Abstract
The present study was undertaken with the objective to assess the effect of distinct stereoisomeric forms of nutrient germinants (selected sugars and amino acids) on the process of germination onset in dormant spores of Bacillus megaterium MTCC 2949. In this respect, epimers of glucose and enantiomers of alanine were employed in current work. When supplemented with these stereoisomers, spores were found germinated only with d-glucose and d-mannose among epimers of glucose and only with l-alanine among enantiomers of alanine. Interestingly, germination in spores was observed to negligible extent with d-galactose and d-alanine. These findings were obtained on the basis of four type of germination assays, namely reduction in absorbance measured at 600 nm (≤5 to ≥30%), refractility examination (phase bright and dark), esterase assay [fluorescence units 0.455–94.62 (×103)] and fluorescent staining (fluorescent/non-fluorescent signals). Understanding of spores germination process and efficacy of different forms of germinants to trigger germination is of immense importance. It aids in development of sensing and sterilization indicating tools employing chiefly spores as biorecognition elements and in uncovering the mechanism of diseases, food contamination and spoilages resulting from the germination of spores. The findings of current work support the possibility to explore such germination mechanism by significantly giving the clue for potential existence of stereospecific receptor sites on the surface of B. megaterium spores. Perhaps, these sites can specifically differentiate and recognize stereoisomerically diverse forms of germinants for induction of germination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Certain species of Gram positive bacteria such as Bacillus and Clostridium and Gram negative bacteria such as Sporomusa ovata, Sporomusa malonica and Coxiella burnetti are able to survive under unfavourable environmental conditions by forming dormant and resistant spores (Nicholson 2002). These spores persistently scrutinize their environment for molecules or conditions that trigger germination followed by outgrowth to generate growing vegetative cells (Paredes-Sabja et al. 2011; Ross and Abel-Santos 2010; Setlow and Johnson 2012).
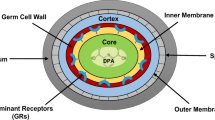
The molecules that trigger germination are called germinants. Germinants can be divided into nutrient types such as sugars, amino acids, purine derivatives and non-nutrient types including chemicals like dodecylamine, Ca-DPA, high pressure and abrasion. The germination of bacterial spores is usually triggered by nutrient germinants. These are generally low molecular weight compounds and are species- and strain specific (Paredes-Sabja et al. 2011). The specificity of the spores for germinants is due to the proteins, termed as ‘germinant receptors’ (GR) (Setlow 2013). Each GR recognizes a distinctive germinant including sugars, amino acids and nucleosides. Interestingly, different GR can cooperate to recognize a single germinant or multiple germinants (Moir et al. 2002; Moir 2006). Generally polycistronic operons encode GRs. These operons are expressed only within developing spore, under regulation of σG which is a forespore-specific transcription factor (Zuberi et al. 1987; Madslien et al. 2014). Spores which lack such GRs have been found to show strongly impaired response towards germinants (Barlass et al. 2002; Hornstra et al. 2006).
In recent years, the concept of germination has been applied as an innovative tool for development of various techniques. Some of these techniques offer spore eradication, while others make the use of spores as biosensing element for detection of contaminants, sterilization and efficacy testing. However, the application of this approach for development of analytical tools relies on selection of right type of germinant that can successfully trigger germination in spores. Germinant binding to specific receptors initiates a series of events. These events in turn lead to degradation of protective structures of spore and recommencement of its metabolism to form vegetative cell. Thus the area employing spores as biorecognition elements highlights the need to explore the specificity of binding mechanism of nutrient germinants with protein receptors to trigger germination in spores. Thereby, it could contribute immensely in screening of germinants required to initiate the process of germination in dormant spores. In this context, the present study was carried out with the goal of determining stereospecificity of binding interaction between spore surface receptors and nutrient germinants, a key factor mediating the onset of germination. In order to fulfil this objective, triggering of germination in dormant spores in response to distinct stereoisomeric forms of different nutrient germinants has been studied. Spores of B. megaterium MTCC 2949 have been used as a representative member of the genera Bacillus. The germination response shown by spores towards stereoisomerically distinct forms of nutrient germinants has been assayed. To assay germination, four widely accepted approaches, namely reduction in absorbance (O.D.) at 600 nm, refractility study, measurement of enzyme activity and fluorescent staining have been used. The findings from current work can elucidate the stereospecific nature of protein receptors present on the surface of B. megaterium spores and hence the onset process of germination.
Materials and methodology
Chemicals and instrumentation
Microbiological media and fluorogenic substrate, i.e. diacetyl fluorescein used is this study, were purchased from Himedia (India). Stereoisomers which included epimers of glucose (d-glucose, d-galactose and d-mannose) and enantiomers of alanine (d-alanine and l-alanine) were of analytical grade (typically >98% pure) and procured from Sigma Aldrich (India). Acetone was obtained from Fisher Scientific (India). Distilled water used in all experiments was produced by Bioage-Labpure ultra plus from BIO-AGE™, Punjab (India). Centrifuge from Eppendorf North America, Inc. (USA) was used. Incubation was done in Innova 42 Incubator Shaker Series, Eppendorf (USA). VictorX3 2030 microbiological plate reader from Perkin Elmer (USA) was used for measurement of absorbance and fluorescence. Heat activation of spores was carried out in AccuBLOCK digital dry bath, Labnet International Inc. (USA). pH meter from Century (India) was used to prepare buffers and growth medium. Microcentrifuge tubes (2.0 mL) were procured from Eppendorf (USA) and 96-well microtitre plates were obtained from M/s Grenier (Germany). Refractility of spores was observed using phase contrast microscope: Olympus BX 51 photomicroscope equipped with UPLAPO PH series of objectives, phase-contrast condenser U-PCD2 and digital camera DP70. For fluorescent staining, Olympus BX 61 equipped with excitation filter wheel CFP exciter 430/24 nm, emission filter wheel CFP emitter 470/24 nm and an electron-multiplying CCD (EMCCD) camera of Andor Technology iXon + (Tokyo, Japan) was used.
Procurement and maintenance of microbial culture
Bacillus megaterium strain MTCC 2949 was procured from Microbial Type Culture Collection (MTCC), Chandigarh, India. The procured strain was revived by inoculation in nutrient broth and incubated overnight at 37 °C. Following incubation, loopful of the culture was streaked on nutrient agar and again allowed to incubate at 37 °C for 12–16 h. The culture was sub-cultured twice, maintained on nutrient agar plates and stored at 4 °C till further use.
Spores production
Spores were produced using nutrient starvation principle-based method as given by Kumar et al. 2010. Loopful of freshly streaked culture of B. megaterium was inoculated in 5.0 mL of trypton glucose yeast extract broth (propagation medium) and incubated at 37 °C for 24 h. Inoculum at 1% was transferred to 100 mL propagation medium and incubated at 37 °C for 48 h. From propagation medium, inoculum at 7.5% was transferred to 100 mL of nutritionally starved sporulation medium and incubation was carried out at 37 °C for 42 h. Subsequently, spores produced were washed twice with 10 mM potassium phosphate buffer (pH 6.8). The final suspension of spores was prepared in same buffer. The O.D. (600 nm) of spore suspension was adjusted to 0.330 ± 0.015 (107 spores/mL) using microbiological plate reader. For every batch of spores prepared, total viable count and spore count were also enumerated (Downes and Ito, 2001). Prepared spore suspensions were free (~95 ± 5%) of cells or germinated spores as observed by phase contrast microscopy. Spore suspensions prepared in buffer were refrigerated at 2–8 °C. Prepared suspensions were used to perform germination assays with stereoisomerically distinct forms of sugar and amino acid taken in presented study.
Heat activation of dormant spores
Two hundred microliters of the spore suspension (107 spores/mL) was taken in a thin-walled glass tube and incubated at 80 °C for 10 min in digital dry bath. Following incubation, spores were cooled rapidly in cold water and were used within 2 h.
Spores viability assay
Viability of spores was determined by pipetting 100 µL aliquots of serially diluted suspension of heat-activated spores onto nutrient agar plates. Plates were incubated for 16–24 h. After incubation, colonies were counted to quantify the abilities of the spores to initiate and complete the process of germination.
Preparation of sugar, amino acid and substrate solution
Six different concentrations, i.e. 50, 100, 150, 200, 250 and 300 mM of each nutrient germinant, i.e. epimers of glucose (d-glucose, d-galactose and d-mannose) and enantiomers of alanine (d-alanine and l-alanine) were prepared in 10 mM potassium phosphate buffer (pH 6.8). Germinant solutions were filter sterilized by 0.22 µm membrane filters. Working solution, i.e. 11 µM of diacetyl fluorescein used to measure esterase activity was prepared by serially diluting the stock solution (1.16 mg per 2.5 mL of acetone) in 10 mM potassium phosphate buffer (pH 6.8).
Germination assays
The protocols used to perform germination assays are explained below. Each germination assay was performed with spores at an O.D. (600 nm) of 0.330 ± 0.015.
Measurement of O.D. (600 nm)
Spore germination was assayed by adding 75 µL of heat-activated spores to 75 µL of stereoisomeric forms of glucose and alanine (six different concentrations in the range of 50–300 mM) in a ratio of 1:1 in 96-well microtitre plate. The control wells contained equal quantity of dormant spores and potassium phosphate buffer (10 mM, pH 6.8) replacing germinant (1:1). Plates were covered with the lid to minimize evaporative losses and then incubated at 37 °C for 150 min. Change in O.D was measured after every 15 min at 600 nm. The extent of germination was measured in terms of total decrease in O.D. 600 nm and expressed as a percentage of the initial value. Experiments were carried out in triplicate, with at least three independent spore preparations.
Determination of refractility
Refractility of spores was studied with phase contrast microscopy. For microscopic examination, 100 μL each of spores and epimers of glucose and enantiomers of alanine (at concentration showing maximum reduction in O.D. 600 nm) were taken in microcentrifuge tubes. The control included 100 μL of dormant spores added to same quantity of 10 mM potassium phosphate buffer (pH 6.8) replacing germinant. Both test and control were incubated at 37 °C for a period of 3.0 h. After incubation, dormant spores as well as spores exposed to stereoisomeric forms of glucose and alanine were spread on a clean glass slide to make the smear as thin as possible. Smears were allowed to air dry and then heat fixed in order to minimize the movement of the spores (dormant or germinated)/cells. Prepared slides were observed for refractility, to study germination in spores of B. megaterium in response to stereoisomerically distinct forms of glucose and alanine by phase contrast microscope.
Esterase assay
Germination was assayed by quantifying activity of esterase in dormant spores and spores incubated with stereoisomerically different forms of glucose and alanine. Fluorogenic assay for esterase activity was carried out in a black-bottom 96-well microtitre plate. Twenty microliters of heat-activated spores was taken along with 70 µL each of the epimers of glucose and enantiomers of alanine (concentration as per refractility study). Ten microlitres of diacetyl fluorescein was added to each well. Control included 20 μL dormant spores and 70 μL of sterile distilled water replacing germinant along with 10 μL of diacetyl fluorescein in one well. Ten microlitres of substrate with 90 µL of sterile water replacing both spores and germinant was taken in another well. The microtitre plate was incubated at 37 °C and esterase activity in terms of fluorescence units was measured after every 10-min interval for a period of 60 min. The activity was measured using microbiological plate reader with excitation and emission filters set to 485 and 535 nm, respectively. Fluorescence measurements were recorded in triplicate for each stereroisomerically distinct form of nutrient germinant.
Fluorescent staining
Fluorescent staining of spores incubated with and without nutrient germinants was performed using EMCCD-based novel microscopy technique designed by Thakur et al. (2013). Two sets each of three glass slides were taken. Spore suspension of 10 μL was added on slides to prepare the smear. Incubation was done at 37 °C for approximately 5 min. On one set of the dried smear slides, 10 μL of d-dextrose, d-mannose, d-galactose and on smear of another set, 10 μL of d-alanine and l-alanine were added and spores were allowed to germinate for 10 min. A negative control was run by adding 10 μL of potassium phosphate buffer (10 mM, pH 6.8) replacing germinant on dried smear. Ten microlitres of diacetyl fluorescein (11 µM) was added on the smears. Each slide was observed and images were taken using an Olympus BX 61 and EMCCD, respectively. All acquired images were observed for fluorescent signals shown by dormant and germinating spores.
Statistical analysis
All results reported are averages of triplicate determinations. Tukey´s estimates of least significant differences were calculated from the ANOVA analysis. Error bars in graphs show standard error within three measurements.
Results and discussion
Germination in spores most commonly initiate in response to nutrient germinants such as l-amino acids, D-sugars and purine nucleosides. These germinants interact in a stereospecific manner with receptors present on the spore’s inner membrane and subsequently trigger events in germination of spores (Igarashi et al. 2004; Black et al. 2007). In this respect, current work was carried out with the objective to study the role of stereospecific nature of germinants on the onset of germination. Germination in B. megaterium spores in response to distinct stereoisomeric forms of glucose and alanine was assayed through four different approaches as described above. The findings obtained for each assay are explained below.
O.D. measurement at 600 nm
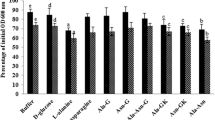
O.D. 600 nm is the optical density or light absorbance at 600 nm and describes transmission of light at this wavelength through a solution or object (Kristin 2012). O.D. measurement of germinating spores, in particular, the maximum rate of fall in the O.D. 600 nm at a given germinant concentration, is a simple and reliable method for comparing rates of spore germination (Cabrera-Martinez et al. 2003). Using this principle, the efficiency of glucose epimers, i.e. d-glucose, d-mannose, d-galactose and alanine enantiomers, i.e. d-alanine and l-alanine for triggering germination in spores was evaluated as per protocol mentioned above. As a result, among three, two epimers of glucose, i.e. d-glucose and d-mannose and out of two, one enantiomer of alanine, i.e. l-alanine, investigated in the current study, indicated the onset of germination at significant level in Bacillus spores. Findings on O.D. 600 nm reduction of spores incubated without (control) and with six different concentrations (50–300 mM) each of glucose epimers (d-glucose, d-mannose, d-galactose) and alanine enantiomers (d-alanine, l-alanine), at varying intervals of time for a period of 150 min, are shown in Fig. 1. Reduction of O.D. 600 nm varied from ~3.90 to 49.18% in a period of 150 min depending upon type and concentration of sugar or amino acid. Spores found germinated well with a reduction in absorbance of 30% or more with d-glucose (50 mM), d-mannose (150 mM) among epimers of glucose and with l-alanine (150 mM) only among enantiomers of alanine. This significant fall in absorbance reflects germination in terms of change in the light scattering property of the multiple individual spores in a suspension, associated with germination events such as the excretion of spore’s depot of Ca2+-DPA, followed by water influx, cortex degradation and core swelling (Cabrera-Martinez et al. 2003; Paidhungat and Setlow 2002; Setlow et al. 2009; Løvdal et al. 2012; Ghosh 2013). On the other hand, spores were found germinated poorly or to a negligible rate (i.e. <6% reduction of absorbance) with d-galactose (100 mM) among glucose epimers and d-alanine (250 mM) among alanine enantiomers. The findings obtained with d-galactose and d-alanine were in parallel to the O.D. 600 nm loss, i.e. <5% shown by dormant spores incubated without any sugar or amino acid (control) and thus proved their non-germinant characteristic.
Phase contrast microscopic observations
The findings obtained on O.D. 600 nm reduction were also confirmed by examination of refractility in spores supplemented with stereoisomerically distinct forms of nutrient germinants. The concentration of germinants which showed maximum loss of O.D. 600 nm was used in this assay (50, 150 and 100 mM for d-glucose, d-mannose and d-galactose, respectively, and 250 and 150 mM for d-alanine and l-alanine, respectively). In order to study refractility, observations were taken with phase contrast microscopy. From the images obtained, germination in spores was observed with d-glucose and d-mannose among glucose epimers and with l-alanine among alanine enantiomers. This was proved by the rapid loss of refractility, as viewed in the images shown in Fig. 2. This change in refractive index of spores as reported by Setlow (2003), Vary and Halvorson (1965), Zhang et al. (2010) occurs dramatically during germination due to both the release of the spore core’s large DPA pool and some water uptake, as well as subsequent cortex hydrolysis that leads to core swelling and further water uptake. In contrast, spores incubated with d-galactose and d-alanine did not show any sign of germination. These spores were quite refractile in appearance similar to dormant spores and thus indicated lack of the spore germination process. Based on these observations, out of three, two epimers of glucose, i.e. d-glucose, d-mannose and out of two, one enantiomer of alanine, i.e. l-alanine exhibited rapid loss of refractility. Hence, these forms of nutrient germinants showed germination in spores. The findings obtained simulate the findings of other researchers who reported a rapid loss in characteristic refractility of spores during onset of germination. Germinated spores give dark appearance when examined by the phase-contrast microscopy (Hitchins et al. 1968; Hashimoto et al. 1969).
Esterase assay
Activity of esterase was taken as a marker for indication of germination onset in spores of B. megaterium in response to nutrient germinants. Esterase activity was determined by fluorogenic assay as described above. Spores incubated with glucose epimers and alanine enantiomers were analysed for esterase expression. As a result, among epimers of glucose, only d-glucose and d-mannose and among enantiomers of alanine, only l-alanine were found to express significant level of esterase, as determined in terms of fluorescence units. On the other hand, spores incubated with d-galactose and d-alanine have shown esterase activity to negligible extent. This activity was found similar to fluorescence units as obtained for dormant spores. Thus the findings obtained, as depicted in Fig. 3, have clearly shown variability in germination response due to distinction in stereoisomeric forms of sugar or amino acid. This indicates stereospecific characteristic of Bacillus spores towards recognition of germinants to trigger the process of germination. Esterase activity has already been targeted as spore germination parameter in Bacillus anthracis (Ferencko et al. 2004).
Fluorescent staining observations
Bacterial spores incubated with and without epimers of glucose and enantiomers of alanine were stained with fluorescent staining technique using diacetyl fluorescein. The results obtained as a result of staining are shown in Fig. 4. In fluorescent staining, the parameter used as marker of germination was measurement of fluorescent intensity of spores incubated with and without germinant. Basically, fluorescent intensity results from the cleavage of fluorogenic substrate, i.e. diacetyl fluorescein by the germinating spores due to release of esterase. Esterase is responsible for converting the dormant spore state to metabolically active vegetative form. The fluorescent staining results clearly revealed that B. megaterium spores are highly specific for germinant requirements. High fluorescent intensity was observed for spores incubated with d-glucose, d-mannose and l-alanine in presented work. On the other hand, fluorescent signal observed for spores was too less or negligible with d-galactose and d-alanine as these were failed to prop up germination in spores at a significant level. The staining observations for spores germinated with d-galactose and d-alanine were analogous to that of dormant spores.
The aforementioned findings as obtained from four types of germination assays indicate diverse response of B. megaterium spores towards stereospecifically distinct form of same germinant (s). The analysis of results obtained from germination assays revealed that (i) among epimers of glucose, i.e. d-glucose, d-galactose and d-mannose, spores were found germinated with d-glucose and d-mannose; and (ii) germination in spores was reported with l-alanine only when enanatiomers of alanine, i.e. d-alanine and l-alanine were used. These different responses suggest that spores carry such receptors that can efficiently distinguish stereoisomerically distinct forms of same biomolecule. Similarly, several other investigators have carried out the studies on the process of germination initiation in response to different germinants for many spore formers including B. megaterium. Germination in spores of B. megaterium QM B1551 in response to d-glucose, d-mannose and l-alanine, while no germination in response to galactose was also reported by Hyatt and Levinson (1964), Racine et al. (1979), Christie and Lowe (2007) in their work which is in line with the findings obtained in current investigation. d-glucose has also been proved to be a powerful germinant inducing rapid germination in spores of Bacillus macerans B70 (Sacks and Thompson 1971), Clostridium botulinum Type E (Ando 1971), while for spores of these bacteria, d-mannose was found effective only at increasing concentration levels. Ando (1971) also reported maximum germination in spores of Clostridium botulinum Type E with galactose. Germination in response to galactose is in contrast to our finding seemingly due to differences in the complement of nutrient germinant receptors in various genera. l-alanine has been known as a common germinant for both Bacilli and Clostridia. l-alanine-mediated germination has been mostly characterized in B. subtilis spores (McCann et al. 1996). However, l-alanine can also induce germination in B. licheniformis (Madslien et al. 2014; Borch-Pedersen et al. 2016), B. cereus (Barlass et al. 2002), B. anthracis (Fisher and Hanna 2005), C. botulinum (Alberto et al. 2003), C. sporogenes (Broussolle et al. 2002) C. perfringens (Paredes-Sabja et al. 2008) and C. sordellii (Ramirez and Abel-Santos 2010). l-alanine appears to be the universal germinant for bacterial spores (Ross and Abel-Santos 2010). On the other hand, d-alanine has been found to be a potent inhibitor of spore germination in many Bacillus species (Cava et al. 2011). Thus, the work reported in this communication demonstrates that the onset of germination in B. megaterium spores is dependent on stereospecific characteristic of nutrient germinants. Enantiomers of alanine, i.e. d and l-alanine showed a distinct behaviour towards germination of B. megaterium spores. l-alanine showed germination of spores while no germination was observed with d-alanine as per the findings obtained from O.D. (600 nm.), refractility, esterase activity and fluorescent staining based germination assays. Observations made with similar techniques showed distinct findings for onset of germination when epimers of glucose, i.e. d-glucose, d-galactose and d-mannose were used as germinants. Spores were germinated with d-glucose and d-mannose while no germination observed with d-galactose. Hence, current study offers significant contribution towards understanding the role of stereospecific nature of germinants on germination of Bacillus spores.
Conclusions
Spore germination is an interesting phenomenon as (i) through germination spores contaminate, spoil foodstuffs and cause diseases in the body; (ii) upon germination, spores lose their resistance and can be easily killed (sterilization) and (iii) germination in spores is sensitive to the presence of biotic or abiotic stuffs present in external environment. Keeping these in view, spore germination approach is finding immense applications in the area of food safety and quality assurance. In recent times, spores, especially of B. megaterium and B. stearothermophilus, have been explored extensively for development of sensing devices. Their working principle takes the advantage of spore’s unique biphasic life cycle for targeting various types of contaminants in food system and also as sterility indicator. In this context, the presented work can contribute immensely to the existing studies in understanding the mechanism of germination in B. megaterium spores employing chiefly sugars and amino acids as germinants. The findings from current work revealed that the stereospecific nature of germinants appeared to influence markedly the germination in dormant spores which in turn can be speculated to link with the presence of stereospecific receptor sites on the surface of bacterial spores. A better understanding of spore germination mechanism may give clue for the selection of germinants, design of either germination inhibitors or artificial germinants that could aid in better design and development of spores-based technologies. Moreover, elucidating such mechanisms by which germinants interact with receptors reveals new paradigms for understanding the germination process.
References
Alberto F, Broussolle V, Mason DR, Carlin F, Peck MW (2003) Variability in spore germination response by strains of proteolytic Clostridium botulinum types A, B and F. Lett Appl Microbiol 36:41–45
Ando Y (1971) The germination requirements of spores of Clostridium botulinum type E Japan. J Microbiol 15:515–525
Barlass PJ, Houston CW, Clements MO, Moir A (2002) Germination of Bacillus cereus spores in response to l-alanine and to inosine: the roles of gerL and gerQ operons. Microbiology 148:2089–2095. doi:10.1099/00221287-148-7-2089
Black EP, Setlow P, Hocking AD, Stewart CM, Kelly AL, Hoover DG (2007) Response of spores to high-pressure processing. Comp Rev Food Sci Food Saf 6:103–119
Borch-Pedersen K, Lindbäck T, Madslien EH, Kidd SW, O’Sullivan K, Granum PE, Aspholm M (2016) The cooperative and interdependent roles of GerA, GerK, and Ynd in germination of Bacillus licheniformis spores. Appl Environ Microbiol 82:4279–4287
Broussolle V, Alberto F, Shearman CA, Mason DR, Botella L, Nguyen-The C, Peck MW, Carlin F (2002) Molecular and physiological characterization of spore germination in Clostridium botulinum and C. sporogenes. Anaerobe 8:89–100
Cabrera-Martinez RM, Tovar-Rojo F, Vepachedu VR, Setlow P (2003) Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis. J Bacteriol 185:2457–2464. doi:10.1128/JB.185.8.2457-2464.2003
Cava F, Lam H, de Pedro MA, Waldor MK (2011) Emerging knowledge of regulatory roles of d-amino acids in bacteria. Cell Mol Life Sci 68:817–831
Christie G, Lowe CR (2007) Role of chromosomal and plasmid-borne receptor homologues in the response of Bacillus megaterium QM B1551 spores to germinants. J Bacteriol 189:4375–4383. doi:10.1128/JB.00110-07
Downes FP, Ito K (eds) (2001) Compendium of methods for the microbiological examination of foods, 4th edn. American Public Health Association, Washington, DC, USA
Ferencko L, Cote MA, Rotman B (2004) Esterase activity as a novel parameter of spore germination in Bacillus anthracis. Biochem Biophys Res Commun 319:854–858. doi:10.1016/j.bbrc.2004.05.062
Fisher N, Hanna P (2005) Characterization of Bacillus anthracis germinant receptors in vitro. J Bacteriol 187:8055–8062
Ghosh S (2013) Understanding the mechanism of Bacillus subtilis spore germination. Doctoral Dissertation, University of Connecticut Graduate School
Hashimoto T, Frieben WR, Conti SF (1969) Germination of single bacterial spores. J Bacteriol 98:1011–1020
Hitchins AD, Kahn AJ, Slepecky RA (1968) Interference contrast and phase contrast microscopy of sporulation and germination of Bacillus megaterium. J Bacteriol 96:1811–1817
Hornstra LM, de Vries YP, Wells-Bennik MHJ, de Vos WM, Abee T (2006) Characterization of germination receptors of Bacillus cereus ATCC 14579. Appl Environ Microbiol 72:44–53. doi:10.1128/AEM
Hyatt MT, Levinson HS (1964) Effect of sugars and other carbon compounds on germination and postgerminative development of Bacillus megaterium spores. J Bacteriol 88:1403–1415
Igarashi T, Setlow B, Paidhungat M, Setlow P (2004) Effects of a gerF (lgt) mutation on the germination of spores of Bacillus subtilis. J Bacteriol 186:2984–2991. doi:10.1128/JB.186.10.2984-2991.2004
Kristin CSR (2012) Diversity in germination response among Bacillus licheniformis strains. Master thesis, Norwegian University of Life Sciences
Kumar N, Singh NA, Singh VK, Bhand S, Malik RK (2010) Development of spore inhibition based-enzyme substrate assay (SIB-ESA) for monitoring aflatoxin M1 in milk. Indian Patent Reg. No. 3064/DEL/2010: Publication No. 46/2012
Løvdal IS, From C, Madslien EH, Romundset KCS, Klufterud E, Rosnes JT, Granum PE (2012) Role of the gerA operon in l-alanine germination of Bacillus licheniformis spores. BMC Microbiol 12:1–12. doi:10.1186/1471-2180-12-34
Madslien EH, Granum PE, Blatny JM, Lindbäck T (2014) L-alanine-induced germination in Bacillus licheniformis the impact of native gerA sequences. BMC Microbiol 14:101–110. doi:10.1186/1471-2180-14-101
McCann KP, Robinson C, Sammons RL, Smith DA, Corfe BM (1996) Alanine germination receptors of Bacillus subtilis. Lett Appl Microbiol 23:290–294
Moir A (2006) How do spores germinate? J Appl Microbiol 101:526–530. doi:10.1111/j.1365-2672.2006.02885.x
Moir A, Corfe BM, Behravan J (2002) Spore germination. Cell Mol Life Sci 59:403–409. doi:10.1007/s00018-002-8432-8
Nicholson W (2002) Roles of Bacillus endospores in the environment. Cell Mol Life Sci 59:410–416. doi:10.1007/s00018-002-8433-7
Paidhungat M, Setlow P (2002) Spore germination and outgrowth. In: Sonenshein AL, Hoch JA, Losick R (eds) Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington
Paredes-Sabja D, Torres JA, Setlow P, Sarker MR (2008) Clostridium perfringens spore germination: characterization of germinants and their receptors. J Bacteriol 190:1190–1201
Paredes-Sabja D, Setlow P, Sarker MR (2011) Germination of spores of Bacillales and Clostridiales species: mechanisms and proteins involved. Trends Microbiol 19:85–94. doi:10.1016/j.tim.2010.10.004
Racine FM, Dills SS, Vary JC (1979) Glucose-triggered germination of Bacillus megaterium spores. J Bacteriol 138:442–445
Ramirez N, Abel-Santos E (2010) Requirements for germination of Clostridium sordellii spores in vitro. J Bacteriol 192:418–425
Ross C, Abel-Santos E (2010) The ger receptor family from sporulating bacteria. Curr Issues Mol Biol 12:47–158
Sacks LE, Thompson PA (1971) Germination requirements of Bacillus macerans spores. J Bacteriol 105:739–746
Setlow P (2003) Spore germination—review article. Curr Opin Microbiol 6:550–556
Setlow P (2013) Summer meeting 2013—when the sleepers wake: the germination of spores of Bacillus species. J Appl Microbiol 115:1251–1268. doi:10.1111/jam.12343
Setlow P, Johnson EA (2012) Spores and their significance. In: Doyle MP, Buchanan RL (eds) Food microbiology: fundamentals and frontiers, 4th edn. ASM Press, Washington
Setlow B, Peng L, Loshon CA, Li YQ, Christie G, Setlow P (2009) Characterization of the germination of Bacillus megaterium spores lacking enzymes that degrade the spore cortex. J Appl Microbiol 107:318–328. doi:10.1111/j.1365-2672.2009.04210.x
Thakur G, Yadav A, Tehri N, Kumar N, Raghu HV, Singh N, Singh VK (2013) Rapid & novel microscopy technique to detect germination initiation and specificity in Bacillus spores. Int J Res Pure Appl Microbiol 3:134–138
Vary J, Halvorson H (1965) Kinetics of germination of Bacillus spores. J Bacteriol 89:1340–1347
Zhang P, Garner W, Yi X, Yu J, Li YQ, Setlow P (2010) Factors affecting variability in time between addition of nutrient germinants and rapid dipicolinic acid release during germination of spores of Bacillus species. J Bacteriol 192:3608–3619. doi:10.1128/JB.00345-10
Zuberi AR, Moir A, Feavers IM (1987) The nucleotide sequence and gene organization of the gerA spore germination operon of Bacillus subtilis 168. Gene 51:1–11
Acknowledgements
Niche Area of Excellence (NAE) is greatly acknowledged for supporting this research work. The Director NDRI is thankfully acknowledged for institute fellowship for pursuing Ph.D. to Nimisha Tehri.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tehri, N., Kumar, N., Raghu, H.V. et al. Role of stereospecific nature of germinants in Bacillus megaterium spores germination. 3 Biotech 7, 259 (2017). https://doi.org/10.1007/s13205-017-0897-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0897-0