Abstract
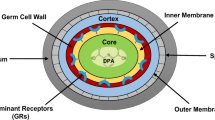
Dormant bacterial spores can sense their environments and under favorable conditions exchange their cycle from spore state to germinated one through the processes of germination and outgrowth. Here, the capability of spore germination is used to design an antibiotic bio-sensing system. Germination assays were carried out by reduction of optical density, release of Dipicolinic acid and respiration test under different germinats and various concentrations of Penicillin as a germination inhibitor. This study showed that although current germinants are not properly useful for germination of Bacillus amyloliquefaciens in starch media, presence of a small amount of cell wall destruction antibiotics (25 µg/ml) can accelerate germination but prevent outgrowth of germinated spores. So, the germinated spores cannot use the starch and stain blue with iodine reagent. This phenomenon is beneficial for detection of antibiotic residues in food and feed which are severe problem for consumers or by giving rise to the expansion of antibiotic resistances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dormant, bacterial spores can sense their environments and under favorable conditions, lose their resistance capabilities in exchange for a regaining of metabolic functions and vegetative growth, through the processes of germination and outgrowth in a species- and strain-specific manner. Germination can be triggered by a variety of factors, including nutrients (amino acids, sugars), non-nutrient germinants (such as calcium dipicolinate, lysozyme, salts and cationic surfactants), and physical factors such as hydrostatic pressure and abrasion. The capability of spore to exchange their cycle between spore state and germinated cell offers them as a biosensing system. The sensitivity of germination to the presence of contaminants such as antibiotics, aflatoxin and some other toxins, affects the life cycle of bacterial spores and this phenomenon can be applied as useful pattern for detection of aforementioned in food and feed [1,2,3].
Biosensors can be classified according to the mode of signal transduction or to the type of recognition molecules. The latter is divided to antibodies, protein receptors, whole cells (mammalian cells, tissues, bacterial cells or endospores), nucleic acids and enzymes.
Using spore based bio-sensing system has some advantages are mentioned in the following:
-
a)
It has a long shelf life of 8 months when kept as dried spores at room temperature,
-
b)
The spore production is a low-priced process and its immobilization is an effortless procedure,
-
c)
Dormant bacterial spores have the capability of sensing their environments and rapidly responding to the presence of specific germinant substances to initiate the process of germination and then outgrowth [3, 4].
Although the recommended levels of antibiotics in feed were 5–10 g/kg in the 1950s, they have increased by 10–20 folds since then. Usage of antibiotics, for example in poultry farms, not only as an anti-microbial agent but also as a growth-promoting agent can increase the rate of weight gains and improve the efficiency of converting feed to meat. On the other hand, increasing usage of antibiotics can cause severe problem for consumers (e.g. penicillin allergy) or by giving rise to the expansion of antibiotic resistances [5,6,7,8].
With the emerging concern regarding antibacterial resistance, the necessity of designing simple but accurate sensors for the detection of antibiotic residues in meat products and poultry feed is felt more than before. So, the purpose of the current study is to introduce spore-based biosensor using Bacillus amyloliquefaciens for the detection of antibiotic residues by means of the starch hydrolysis.
Materials and methods
Preparation of bacterium: Bacillus amyloliquefaciens PTCC 23350 obtained from Persian Type Culture Collection.
Preparation of spore suspension
Spore suspension was prepared as described before [9]. To assess the level of sporulation, culture was examined microscopically, when the ratios of spores to vegetative cells reached > 95%, the biomass was collected in distilled water and centrifuged at 6000 rpm for 30 min. This washing method repeated two more times. Final pellet was re-suspended in deionized water, and then suspension subjected to heat shock treatment (80 °C, 10 min).
Germinants preparation
All germinant solutions were prepared in 50 mM HEPES pH 7.4. Germinants were selected from the literature and included d-glucose (G), l-alanine (Ala), l-asparagine (Asn), KCl (K) and the germinant combinations Ala-G (equimolar solution of alanine and glucose), Asn-GK (equimolar solution of asparagine, glucose and KCl) and Ala-GK (equimolar solution of alanine, glucose and KCl). Unless otherwise noted, all germinants were prepared to a final concentration of 10 mM [2].
Biosensing medium and spore germination assay
The bio-sensing medium was consisted of specific formula of Basal Saline Medium consisted of K2HPO4, ammonium sulfate, CaCl2, FeCl3 and soluble starch (BHD Co.), Bacillus amyloliquefaciens PTCC 23350 spores (1.5 × 108 spores/ml). Germination assay of the spore in this medium was investigated during the time by means of two tests as mentioned in the following:
-
a)
Reduction of the optical density: Spore germination was monitored by measuring the reduction of the optical density at 600 nm (OD600). Analysis of spore germination were measured in a 96-well plate (USA Scientific, Orlando, FL) by mixing 10 µl spore suspension, 70 µl soluble starch with 20 µl of germinant or HEPES. Spores and germinants were incubated at room temperature (28 °C), and the optical density at 600 nm (OD600) was recorded periodically. Spores and germinants were incubated for 2 h. Three replicates per treatment were analyzed and assays were performed twice, with separate spore preparation [1, 2, 10].
-
b)
Release of DPA Spore germination was also assessed (in test tube with the same proportion in microtiter plate method as mentioned above) by measuring the release of DPA as spore germination indicator at 270 nm from 1 ml cell free supernatant. The total DPA of the spores was determined from the supernatant of the 20-min boiled culture [11].
Cellular Respiration Assay in presence of different germinants by using Microtiter Plate Method: In order to assess the possibility of respiration test for the detection of germinating spores, spores were exposed to the germinant in absence of starch for maximum reaction between them. Analysis of spore germination were measured in a 96-well plate (USA Scientific, Orlando, FL) by mixing the proportion of 1:1 spore suspension and germinants or HEPES. For each germinant tested, two spore-germinant mixtures were loaded per incubation time. Samples were prepared so that all incubations finished simultaneously and were analyzed together. Following addition of 100 µl TTC reagent to all samples, the resulted pink color was measured after three hours incubation time. The accumulation of the pink color is proportional to the rate of respiration by germinated spores. So, the OD of each well was measured at 450 nm by using an automated Eliza counter [12, 13].
Investigation of starch hydrolysis in the presence of different germinants
It was proposed that germinated spores hydrolyze the starch, so the remaining starch can be detected in the presence of different germinants by iodine test [14].
Germination assay in the presence of different concentration of Penicillin
Different concentrations of Penicillin G (25, 50 and 100 µg/ml) were added to the medium and spore germination assay was carried out as mentioned above.
Confirmation of DPA release in presence of Penicillin by means of FTIR analysis
The biosensor was prepared in starch media and treated with and without Penicillin. After 3 h, the suspensions were centrifuged and pellet washed two times with deionized water. Then pellet re-suspended in deionized water. Prior to analysis, the samples were oven-dried at 50 °C for 30 min [15].
Statistical analysis
The data were assessed using the analysis of variance (ANOVA) at P ≤ 0.05 level of significance using the GraphPad Prism 7 software. Graphs were plotted by Excel 2016, and all of treatments were done in triplicate.
Results
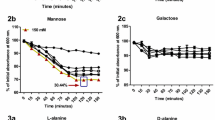
Germination rates were demonstrated by reduction of optical density (OD600) for different germinants at 28 °C (Fig. 1). As it was shown germinants caused reduction in optical density during the time, with nearly the same pattern however the most reduction was significantly occurred in the presence of l-alanine and Ala-Asn after 120 min.
Germination assay of B. amyloliquefaciens (using reduction of OD600nm) under different germinants in starch media during incubated at 28 °C (Ala-G: an equimolar solution of l-alanine and d-glucose, Asp-G: an equimolar solution of l-asparagine and d-glucose; Ala-Asn-G: an equimolar solution of l-alanine, l-asparagine and d-glucose; Ala-GK: an equimolar solution of l-alanine, d-glucose and KCl; Asn-GK: an equimolar solution of l-asparagine, d-glucose and KCl; Ala-Asn: an equimolar solution of l-alanine and l-asparagine). Data points represent the mean percent spore germination of three replicates per sample, and vertical lines represent the standard error of the mean for each treatment. Means not followed by the same letter were statistically different (P < 0.05)
Spore germination was measured by monitoring DPA which released in the supernatant of the cell free samples in the presence of different germinant (Fig. 2). The figure shows that Ala-Asn germinant significantly promotes spore germination among samples after 120 min.
Germination assay of B. amyloliquefaciens (using DPA release) under different germinants in starch media incubated at 28 °C (Ala-G: an equimolar solution of l-alanine and d-glucose, Asp-G: an equimolar solution of l-asparagine and d-glucose; Ala-Asn-G: an equimolar solution of l-alanine, l-asparagine and d-glucose; Ala-GK: an equimolar solution of l-alanine, d-glucose and KCl; Asn-GK: an equimolar solution of l-asparagine, d-glucose and KCl; Ala-Asn: an equimolar solution of l-alanine and l-asparagine). Data points represent the mean percent spore germination of three replicates per sample, and vertical lines represent the standard error of the mean for each treatment. Means not followed by the same letter were statistically different (P < 0.05)
The respiration was measured using dehydrogenase assay by TTC reagent. Dehydrogenase enzymes have an important role in aerobic respiration. The technique described here depends upon the fact that these dehydrogenase enzymes can donate the hydrogen ions to a color-less compound, causing it to change color. When the color-less chemical 2,3,5-Triphenyl tetrazolium chloride (TTC) diffuses into bacteria, it accepts electrons and reduced to a pink compound, known as formazan. The accumulation of this pink compound is proportional to the rate of bacterial respiration in inoculated microplate. So, the OD of each well was measured at 450 nm by using an automated Eliza counter [13]. Since the vegetative forms of B. amyloliquefaciens were responded to TTC test, this test was designed for assessment of respiration as an index of spore germination in B. amyloliquefaciens spores. So here, the accumulation of the pink compound is proportional to the rate of respiration by germinating spores. Although the statistical analysis was not significantly shown the differences among samples, germinating spores were visually detectable (pink color) in the presence of both d-Glucose and Asn-GK after 6 and 24 h (Fig. 3).
Respiration assay of germinated spores under different germinants incubated at 28 °C (Ala-G: an equimolar solution of l-alanine and d-glucose, Asp-G: an equimolar solution of l-asparagine and d-glucose; Ala-Asn-G: an equimolar solution of l-alanine, l-asparagine and d-glucose; Asn-GK: an equimolar solution of l-asparagine, d-glucose and KCl). The experiment was carried out in triplicate and error bars represent the standard error of the mean for each treatment. In some cases, the error bars are too small to be visible
Finally, the rate of starch hydrolysis was investigated in the presence of different germinants (Fig. 4). Statistical analysis showed that the rate of starch hydrolysis in buffer (blank) is better than all germinants. It seems that the proper action of germinants was inhibited in the presence of starch as the only carbon source of the media.
The percentage of remaining starch in the presence of different germinants incubated at 28 °C (Ala-G: an equimolar solution of l-alanine and d-glucose, Asp-G: an equimolar solution of l-asparagine and d-glucose; Ala-Asn-G: an equimolar solution of l-alanine, l-asparagine and d-glucose; Ala-GK: an equimolar solution of l-alanine, d-glucose and KCl; Asn-GK: an equimolar solution of l-asparagine, d-glucose and KCl). Data points represent the mean percent spore germination of three replicates per sample, and vertical error bars represent the standard error of the mean for each treatment
In the next stage, spore germination assay was repeated in the presence of different concentration of Penicillin G. Since it was shown (in the previous stage) that the presence of germinants does not have any significant effect on starch hydrolysis, this stage was carried out only in the presence of buffer as a blank. Statistical analysis showed that different concentrations of Penicillin (Fig. 5) did not cause significant reduction in OD600nm and therefore had no significant effect on germination.
Figure 6 showed that the rate of DPA release in the presence of different concentration of Penicillin was significantly high rather than buffer. This phenomenon is because of hydrolysis of outer layers of spore (e.g. Cortex) in the presence of Penicillin. It seems that although the presence of Penicillin can accelerate the germination, prohibited out-growth. Consequently, germinating spores faced to Penicillin were killed and starch hydrolysis could not occur properly depend on Penicillin concentration (Fig. 7). Figure 8 visibly shows the possibility of iodine and respiration test to detect the presence of Penicillin after 5 h at room temperature.
The effect of Penicillin concentrations on DPA release as an index of spore germination. Data points represent the mean percent spore germination of three replicates per sample, and error bars represent the standard error of the mean for each treatment. In some cases, the error bars are too small to be visible. Means not followed by the same letter were statistically different (P 0.05)
In order to confirm significant amount of DPA release from germinating spores in the presence of Penicillin, FTIR analysis of spore based biosensor with and without Penicillin was carried out. As the arrow was shown in Fig. 9, the picks between 1435 and 1470 cm−1 (regarding to the pyridine ring of DPA) were disappeared after treatment with Penicillin [15].
Discussion
Germination of bacterial spore was extensively studied [1,2,3, 16,17,18,19,20]. Here we introduced a new way for detection of germinating spores by using TTC in buffer, in the presence of some germinants and germination inhibitor (Penicillin). According to these data a biosensing system was designed for the detection of cell wall destruction antibiotics in food and feed. For this propose, the spores of B. amyloliquefaciens was inoculated in a minimal medium with starch as carbon source. Response time of the biosensor depends on the rate of starch hydrolysis by the germinating spores. In order to manipulate the response time of the biosensor, spores were exposed to the germinants to select the best ones for using in the designed medium.
As it was reported by other lectures, Bacillus nutrient-germination occurred via germination receptors [1]. Crane et al. (2014) found that a combination of d-glucose, d-fructose and potassium chloride (GFK), in addition to either l-asparagine (Asn-GFK) or l-alanine (Ala-GFK), induced maximal levels of TrigoCor spore germination in vitro. Although the induction of germination occurred in the presence of Ala-Gk or Asn-Gk, the combination of Ala-Asn significantly enhanced germination of B. amyloliquefaciens spores. Reduction of OD600nm showed that B. amyloliquefaciens germinate with the most common Bacillus germinant, l-alanine, but at lower extent. Since the starch hydrolysis was not carried out properly in the presence of germinants, it can be concluded that the presence of starch in the medium may interfere with the access of nutrient-receptors to the germinants. So, it seems that the minimal starch medium without any germinants was the best choice as the designed medium for the biosensor.
Laflamme et al. [12] showed that CTC (5-cyano-2, 3-diotolyl tetrazolium chloride) was used for germinating study by means of fluorescent microscope. Here the respiration test (TTC) was applied for detection of germinating spores in the presence of germinants by microtiter plate. Since this test was carried out in absence of starch, the result (Asn-GK) qualitatively was more similar to other studies [2].
In the next stage, the performance of biosensor in the presence of different concentrations of Penicillin was assayed. As it was mentioned in other texts, because of impressing spore germination by environmental conditions, it can be a useful tool for the detection of contaminations such as antibiotic residues in feed. On the other hand, the presence of antibiotics prohibited the out-growth of spores [3, 7, 21]. Interestingly when spores were exposed to the different concentration of Penicillin, high concentration of DPA (especially in the presence of 100 µg/ml of Penicillin) was released. Although the presence of different concentration of Penicillin may accelerate the germination process, the starch hydrolysis of the designed biosensor was significantly delayed depending on Penicillin concentration. Investigation of germination in the presence of Penicillin by means of FTIR analysis and respiration test was also confirmed the Penicillin effect on germination process, however prohibited out-growth of germinating spores.
Conclusion
In current research, spore germination of B. amyloliquefaciens was investigated by several methods and suggests that each of them can be a suitable tool for designing a bio-sensing system for the detection of substances that affect germination and out-growth such as antibiotics.
References
P.J. Barlass, C.W. Houston, M.O. Clements, A. Moir, Germination of Bacillus cereus spores in response to l-alanine and to inosine: the roles of gerL and gerQ operons. Microbiology 148, 2089–2095 (2002)
J.M. Crane, M.E. Frodyma, G.C. Bergstrom, Nutrient-induced spore germination of a Bacillus amyloliquefaciens biocontrol agent on wheat spikes. J. Appl. Microbiol. 116, 1572–1583 (2014)
N. Kumar, G. Thakur, H.V. Raghu, N. Singh, P.K. Sharma, V.K. Singh, A. Khan, M. Balhara, R. Avinash Lawaniya, S. Kouser, N. Tehri, R. Gopaul, A. Shivani, Bacterial spore based biosensor for detection of contaminants in milk. J. Food Process. Technol. 4(11), 1–6 (2013)
N.A. Mungroo, S. Neethirajan, Biosensors for the detection of antibiotics in poultry industry. Biosensors 4, 472–493 (2014)
D.F. Apata, Antibiotic resistance in poultry. Int. J. Poult. Sci. 8(4), 404–408 (2009)
M. Clauβen, D. Bahmann, S. Schmidt, Detection of antibiotic residues in food—pitfalls and optimization of agar diffusion tests in comparison with commercial test kits. in Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education, ed. by A. Méndez-Vilas (Formatex Research Center, Badajoz, 2013) pp. 359–366
N. Kumar, H.V. Raghu, A. Kumar, L. Haldar, A. Khan, S. Rane, R. Kumar Malik, Spore germination based assay for monitoring antibiotic residues in milk at dairy farm. World J. Microbiol. Biotechnol. 28, 2559–2566 (2012)
W.M. Wachira, A. Shitandi, R. Ngure, Determination of the limit of detection of penicillin G residues in poultry meat using a low cost microbiological method. Int. Food Res. J. 18(3), 1203–1208 (2011)
S. Shahrokh Esfahani, G. Emtiazi, R. Shafiei, N. Ghorbani, S.H. Zarkesh Esfahani, Tolerance induction of temperature and starvation with tricalcium phosphate on preservation and sporulation in Bacillus amyloliquefaciens detected by flow cytometry. Curr. Microbiol. (2016). doi:10.1007/s00284-016-1066-0
L.M. Hornstra, YPD Vries, WMD Vos, T. Abee, Influence of sporulation medium composition on transcription of ger operons and the germination response of spores of Bacillus cereus ATCC 14579. Appl. Environ. Microbiol. 72(5), 3746–3749 (2006)
W.H. Coleman, D. Chen, Y.Q. Li, A.E. Cowan, P. Setlow, How moist heat kills spores of Bacillus subtilis. J. Bacteriol. 189(23), 8458–8466 (2007)
C. Laflamme, S. Lavigne, J. Ho, C. Duchaine, Assessment of bacterial endospore viability with fluorescent dyes. J. Appl. Microbiol. 96, 684–692 (2004)
S. Shahrokh, G. Emtiazi, Toxicity and unusual biological behavior of nanosilver on gram positive and negative bacteria assayed by microtiter-plate. Eur. J. Biol. Sci. 1(3), 28–31 (2009)
Z. Xiao, R. Storms, A. Tsang, A quantitative starch–iodine method for measuring alpha-amylase and glucoamylase activities. Anal Biochem 351(1), 146–148 (2006)
R. Goodacre, B. Shann, R.J. Gilbert, E.M. Timmins, A.C. McGovern, B.K. Alsberg, D.B. Kell, N.A. Logan, Detection of the dipicolinic acid biomarker in bacillus spores using curie-point pyrolysis mass spectrometry and fourier transform infrared spectroscopy. Anal. Chem. 72, 119–127 (2000)
L.B. Kong, P.F. Zhang, G.W. Wang, P. Setlow, Y.Q. Li, Characterization of bacterial spore germination using phase contrast microscopy, fluorescence microscopy, Raman spectroscopy and optical tweezers. Nat. Protoc. 6, 625–639 (2011)
L.B. Kong, P.F. Zhang, J. Yu, P. Setlow, Y.Q. Li, Monitoring the kinetics of uptake of a nucleic acid stain during the germination of single spores of Bacillus species”. Anal. Chem. 82, 8717–8724 (2010)
L.B. Kong, P.F. Zhang, P. Setlow, Y.Q. Li, Characterization of bacterial spore germination using integrated phase contrast microscopy, Raman spectroscopy and optical tweezers. Anal. Chem. 82, 3840–3847 (2010)
P.F. Zhang, W. Garner, X. Yi, J. Yu, Y.Q. Li, P. Setlow, Factors affecting the variability in the time between addition of nutrient germinants and rapid DPA release during germination of spores of Bacillus species. J. Bacteriol. 192, 3608–3619 (2010)
A. Magge, A.C. Granger, P.G. Wahome, B. Setlow, V.R. Vepachedu, C.A. Loshon, L. Peng, D. Chen, Y.Q. Li, P. Setlow, Role of dipicolinic acid in the germination, stability and viability of spores of Bacillus subtilis. J. Bacteriol. 190, 4798–4807 (2008)
C.A. Allen, F. Babakhani, P. Sears, L. Nguyen, J.A. Sorg, Both fidaxomicin and vancomycin inhibit outgrowth of Clostridium difficile spores. Antimicrob. Agents Chemother. 57(1), 664–667 (2012)
Acknowledgements
The author gratefully acknowledges financial support of University of Isfahan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Rights and permissions
About this article
Cite this article
Esfahani, S.S., Emtiazi, G. & Rabbani, M. Germination assay of Bacillus amyloliquefaciens as a spore-based biosensing method for detection of cell wall destruction antibiotics. Food Measure 12, 441–448 (2018). https://doi.org/10.1007/s11694-017-9657-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-017-9657-4