) Mycoflora erythraea somala et aethipica suppl. Atti Ist Bot Lab Crittog Univ, Pavia
Castellani E (1951) Una nuova specie di Cochliobolus. Mycopathologia 6:51–57
Chandra S (1974) Some new leaf-spot diseases from Allahabad (India). Beih Nova Hedwigia 47:35–102
Chang SH (1992) Cochliobolus zea sp. nov the teleomorph of Bipolaris zea. Bot Bull Acad Sin Taipei 33(2):175
Chand R, Singh HV, Joshi AK, Duveiller E (2002) Physiological and morphological aspects of Bipolaris sorokiniana conidia surviving on wheat straw. Plant Pathol J 18(6):328–332
Chalet M, Howard DH, McGinnis MR, Zapatero I (1986) Isolation of Bipolaris australiensis from a lesion of viral vesicular dermatitis on the scalp 24: 461–465 doi:10.1080/02681218680000731
Chardon CE, Toro RA (1930) Mycological explorations of Colombia. J Dept Agr Porto Rico 14:195–369
Charudattan R (2001) Biological control of weeds by means of plant pathogens: significance for integrated weed management in modern agro-ecology. Bio Control 46:229–260
Chase AR (1982) Dematiaceous leaf spots of Chrysalidocarpus lutescens and other palms in Florida. Pl Dis 66:697–699
Chauhan S, Pandey BN (1992) A new leaf blight of Populus deltoides caused by Bipolaris maydis. Indian Phytopathology 45:130–133
Chen WQ, Ntahimpera N, Morgan DP, Michailides TJ (2002) Mycoflora of Pistacia vera in the central valley, California. Mycotaxon 83:147–158
Cho WD, Shin HD (2004) List of plant diseases in Korea. Korean Society of Plant Pathology
Cholil A, De Hoog GS (1982) Variability in Drechslera oryzae. Trans Br Mycol Soc 79:491–496
Chomcheon P, Wiyakrutta S, Aree T, Sriubolmas N, Ngamrojanavanich N, Mahidol C, Ruchirawat S, Kittakoop P (2010) Curvularides A–E: antifungal hybrid peptide–polyketides from the endophytic fungus Curvularia geniculata. Chem Eur J 16:11178–11185
Cheewangkoon R, Groenewald JZ, Verkley GJM, Hyde KD, Wingfield MJ, Gryzenhout M, Summerell BA, Denman S, Toanun C, Crous PW (2010) Re-evaluation of Cryptosporiopsis eucalypti and Cryptosporiopsis-like species occurring on eucalyptus leaves. Fungal Divers 44:89–105. doi:10.1007/s13225-010-0041-
Christensen CM, Meronuck RA (1986) Quality maintenance in stored grains and seeds. Chapter 3: quality of fungi and quality of grains and feeds: 18–40
Christiansen SK, Wirsel S, Yun SH, Yoder OC, Turgeon BG (1998) The two Cochliobolus mating type genes are conserved among species but one of them is missing in C. victoriae. Mycol Res 102:919–929
Ciegler A, Lee S, Dunn JJ (1981) Production of naphthoquinone mycotoxins and taxonomy of Penicillium viridicatum. Appl Environ Microbiol 42:446–449
Ciferri R (1961) Mycoflora domingensis integrata. Quaderno 19:1–539
Clark RV, Wallen VR (1969) Seed infection of barley by Cochliobolus sativus and its influence on yield. Can Plant Dis Surv 49:60–64
Cook RP, Dubé AJ (1989) Host-pathogen index of plant diseases in south Australia. South Australian Department of Agriculture
Conners IL (1967) An Annotated index of plant diseases in Canada and fungi recorded on plants in Alaska, Canada and Greenland. Res Bra Canada Dept Agr 1251:1–381
Costa AR, Porto E, Tabuti AH, Lacaz CS, Valente NYS, Maranhâo WM, Rodrigues MC (1991) Subcutaneous pheaohyphomycocis caused by Bipolaris hawaiiensis. A case report. Rev Inst Med Trop Sâo Paulo 33:74–79
Cooke WB (1971) The 1967 foray in Texas. Mycologia 63:1063–1067
Crepel C, Inghelbrecht S, Baeyen S, Maes M (2006) First report of Cochliobolus sativus on Guzmania sp. in Belgium. Pl Dis 90:1361
Crous PW, Petrini O, Marais GF, Pretorious ZA, Rehder F (1995) Occurrence of fungal endophytes in cultivars of Triticum aestivum in South Africa. Mycoscience 36:105–111
Crous PW, Phillips AJL, Baxter AP (2000) Phytopathogenic fungi from south Africa. University of Stellenbosch, Department of Plant Pathology Press
Dade HA (1940) A revised list of Gold coast fungi and plant diseases. XXIX. Bull Misc Inform Kew 6:205–247
Dadwal VS, Verma RK (2009) A new blotch disease (Curvularia pallescens boedijn) of gloriosa superba linn. J Mycol Pl Pathol 39:156–157
Dahal G, Amatya P, Manandhar H (1992) Plant diseases in Nepal. Rev Pl Pathol 71:797–807
Danquah OA (1975) New new species of Drechslera. Trans Br Mycol Soc 64:544–546
Dasgupta S, Saha D, Saha A (2005) Levels of common antigens in determining pathogenicity of Curvularia eragrostidis in different tea varieties. J Appl Microbiol 98:1084–1092. doi:10.1111/j.1365-2672.2005.02538.x
Dastur JF (1942) Notes on some fungi isolated from ‘black point’ effected kernels in the central provice. Indian J Agr Res 12:731–742
Davis TC, Kelley WD, Goggans JF (1972) Curvularia intermedius associated with seedling tip blight of Arizona cypress and eastern red cedar. Pl Dis Reporter 56:192
Dehne HW, Oerke EC (1985) Investigations on the occurrence of Cochliobolus sativus on barely and wheat. II. Infection, colonization and damage of stems and leaves. Zeitschrift fuer Pflanzenkrankheiten und Pflanzenschutz 92:606–617
Deighton FC (1936) Preliminary list of fungi and diseases of plants in Sierra Leone and list of fungi collected in Sierra Leone. Bull. Misc. Inform
Delgado-Rodriguez G, Mena-Portales J, Calduch M, Decock C (2002) Hyphomycetes (hongos mitosporicos) del area protegida mil cumbres, Cuba Occidental. Cryptog Mycolog 23:277–293
Deng H, Zhang TY (2002) Taxonomic studies of Bipolaris (hyphomycetes) from China I. Mycosystema 21:327–333
Dennis RWG (1970) Kew bulletin additional series III. Fungus Flora of Venezuela and Adjacent Countries. Verlag von J. Cramer
De Luna LZ, Watson AK, Paulitz TC (2002) Reaction of rice (Oryza sativa) cultivars to penetration and infection by Curvularia tuberculata and C. oryzae. Plant Dis 86:470–476
Doidge EM (1950) The South African fungi and lichens to the end of 1945. Bothalia 5:1–1094
Dosdall L (1923) Factors influencing the pathogenicity of Helminthosporium sativum. Univ. Minn. Agric Exp Stn Tech Bull 17
Drechsler C (1923) Some graminicolous species of Helminthosporium. J Agric Res 24:641–739
Drechsler C (1934) Phytopathological and taxonomical aspects of Ophilobolus, Pyrenophora, Helminthosporium and a new genus Cochliobolus. Phytopathology 24:973
Drechsler C (1942) Cochliobolus kusanoi (Y. Nisik.) Drechsler ex Dastur. Indian J Agr Res 12:733
Duveiller E, Gilchrist LI (1994) Production constraints due to Bipolaris sorokiniana in wheat: current situation and future prospects. In: Saunders D, Hettel G (ed) Wheat in heat-stressed environments: irrigated, dry areas and rice-wheat farming systems. Proceedings of the CIMMYT/UNDP workshop, Nashipur (Dinajpur), Bangladesh, February: 343–52
Duveiller E, Kandel YR, Sharma RC, Shrestha SM (2005) Epidemiology of foliar blights (spot blotch and tan spot) of wheat in the plains bordering the Himalayas. Phytopathology 95:248–256
Dyer PS, Paoletti M, Archer DB (2003) Genomics reveals sexual secrets of Aspergillus. Microbiology 149:2301–2303. doi:10.1099/mic.0.C0119-0
Dyer ZA, Wright RS, Rong IS, Jacobs A (2008) Back pain associated with endobronchial mucus impaction due to Bipolaris australiensis colonization representing atypical allergic bronchopulmonary. Mycosis 46:589–594. doi:10.1080/13693780801968563
Earhart RW (1950) Small grain diseases in the southeastern coastal plain. Pl Dis Reporter 34:257–258
Earhart RW (1952) Small grain diseases of the southeastern coastal plain. Pl Dis Reporter 36:420–422
Earhart RW (1955) South Carolina small grain diseases1954-55. Pl Dis Reporter 39:947–948
Earhart RW (1957) Small grain diseases of South Carolina. Pl Dis Reporter 41:863–870
Ebbels DL, Allen DJ (1979) A supplementary and annotated list of plant diseases, pathogens and associated fungi in Tanzania. Phytopathol Pap 22:1–89
Elliott ES (1955) Notes on diseases of cereals and ornamentals recorded in West Virginia during 1953 and 1954. Pl Dis Reporter 39:332–333
Ellis MB (1966) Dematiaceous hyphomycetes VII. Curvularia Brachysporium etc. Mycol Pap 106:1–57
Ellis MB (1971) Dematiaceous hyphomycetes. Commonwealth Mycological Institute, Kew, p 608
Ellis MB, Gibson IAS (1975) Cochliobolus lunatus. CMI Descr Path Fungi and Bact: 474
El Shafie AE, Webster J (1980) Ascospore liberation in Cochliobolus cymbopogonis. Trans Brit Mycol Soc 75:141–146. doi:10.1016/S0007-1536(80)80204-X
Endo RM (1961) Turfgrass diseases in southern California. Pl Dis Reporter 45:869–873
Emami K, Hack E (2002) Conservation of XYN11A and XYN11B xylanase genes in Bipolaris sorghicola, Cochliobolus sativus, Cochliobolus heterostrophus, and Cochliobolus spicifer. Curr Microbiol 45:303–306. doi:10.1007/s00284-002-3618-8
Epsteina L, Lockwooda JL (1984) Effect of soil microbiota on germination of Bipolaris victoriae conidia. Trans Brit Mycol Soc 82:63–69. doi:10.1016/S0007-1536(84)80212-0
Eriksson OE, Yue JZ (1998) Bambusicolous pyrenomycetes, an annotated check-list. Myconet 1:25–78
Eschen R, Hunt S, Mykura C, Gange AC, Sutton BC (2010) The foliar endophytic fungal community composition in Cirsium arvense is affected by mycorrhizal colonization and soil nutrient content. Fung Biol 114:991–998
Evans HC, Greaves MP, Watson AK (2001) Fungal biological control agents of weeds: fungi as biocontroling agents progress, problem and potential. CABI Publishing 2001:1–9
Farrar LL, Gore UR (1957) Diseases of small grains observed in Georgia during the 1956–57 season. Pl Dis Reporter 41:986–987
Farrar LL, Stacy SV (1958) Diseases of small grains observed in Georgia during the 1957–58 season. Pl Dis Reporter 42:1262–1267
Farr ML, Stevenson JA (1963) Eine erganzungsliste bolivianischer pilze. Sydowia 17:37–69
Farias CRJ, Afonso APS, Pierobom CR, Ponte EMD (2011) Regional survey and identification of Bipolaris spp. associated with rice seeds in Rio Grande do Sul State, Brazil Cienc. Rural 41:3. doi:10.1590/S0103-84782011000300001
Fell JW, Hunter IL (1979) Fungi associated with the decomposition of the black rush, Juncus roemerianus, in south Florida. Mycologia 71:322–342
Fernandez MR (1991) Recovery of Cochliobolus sativus and Fusarium graminearum from living and dead wheat and nongramineous winter crops in southern Brazil. Can J Bot 69:1900–1906
Filonow AB, Akueshi CO, Lockwood JL (1983) Decreased virulence of Cochliobolus victoriae conidia after incubation on soils or on leached sand. Phytopathology 73:1632–1636
Figliola SS, Camper ND, Ridings WH (1988) Potential biological control agents for goose grass (Eleusine indica) 36: 830–835
Firman ID (1972) A list of fungi and plant parasitic bacteria, viruses and nematodes in Fiji. Phytopathol Pap 15:1–36
Fisher PJ, Petrini O (1992) Fungal saprobes and pathogens as endophytes of rice (Oryzasativa L.). New Phytol 120:137–143
Foister CE (1961) The economic plant diseases of Scotland. Tech Bull Dept Agr Fish Scotland 1:1–210
Fothergill AW (1996) Identification of dematiaceous fungi and their role in human disease. Clin Infect Dis 22:S179–S184. doi:10.1093/clinids/22.Supplement_2.S1
Fournier J, Stadler M, Hyde KD, Duon ML (2010) The new genus rostrohypoxylon and two new annulohypoxylon species from Northern Thailand. Fungal Divers 40:23–36. doi:10.1007/s13225-010-0026-4
French AM (1989) California plant disease host index. California Department of Food and Agriculture, Sacramento
Freeman TE (1957) A new Helminthosporium disease of Bermuda grass. Pl Dis Reporter 41:389–391
Futrell MC, Atkins IM (1954) Diseases of small grains in Texas in 1953. Pl Dis Reporter 38:167–168
Futrell MC, Atkins IM, Hobbs CD (1959) Unusual occurrence of small-grain diseases in Texas in 1957 and 1958. Pl Dis Reporter 43:777–781
Gazis R, Chaverria P (2010) Diversity of fungal endophytes in leaves and stems of wild rubber trees (Hevea brasiliensis) in Peru. Fungal Ecol 3:240–254
Gehlot P, Attitalla IH, Salleh B (2010) Anamorphic fungi: an overview. Middle East J Sci Res 6:201–208
Georghiou GP, Papadopoulos C (1957) A second list of Cyprus fungi. Government of Cyprus, Department of Agriculture, 38 pp
Ghimire SR, Charlton ND, Bell JD, Krishnamurthy YL, Craven KD (2011) Biodiversity of fungal endophyte communities inhabiting switchgrass (Panicum virgatum L.) growing in the native tall grass prairie of northern Oklahoma. Fungal Divers 47:19–27
Ghisalberti EL, Rowland CY (1993) 6-chlorodehydrocurvularin, a new metabolite from Cochliobolus spicifer. J Nat Prod 56:2175–2177
Ginns JH (1986) Compendium of plant disease and decay fungi in Canada 1960–1980. Res Br Can Agr Publ 1813:416
Gilman JC (1949) Second supplementary list of parasitic fungi from Iowa. Iowa State Coll J Sci 23:261–272
Goh KT, Hyde KD, Lee KLD (1998) Generic distinction in Helminthosporium complex based on restriction analysis of the nuclear ribosomal RNA gene. Fungal Divers 1:85–107
Gore UR, Morrey DD, Brown AR, Miller JH (1956) Diseases of small grains in Georgia. Pl Dis Reporter 40:224
Grand LF (1985) North Carolina plant disease index. North Carolina Agr Res Serv Tech Bull 240:1–157
Greene HC (1944) Notes on Wisconsin parasitic fungi. III. Trans Wis Acad Sci 35:113–135
Greene HC (1950) Notes on Wisconsin parasitic fungi. XIV. Amer Midl Nat 44:630–642
Greene HC (1960) Notes on Wisconsin parasitic fungi. XXVI. Trans Wis Acad Sci 49:85–111
Green JR (1958) Observations on fungus diseases of rice in Florida 1951–1957. Pl Dis Reporter 42:624–628
Goos RD (1963) Further observations of soil fungi in Hondurus. Mycologia 55:142
Gorter GJMA (1977) Index of plant pathogens and the diseases they cause in cultivated plants in South Africa. Republic S Af Depart Agr Tech Serv Pl Protect Res Inst Sci Bull 392:1–177
Gorter GJMA (1981) Index of plant pathogens (II) and the diseases they cause in wild growing plants in South Africa. Republic S Af Depart Agr Fish Sci Bull 398:1–84
Guarro J, Gené J, Stchigel AM (1999) Developments in fungal taxonomy. Clin Microbiol Rev 12:454–500
Gulya TJ Jr, Martinson CA, Tiffany LH (1979) Ear-rotting fungi associated with opaque-2 maize. Pl Dis Reporter 63:370–373
Guo YL (1999) Fungal flora of tropical guangxi, China: hyphomycetes I. Mycotaxon 72:349–358
Hafez SIIA (1984) Mycoflora of bean, broad bean, lentil, lupine and pea seeds in Saudi Arabia. Mycopathologia 88:45–49
Hanlin RT, Foudin LL, Berisford Y, Glover SU, Jones JP, Huang LH (1978) Plant disease index for maize in the United States, part I: host index. Agric Exp Sta Univ Georgia Res Rep 277:1–62
Hanlin RT (1963) A revision of the ascomycetes of Georgia. Georgia Agric Exp Sta Mimeo Ser ns 175:1–65
Harding H, Tinline RD (1983) The existence of differentially fertile strains in two populations of Cochliobolus sativus. Can J Plant Pathol 5:17–20
Hawa MM, Salleh B, Latiffah Z (2009) First report of Curvularia lunata on red-fleshed dragon fruit (Hylocereus polyrhizus) in Malaysia. Plant Dis 93:971. doi:10.1094/PDIS-93-9-0971C
Hawksworth DL, Crous PW, Redhead SA et al (2011) The Amsterdam declaration on fungal nomenclature. IMA Fungus 2:105–112
Herrero N, Márquez SS, Zabalgogeazcoa I (2009) Mycoviruses are common among different species of endophytic fungi of grasses. Arch Virol 154:327–330
Hetherington SD, Irwin JAG (1999) Pathological and molecular genetic variation in the interaction between Sporobolus spp. and Bipolaris spp. Aust J Agr Res 50:583–588
Hetzler J, Eyal Z, Mehta YR, Campos LA, Fehrmann H, Kushnir U, Zekaria-Oren J, Cohen L (1990) Interaction between Spot blotch (Cochliobolus sativus) and wheat cultivars. In: Saunders DA (ed) Wheat for the nontraditional warm areas: a proceedings of the International Conference, (Centro Internacional de Mejoramiento de Maiz y Trigo, Asuncion (Paraguay). Wheat Program). CIMMYT, Mexico, DF (Mexico), 1990, pp 146–164
Hino I, Katumoto (1960) Cochliobolus sasae. Bull. Faculty of Agriculture, Yamaguchi University 11:26
Hino MI, Katum (1966) Cochliobolus. J Jap Bot 41:292
Hilton S (2000) Canadian plant disease survey. Agr Agr Food Canada 80:151
Homma Y (1984) Perforation and lysis of hyphae of Rhizoctonia solani and conidia of Cochliobolus miyabeanus by soil myxobacteria. Phytopathology 74:1234–1239
Hooker AL, Smith DR, Lim SM, Becktt JB, Hooker (1970) Reaction of corn seedlings with male-sterile cytoplasm to Helminthosporium mydis. Plant Dis Reptr 54:708–712
Huang WY, Cai YZ, Surveswaran S, Hyde KD, Corke H, Sun M (2009) Molecular phylogenetic identification of endophytic fungi isolated from three Artemisia species. Fungal Divers 36:69–88
Hughes SJ (1952) Fungi from the gold coast. I. Mycol Pap 48:1–91
Hughes SJ (1953) Fungi from the gold coast. II. Mycol Pap 50:1–104
Hyde KD, Alcorn JL (1993) Some disease-associated microorganisms on plants of Cape York Peninsula and Torres Strait Islands. Australas Pl Pathol 22:73–83
Hyde KD, Soytong K (2008) The fungal endophyte dilemma. Fungal Divers 33:163–173
Hyde KD, Zhou DQ, McKenzie EHC, Ho WH, Dalisay T (2002) Vertical distribution of saprobic fungi on bamboo culms. Fungal Divers 11:109–118
Hyde KD, Cai L, Cannon PF et al (2009) Colletotrichum–names in current use. Fungal Divers 39:147–182
Hyde KD, Chomnunti P, Crous PW, Groenewald JZ, Damm U, Ko TW, Shivas RG, Summerell BA, Tan YP (2010) A case for re-inventory of Australia’s plant pathogens. Persoonia 25:50–60. doi:10.3767/003158510X548668
Hyde KD, Mckenzie EHC, Koko TW (2011) Towards incorporating anamophic fungi in a natural classification- checklist and notes for 2010. Mycosphere 2:1–88
Imolehin ED (1983) Rice seedborne fungi and their effect on seed germination. Plant Dis 67:1334–1336
Isenor M, Kaminskyj SGW, Rodriguez RJ, Redman RS, Gough KM (2010) Characterization of mannitol in Curvularia protuberata hyphae by FTIR and Raman spectromicroscopy. Analyst 135:3249–3254. doi:10.1039/C0AN00534G
Ito S (1930) On some new ascigerous stages of species Helminthosporium parasitic on cereals. Proc Imp Acad (Japan) Suppl 6:352–355
Ivanoff SS, Bowman DH, Rothman PG (1958) Oat diseases in Mississippi. Pl Dis Reporter 42:521–523
Jadulco R, Brauers G, Edrada RA, Ebel R, Wray V, Sudarsono S, Proksch P (2002) New metabolites from sponge-derived fungi Curvularia lunata and Cladosporium herbarum. J Nat Prod 65:730–733
Jiang SJ, Qiang S, Zhu YZ, Dong YF (2008) Isolation and phytotoxicity of a metabolite from Curvularia eragrostidis and characterisation of its modes of action. Ann Appl Biol 152:103–111
Johnston A (1960) A supplement to a host list of plant diseases in Malaya. Mycol Pap 77:1–30
Johansen I, Percich JA (1992) Wild rice domestication, fungal brown spot disease, and the future of commercial production in Minnesota. Pl Dis 76:1193–1198
Jones GM, Wiggins EA (1974) Fungi of Alabama II. Dematiaceous hyphomycetes. J Ala Acad Sci 45:201–211
Jones GM (1976) Fungi of Alabama. III. dematiaceous hyphomycetes. J Ala Acad Sci 47:38–48
Jones MP, Jeutong F, Tchatchoua J (1993) A survey of rice disease in Cameroon. Pl Dis 77:133–136
Jones MJ, Dunkle LD (1993) Analysis of Cochliobolus carbornum races by PCR amplification with arbitarary and gene-specific primers. Phytopathology 83:366–370
Joshi AK, Chand R, Kumar S, Singh RP (2004) Leaf tip necrosis: a phenotypic marker associated with resistance to spot blotch disease in wheat. Crop Sci 44:792–796
Josephson LM, Reid DA (1950) Small grain diseases in Kentucky in 1949. Pl Dis Reporter 34:24–26
Jung HJ, Lee HB, Lim CH, Kim CJ, Kwon HJ (2003) Cochlioquinone A1, a new anti-angiogenic agent from Bipolaris zeicola. Bioorg Med Chem 11:4743–4747
Jurjevic Z, Wilson JP, Wilson DM, Casper HH (2006) Changes in fungi and mycotoxins in pearl millet under controlled storage conditions. Mycopathologia 164:229–239. doi:10.1007/s11046-007-9042-7
Keller NP, Turner G, Bennett JW (2005) Fungal secondary metabolism–from biochemistry to genomics. Nature reviews microbiology. Nat Publish Group 3:937–947
Kernkamp MF, Kroll R, Woodruff WC (1976) Diseases of cultivated wild rice in Minnesota. Pl Dis Reporter 60:771–775
Khan S, Kamal M (1974) Additions to the parasitic fungi of West Pakistan–III. Mycopathol. Mycol Appl 52:29–43
Khare MN (1991) Lentil diseases with special reference to seed quality. Indian J Mycol Pl Pathol 21:1–13
Kihara J, Ishikawa S, Sato A, Kumagai T (1998) Inheritance of photo-control of conidial development in the fungus Bipolaris oryzae. Mycoscience 39:89–91
Kim SK, Yang JS (1998) In vitro formation of Cochliobolus nisikadoi, the perfect state of Bipolaris coicis. Korean J Mycol 26:287–292
Kirk PM, Cannon PF, David JC, Stalpers JA (2008) Dictionary of the fungi, 10th edn. CABI International, UK
Kleczewski NM, Flory SL (2010) Leaf blight disease on the invasive grass Microstegium vimineum caused by a Bipolaris sp. Plant Dis 94:807–811
Klittich CJR, Bronson CR (1986) Reduced fitness associated with TOX 1 of Cochliobolus heterostrophus. Phytopathology 76:1294–1298
Kline DM, Nelson RR (1963) Pathogenicity of isolates of Cochliobolus sativus from cultivated and wild gramineous hosts from the Western Hemisphere to species of the Gramineae. Plant Dis Rep 47:890–894
Kobayashi T (2007) Index of fungi inhabiting woody plants in Japan. Host, distribution and literature. Zenkoku-Noson-Kyoiku Kyokai Publishing Co. Ltd
Kodsueb R, McKenzie EHC, Lumyong S, Hyde KD (2008) Diversity of saprobic fungi on Magnoliaceae. Fungal Divers 30:37–53
Komoto Y, Nishihara N, Yokoyama T (1980) A new disease of Sudan grass caused by Curvularia lunata and C. intermedia. Bull Chugoku Nat Agr Exp Stat 17:1–15
Koshi G, Anandi V, Kurien M, Kirubakaran MG, Padhye AA, Ajello L (1987) Nasal phaeohyphomycosis caused by Bipolaris hawaiiensis. J Med Vet Mycol 25:397–402. doi:10.1080/02681218780000481
Kowalik M, Sagan A (2005) Fungi causing dying out of heather in permanent plantings. Acta Mycol 40:191–195
Kranz J (1963) Fungi collected in the Republic of Guinea, collections from the Kindia area in 1962. Sydowia 17:174–185
Kranz J (1965) Fungi collected in the Republic of Guinea, III. Collections from the Kindia area in 1963/64, and host index. Sydowia 19:92–107
Krupinsky JM, Berdahl JD (1984) Septoria spraguei, Pyrenophora trichostoma, and Cochliobolus sativusincidence on Russian wild rye grass leaves and S. spragueihost range. Pl Dis 68:13–16
Krupinsky JM (1986) Pyrenophora tritici-repentis, P. bromi, and Leptosphaeria nodorum on Bromus inermis in the northern Great Plains. Pl Dis 70:61–64
Krupinsky JM, Berdahl JD, Schoch CL, Rossman AY (2004) Leaf spot on switch grass (Panicum virgatum), symptoms of a new disease caused by Bipolaris oryzae. Can J Plant Pathol 26:371–378
Kubo Y, Tsuda M, Furusawa I, Shishiyama J (1989) Genetic analysis of genes involved in melanin biosynthesis of Cochliobolus miyabeanus. Exp Mycol 13:77–84. doi:10.1016/0147-5975(89)90010-8
Kulkarni S, Ramakrishnan K, Hegde RK (1980) Genetic analysis of genes involved in melanin biosynthesis of Cochliobolus miyabeanus. Int Rice Res Newslett 5:13–14
Kumar V, Dwivedi RS (1981) Mycoflora associated with floral parts of sunflower. Indian Phytopathol 34:314–317
Kuribayashi K (1929) The ascigerous stage of Helminthosporium sativum. Trans Sapporo Nat Soc 10:138–145, In japanese with English summery
Lang J, Tisserat N (2005) First report of brown stripe of saltgrass caused by Bipolaris heveae in Colorado. Plant Dis 89:913. doi:10.1094/PD-89-0913A
Lanoiselet V, Cother EJ, Ash GJ (2005) Risk assessment of exotic plant diseases to the Australian rice industry, with emphasis on rice blast. Farrer Centre Charles Sturt University, 29–30
Larran S, Perelló A, Simón MR, Moreno V (2007) The endophytic fungi from wheat (Triticum aestivum L.). World J Microbiol Biotechnol 23:565–572. doi:10.1007/s11274-006-9266-6
Larran S, Monaco C (2010) Status and progress of research in endophytes from agricultural crops in Argentina. Management of Fungal Plant Pathogens. 149–160
Larter LNH, Martyn EB (1943) A preliminary list of plant diseases in Jamaica. Mycol Pap 8:1–16
Leath S, Leonard KJ (1986) Distribution of race 3 of Cochliobolus carbonus on corn in North Carolina. Pl Dis 70:800
Ledingham RJ, Sallans BJ, Wenhardt A (1960) Influence of cultural practices on incidence of common root rot of wheat in Saskatchewan. Can J Plant Sci 40:310–316
Lele VC, Raychaudhuri SP, Bhalla RB, Ram A (1968) Curvularia tuberculata, a new fungus causing die-back disease of Citrus in India. Indian Phytopathol 21:66–72
Lenne JM (1990) World list of fungal diseases of tropical pasture species. Phytopathol Pap 31:1–162
Leonard KJ, Suggs EG (1974) Setosphaeria prolata, the ascigerous state of Exserohilum prolatum. Mycologia 66:197–281
Lev S, Sharon A, Hadar R, Ma H, Horwitz BA (1999) A mitogen-activated protein kinase of the corn leaf pathogen Cochliobolus heterostrophus is involved in conidiation, appressorium formation, and pathogenicity: diverse roles for mitogenactivated protein kinase homologs in foliar pathogens. PNAS 96:13542–13547
Lijia L, Yunchun S, Huimin Y, Lihua L (1998) Physical location of Helminthosporium carbonum susceptibility gene hm1 by FISH of a RFLP marker umc119 in maize. Whuan Univ J Nat Sc 3:495–498. doi:10.1007/BF028300591998
Litzenberger SC, Farr ML, Lip HT (1962) A preliminary list of Cambodian plant diseases. Div Agric Nat Res, USAID, Minist. Agric. Phnom-Penh, Cambodia
Liu PSW (1977) A supplement to a host list of plant diseases in Sabah, Malaysia. Phytopathol Pap 21:1–49
Liu WC, Li CQ, Zhu P, Yang JL, Cheng KD (2010) Phylogenetic diversity of culturable fungi associatedwith two marine sponges: Haliclona simulans and Gelliodes carnosa, collected from the Hainan Island coastal waters of the South China Sea. Fungal Divers 42:1–15
Liu H-M, Zhang T-Y (2004) A preliminary report of soil dematiaceous hyphomycetes from the Yellow River Delta I. Mycosystema 23:338–344
Livingston JE (1950) Small grain diseases in Nebraska in 1950. Pl Dis Reporter 34:232
Lu B, Hyde KD, Ho WH, Tsui KM, Taylor JE, Wong KM, Yanna ZD (2000) Checklist of Hong Kong fungi. Fungal Diversity Press, Hong Kong
Luttrell ES, Rogerson CT (1959) Homothallism in an undescribed of Cochliobolus and Cochliobolus kusoni. Mycologia 51:195–202
Luttrell ES (1955) A taxonomic revision of helminthosporium sativum and related species. Amer J Bot 42:57–68
Luttrell ES (1954) Diseases of pearl millet in Georgia. Pl Dis Reporter 38:507–514
Luttrell ES (1952) Diseases of brome grasses in Georgia. Pl Dis Reporter 36:283–286
Luttrell ES (1957) Helminthosporium nodulosum and related species. Phytopathology 47:540–548
Luttrell ES (1963) Taxonomic criteria in Helminthosporium. Mycologia 55:643–674
Luttrell ES (1964) Systematics of Helminthosporium and related genera. Mycologia 56:119–132
Luttrell ES (1976) Ovarian infection of sporobolus poiretii by Bipolaris ravenelii. Phytopathology 66:260–268
Luttrell ES, Harris HB, Wells HD (1974) Bipolaris leaf blight of Panicum fasciculatum: effects of host age and photoperiod on susceptibility. Phytopathology 64:476–480
Lyle JA (1950) Diseases of small grains in Alabama. Pl Dis Reporter 34:318–320
Macmillan RH, Cooper PH, Body BA, Mills AS (1987) Allergic fungal sinusitis due to Curvularia lunata. Hum Pathol 18:960–964
Madhukar J, Reddy SM (1988) Some new leaf spot diseases of pomegranate. Indian J Mycol Pl Pathol 18:171–172
Majumdar VL (1997) Vigna aconitifolia—a new host for Curvularia tuberculata. Indian Phytopathol 50:300
Majumdar VL, Bhatanagar K, Sharma H, Verma OP (2007) Three new diseases of aloe barbadensis mill. J Mycol Pl Pathol 37:124–125
Makun HA, Gbodi TA, Akanya OH, Salako EA, Ogbadu GH (2007) Fungi and some mycotoxins contaminating rice (Oryza Sativa) in Niger State, Nigeriafrican. J Biotech 6:99–108
Malaker PK, Mian IH, Khandaker M, Reza MMA (2007) Survival of Bipolaris sorokiana(Sacc.) shoemaker in soil and residues of wheat. Bangladesh. J Bot 36:133–137
Mallaiah KV, Vijayalakshmi M, Rao AS (1981) New records of some foliar diseases. Indian Phytopathol 34:247
Manoch L, Tokumasu S, Tubaki K (1986) A preliminary survey of micro fungal flora of pine leaf litter in Thailand. Trans Mycol Soc Jpn 27:159–165
Manamgoda DS, Cai L, Hyde KD (2011) Unpublished data
Manandhar HK, Jørgensen HJL, Mathur SB, Petersen VS (1998) Suppression of rice blast by preinoculation with avirulent Pyricularia oryzae. Phytopathology 88:735–739
Mankin CJ (1969) Diseases of grasses and cereals in South Dakota. Agric Exp Sta South Dakota State Univ Tech Bull 35:1–27
Marquez LM, Redman RS, Rodriguez RJ (2007) A virus in a fungus in a plant: three-way symbiosis required for thermal tolerance. Science 315:513–515. doi:10.1126/science.1136237
Matsumoto T, Yamamoto W (1936) On the perfect and imperfect stages of the fungi causing sugar cane disease. J Plant Protect Tokyo 23:9–14
Matsushima T (1975) Icones microfungorum a matsushima lectorum. Nippon Printing Publishing Co
Matsushima T (1980) Matsushima mycological memoirs no. 1. Saprophytic microfungi from Taiwan, part 1. Hyphomycetes. Matsushima fungus collect, Kobe, Japan
Matsushima T (1989) Matsushima mycological memoirs no. 6. Matsushima fungus collect, Kobe, Japan
Matsushima T (1983) Matsushima mycological memoirs no. 3. Matsushima fungus collect, Kobe, Japan
McAleer R, Kroenert DB, Elder JL, Froudist JH (1981) Allergic bronchopulmonary disease caused by Curvularia lunata and Drechslera hawaiiensis. Thorax 36:338–344. doi:10.1136/thx.36.5.338
McGinnis MR, Rinaldi MG, Winn RE (1986) Emerging agents of phaeohyphomycosis: pathogenic species of Bipolaris and Exserohilum. J Clin Microbiol 24:250–259
Mc Guire Jr JU, Crandall BS (1967) Survey of insect pests and plant diseases of selected food crops of Mexico, Central America and Panama
McKenzie EHC (1992) Fungi of the Kermadec islands. Mycotaxon 45:149–170
McKenzie EHC (1996) Fungi, bacteria and pathogenic algae on plants in American Samoa, South Pacific Commission Information 206:1–77
McPartland JM, Cubeta MA (1997) New species, combinations, host associations, and location records of fungi associated with hemp (Cannabis sativa). Mycol Res 101:853–857
Mehta YR (1993) Seed borne disease and seedhealth testing of wheat. Copenhagen, Denmark
Mendes MAS, da Silva VL, Dianese JC (1998) Fungos em plants no Brasil. Embrapa-SPI/Embrapa-Cenargen, Brasilia: 555
Mhadri ME, Benkirane R, Touhami AO, Douira A (2009) New or unusual disease reports Citrullus lanatus, a new host of Bipolaris spicifera in Morocco. Phytopathol Mediterr 48:291–293
Michereff SJ, De Noronha MA, Maffia LA (2008) Sample size for assessment of yam leaf blight severity Tamanho de amostras para avaliação da severidade da queima das folhas do inhame. Summa Phytopathologica 34:189–191
Michereff SJ, Silveira NSS, Reis A, Mariano RLR (1994) Epiphytic bacteria antagonistic to Curvularia leaf spot of yam. Microb Ecol 28:101–110. doi:10.1007/BF00170250
Miller JH, Brown A, Johnson JR (1952) Notes on small grain diseases in Georgia for 1951–1952. Pl Dis Reporter 36:287
Miller JH, Gore UR, Brown AR, Morey DD (1955) Small grain diseases in Georgia in 1953–54. Pl Dis Reporter 39:17–19
Miller JW (1971) Tan leaf spot of Rhoeo discolor caused by Curvularia eragrostidis. Pl Dis Reporter 55:38–40
Miller JW (1991) Bureau of plant pathology. Tri-ology Tech Rep Div Pl Indust Florida 30:4–5
Minter DW, Hernández MR, Portales JM (2001) Fungi of the Caribbean: an annotated checklist. PDMS Publishing
Misra AP, Prakash O (1972) Helminthosporium species occurring on graminaceous hosts in India. Indian J Mycol Pl Pathol 2:95–97
Misra AP (1973) Helminthosporium species occurring on cereals and other Gramineae. Tirhut College of Agriculture (Dholi), The Catholic Press, Ranchi
Mishra AN, Kaushal K, Varma PK, Pandey HN (2001) Incidence of head blight of wheat in madhya pradesh during 1997–98. Indian Phytopath 54:476–478
Mishra D, Jena S, Mohanty RN (1981) A new leaf spot disease of banana caused by Drechslera hawaiiensis in India. J Ind Phytopathol 33:625–626
Mitra M (1930) A comparative study of species and strains of Helminthosporium on certain Indian cultivated crops. Trans Br Mycol Soc 15:254–293
Mitra M, Meheta PR (1934) Disease of Elusine indica Gaertn. and E. aegyptica Desf. caused by species of Helminthosporium. Indian J Agr Res 4:943–975
Mulenko W, Majewski T, Ruszkiewicz-Michalska MR (2008) A preliminary checklist of micromycetes in Poland. W. Szafer Institute of Botany, Polish Academy of Sciences 9:752
Mohamed R, Jong PL, Zal MS (2010) Fungal diversity in wounded stems of Aquilaria malaccensis. Fungal Divers 43:67–74. doi:10.1007/s13225-010-0039
Morejon KR, Kimati H, Fancelli MI (1998) Bipolaris bicolor (Mitra) Shoemaker: species associated to folial spot in pupunha palm (Bactris gasipaes Kunth) in Brazil. Rev Iberoam Micol Rev Iberoam Micol 15:55–57
Morsy MR, Oswald J, He J, Tang Y, Roossinck MJ (2010) Teasing apart a three-way symbiosis: transcriptome analyses of Curvularia protuberata in response to viral infection and heat stress. Biochem Bioph Res Co 401:225–230
Moseman JG, Hebert TT (1950) Small grain diseases in North Carolina in 1949–50. Pl Dis Reporter 34:392–394
Motlag MRS (2011) Evaluation of Curvularia lunata as an biological control agent in major weeds of rice paddies. Life Sci J 8:82–91
Msikita W, Yaninek JS, Ahounou M, Baimey H, Fagbemissi R (1997) First report of Curvularia lunata associated with stem disease of cassava. Pl Dis 81:112. doi:10.1094/PDIS.1997.81.1.112A
Nakada M, Tanaka C, Tsunewaki K, Tsuda M (1994) RFLP analysis for species separation in the genera Bipolaris and Curvularia. Mycoscience 35:271–278. doi:10.1007/BF02268449
Nakajima H, Fujimoto H, Matsumoto R, Hamasaki T (1993) Biosynthesis of spiciferone A and spicifernin, bioactive metabolites of the phytopathogenic fungus, Cochliobolus spicifer. J Org Chem 58:4526–4528. doi:10.1021/jo00069a008
Nakajima H, Isomia K, Hamasakia T, Ichinoeb M, Sorokinianin (1994) A novel phytotoxin produced by the phytopathogenic fungus Bipolaris sorokiniana 35: 9597–9600. doi:10.1016/0040-4039(94)88520-6
Nattrass RM (1961) Host lists of Kenya fungi and bacteria. Mycol Pap 81:1–46
Nelson RR (1957) Heterothallism in Helminthosporium maydis. Phytopathology 47:191–192
Nelson RR (1959) Cochliobolus carbornum, the perfect stage of Helminthosporium carbornum. Phytopathology 49:809
Nelson RR (1960a) Cochliobolus intermedius, the perfect stage of Curvularia intermedia. Mycologia 52:775–778
Nelson RR (1960b) The genetic compatible in Cochliobolus carbornum. Phytopathology 50:158–160
Nelson RR (1960c) Cochliobolus victoriae, the perfect stage of Helminthosporium victoriae. Phytopathology 50:774–775
Nelson RR (1964a) The perfect stage of Helminthosporium cynodontis mycologia. Mycol Soc Am 56:64–69
Nelson RR (1964b) The perfect stage of Curvularia geniculata. Mycologia 56:777–779
Nelson RR (1964c) The perfect state of Helminthosporium spiciferum. Mycologia 56:196–201
Nelson RR, Haasis FA (1964) The perfect stage of Curvularia lunata. Mycologia 56:316
Newell CK, Steinmetz RL, Brooks HL (2006) Chronic postoperative endophthalmitis caused by Bipolaris Australiensis. Retina 26:109–110
Nikolskaya AN, Pitkin JW, Schaeffer HJ, Ahn JH, Walton JD (1998) EXG1p, a novel exo-β1,3-glucanase from the fungus Cochliobolus carbonum, contains a repeated motif present in other proteins that interact with polysaccharides. Biochimica et Biophysica Acta (BBA) 1425:632–636. doi:10.1016/S0304-4165(98)00117-2
Nisikado Y (1928) Studies on Helminthosporium diseases of graminae in Japan. Sp. Rep.Ohara Inst. Agric.Res. 4:1–384 (in Japanese). English translation in Ber Ohara Inst Landw Forsch 4:111–126
Norse D (1974) Plant diseases in Barbados. Phytopathol Pap 18:1–38
Nyvall RF, Percich JA, Porter RA, Brantner JR (1995) Comparison of fungal brown spot severity to incidence of seedborne Bipolaris oryzae and B. sorokiniana and infect floral sites on cultivated wild rice. Pl Dis 79:249–250
Orieux L, Felix S (1968) List of plant diseases in Mauritius. Phytopathol Pap 7:1–48
Ostazeski SA (1959) A Curvularia leaf spot of Alyce clover. Pl Dis Reporter 43:350–351
Ou SH (1985) Rice diseases, 2nd edn. CAB International, Great Britain
Page RD (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Panaccione DG, Craig JSS, Pocard JA, Walton J (1992) A cyclic peptide synthetase gene required for pathogenicity of the fungus Cochliobolus carbonum on maize. Proc Nadl Acad Sci U S A 89:6590–6594
Pantidou ME (1973) Fungus-host index for Greece. Benaki Phytopathological Institute, Kiphissia
Parmelee JA (1956) Identification of the Curvualria parasite on gladiolus. Mycologia 48:557–567
Parris GK (1959) A revised host index of Mississippi plant diseases. Mississippi State Univ. Bot Dept Misc Publ 1:1–146
Patil SD, Ramesh C (1986) Some loculoascomycetes from Maharashtra (India). Indian Bot Reporter 5:27–31
Paul R, Parbery DG (1966) The perfect stage of Helminthosporium bicolor. Trans Br Mycol Soc 49:385–386
Paz MAGD, Goodwin PH, Raymundo AK, Ardales EY, Vera Cruz CM (2006) Phylogenetic analysis based on ITS sequences and conditions affecting the type of conidial germination of Bipolaris oryzae. Pl Pathol 55:756–765
Paz Z, Zelazowska MK, Druzhinina IS, Aveskamp MM, Shnaiderman A, Aluma Y, Carmeli S, Ilan M, Yarden O (2010) Diversity and potential antifungal properties of fungi associated with a Mediterranean sponge. Fungal Divers 42:17–26
Percich JA, Nickleson LJ (1982) Evaluation of several fungicides and adjuvant materials for control of brown spot of wild rice. Pl Dis 66:1001–1003
Peterson MT, Balbalian CJ (2010) First report of Bipolaris oryzae causing leaf spot of switchgrass in Mississippi. Pl Dis 94:643
Peregrine WTH, Siddiqi MA (1972) A revised and annotated list of plant diseases in Malawi. Phytopathol Pap 16:1–51
Peregrine WTH, Ahmad KB (1982) Brunei: a first annotated list of plant diseases and associated organisms. Phytopathol Pap 27:1–87
Pennycook SR (1989) Plant diseases recorded in New Zealand. Pl Dis Div, D.S.I.R., Auckland
Piepenbring M (2006) Checklist of fungi in Panama. Preliminary version. Puente Biol 1:1–190
Pitt JI, Hocking AD (1995) Current knowledge of fungi and mycotoxins associated with food commodities in Southeast Asia. Mycotoxin Contamination in Grains. Papers presented at the 17th ASEAN Technical Seminar on Grain Postharvest Technology: 5–11
Pirozynski KA (1972) Microfungi of Tanzania. I. Miscellaneous fungi on oil palm. Mycol Pap 129:1–39
Phillips DJ, Mackey B, Ellis W, Hansen TN (1979) Occurrence and interaction of Aspergillus flavus with other fungi on almonds. Phytopathology 69:829–831
Photita W, Lumyong S, Lumyong P, McKenzie EHC, Hyde KD (2004) Are some endophytes of Musa acuminata latent pathogens? Fungal Divers 16:131–140
Phuwapraisirisan P, Sawang K, Siripong P, Tip-pyanga S (2007) Anhydrocochlioquinone A, a new antitumor compound from Bipolaris oryzae. Tetrahedron Lett 48:5193–5195
Podkin OV, Chanukvadze RG, Frolova VS (1981) Farmers’ weed control technology for water seeded rice in the Eastern Europe. Proceedlings of the conference on the weed control in rice: 179–183
Pourabdollah S, Ershad D (1997) An investigation on mycoflora of peanut seeds in Iran. Iran J Pl Pathol 33:64–68
Portales JM, Abarca GH, Sierra MA (1995) Species of Bipolaris and Curvularia on leaves of Quercus and liquidambar from the state of Veracruz, Mexico Revista. Mex Micol 11:109–121
Pratt RG (2006) Johnsongrass, yellow foxtail, and broadleaf signalgrass as new hosts for six species of Bipolaris, Curvularia, and Exserohilum pathogenic to Bermudagrass. Pl Dis 90:528
Preston DA (1945) Host index of Oklahoma plant diseases. Oklahoma Agric. Coll. Agric. Exp. Sta. Techn Bull T-21:1–168
Raabe RD, Conners IL, Martinez AP (1981) Checklist of plant diseases in Hawaii. College of Tropical Agriculture and Human Resources, University of Hawaii
Raemaekers RH (1988) Helminthosporium sativum: disease complex on wheat and sources of resistance in Zambia. In: Klatt AR (ed) Wheat production constraints in tropical environments, 175–185
Rao VG (1969) Fungi on citrus from India. Sydowia 23:215–224
Rao PN, Chaudhury R (1964) A new species of Cochliobolus from Hyndrabad- India. Mycopathologia 23(1):38
Raviraja NS (2005) Fungal endophytes in five medicinal plant species from Kudremukh Range, Western Ghats of India. J Basic Microbiol 45:230–235. doi:10.1002/jobm.200410514
Reddy SM (1977/1976) Cochliobolus sitharamii. Indian Phytopath 29(2): 199
Reis ER, Wunschr WA (1984) Sporulation of Cochliobolus sativus on residues of winter crops and its relationship to increase inoculums density in soil. Plant Dis 68:411–412
Reis EM (1991) Integrated disease management—the changing concepts of controlling head blight and spot blotch. In: Saunders DA (ed) Wheat for the Nontraditional Warm Areas: 165–177
Resplandy R, Chevaugeon J, Delassus M, Luc M (1954) Premiere liste annotee de champignons parasites de plantes cultivees en Cote d’Ivoire. Ann Epiphyt 1:1–61
Richardson MJ (1990) An annotated list of seed-borne diseases, 4th edn. International Seed Testing Association, Zurich
Riess H (1854) Neue Kerenpilze. Hedwigia 1:25–28
Rizner TL, Moeller G, Thole HH, Mavric MZ, Adamski J (1999) A novel 17 beta-hydroxysteroid dehydrogenase in the fungus Cochliobolus lunatus: new insights into the evolution of steroid-hormone signaling. Biochem J 337:425–431
Riley EA (1960) A revised list of plant diseases in Tanganyika Territory. Mycol Pap 75:1–42
Roane CW, Starling TM (1958) Miscellaneous notes on small grain diseases in Virginia. Pl Dis Reporter 42:1268–1271
Roane CW, Roane MK (1996) Graminicolous fungi of Virginia: fungi associated with genera aegilops to Digitaria. Virginia J Sci 47:197–224
Roane CW, Roane MK (1997) Graminicolous fungi of Virginia: fungi associated with genera Echinochloa to Zizania. Virginia J Sci 48:11–46
Roane CW (2004) Graminicolous fungi of Virginia: fungi in collections 1995–2003. Virginia J Sci 55:139–157
Roane CW (2009) Graminicolous fungi of Virginia: fungi in collections 2004–2007. Virginia J Sci 60:13–50
Rodriguez M, Gloriner MS (2008) Potential of fungal endophytes from Thalassia testudinum Bank ex K.D. Koenig as producers of bioactive compounds Master thesis University of Puerto Rico, Mayaguez: 92
Rogerson CT (1958) Diseases of grasses in Kansas: 1956–1957. Pl Dis Reporter 42:346–353
Rosen HR (1950) Oat diseases in Arkansas 1948–49. Pl Dis Reporter 34:43–44
Rohwedder JJ, Simmons JL, Colfer H, Gatmaitan B (1979) Disseminated Curvularia lunata infection in a football player. Arch Intern Med 139:940–941
Ruppel EG (1974) Factors effecting the conidial dimensions of a Dreschera species. Mycologia 66:803–807
Saha A, Dasgupta S, Saha D (2001) Discovery of Curvularia eragrostidis on tea (Camellia sinensis (L.) O. Ktze.) leaves from clonal-cutting nurseries in North Bengal. Environ Ecol 19:846–848
Sakayaroj J, Preedanon S, Supaphon O, Jones EBG, Phongpaichit S (2010) Phylogenetic diversity of endophyte assemblages associated with the tropical seagrass Enhalus acoroides in Thailand. Fungal Divers 42:27–45. doi:10.1007/s13225-009-0013-9
Sanchez Marquez S, Bills GF, Acuna LD, Zabalgogeazcoa I (2010) Endophytic mycobiota of leaves and roots of the grass Holcus lanatus. Fungal Divers 41:115–123
Santamaría J, Bayman P (2005) Fungal epiphytes and endophytes of coffee leaves (Coffea arabica). Microb Ecol 50:1–8. doi:10.1007/s00248-004-0002-1
Sarbhoy AK, Lal G, Varshney JL (1971) Fungi of India (1967–71). Navyug Traders, New Delhi
Sawada K (1959) Descriptive catalogue of Taiwan (formosan) fungi. XI. Spec Publ Coll Agr Natl Taiwan Univ 8:1–268
Scheffer RP, Nelson RR (1967) Geographical distribution and prevalence of Helminthosporium victoriae. Pl Dis Reporter 51:110–111
Scheffer RP (1997) The nature of disease in plants. Cambridge University Press, New York
Schoch CL, Crous PW, Groenewald JZ (2009) A class-wide phylogenetic assessment of dothideomycetes. Stud Mycol 64:1–15
Schroeder HW (1964) Grain discoloration in Belle Patna rice. Pl Dis Reporter 48:288–291
Sharma ND, Singh R, Jain AC (1981) Some new fungi recorded on pineapple. Indian Phytopathol 34:245
Shaw DE (1984) Microorganisms in Papua New Guinea. Dept Primary Ind Res Bull 33:1–344
Shenoy BD, Jeewon R, Hyde KD (2007) Impact of DNA sequence-data on the taxonomy of anamorphic fungi. Fungal Divers 26:1–54
Shenoy BD, Jeewon R, Wang H, Amandeep K, Ho WH, Bhat DJ, Crous PW, Hyde KD (2010) Sequence data reveals phylogenetic affinities of fungal anamorphs bahusutrabeeja, diplococcium, natarajania, paliphora, polyschema, rattania and spadicoides. Fungal Divers 44:161–169. doi:10.1007/s13225-010-0059-8
Shi T, Li CP, Li JF, Cai JM, Huang GX (2010) First report of leaf spot caused by Bipolaris setariae on cassava in China. Pl Dis 94:919
Shimizu K, Tanaka T, Peng YL, Tsuda M (1998) Phylogeny of Bipolaris inferred from nucleotide sequences of Brn1, a reductase gene involved in melanin biosynthesis. J Gen Appl Microbiol 44:251–258
Shivas RG (1989) Fungal and bacterial diseases of plants in Western Australia. J Roy Soc W Australia 72:1–62
Shivas RG, Alcorn JL (1996) A checklist of plant pathogenic and other microfungi in the rainforests of the wet tropics of northern Queensland. Australas Plant Pathol 25:158–173
Shoemaker RA (1955) Biology, cytology, and taxonomy of Cochliobolus sativus. Can J Bot 33:562–567
Shoemaker RA (1959) Nomenclature of Drechslera and Bipolaris, grass parasites segregated from Helminthosporium. Can J Bot 37:879–887
Shu-jun J, Sheng Q (2005) The effect of α,β-dehydrocurvularin, a toxin from a bioherbicidal candidate Curvularia eragrostidis, on chloroplast function of Digitaria sanguinalis. Acta Phytopathologica Sinica
Sierra MA (1984) Hifomicetes Demaciaceos de Sierra del Rosario, Cuba. Editorial Academica, Havana
Singh I, Chohan JS (1974) Seed-borne fungi on cowpea (Vigna sinensis). Indian Phytopathol 27:239–240
Singh RA, Misra AP (1979) Some graminicolous species of Helminthosporium occurring in India. Indian J Mycol Pl Pathol 9:262–264
Singh RA, Misra AP, Prakash O (1981) A new host of Drechslera setariae from India. Indian J Mycol Pl Pathol 11:107
Singh SR, Singh NI (1986) Seed mycoflora of broad bean and its control. Indian Phytopathol 39:541–543
Simone GW, Brunk DD (1983) New leaf spot disease of Calathea and Maranta spp. incited by Drechslera setariae. Pl Dis 67:1160–1161
Sivanesan A, Holliday P (1982) Cochliobolus cymbopogonis. CMI Descr Pathog Fungi Bact 726:1–2
Sivanesan A (1984) The bitunicate Ascomycetes and their anamorphs. J. Cramer Vaduz
Sivanesan A (1985) New species of Bipolaris. Trans Brit Mycol Soc 84:403–421
Sivanesan A (1987) Graminicolous species of bipolaris, curvularia, drechslera, exserohilum and their teleomorphs. Mycol 158:1–261
Sivanesan A, Holliday P (1981) Cochliobolus victoriae. CMI Descr Pathog Fungi Bact 703:1–2
Simmonds JH (1966) Host index of plant diseases in Queensland. Queensland Department of Primary Industries, Brisbane: 111
Sobti AK, Bansal RK (1989) A new report of Drechslera leaf spot of groundnut from India. Indian Phytopathol 42:479–480
Sprague R (1950) Diseases of cereals and grasses in North America. Ronald Press Company, New York
Sprague R (1951) Some leaf spot fungi on western Graminae–VI. Mycologia 43:550
Srivastava RN, Gupta JS (1981a) Seed mycoflora of Zinnia from India. Indian Phytopathol 34:159–161
Srivastava RN, Gupta JS (1981b) Seed mycoflora from Indian seed lots of Cosmos bipinnatus and their control. Indian Phytopathol 34:383–385
Stadler M (2011) Importance of secondary metabolites in the xylariaceae as parameters for assessment of their taxonomy, phylogeny, and functional biodiversity. Mycosphere (Inpress)
Stevenson JA (1975) Fungi of Puerto Rico and the American Virgin Islands. Contr Reed Herb 23:743
Stevens FL (1925) Hawaiian fungi. Bernice P Bishop Mus Bull 19:1–189
Stoner WN (1951) A list of some of the plant diseases occurring in Everglades region of Florida during the 1949–1950 season. Pl Dis Reporter 35:170–172
Stone JK, Bacon CW, White Jr (2000) An overview of endophytic microbes: endophytism defined. In: Bacon CW, White JF Jr (eds) Microbial endophytes. Dekker, New York, pp 3–30
Strobel G, Kenfield D, Bunkers G, Sugawara F, Clardy J (1991) Phytotoxins as potential herbicides. Experientia 47:819–826
Subramanian CV, Jain BL (1966) A revision of some graminicolus Helminthospora. Curr Sci 35:352–355
Subramanyam K, Hegde RK, Kulkarni S, Nargund VB (1990) Effect of leaf blight infection caused by Drechslera hawaiiensis Subram and Jain Ex. M.B. Ellis on biochemical constituents of wheat varieties. Curr Res 19:188–189
Sugawara F, Strobel G, Fisher LE, Van Duyme GD, Clardy J (1985) Chemistry bipolaroxin, a selective phytotoxin produced by Bipolaris cynodontis. Proc Natl Acad Sci USA 82:8291–8294
Summers TE, Bowman DH (1953) The cereal rusts and other diseases of small grains in Mississippi. Pl Dis Reporter 37:142–147
Sun GY, Zhang R, Zhou W (2003) Two new records of the genus Curvularia from China. Mycosystema 22:508–509
Sun X, Guo L-D, Hyde KD (2011) Community composition of endophytic fungi in Acer truncatum and their role in decomposition. Fungal Divers 47:85–95
Surridge AKJ, Viljone A, Wehner FC (2002) Fungi associated with banana foliage in South Africa. Mycospharella leaf spot disease of bananas: present status and outlook. Proceedings of the 2nd international work shop on Mycospharella leaf spot disease. San Jose, Costa rica: 99–104
Suryanarayanan TS, Murali TS, Venkatesan G (2002) Occurrence and distribution of fungal endophytes in tropical forests across a rainfall gradient. Can J Bot 80:818–826
Swofford DL (2002) PAUP*. Phylogenetic analysis using parsimony (*and other methods). Sinauer, Sunderland
Tai FL (1979) Sylloge fungorum sinicorum. Sci. Press, Acad. Sin., Peking
Tatum LA (1971) The southern corn leaf blight epidemic. Science 171:1113–1116. doi:10.1126/science.171.3976.1113
Taylor JE, Hyde KD (2003) Microfungi of tropical and temperate palms. Fungal Diversity Press, Hong Kong
Teng SC (1996) Fungi of China. Mycotaxon, Ltd, Ithaca
Teodoro NG (1937) An enumeration of Philippine fungi. Tech Bull Dept Agr Comm Manila 4:1–585
Than PP, Shivas RG, Jeewon R, Pongsupasamit S, Marney TS, Taylor PWJ, Hyde KD (2008) Epitypification and phylogeny of Colletotrichum acutatum J.H. Simmonds. Fungal Divers 28:97–108
Thaung MM (2008) Pathologic and taxonomic analysis of leaf spot and tar spot diseases in a tropical dry to wet monsoon ecosystem of lowland Burma. Australas Pl Pathol 37:180–197
Thongkantha S, Lumyong S, McKenzie EHC, Hyde KD (2008) Fungal saprobes an pathogens occurring on tissues of Dracaena lourieri and Pandanus spp. in Thailand. Fungal Divers 30:149–169
Thompson A, Johnston A (1953) A host list of plant diseases in Malaya. Mycol Pap 52:1–38
Tilley AM, Walker HL (2002) Evaluation of Curvularia intermedia (Cochliobolus intermedius) as a potential microbial herbicide for large crabgrass (Digitaria sanguinalis). Bio Control 25:12–21
Tinline RD (1951) Studies on the perfect stage of Helminthosporium sativum. Can J Botany 29:467–478
Tinline RD, Dickson JG (1958) Cochliobolus sativus. I. Perithecial development and the inheritance of spore colour and mating type. Mycologia 50:697–706
Tisdale WB (1952) What’s new in plant pathology. Pl Dis Reporter 36:208–210
Tokumasu S, Tubaki K, Manoch L (1990) A preliminary list of hyphomycetes isolated from pine leaf litter of Thailand. Rep Tottori Mycol Inst 28:185–190
Tsuda M, Uyama A (1977) Pseudocochliobolus nisikadoi the perfect stage of Bipolaris coicis. Mycologia 69:1109–1120
Tsuda M, Ueyama A (1981) Pseudocochliobolus australiensis, the ascigerous state of Bipolaris australiensis. Mycologia 73:88–96
Tsuda M, Ueyama A (1982) Pseudocochliobolus verruculosus and variability of conidium morphology. Mycologia 74:563–568
Tsuda M, Ueyama A (1985) Two new Pseudocochliobolus and a new species of Curvularia. Trans Mycol Soc Japan 26:321–330
Tsuda M, Ueyama A, Nishihara N (1977, publ. 1978) Pseudocochliobolus nisikadoi, the perfect stage of Helminthosporium coicis. Mycologia 69: 1109–1120
Tsukamoto H, Gohbara M, Tsuda M, Fujimori T (1997) Evaluation of fungal pathogens as biological control agents for the paddy weed, Echinochloa species by drop inoculation. Ann Phytopathol Soc Jpn 63:366–372
Tsukiboshi T, Chung WH, Yoshid S (2005) Cochliobolus heveicola sp. nov. (Bipolaris heveae) causes brown stripe of bermudagrass and Zoysia grass. Mycoscience 46:17–21. doi:10.1007/s10267-004-0204-x
Tsuda M, Ueyama A (1975) Culture condition and the formation of perfect state of Helminthosporium oryzae, Cochliobolus miyabeanus. Ann Phytopathol Soc Jpn 41:447–452
Turner PD (1971) Microorganisms associated with oil palm (Elaeis guineensis Jacq.). Phytopathol Pap 14:1–58
Ueyama A, Tsuda M (1977) Leaf-spot disease fungus of Chikusichloa aquatica, a new host plant of Cochliobolus miyabeanus [imperfect state: Helminthosporium oryzae]. Trans Mycol Soc Jpn 18:245–250
Ullstrup AJ (1972) The impacts of the southern corn leaf blight epidermics of 1970–1971. Annu Rev Phytopathol 10:37. doi:10.1146/annurev.py.10.090172.000345
Urtiaga R (1986) Indice de enfermedades en plantas de Venezuela y Cuba. Impresos en Impresos Nuevo Siglo. S.R.L, Barquisimeto
van Ginkel M, Rajaram S (1998) Breeding for resistance to spot blotch in wheat: global perspective. In: Duveiller E, Dubin HJ, Reeves J, McNab A (eds) Helminthosporium Blights of wheat: spot blotch and tan spot, 162–170
Verma VC, Kharwar RN (2006) Efficacy of neem leaf extract against it’s own fungal endophyte Curvularia lunata. J Agr Tech 2:329–335
Vittal BPR, Dorai M (1994/1995). Studies on litter fungi VIII. Quantitative studies of the mycoflora colonizing Eucalyptus tereticornis Sm. Litter. Kavaka 22/23: 35–41
Von Arx JA, Oliviera DL (1952) The taxonomy of Ophiobolus graminis Sacc. Trans Br Mycol Soc 35:29–33
Von Arx JA, Luttrell ES (1979) Whole fungus ed B. Kendrick. National museum of natural sciences and the Kananaskis foundation. Ottawa 1:260–261
Wakker JH, Went FAFC (1898) De Zickten van het suikerrict op java. Boekhandel en Drukkerij voorheen. Ej Brill. Leiden
Wallbridge A, Pinegar JA (1975) Fungi associated with crown-rot disease of bananas from St. Lucia in the windward islands. Trans Brit Mycol Soc 64:247–254
Wellman FL (1977) Dictionary of tropical American crops and their diseases. Scarecrow Press, Inc, Metuchen
Wells JM, Cole RJ, Cutter HC, Spaldin DH (1981) Curvularia lunata, a new source of cytochalasin B. Appl Environ Microb 41: 967–971 0099-2240/81/040967-05$02.00/0
White JP, Johnson GT (1971) Zinc effects on growth and cynodontin production of Helminthosporium cynodontis. Mycologia 63:548–561
Whiteside JO (1966) A revised list of plant diseases in Rhodesia. Kirkia 5:87–196
Wiehe PO (1948) The plant diseases and fungi recorded from Mauritius. Mycol Pap 24:1–39
Williams TH, Liu PSW (1976) A host list of plant diseases in Sabah, Malaysia. Phytopathol Pap 19:1–67
Wolf FA, Garren KH, Miller JK (1938) Fungi of the Duke Forest and their relation to forest pathology. Bull School Forest Duke Univ 2:1–122
Wolpert TJ, Macko V, Acklin W, Arigoni D (1988) Molecular features affecting the biological activity of the host-selective toxins from Cochliobolus victoriae. Plant Physiol 88:37–41
Worapattamasri TJ, Ninsuwan N, Chuenchit S, Petcharat V (2009) Anamorphs of Cochliobolus on disease plants in Southern. J Agr Tech 5:143–155
Xi PG, Chi PK, Jiang ZD (2000) Identification of the fungal diseases in Pachira macrocarpa. J S China Agr Univ 21:30–32
Xio JZ, Tsuda M, Doke N, Nishimura S (1991) Phytotoxins produced by germination spores of Bipolaris oryzae. Phytopathology 81:58–64
Xu J, Ebada ES, Proksc P (2010) Pestalotiopsis a highly creative genus: chemistry and bioactivity of secondary metabolites. Fungal Divers 44:15–31. doi:10.1007/s13225-010-0055
Yadav BS (1981) Behaviour of Cochliobolus sativus during its infection of barley and wheat leaves. Aust J Bot 29:71–79
Yassin MA, El-Samawaty AR, Bahkali A, Moslem M, Abd-Elsalam KA, Hyde KD (2010) Mycotoxin- producing fungi occurring in sorghum grains from Saudi Arabia. Fungal Divers 44:45–52. doi:10.1007/s13225-010-0058-9
Yun SH, Berbee ML, Yoder OC, Turgeon BG (1999) Evolution of the fungal self-fertile reproductive life style from self-sterile ancestor. Proc Natl Acad Sci 96:5592–5597
Zhang ZB, Burgos NR, Zhang JP, Yu LQ (2007) Biological control agent for rice weeds from protoplast fusion between Curvularia lunata and Helminthosporium Gramineum. Weed Sci 55:599–605. doi:10.1614/WS-07-061.1
Zhang Y, Crous PW, Schoch CL, Hyde KD (2012) Pleosporales. Fungal Divers (in press)
Zhuang W-Y (ed) (2001) Higher fungi of tropical China. Mycotaxon, Ltd, Ithaca
Zhuang W-Y (ed) (2005) Fungi of northwestern China. Mycotaxon, Ltd, Ithaca
Zhu Y, Qiang S (2004) Isolation, pathogenicity and safety of Curvularia eragrostidis Isolate QZ-2000 as a bioherbicide agent for large crabgrass (Digitaria sanguinalis). Biocontrol Sci Tech 14:769–782. doi:10.1080/09583150410001720699
Zhong S, Steffenson BJ (2001) Virulence and molecular diversity in Cochliobolus sativus. Phytopathology 91:469–476
Zillinsky FJ (1983) Common diseases of small grain cereals: a guide to identification. CIMMYT, Mexico
Acknowledgements
This work is financially supported by NSFC 31110103906 and CAS KSCX2-YW-Z-1026 (China) and Global Research Network for Fungal Biology, King Saud University. Dimuthu S. Manamgoda thanks the State Key Laboratory of Mycology, Institute of Microbiology, Chinese Academy of Sciences, Beijing and the Mushroom Research Foundation, Chiang Mai, Thailand for a postgraduate scholarship. The authors thank Roger Shivas (BRIP, Queensland Plant Pathology Herbarium, Australia) for lending specimens and helpful comments on the manuscript. D. Udayanga (Mae Fah Luang University, Thailand) is thanked for assistance.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Manamgoda, D.S., Cai, L., Bahkali, A.H. et al. Cochliobolus: an overview and current status of species. Fungal Diversity 51, 3–42 (2011). https://doi.org/10.1007/s13225-011-0139-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-011-0139-4