Abstract
Exposure to specific germinant can induce germination in dormant bacterial spores converting them into vegetative cells which are metabolically active and fragile. This phenomenon of conversion of spores from one phase to another could be a keynote potential strategy for development of different type of techniques ranging from spore detection to their eradication and spore-based biosensing. To extend this biphasic spore germination-based approach in order to facilitate development of detection systems, mechanistic details of markers that signals the process of germination initiation in bacterial spores are required. These markers possess a high level of specificity for differentiation in germinating and dormant spores. In this line, present review underscores the structural properties, sporulation and germination in bacterial spores, and covers a detailed description on various biomarkers namely absorbance (600 nm), dipicolinic acid (DPA), refractility of spores, nucleic acid, ATP, spore’s heat resistance, and enzymes which could be valuable in perceiving germination in bacterial spores. Assaying germination using these markers can further explore the efficient use of spores in development of detection devices for food and environment safety.
Similar content being viewed by others
Introduction
Bacterial spores are robust and metabolically dormant structures that are produced by a process of sporulation to prevail over harsh and unfavorable climatic conditions of starvation and stress (Nicholson 2002; Thakur et al. 2013; Tehri et al. 2017). Being a survival strategy, spores are well resistant to varying range of temperature and pressure, U.V. radiation, many noxious chemical substances such as hypochlorite, aldehydes, ethylene oxide, and several other extreme environmental conditions (Setlow 1994; Nicholson et al. 2000; Tennen et al. 2000). Spores can remain in dormant form for long time periods; however, they persistently scrutinize their environment for the presence of germinants that trigger germination (Setlow 2003). During initiation of germination, metabolic activities commence resulting in ATP formation, synthesis of RNA, and proteins. Eventually, replication of DNA occurs which in turn results in vegetative cell growth (Setlow 1983; Paidhungat and Setlow 2002).
Various food spoilages and food-borne diseases are caused by some Bacillus and Clostridium spp. spores that have been found very resistant to several killing agents (Setlow 2006; Setlow and Johnson 2007; Coleman et al. 2010). These are liable to cause threatening impacts on human life and have ability to cause great economic loss to food industries. Therefore, detection and eradication of spores are crucial for public health, food, and environment safety. But in recent years, their detection has become a challenging task for regulatory authorities in various developed and developing countries. Various techniques available for elimination of spores which mainly include U.V., heat, high pressure, and other stress treatments, etc. are failed due to dormant and robust nature of spores. This indicates an immense need for new tools in order to combat drawbacks of existing techniques.
Physiological behavior of spores that occurs as a part of their life cycle holds great promise to resolve the constraints of available methods. It is well known that the life of spore-forming bacteria turns around two phases, the dormant and the metabolically active vegetative state, and perhaps, this exclusive biphasic phenomenon of bacterial spores could have great potential to form the basis of development of various techniques ranging from spores eradication, sterilization, and efficacy testing to spore-based biosensing. Development of such techniques based on the use of spores takes the advantage of the process of germination by examining the physiological changes occurring upon addition of endospore-specific dormancy breaking signals (Yung 2008).
Induction of germination in spores by exposure to specific germinant converts them into vegetative cells which are metabolically very active and fragile and can easily be eliminated from food products and environmental samples. Thus germination-based approach could be a potential strategy to facilitate eradication of bacterial spores more efficiently. It is well known that conversion from dormant to vegetative forms takes place when spores sense favorable environmental conditions. Although, the presence or absence of contaminants directly or indirectly affects this conversion indicating the potential of spores for their use as biosensing element.
Thus, the efficient use of spore germination-based approach in order to develop aforementioned sensitive and reliable techniques requires detection of germination in spores that commences within minutes after return of favorable conditions and is a major challenge. The advent of biomarkers has been a revival of interest in practical utility of germination process in bacterial spores. Presented article is the first review that covers description of all type of biomarkers of spore germination such as absorbance, DPA, refractility of spores, nucleic acid, ATP, spore’s heat resistance, and enzymes used to date. Detailed knowledge of these markers can aid to better understanding of machinery of germination process and improvement of existing and emergence of novel spore eradication and spore-based biosensing techniques in order to guarantee food and consumer safety.
Bacterial spores, their structural properties and sporulation
Spores are produced by certain species of Gram-positive and Gram-negative bacteria such as Bacillus spp., Clostridium spp., Sporomusa malonica, Sporomusa ovata to prevail over unfavorable environmental conditions (Nicholson 2002). Being a survival strategy, endospores have very hardy and robust structure comprises of many layers such as spore coat, outer membrane, cortex, germ cell wall, inner membrane, core, and sometimes outer exosporium as depicted in Fig. 1. Core is the innermost layer of intact spores consisting of nucleic acids, i.e., DNA, RNA, small acid soluble proteins associated with DNA, enzymes, Ca-DPA (contributing to 25% dry weight of core), and moisture content around 25–50% of wet weight. Inner membrane surrounding core region is composed of lipids, act as barrier to many undesirable substances such as toxic chemicals. Inner membrane is further surrounded by germ cell wall composed of peptidoglycan (Setlow 2006, 2007; Zhang et al. 2010). Outside the germ cell wall is the cortex region containing peptidoglycan with modified composition as compared to vegetative cells. An outer membrane surrounds this cortex region and plays its unique role in process of spore formation. A spore coat that is of proteinaceous nature further surrounds this outer membrane and protects the spore from the attack of lytic enzymes and other chemical compounds. Most of the species have spore coat as its outermost layer; while in other, coat is further surrounded by an outer layer known as exosporium (Warth and Strominger 1972; Laaberki and Dworkin 2008; Henriques et al. 2007). These unique structural properties of spores well preserve their genetic material and allow them to resist radiations, very low and high range of temperature and pressure, toxic chemical compounds, and several other extreme unfavorable environmental conditions.
The process by which spores are formed under unfavorable environmental condition is known as sporulation. In sporulation, a vegetative cell is divided into two unequal compartments: one small forespore and another large mother cell by the formation of a polar septum. Successively, the mother cell engulfs the forespore, and its cytoplasm constitutes the core of spore (Piggot and Hilbert 2004). Synthesis of core is followed by formation of cortex, a thick wall material around it. Subsequently, a proteinaceous multilayered coat is formed by mother cell on forespore surface. In recent times, an additional outermost layer on spore, known as crust, has been recognized (McKenney et al. 2010). Spore’s outer layers, as also mentioned above under structural properties, provide them mechanical strength and exclude undesirable substances. Eventually, upon completion of sporulation process, mother cell lysis takes place which in turn is followed by liberation of mature spore (Piggot and Hilbert 2004).
Spores germination
Spores are dormant; with their resistant forms still they scrutinize their surroundings for molecules and/or conditions that trigger germination within minutes followed by outgrowth to generate growing vegetative cells (Setlow 2003; Paredes-Sabja et al. 2010; Ross and Abel-Santos 2010; Setlow and Johnson 2012).
Germinants
Germinants are those molecules which trigger germination in spores. They can be classified into two types, i.e., nutrient and non-nutrient. Further, both types of germinants initiate the process of germination by different approaches.
Nutrient germinants
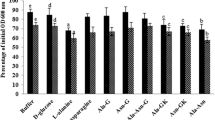
These are the most common type of germinants known to trigger germination in bacterial spores. They are mostly species and strain specific and mainly include sugars, amino acids, purines, and other low molecular weight compounds (Gould 1969; Paredes-Sabja et al. 2010; Tehri et al. 2018). Onset of germination in spores has also been found to be dependent on the stereospecific nature of germinants. For example, l-alanine is the most common form of alanine used as germinant, while d-alanine is often an inhibitor of germination triggered by l-alanine. This unique specificity associated with germinants has been found to be associated with proteins, called as germinant receptors that bind to germinants. In addition to nutrient germinants, some monovalent cations like K+ that alone can trigger germination can also act as obligatory co-germinants for nutrient germinants (Setlow 2013; Tehri et al. 2017).
Non-nutrient germinants
Apart from nutrient germinants, several other physical and chemical agents such as calcium dipicolinate (CaDPA) (at increased concentrations), alkylamines, and high pressure are also known to initiate the process of germination in bacterial spores. However, the mechanism they use to stimulate germination is extremely different from nutrient type of germinants (Setlow 2013). Mechanism for onset of germination in spores varies depending on the type and level of non-nutrient means of germination. Involvement of germinant receptors in triggering germination has been observed at 100 MPa, however not at 600 MPa. On the other hand, pressure of 600 MPa leads to the release of CaDPA from spore’s core which itself possess germinant properties and therefore not involving germinant receptors (Wuytack et al. 2000; Black et al. 2005; Moir 2006). The spores have also been found germinated in response to heat activation (Luu et al. 2015). Interestingly, germination can also be initiated upon exposure to glycine and certain bile acids, e.g., taurocholate. Spores of Clostridium difficile have been reported to interact with bile acids along the gastrointestinal tract in such a way that spores use a host-derived signal to initiate germination (Wilson 1983; Weese et al. 2000; Sorg and Sonenshein 2010).
Germinant receptors
Germinant receptors, termed as GRs, are the proteins that bind germinants (Setlow 2013). Each GR can bind a specific germinant, i.e., sugars, amino acids, and cations, and thus provides nutrient germinant specificity to bacterial spores. However, structurally distinct biomolecules are responded by spores by expression of multiple GRs. Moreover, a unique set of GRs is encoded by each spore-forming bacterial species for recognizing different types of germinants. Remarkably, a single or multiple germinants can also be responded by cooperation of diverse range of GRs. Under forespore-specific transcription factor, i.e., σG regulation, polycistronic operons are mainly responsible for expression of GRs only within late sporulation phase (Ross and Abel-Santos 2010; Madslien et al. 2014). Each type of GRs is found with only about ten molecules in the inner membrane of per spore and is therefore less abundance protein (Paredes-Sabja et al. 2010). Some of the major GRs involved in germination of Bacillus and Clostridium species are listed in Table 1.0. This reveals importance of GRs as key player in the process of spore germination. On the other hand, an impaired response to recognize germinants has been observed in spores lacking GRs (Hornstra et al. 2006; Barlass et al. 2002).
Steps involved in spore germination
Interactions between germinant and GRs play as key role in triggering of germination. Upon binding to specific germinant-like amino acid, inorganic salts, and sugars, GRs are activated and trigger spore germination irreversibly and therefore known as commitment step. Subsequent to this step, monovalent cations along with pyridine-2,6-dicarboxylic acid (dipicolinic acid, DPA) and divalent cations, especially Ca2+, in chelated form, i.e., CaDPA are released from spore core. This is followed by entry of water inside core region to initiate the process of rehydration. Further, release of CaDPA in Bacillus spores has also been found to contribute to peptidoglycan hydrolysis in spore cortex due to activating any of the two types, i.e., CwlJ and SleB of cortex lytic enzymes (CLEs). Subsequently, hydrolysis of spore cortex permits the core region for expansion and rehydration, which in turn activates enzymes and hence initiate the metabolic activities in spore core (Moir et al. 2002; Yi and Setlow 2010). During the phase of outgrowth, synthesis of RNA, proteins, and DNA recommences and thus results in conversion of spore to vegetative cell (Gupta et al. 2013). Under optimal conditions, this process occurs very rapidly, most probably within minutes (Carr et al. 2010). Several factors such as medium composition, temperature, pH, germinant type and concentration, and heat treatment are known to influence the rate and thus time of spore germination (Caipo et al. 2002). Further enhancement of germination, i.e., activation has been found to be associated with additional treatments like type of time-temperature combination, certain chemicals, etc. (Foster and Johnstone 1990). However, the mechanisms underlying in activation have not been clearly understood.
Biomarkers for detection of spore germination
Germination is a process that can be measured and detected in various ways. A number of physical, biological, and chemical changes occur in germinating spores at the time of transition from dormant to vegetative cells. As germination is an irreversible event, therefore, germination can be detected by the use of different types of markers also known as biomarkers or signature markers of germination process which can directly correlate the changes in the aforementioned properties, occurring during germination, to the process of germination (Stewart et al. 1981; Yi and Setlow 2010; Katherine 2010). To date, various such markers have successfully been used as unique tools to assess germination in bacterial spores and are mainly of seven different types as depicted in Fig. 2. Detailed description of each of the seven potential markers of spore germination is explained below.
Dipicolinic acid
The core region of spores contains low water content, i.e., 25–50% of wet weight and large quantity of DPA, i.e., dipicolinic acid (~ 10% dry weight of spore) which is unique to spores, as well as huge amount of divalent cations. DPA in spores is normally found in chelated form with divalent cations chiefly with Ca2+, i.e., CaDPA (Paidhungat et al. 2000; Gerhardt and Marquis 1989). Germinant binding to its respective receptor site triggers germination with concomitant change in inner membrane permeability allowing the release of monovalent cations, i.e., K+, Na+, and H+ with subsequent release of CaDPA (Setlow 2013; Swerdlow et al. 1981; Peng et al. 2009; Cabrera-Martinez et al. 2003). Multiple spore-specific proteins, i.e., SpoVA, are probable candidates known to form channels in membrane of spore to allow DPA release from the core region (Setlow 2014; Cabrera-Martinez et al. 2003). Thus, release of DPA is early event in the process of germination and therefore possesses great potential for its use as one of the best biomarker to detect germination in dormant spores. To date, several methods have been also developed to assay DPA released during the process of germination. The most commonly used and highly sensitive method for detection of DPA is Tb3+ luminescence assay. This assay works on the principle of transfer of excitation energy from DPA to bound Tb3+ (Clear et al. 2013). Another DPA assay namely dual luminescent and colorimetric assay based on the concept of dye displacement has been also developed by Clear and co-workers. Recently, a highly sensitive and selective DPA assay employing ratiometric colorimetric and fluorescent dual probe based on Eriochrome Black T (EBT)–Eu3+ complex has been designed. The assay works on the principle of formation of magenta colored complex between Eu3+ ions and EBT that upon the addition of DPA changed to blue immediately. The performance of developed assay was evaluated using spores of Geobacillus stearothermophilus that resulted in detection of as low as 2.5 × 105 spores in the test sample (Yilmaz and Oktem 2018).
DPA-based methods are highly reliable and known traditionally for their use to assay germination in spores. However, studies exist that have reported the presence of DPA-less spores of Bacilli and Clostridia spp. An asporogenous phenotype of Bacillus subtilis resulting from the loss of any SpoVA protein (except SpoVAEa or SpoVAF) has been observed where DPA was synthesized normally in sporulation, but it was not taken up and the unstable DPA-less spores germinated in the sporangium and lyse (Errington 1993; Setlow 2013). Another study carried out with Clostridium perfringens DspoVA showed no DPA accumulation in spores; however, DPA-less spores were stable (Paredes-Sabja et al. 2008; Setlow 2013). This raises a question on the applicability of DPA as a marker for germination of spores from different spore formers.
Absorbance at 600 nm
OD600 is the optical density, or light absorbance, at 600 nm, and describes transmission of light at this wavelength through a solution or object (Kristin 2012). In germinating spores, OD600 measurement, i.e., maximum rate of fall in OD600, is a simple and reliable way that is used to study kinetic analysis of germination and commitment, and to monitor different spore populations in terms of comparing and quantitating germination rates, at particular concentration of germinant (Powell 1950; Paidhungat and Setlow 2002; Løvdal et al. 2012; Cabrera-Martinez et al. 2003). The absorbance loss reflects the change (decrease) in refractive index of core in multiple spores present in a suspension, following germination steps occurring well after commitment step such as Ca-DPA release, core swelling, and rehydration which accompanies hydrolysis of cortex causing loss in OD600 (Ghosh 2013), and therefore is not the earliest event in the spore germination process (Yi and Setlow 2010; Løvdal et al. 2012). However, during spore germination, significant OD600 loss can occur even when there is no hydrolysis of cortex; therefore, when this assay is used, it is essential always to use either flow cytometry or phase contrast microscopy to study degree of spore germination completion (Ghosh 2013). Thus, at a particular germinant concentration, rate of spore germination is calculated from maximum rate in OD600 fall versus time which is represented graphically and measured from the maximum slope of OD600 vs. time plot (Cabrera-Martinez et al. 2003). OD600 is usually determined after every 10 min. to capture the typical refractility loss during initial phase, i.e., in first few minutes of spore germination (McCormick 1965; Katherine 2010). Studying germination in populations by use of OD600 is also associated with some disadvantages. In a population, all the spores are not identical and will respond differently to germinants and germinant environments. In a population, only a germination tendency can be studied in the population as a whole, with a probability of each spore germinating within a given time. In populations with low germination, OD600 measurements may not even show the response. Spores can also clot together or adhere to the surfaces in the testing container where OD600 is measured, thereby complicating measurements and interpretation of these. When germination exceeds 90%, it is also difficult to show differences between populations in the assay. In other words, OD600 is a good biomarker for observing germination in populations which show a relatively good germination response. When comparing different strains, the response should differ at least 10–20% for this method of observation to be effective. Additionally, other methods such as phase contrast microscopy and DPA release can also be used as a means of control when studying germination by measuring OD600 in a population. During germination conversion of the spores from bright to dark phase translates an OD600 loss of up to 60% of the original OD600 for a spore population (Kristin 2012). Nearly, 70% loss in OD600 has also been found when DPA release occurs during spore germination (Cabrera-Martinez et al. 2003).
Refractility of spores
Loss of refractility in spores occurs due to the influx of water, swelling of cell, and excretion of dry matter during their germination. This change in refractility of germinating spores can be easily studied by phase contrast microscopy using an oil-immersion objective with × 60 or × 100 magnifications. Water uptake in spores occurring during germination in terms of refractility loss is observed as transition from phase bright dormant spores to phase dark germinating spores under phase contrast microscopy as shown in Fig. 3 (Tehri 2015). The degree of spore refractility can be correlated to the course of germination, which follows biphasic kinetics. In the first phase, spores change into partial phase dark and lose part of the heat resistance. The proteinaceous coat becomes more porous, leading to water hydrolysis and release of calcium dipicolinate from the spore core. In second phase, a complete hydration of the core and degradation of the cortex in spore take place, which render the spore phase dark. Phase contrast microscopy has successfully been applied to observe germination in both Bacillus and Clostridium spores. This can be carried out using a normal microscope slide-cover slip setup or on solidified agar covered with a cover slip. Acid popping of spores is another method of choice to study germination in bacterial spores using this technique at laboratory level. In this method, strong acid is passed through the spore suspension in a fixed microscopic mount allowing the spores to rehydrate and lose their high refractility and turning them immediately from bright to dark. An inverse relationship has been reported between the spore inoculum size and germination time. Several factors such as species, inoculum size, germinants, temperature, and the optics used for observation are known to affect the duration of germination. The duration of phase transition for individual bacterial spores ranges from 75 s to approximately an hour. Use of phase contrast microscopy is also advantageous in studying the process of germination at single spore level. Recently, this technique has been used to study the live imaging of germination in individual Bacillus subtilis spores. For this, a novel phase contrast microscopy-based closed air containing chamber was used to analyze B. subtilis spore germination, outgrowth, and subsequent vegetative growth. A program named Spore Tracker was used for image analysis which allowed automated data processing from germination to outgrowth and vegetative cell doubling (Pandey et al. 2013). Limitations inherent with the use of phase contrast technique is that lipid inclusions may be mistakenly assigned as spores because they are phase bright and are about micron size. Still, phase transition observed under the microscope provides a strong evidence for spore germination. When used in conjunction with other markers of spore germination, phase contrast microscopy proves to be a very useful validation test and provides total and germinable counts of spore suspensions (Yung 2008).
Enzymes
In the course of germination, a series of complex biophysical processes occur upon activation of germinant receptors. As a result of which, several intracellular proteases and extracellular hydrolases that facilitate cellular differentiation to the vegetative form are activated (Fisher and Hanna 2005; Ferencko et al. 2004). Perhaps, these enzyme released during germination process possesses great potential for their use as marker in order to detect onset of germination in spore formers. Recently, activation of esterases has been used as a successful parameter to quantitatively measure the process of germination in Bacillus spores. Expression of esterases during germination comes under the umbrella of biochemical events presumably related to molecular mechanisms associated with germinant-mediated activation of silent hydrolytic enzyme(s). This enzyme has been used as a marker to detect germination in several species of Bacilli. To measure the activity of this enzyme, a fluorogenic substrate named diacetyl fluorescein (DAF), an acetylated derivative of the green fluorescent dye fluorescein, has been used. In DAF, dye remains in its non-fluorescent state as acetyl groups are attached on the xanthene group (Boyd et al. 2008). Once spore undergoes germination, DAF is hydrolyzed non-specifically by esterases, lipases, and proteases and converted to deacetylated form. The main product of the reaction is fluorescein, i.e., deacetylated form of molecule, a highly fluorescent compound and by-products include acetic acid/acetaldehyde (Thakur et al. 2013). Thus, fluorescent compound DAF is a cell viability stain which is cleaved effectively by germinating spores as compared to dormant spores. DAF has been used to measure activity of esterase which has been proved as a successful marker to study the onset of germination in spores of Bacillus anthracis. The expression of esterase in this work was also reported as an early event occurring consistently under conditions known to date to induce germination in spores. The findings of this work based on the use of esterase as biochemical marker of spore germination were also validated by measurement of refractility using phase contrast microscopy (Ferencko et al. 2004). The similar principle based on the use of enzymes and DAF has been used to develop a simple and rapid technique for detection of germination in Bacillus megaterium spores. The spores were allowed to germinate in the presence of nutrient germinant-like d-dextrose, and fluorescent signal was captured using EMCCD that allowed automated data processing in terms of detection of distinct fluorescent pattern for dormant and germinating spores (Thakur et al. 2013). Furthermore, 2,3,5-triphenyltetrazolium chloride (Tzm) has also been used to assay the activity of enzymes possessing the potential to use as markers upon onset of germination in spores. Colorless form of Tzm is reduced to a red formazon derivative by an array of enzymes released during germination of spores. Thus, use of enzymes as marker is a convenient way to measure germination signal in spores and also advantageous over other existing markers. As measurement of germination, using enzymes neither require vegetative growth nor it suffer from spore clumping which can interfere to some extent in O.D. measurements (Gupta et al. 2013).
Spore’s heat resistance
The core of bacterial spore contains less amount of water. Large peptidoglycan cortex that surrounds the spore inner membrane and core restricts the uptake of water in the spore core. Secondly, the presence of huge amount of CaDPA displaces significant amounts of water from the maturing spore core. This low water content of spore’s core is the major factor known to contribute heat resistance to bacterial spores. The process of germination in bacterial spores leads to loss of heat resistance. During germination of B. megaterium and B. subtilis spores, the loss of heat resistance has been reported to precede CaDPA release and occurs essentially in parallel with commitment (Luu and Setlow 2014). Thus, loss of heat resistance is a potential marker to assay the extent of germination in bacterial spores exposed to either nutrient or non-nutrient germinant. Germination of B. anthracis in the presence of nutrient germinants was studied by determination of loss of heat resistance. For this, spore samples that had been exposed for various periods of time to germinant were incubated at 65 °C for 30 min. The samples were then rapidly chilled on ice, diluted, and spread on trypticase soy agar plates to determine viable counts (Levinson and Hyatt 1966; Welkos et al. 2001). The percentage of survival was determined by comparing the colony count of each germinant-exposed sample to that of the sample collected after exposure to germinant (Welkos et al. 2004).
Stainability of nucleic acid
Both intact dormant and decoated dormant spores that have lost their outer membrane and much of their coat show poor stainability because of their complex structural properties and therefore do not easily take up and bind many types of reagents particularly nucleic acid dyes, as nucleic acid of dormant spores remains in the central core region and is not readily assessed and penetrated by binding dyes (Gould 1969; Setlow et al. 2002; Magge et al. 2009; Ragkousi et al. 2000; Black et al. 2005). On the other hand, spores upon germination convert into vegetative cells and are easily get stained using similar dyes. Thus, binding of nucleic acid with its specific dyes shows great potential for its use as significant marker for detection of germination induced in dormant spores under favorable conditions. However, staining of nucleic acids by dyes has been found to occur after the loss of most spore heat resistance and thus, the germination step that is measured by this marker would not be expected to be an early event (Setlow et al. 2002; Welkos et al. 2004). Work has been carried out with various dyes such as Syto 9, Syto 11, Syto 12, Syto 13, Syto 14, Syto 15, and Syto 16 to study staining pattern of dormant and germinating spores of B. subtilis. Among all dyes, Syto 16 was reported to give more distinct separation in fluorescence between dormant and germinated spores using flow cytometer (Black et al. 2005). Kong et al. (2010) studied the kinetics of uptake of this dye during germination of Bacillus cereus and Bacillus subtilis spores using a methodology that combines fluorescence microscopy, phase contrast microscopy, and laser tweezers Raman spectroscopy. The findings of this work showed that the uptake of this dye will occur following release of CaDPA and degradation of DNA binding proteins. Degradation of spore cortex by CLEs also reported to play a crucial role in uptake of Syto 16. Significant findings obtained when this dye, i.e., Syto 16, has been used to study germination induced under different conditions in dormant spores of various species of Bacillus. Spores of B. subtilis when germinated by a pressure of 150 MPa have shown strong stainability with Syto 16 using flow cytometry (Black et al. 2005). A dye named Syto 9 has also been used to study the process of germination in B. anthracis spores induced by two different combinations of nutrient germinants. Germination was detected in terms of change in fluorescence (RFU) upon addition of Syto 9 with both combinations of germinants comprising of l-alanine, inosine and l-alanine, adenosine, and casamino acids. The findings of this Syto 9-based assay were further validated with other established procedures. As a result, a significant level of correlation was obtained between germination as measured by different methods, i.e., increase in RFU, increase in percent of phase dark spores, and decline in heat resistance of spores (Welkos et al. 2004).
Impact of different treatments used for killing of spores could have drastic impacts in some spore permeability barrier and in turn, changes should be reflected in staining of core region by nucleic acid binding agents. One such study was carried out by using two different dyes namely DAPI and acridine orange, when killing of spores was observed by acid or ethanol but not by alkali. A peripheral staining of untreated spores, i.e., of many external layers but neither of coat nor core, observed with these dyes. However, after onset of germination, the peripheral staining in dormant spores disappeared in 20 min. and was replaced by an intense staining of the germinated spore core, (Gould 1969; Ragkousi et al. 2000). Thus, suggesting that the peripheral staining in dormant spores is due to the staining of cortex region, which is hydrolyzed in the first minutes of germination. Staining of spores treated with NaOH was found similar to staining untreated dormant spores. On the other hand, spores treated with both ethanol and acid showed staining of the core region (Setlow et al. 2002). Thus, as a marker, stainability of spores offers significant advantages such as easy and rapid mode to detect the process of germination in bacterial spore.
ATP
ATP is found in all living microorganisms, and therefore, it has been used as an outstanding marker for detection of cell viability. ATP bioluminescence assays based on luciferine/luciferase reaction have been developed to detect various microorganisms. The working principle of bioluminescence assay is based on the oxidation of luciferine in the presence of ATP by luciferase with simultaneous production of enzyme-bound luciferil adenylate complex. This complex is further oxidized to oxyluciferin that is converted from excited to stationary state resulting in the emission of light due to rapid loss of energy of this molecule (Fig. 4). In this reaction, photons are emitted with quantum yield of about 90% (Seliger 1989; Wilson and Hastings 1998; Chollet and Ribault 2012). Similar approach of bioluminescence employing ATP as marker has also been extended for detection of the process of germination in bacterial spores. The amount of ATP is very low in dormant spores, and germination is a required process to increase the content of this molecule (Kodaka et al. 1996; Chollet and Ribault 2012). Most of the nucleotides in dormant form of spores remain stored in the form of 3-phosphoglyceric acid. Thus, less than 1% of the adenine nucleotide pool in dormant spores is ATP. On the other hand, spores undergoing germination and converting into vegetative cells account for 80% of ATP. This is because upon onset of germination, the stored 3-phosphoglyceric acid is catabolized into ATP, and increase in porosity of spore coat permits an easy extraction of intracellular pool of ATP (Santo and Doi 1974; Singh et al. 1977). Increase in light intensity by germinating spores of Bacillus in ATP bioluminescence assays has been reported in various works. Germination in spores of B. subtilis incubated in nutrient broth containing l-alanine for 30 min. have been observed in terms of increased RLU of spores (Fujinami et al. 2004; Chollet and Ribault 2012). Increase of ATP content was also reported during nutrient-induced germination in anaerobic Clostridium spores (Hausenbauer et al. 1977). A rapid production of ATP in spores of B. subtilis upon germination by a pressure of 100 MPa was observed, but no ATP produced during germination induced at 600 MPa (Wuytack et al. 1998; Yung 2008). Until today, ATP assays have mainly been used for detection and enumeration of vegetative cells of pathogenic bacteria for food quality control and hygiene testing. But because of aforementioned distinctive properties of dormant and germinating spores in relation to ATP and earlier studies, ATP-based assay could also find immense application in detection of the process of germination. Bioluminescence assay based on measurement of ATP for detection purpose offers several advantages in terms of high sensitivity and rapidity. However, in some cases this assay can suffer from the problem associated with the presence of substances in some food samples which can pose inhibitory effect or interfere with luciferase activity (Leach and Webster 1986).
Conclusions
Spore-based sensing technologies are ideal tools for the detection of microbial and non-microbial contaminants in order to assure quality of various types of food and environmental samples. They offer several advantages like high sensitivity, selectivity, easy measurements, and low cost, high throughput analysis and are able to resolve problems encountered by conventional methods. Use of spores for development of such sensors requires a detailed understanding of appropriate markers that can be used to detect the process of germination. Keeping in view, the major objective of presented article is to highlight the scope of various biomarkers (absorbance, DPA, refractility of spores, nucleic acid, ATP, spore’s heat resistance, and enzymes) that have the potential to assay germination in dormant spores. The better understanding of germination markers will be helpful for development of new as well as improvement of developed spore sensors which in turn can lead to their faster commercialization by transferring them from lab to industry. Moreover, knowledge of markers can immensely contribute also towards understanding of spore germination process and in development of novel spore eradication techniques in order to guarantee food and consumer safety.
References
Barlass PJ, Houston CW, Clements MO, Moir A (2002) Germination of Bacillus cereus spores in response to L-alanine and to inosine: the roles of gerL and gerQ operons. Microbiology 148:2089–2095
Bhattacharjee D, McAllister KN, Joseph A, Sorg JA (2016) Germinants and their receptors in Clostridia. J Bacteriol 198:2767–2775
Black EP, Koziol-Dube K, Guan DS, Wei H, Setlow B, Cortezzo DE, Hoover DG, Setlow P (2005) Factors influencing germination of Bacillus subtilis spores via activation of nutrient receptors by high pressure. Appl Environ Microbiol 71:5879–5887
Boyd V, Cholewa OM, Papas KK (2008) Limitations in the use of fluorescein diacetate/propidium iodide (FDA/PI) and cell permeable nucleic acid stains for viability measurements of isolated islets of Langerhans. Curr Trends Biotechnol Pharm 2:66
Broussolle V, Alberto F, Shearman CA, Mason DR, Botella L, Nguyen-The C, Peck MW, Carlin F (2002) Molecular and physiological characterisation of spore germination in Clostridium botulinum and C. sporogenes. Anaerobe 8:89–100
Cabrera-Martinez RM, Tovar-Rojo F, Vepachedu VR, Setlow P (2003) Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis. J Bacteriol 185:2457–2464
Caipo ML, Duffy S, Zhao L, Schaffner DW (2002) Bacillus megaterium spore germination is influenced by inoculum size. J Appl Microbiol 92:879–884
Carr KA, Lybarger SR, Anderson EC, Janes BK, Hanna PC (2010) The role of Bacillus anthracis germinant receptors in germination and virulence. Mol Microbiol 75:365–375
Chollet R, Ribault S (2012) Use of ATP bioluminescence for rapid detection and enumeration of contaminants: the Milliflex rapid microbiology detection and enumeration system. In: Lapota D (ed) Bioluminescence. IntechOpen. https://doi.org/10.5772/37055
Christie G, Lowe CR (2007) Role of chromosomal and plasmid-borne receptor homologues in the response of Bacillus megaterium QM B1551 spores to germinants. J Bacteriol 189:4375–4383
Clear KJ, Sarah Stroud S, Smith BD (2013) Dual colorimetric and luminescent assay for dipicolinate, a biomarker of bacterial spores. Analyst 138:1–9
Coleman WH, Zhang P, Yq L, Setlow P (2010) Mechanism of killing of spores of Bacillus cereus and Bacillus megaterium by wet heat. Lett Appl Microbiol 50:507–514
Errington J (1993) Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev 57:1–33
Ferencko L, Cote MA, Rotman B (2004) Esterase activity as a novel parameter of spore germination in Bacillus anthracis. Biochem Biophys Res Commun 319:854–858
Fisher N, Hanna P (2005) Characterization of Bacillus anthracis germinant receptors in vitro. J Bacteriol 187:8055–8062
Foster SJ, Johnstone K (1990) Pulling the trigger: the mechanism of bacterial spore germination. Mol Microbiol 4:137–141
Fujinami Y, Kataoka M, Matsushita K, Sekigushi H, Itoi T, Tsuge K, Seto Y (2004) Sensitive detection of bacteria and spores using a portable bioluminescence ATP measurement. Assay System Distinguishing from White Powder Materials. J Health Sci 50:126–132
Gerhardt P, Marquis RE (1989) Spore thermoresistance mechanisms. In: Smith I, Slepecky RA, Setlow P (eds) Regulation of prokaryotic development: structural and functional analysis of bacterial sporulation and germination. American Society for Microbiology, Washington
Ghosh S (2013) Understanding the mechanism of Bacillus subtilis spore germination. Doctoral Thesis. Submitted to University of Connecticut Graduate School
Gould GW (1969) Germination. In: Gould GW, Hurst A (eds) The bacterial spore. Academic Press, London
Griffiths KK, Zhang J, Cowan AE, Yu J, Setlow P (2011) Germination proteins in the inner membrane of dormant Bacillus subtilis spores colocalize in a discrete cluster. Mol Microbiol 81:1061–1077
Gupta S, U¨stok FI, Johnson CL, DMD B, Lowe CR, Christie G (2013) Investigating the functional hierarchy of Bacillus megaterium PV361 spore germinant receptors. J Bacteriol 195:3045–3053
Hausenbauer JM, Waites WM, Setlow P (1977) Biochemical properties of Clostridium bifermentans spores. J Bacteriol 129:1148–1150
Henriques AO, Moran J, Charles P (2007) Structure, assembly, and function of the spore surface layers. Annu Rev Microbiol 61:555–588
Hornstra LM, de Vries YP, Wells-Bennik MHJ, de Vos WM, Abee T (2006) Characterization of germination receptors of Bacillus cereus ATCC 14579. Appl Environ Microbiol 72:44–53
Katherine AC (2010) Early events of spore germination and their role in Bacillus anthracis virulence. Doctoral Thesis.University of Michigan
Kodaka H, Fukuda K, Mizuochi S, Horigome K (1996) Adenosine triphosphate content of microorganisms related with food spoilage. Jpn J Food Microbiol 13:29–34
Kong L, Zhang P, Yu J, Setlow P, Li Y (2010) Monitoring the kinetics of uptake of a nucleic acid dye during the germination of single spores of Bacillus species. Anal Chem 82:8717–8724
Kristin CSR (2012) Diversity in germination response among Bacillus licheniformis strains. Master Thesis. Submitted to Norwegian University of Life Sciences
Laaberki MH, Dworkin J (2008) Role of spore coat proteins in the resistance of Bacillus subtilis spores to Caenorhabditis elegans predation. J Bacteriol 190:6197–6203
Leach FR, Webster JJ (1986) Commercially available firefly luciferase reagents. Methods Enzymol 133:51–70
Levinson HS, Hyatt MT (1966) Sequence of events during Bacillus megaterium spore germination. J Bacteriol 91:811–818
Li Y, Ma X, Zhao M, Qi P, Zhong J (2014) Quick and label-free detection for coumaphos by using surface plasmon resonance biochip. PLoS One 9:1–7
Løvdal IS, From C, Madslien EH, Romundset KCS, Klufterud E, Rosnes JT, Granum PE (2012) Role of the gerA operon in L-alanine germination of Bacillus licheniformis spores. BMC Microbiol 34:1–12
Luu S, Setlow P (2014) Analysis of the loss in heat and acid resistance during germination of spores of Bacillus species. J Bacteriol 196:1733–1740
Luu S, Cruz-Mora J, Setlow B, Feeherry FE, Doona CJ, Setlow P (2015) The effects of heat activation on Bacillus spore germination, with nutrients or under high pressure, with or without various germination proteins. Appl Environ Microbiol 81:2927–2938
Madslien EH, Granum PE, Blatny JM, Lindbäck T (2014) L-alanine-induced germination in Bacillus licheniformis the impact of native gerA sequences. BMC Microbiol 14:101–110
Magge A, Setlow B, Cowan AE, Setlow P (2009) Analysis of dye binding by and membrane potential in spores of Bacillus species. J Appl Microbiol 106:814–824
McCormick NG (1965) Kinetics of spore germination. J Bacteriol 89:1180–1185
McKenney PT, Driks A, Eskandarian HA, Grabowski P, Guberman J, Wang KH, Gitai Z, Eichenberger P (2010) A distance-weighted interaction map reveals a previously uncharacterized layer of the Bacillus subtilis spore coat. Curr Biol 20:934–938
Moir A (2006) How do spores germinate? J Appl Microbiol 101:526–530
Moir A, Corfe BM, Behravan J (2002) Spore germination. Cell Mol Life Sci 59:403–409
Nicholson W (2002) Roles of Bacillus endospores in the environment. Cell Mol Life Sci 59:410–416
Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P (2000) Resistance of Bacillus endospores to extreme terrestrial and extra-terrestrial environments. Microbiol Mol Biol Rev 64:548–572
Paidhungat M, Setlow P (2002) Spore germination and outgrowth. In: Sonenshein AL, Hoch JA, Losick R (eds) Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington
Paidhungat M, Setlow B, Driks A, Setlow P (2000) Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J Bacteriol 182:5505–5512
Pandey R, Ter Beek A, Vischer NO, Smelt JP, Brul S, Manders EM (2013) Live cell imaging of germination and outgrowth of individual Bacillus subtilis spores; the effect of heat stress quantitatively analyzed with SporeTracker. PLoS One 8:e58972
Paredes-Sabja D, Setlow B, Setlow P, Sarker MR (2008) Characterization of Clostridium perfringens spores that lack SpoVA proteins and dipicolinic acid. J Bacteriol 190:4648–4659
Paredes-Sabja D, Setlow P, Sarker MR (2010) Germination of spores of Bacillales and Clostridiales species: mechanisms and proteins involved. Trends Microbiol 19:85–94
Peng L, Chen D, Setlow P, Y-q L (2009) Elastic and inelastic light scattering from single bacterial spores in an optical trap allows the monitoring of spore germination dynamics. Anal Chem 81:4035–4042
Piggot PJ, Hilbert DW (2004) Sporulation of Bacillus subtilis. Curr Opin Microbiol 7:579–586
Powell JF (1950) Factors affecting the germination of thick suspension of Bacillus subtilis spores in L-alanine solution. J Gen Microbiol 4:330–339
Ragkousi K, Cowan AE, Ross MA, Setlow P (2000) Analysis of nucleoid morphology during germination and outgrowth of spores of Bacillus species. J Bacteriol 182:5556–5562
Ross C, Abel-Santos E (2010) The Ger receptor family from sporulating bacteria. Curr Issues Mol Biol 12:147–158
Santo LY, Doi RH (1974) Ultrastructural analysis during germination and outgrowth of Bacillus subtilis spores. J Bacteriol 120:475–481
Seliger HH (1989) Some reflections on McElroy and bioluminescence. J Biolumin Chemilumin 4:26–28
Setlow P (1983) Germination and outgrowth. In: Gould GW, Hurst A (eds) The bacterial spore. Academic Press, London
Setlow P (1994) Mechanisms which contribute to the long term survival of spores of bacillus species. J Appl Bacteriol 76:49S–60S
Setlow P (2003) Spore germination- review article. Curr Opin Microbiol 6:550–556
Setlow P (2006) Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol 101:514–525
Setlow P (2007) I will survive: DNA protection in bacterial spores. Trends Microbiol 15:172–180
Setlow P (2013) When the sleepers wake: the germination of spores of Bacillus species. J Appl Microbiol 115:1251–1268
Setlow P (2014) Germination of spores of Bacillus species: what we know and do not know. J Bacteriol 196:1297
Setlow P, Johnson EA (2007) Spores and their significance. In: Doyle MP, Beuchat LR (eds) Food microbiology, fundamentals and frontiers, 3rd edn. American Society for Microbiology, Washington
Setlow P, Johnson EA (2012) Spores and their significance. In: Doyle MP, Beuchat LR (eds) Food microbiology, fundamentals and frontiers, 4th edn. American Society for Microbiology, Washington
Setlow B, Loshon CA, Genest PC, Cowan AE, Setlow C, Setlow P (2002) Mechanisms of killing of spores of Bacillus subtilis by acid, alkali, and ethanol. J Appl Microbiol 92:362–375
Singh RP, Setlow B, Setlow P (1977) Levels of small molecules and enzymes in the mother cell compartment and the forespore of sporulating Bacillus megaterium. J Bacteriol 130:1130–1138
Sorg JA, Sonenshein AL (2010) Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid. J Bacteriol 192:4983–4990
Stewart GS, Johnstone K, Hagelberg E, Ellar DJ (1981) Commitment of bacterial spores to germinate: a measure of the trigger reaction. Biochem J 198:101–106
Swerdlow BM, Setlow B, Setlow P (1981) Levels of H+ and other monovalent cations in dormant and germinated spores of Bacillus megaterium. J Bacteriol 148:20–29
Tehri N (2015) Spore based sensor for pesticide residues in milk. Doctoral thesis submitted to NDRI, Karnal. http://krishikosh.egranth.ac.in/handle/1/5810039846. Accessed 7 Jan 2018
Tehri N, Kumar N, Raghu HV et al (2017) Role of stereospecific nature of germinants in Bacillus megaterium spores germination. 3 Biotech 7:259
Tehri N, Kumar N, Yadav A, Raghu HV, Singh NA (2018) Sugars mediated germination in spores of Bacillus megaterium. Int J Microbiol Res 10:1058–1061
Tennen R, Setlow B, Davis KL, Loshon CA, Setlow P (2000) Mechanisms of killing of spores of Bacillus subtilis by iodine, glutaraldehyde and nitrous acid. J Appl Microbiol 89:330–338
Thakur G, Yadav A, Tehri N, Kumar N, Raghu HV, Singh N, Singh VK (2013) Rapid & novel microscopy technique to detect germination initiation and specificity in Bacillus spores. Int J Res Pure Appl Microbiol 3:134–138
Warth A, Strominger J (1972) Structure of the peptidoglycan from spores of Bacillus subtilis. Biochem 11:1389–1396
Weese JS, Staempfli HR, Prescott JF (2000) Isolation of environmental Clostridium difficile from a veterinary teaching hospital. J Vet Diagn Investig 12:449–452
Welkos S, Little S, Friedlander A, Fritz D, Fellows P (2001) The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores. Microbiology 147:1677–1685
Welkos SL, Cote CK, Rea KM, Gibbs PH (2004) A microtiter fluorometric assay to detect the germination of Bacillus anthracis spores and the germination inhibitory effects of antibodies. J Microbiol Methods 56:253–265
Wilson KH (1983) Efficiency of various bile salt preparations for stimulation of Clostridium difficile spore germination. J Clin Microbiol 18:1017–1019
Wilson T, Hastings JW (1998) Bioluminescence. Annu Rev Cell Dev Biol 14:197–230
Wuytack EY, Boven S, Michiels CW (1998) Comparative study of pressure-induced germination of Bacillus subtilis spores at low and high pressures. Appl Environ Microbiol 64:3220–3224
Wuytack EY, Soons J, Poschet F, Michiels CW (2000) Comparative study of pressure- and nutrient induced germination of Bacillus subtilis spores. Appl Environ Microbiol 66:257–261
Yi X, Setlow P (2010) Studies of the commitment step in the germination of spores of Bacillus species. J Bacteriol 192:3424–3433
Yilmaz MD, Oktem HA (2018) Eriochrome black T–Eu3+ complex as a ratiometric colorimetric and fluorescent probe for the detection of dipicolinic acid, a biomarker of bacterial spores. Anal Chem 90:4221–4225
Yung (2008) Detection of aerobic bacterial endospores: from air sampling, sterilization validation to astrobiology. Doctoral Thesis. Submitted to California Institute of Technology Pasadena, California
Zhang P, Garner W, Yi X, Yu J, Y-q L, Setlow P (2010) Factors affecting variability in time between addition of nutrient germinants and rapid dipicolinic acid release during germination of spores of Bacillus species. J Bacteriol 192:3608–3619
Acknowledgements
The authors are extremely grateful to all members of Microbial Biosensors and Food Safety Laboratory, ICAR-NDRI, Karnal (Haryana), India, for their contribution in understanding types of biomarkers of spore germination.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tehri, N., Kumar, N., Raghu, H. et al. Biomarkers of bacterial spore germination. Ann Microbiol 68, 513–523 (2018). https://doi.org/10.1007/s13213-018-1361-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-018-1361-z