Abstract
Lactic acid bacteria (LAB) and non-pathogenic staphylococci from naturally fermented sausages manufactured in northeastern Argentina were isolated and selected on the basis of physiological, technological, and safety properties. Eighty-seven isolates recovered from four small-scale facilities were studied to evaluate growth and acidification kinetics, nitrate-reductase, proteolytic, lipolytic, decarboxylase, and antagonistic activities, as well as growth ability at different temperatures, pH, and NaCl concentrations. Based on these characteristics as well as antibiotic resistance for CNC isolates, a selection and identification by sequencing the 16S rRNA gene was carried out. As a result, LAB isolates were identified as Lactobacillus sakei (seven isolates) and Lactobacillus farciminis (one isolate), while CNC revealed isolates as being Staphylococcus xylosus (two isolates), Staphylococcus vitulinus (one isolate), and Staphylococcus hominis (one isolate). Properties exhibited by selected isolates would make them eligible as starter cultures to enhance both sensorial and safety qualities of artisanal fermented sausages while keeping manufacturing traditions.
Similar content being viewed by others
Introduction
Fermentation and drying are some of the oldest technologies used to preserve food (e.g., meat) for long periods. The knowledge of preserving meat was inherited by Romans, and from then on, these products spread to central, eastern, and northern European countries, as well as to America and Australia (Fadda and Vignolo 2007). After both world wars, Argentina became the shelter for a number of European immigrants who brought their lifestyle and cooking habits to the country; particularly in the northeastern region, many of the typical fermented meat products are still manufactured in their traditional style. As outlined by recent abundant literature, there is an increased interest in traditional naturally fermented sausages. The fermentation of these products is known to rely on natural contamination by environmental microbiota occurring during slaughtering and manufacturing, the specific composition of the “house microbiota” being responsible for the distinctive qualities of artisanal products from small-scale facilities (Ammor et al. 2005; Lebert et al. 2007). Although this autochthonous microbiota plays a major role on flavor, texture, quality, and safety of the final product, the high variability in bacterial amount and species may induce quality problems due to lack of normalization and/or homogenization.
The most promising microorganisms for starter cultures are those selected from indigenous microbiota, which are competitive enough to dominate during fermentation, well adapted to the particular product and to the specific production technology, and with high metabolic capacities which can beneficially affect product quality and safety, preserving their typicity (Leroy et al. 2006). In fermented sausages, starter cultures consist of lactic acid bacteria (LAB), coagulase-negative cocci (CNC), and yeasts and molds, depending on the sausage type (Drosinos et al. 2007). In recent years, the use of molecular methods has allowed establishing the picture of fermented sausage microbiota. Among LAB, the predominance of Lactobacillus sakei, L. curvatus, and to a lesser extent L. plantarum, has been described. In addition, Staphylococcus xylosus and S. saprophyticus emerged as the most commonly isolated CNC, independently of the country and the technology used (Vignolo et al. 2010a). The role of the dominant microbiota was studied to identify enzymatic activities such as proteolysis and lipolysis whose end-products directly or indirectly are responsible for the sensory attributes of fermented sausages (Casaburi et al. 2005, 2008; Ravyts et al. 2010). LAB play a major role during fermentation affecting both the technological properties and the microbial stability of the final product through acid production and the consequent pH decrease. The production of antimicrobial substances by LAB isolated from fermented sausages has been reported (Vignolo et al. 2010b), which can be used either directly as starter cultures, as adjunct or co-cultures to enhance hygienic quality. On the other hand, the main role of CNC during sausage fermentation is their ability to reduce nitrate to nitrite, which is important for nitrosylmyoglobin formation, the compound responsible for the red color characteristic of fermented meats (Gøtterup et al. 2008; Hammes 2012). Regarding safety concerns, dry fermented sausages could be a natural reservoir of biogenic amines due to their high content of proteins and the proteolytic activity displayed by starter cultures and wild microflora, providing the precursors for decarboxylase activity (Suzzi and Gardini 2003). Hence, increasing attention is given to biogenic amines production. Fermented meat products may also act as a vehicle for high amounts of living bacteria into the human body, carrying transferable antibiotic resistance which may be transferred to commensal or pathogenic bacteria. Therefore, the presence of transmissible antibiotic resistance genes in starter culture isolates constitutes other important safety criterion. Nevertheless, the main challenge in developing starter cultures is to improve safety, but also to preserve the typical sensory quality of traditional sausages. The aim of this study was to characterize LAB and CNC isolated from traditional dry sausages manufactured in northeastern Argentina (Chaco province) on the basis of their biochemical, physicochemical, and technological properties relevant to sausage manufacture in view to select potential autochthonous starter culture.
Materials and methods
Production technology and sampling
Fermented dry sausages were prepared in four different small-scale factories (SSF) from northeastern Argentina (Chaco province) using traditional techniques without the use of starter cultures. Sausages were all alike in their formulations: pork, beef, and bacon in an average proportion of 60:30:10 thoroughly mixed together with salt (~2 %), wheat starch or milk powder (~1 %), sucrose (~2 %), spices (~2 %), and nitrite/nitrate salt (0.03 %). The meat dough obtained after mixing was used to fill natural casings (sheep gut). Sausages were fermented and ripened under non-standardized environmental conditions. From each of the four SSF (identified as A, B, C, and D), three samples were collected on three different instances in order to have samples from three different batches; they were transported to the laboratory in portable, insulated cold-boxes and stored at 4 °C until they were analyzed (usually, between 1 and 2 days after collection).
Physicochemical and microbiological analyses
Potentiometric measurements of pH were made by inserting a digital pH meter (Oakton®, Eutech Instruments, Singapore) in a diluted and homogenized sample containing 10 g of sausage and 90 ml of distilled water (Gounadaki et al. 2008). Moisture, ash, NaCl, nitrite, and nitrate determinations were performed according to AOAC procedures (AOAC 1995).
For microbiological determinations, sausage casing was aseptically removed, and 10 g of each sample derived as cross sections were homogenized with 90 ml of sterile solution containing 0.1 % (w/v) peptone (Oxoid, UK) and 0.85 % (w/v) NaCl (Anedra, Argentina) using a domestic blender. Decimal dilutions of the samples were prepared using the same diluent and plated in duplicate on different growth media for enumeration of lactic acid bacteria on de Man, Rogosa, Sharpe Agar (MRS, Biokar Diagnostics, France), incubated at 30 °C for 72 h; coagulase-negative cocci (CNC) and staphylococci on mannitol salt agar (MSA, Biokar Diagnostics), incubated at 37 °C for 72 h; total mesophilic aerobes on Plate Count Agar (PCA, Oxoid), incubated at 32 °C for 72 h; yeasts and molds on Sabouraud agar supplemented with 0.1 g L−1 of chloramphenicol (Oxoid), incubated at 25 °C for 5 days; Enterobacteriaceae on crystal Violet Red Bile Glucose Agar (VRBG, Oxoid) incubated at 37 °C for 24 h; and Staphylococcus aureus on Baird–Parker agar (BPA; Oxoid) supplemented with Tellurite Egg Yolk, incubated at 37 °C for 48 h. Sulphur-reducing clostridia were determined by pouring 10-ml aliquots from the first dilution (equal to 1 g sausage) in 20 ml of molten SPS agar (Sulphite Polymyxin Sulfadiazine agar; Merck, Germany); after solidification, the agar was overlaid with 5 ml of sterile paraffin (Merck) and incubated at 35 °C for 24 h. Results were expressed as log10 number of colony forming units per gram (log10 CFU g−1). Qualitative determination of Salmonella was assessed by the BAM-FDA method (Andrews et al. 2007) and absence of Listeria monocytogenes was checked according to BAM-FDA method (Hitchins and Jinneman 2011).
Preliminary characterization of LAB and CNC
For each plated sample, colonies were selected according to their morphological characteristics, Gram staining, and catalase test. Isolates were purified by successive subculturing on MRS and MSA for presumptive LAB and CNC, respectively. Colonies showing lecithinase activity on BPA (presumptive staphylococci) were tested for coagulase activity using rabbit plasma with EDTA (Beckton, Dickinson & Co., USA). Gas production from glucose was determined as described by Sperber and Swan (1976). The isolates were then maintained at −20 °C in MRS or Tryptic Soy Broth (TSB, Biokar Diagnostics) added with 20 % (v/v) glycerol (Merck) as a cryoprotective agent.
Effect of temperature, pH, and NaCl on the growth of presumptive LAB and CNC. Acidification kinetics of LAB
LAB growth was evaluated at 10, 15, 20, and 45 °C during 3 days, and at 4 °C during 5 days in MRS medium. As described by Ammor et al. (2005), growth at different pH values was investigated after 3 days of incubation at 30 °C in MRS agar with the addition of HCl (1 N) or NaOH (1 N) to reach pH 3.5, 4.5, 5.5, and 9.0. The effect of salt concentration was evaluated after 3 days of incubation at 30 °C in MRS agar added with 4.0, 6.5, or 10 % NaCl; after incubation, the presence of colonies on the plates was registered as a positive result. On the other hand, CNC growth was evaluated at 10, 15, and 20 °C in Yeast Triptone agar (YTA), a medium containing (per liter) triptone (Oxoid), 10 g; yeast extract (Merck), 5 g; NaCl, 4 g; agar (Britania, Argentina), 15 g (pH 7.0). The effect of NaCl was determined in YTA supplemented with 4.0, 6.5, and 8.0 % (w/v) NaCl. The effect of pH on bacterial growth was performed in YTA adjusted to pH 4.5, 5.0, and 5.5 by addition of HCl (0.1 M). Ten microliters of an overnight culture of each isolate were inoculated into the different described media and after incubation (3 days at 30 °C), the presence of colonies on the plates was registered as a positive result (Babić et al. 2011).
A culture medium designed for simulating technological conditions of sausage manufacturing, named after SB by Ammor et al. (2005), was used to evaluate acidification kinetics of presumptive LAB at 15 °C. The optical density (OD600) and pH values of each culture were monitored at 0, 8, 11, 14, 17, 20, 24, 32, and 48 h of incubation. Experiments were performed in duplicate and the obtained data were used to estimate the following kinetic parameters: growth lag phase (GL) and acidification lag phase (AL), growth rate (GK) and acidification rate (AK), OD600 , and pH at 48 h and ΔpH (AD) defined as the difference between initial and estimated final pH. Results were expressed as the estimated value ± standard deviation.
Nitrate-reductase assay
Nitrate broth containing meat extract 0.3 % (Biokar Diagnostics), peptone 0.5 %, and KNO3 0.1 % was used to determine nitrate reduction ability of isolates. After incubation for 24 h, drops of Griess A reagent (sulphanilic acid 0.6 % in acetic acid 5 N) and Griess B reagent (α-naphthylamine 0.5 % in acetic acid 5 N) were added for nitrite detection. Incubation was carried out at 30 and 37 °C for presumptive LAB and CNC, respectively. A red color in the broth indicated the presence of nitrate-reductase activity. Chemical substances not previously specified were from Merck.
Screening for proteolytic and lipolytic activity
Proteolytic activity on sarcoplasmic protein fractions was evaluated as described by Drosinos et al. (2007). The presence of clear zones around the wells indicated proteolytic activity. Lipolytic activity was assayed by inoculation of overnight bacterial cultures in nutrient broth (Britania) supplemented with 15 g l−1 agar and 10 g l−1 triolein (Sigma-Aldrich, Germany) and incubated for 5 days at 30 °C (LAB) and 37 °C (CNC). The appearance of transparent zone around the colonies was considered as an indicator for lipolysis.
Screening and characterization of antagonistic activity
Antagonistic activity was screened by means of an agar well diffusion assay (AWDA) as described by Castro et al. (2011). The following bacteria were used as indicators: Listeria innocua 7 (from Unité de Recherches Laitieres et Genetique Appliqué, INRA, France), Staphylococcus aureus ATCC 6538, Pseudomonas aeruginosa FBUNT, Staphylococcus aureus FBUNT (clinical isolates from Departamento de Microbiología, Facultad de Bioquímica, Química y Farmacia, Universidad Nacional de Tucumán, Argentina). Isolates exhibiting antagonistic activities against indicator microorganisms were further investigated for the nature of the produced antibacterial compounds, using Listeria innocua 7 and S. aureus ATCC 6538 as indicator microorganisms. After growth overnight in MRS at 30 °C (LAB) or TSB a 37 °C (CNC), bacterial cultures were harvested by centrifugation (4000 × g, 10 min at 4 °C) to obtain cell-free supernatants (CFS) and filtered through a 0.22-μm pore-size cellulose acetate filter (Sartorius, Germany). Samples were adjusted to pH 6.5 with 1 N of NaOH and catalase (300 IU ml-1) (Sigma-Aldrich) was added to rule out acid and H2O2 inhibition. Sensitivity of the antimicrobial compounds to proteolytic enzymes was investigated by the addition of trypsin (Sigma-Aldrich) and proteinase K (Sigma-Aldrich) at a final concentration of 1 mg ml−1 to the culture supernatants. The samples were incubated for 3 h at 37 °C and immediately after, the residual activity was determined by the AWDA.
Biogenic amine production
Production of biogenic amines was assessed by using the medium previously described by Bover-Cid and Holzapfel (1999). All isolates were streaked in duplicate onto the decarboxylation medium plates with and without amino acids (as control) and incubated for 3 days at 30 °C (LAB) or 37 °C (CNC). Growth of decarboxylating isolates was easily recognizable because of their purple halo in the yellow medium.
Antibiotic susceptibility
Petri dishes containing 25 ml of MRS or MHA (Mueller Hinton Agar, Biokar Diagnostics) were used for LAB and CNC, respectively. Cellular suspensions of DO625 = 0.08–0.1 were streaked onto the agar plates, and antibiotic discs (Oxoid) were placed on the agar which were incubated for 20–24 h at 30 °C (LAB) or 37 °C (CNC). LAB isolates were screened for their susceptibility to ampicillin (10 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), gentamicin (10 μg), erythromycin (15 μg), linezolid (30 μg), nitrofurantoin (300 μg), penicillin G (10 units), rifampicin (5 μg), tetracycline (30 μg), and vancomycin (30 μg), according to Charteris et al. (1998). For CNC isolates, the aforementioned antibiotics were assayed and the following were also included: amoxicillin/clavulanic 2:1 (30 μg), cefotaxime (30 μg), clindamycin (2 μg), imipenem (10 μg), quinupristin/dalfopristin (15 μg), and sulphametoxazole/trimethoprim 19:1 (cotrimoxazole, 25 μg) (Martín et al. 2006). Results were analyzed using the breakpoints recommended by the Clinical and Laboratory Standards Institute (CLSI 2010). Staphylococcus aureus ATCC 25923 was used as control bacterium for monitoring the performance of study conditions.
PCR detection of genes coding for staphylococcal enterotoxins
Genes for “classical” Staphylococcal enterotoxins (SE) (sea, seb, sec, sed, and see) were detected by a modified multiplex PCR method described by Løvseth et al. (2004). PCR was performed in a final volume of 50 μl containing 5 μl of PCR Buffer, 400 μM of each dNPT, 0.3 μM of each primer, 0.04 U μl−1 of TAQ polymerase. The amplification program consisted of an initial denaturation at 95 °C for 5 min, 14 cycles of denaturation at 95 °C for 30 s, annealing at 68 °C for 30 s, and extension at 72 °C for 30 s, 19 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s, and a final extension at 72 °C for 2 min.
Molecular identification of isolates
Genomic DNA was isolated from pure cultures according to Pospiech and Neumann (1995). Oligonucleotide primers (PLB16, 50-AGAGTTTGATCCTGGCTCAG-30; and MLB16, 50-GGCTGCTGGCACGTAGTTAG-30) were used to amplify the variable (V1) region of the 16S ribosomal RNA gene (Kullen et al. 1999). PCR was accomplished as described by Drake et al. (1996). PCR was carried out in a DNA Thermal cycler 480 (Perkin-Elmer Co., USA) and the reaction started by denaturation for 5 min at 94 °C followed by 30 cycles consisting of 1 min at 94 °C, 30 s at 48 °C, and 30 s at 72 °C for 30 s, and then a final extension at 72 °C for 10 min. PCR products were electrophoresed in 1.2 % agarose gels, stained with ethidium bromide, and photographed. Amplicons were excised from the gel and purified using a Prep A Gene DNA Purification Kit (Bio-Rad, USA). Purified PCR products were sequenced at the BioResource Center of Cornell University (USA). DNA homology searches were performed on line with the BLAST program (Altschul et al. 1997). The sequence of the partial 16S rRNA of Lactobacillus and Staphylococcus were submitted to the GenBank database (accession numbers AF429523, AY865606, AF429523, AY357581, AY341562, HQ315860, AM882700, EU580467, and Z26905).
Statistical analysis
Microbiological analyses were performed in duplicate while physicochemical determinations were performed in quadruplicate. In order to evaluate the homogeneity of the production within each facility, data were compared statistically using one-way analysis of variance (ANOVA). When the F-test was significant, the comparison of means was assessed by the Tukey test at a significance level of 5 %. Statgraphics Plus for Windows, Version 4.0 (Manugistics Inc., USA) was used for statistical analysis. A principal component analysis (PCA) was applied to growth and acidification data from LAB isolates. The computer software used was INFOSTAT 2009 (Córdoba, Argentina).
Results
Physicochemical and microbiological characteristics of sausages
Traditional dry fermented sausages manufactured without the addition of starter cultures in four SSF from northeastern Argentina were investigated. Three batches from each processing plant were analyzed. The results obtained from physicochemical and microbiological analyses are reported in Table 1. Nitrate and nitrite concentrations for all samples were lower than 300 and 150 mg kg−1 , respectively, these values being within the acceptable limits imposed by the legislation (data not shown). As a whole, the results showed physicochemical parameters to be significantly different between batches of the same SSF. Concerning microbiological analysis, despite their different origins, a predominance of LAB was detected in all analyzed samples, while CNC was the second largest microbiota. Regarding the hygienic status of sausages, Enterobacteriaceae counts were lower than 2 log CFU g−1, and no Staphylococcus aureus, Salmonella sp., Listeria sp., or sulfite-reducing clostridium were detected in any of the analyzed batches (data not shown).
Isolation of LAB and CNC. Effect of temperature, pH, and NaCl concentration on isolates
Ninety-three colonies were recovered from MRS and MSA enumeration plates, and the average number of LAB and CNC isolates from each SSF was similar. Among these colonies, 63 were considered presumptive LAB, being Gram-positive and catalase-negative, while 30 colonies from MSA were characterized as catalase-positive and coagulase-negative cocci. Six LAB isolates were discarded because of their heterofermentative profile, i.e., not suitable for sausage fermentation. The growth of LAB and CNC isolates under different temperature, pH, and NaCl conditions were evaluated and the results are presented in Table 2. All LAB isolates were able to grow at 10, 15, and 20 °C while only 20 isolates registered growth at 45 °C and nine isolates grew at 4 °C. Concerning the influence of pH, all LAB isolates grew at pH 4.5, 5.5, and 9.0 while only eight of them were able to grow at pH 3.5. In addition, LAB isolates showed growth in the presence of 4.0 and 6.5 % (w/v) NaCl, but only ten of them grew when salt concentration was 10 %. Among the 30 presumptive CNC, although all isolates grew at pH 5.0 and 5.5, only 12 showed growth at pH 4.5. Nevertheless, all CNC isolates grew in the presence of 4.0, 6.0, and 8.0 % (w/v) NaCl and temperatures of 10, 15, and 20 °C.
Technological and safety properties of the isolates
When the technological characterization of isolates was performed, seven out of 57 LAB and 20 out of 30 CNC isolates exhibited nitrate-reductase activity, while 35 LAB and three CNC isolates displayed proteolytic activity under the assayed conditions (Table 2). In addition, ten CNC isolates showed lipolytic ability when triolein was used as substrate, but none of the LAB isolates were able to hydrolyze this triglyceride. On the other hand, among the safety traits evaluated, cell-free supernatants from 52 LAB isolates were shown to exert antagonistic activity against S. aureus ATCC 6538; 38 of them were also effective in inhibiting S. aureus FBUNT, and only five isolates exhibited antilisterial activity. Among CNC, two isolates showed antagonistic activity against tested S. aureus strains while four and five isolates inhibited Listeria innocua and P. aeruginosa, respectively. When the proteinaceous nature of the inhibitory compounds produced by LAB and CNC isolates was investigated, resistance to the proteolytic enzymes (trypsin and proteinase K) was observed indicating inhibition was due to non-protein compounds. When biogenic amine production was evaluated, 25 out of 30 CNC isolates exhibited decarboxylase activity producing tyramine (11), histamine (13), putrescine (16), and cadaverine (12). Based on acidification kinetics data of presumptive LAB, ability to produce antagonistic substances and lack of decarboxilating activity of presumptive CNC, eight LAB and five CNC isolates were selected for antibiotic resistance and enterotoxin production investigation (Table 2). LAB isolates were found to be resistant to ciprofloxacin, gentamicin, and vancomycin, and one out of five CNC isolates exhibited tetracycline resistance. In addition, no enterotoxigenic genes were detected for any of the five CNC isolates (data not shown).
Growth and acidification kinetics
Principal component analysis (PCA) based on growth and acidification data obtained for all LAB isolates was performed in order to find the isolates with the best acidification kinetics. Data and LAB isolates represented on two axes (shown in Fig. 1) showed a cophenetic correlation coefficient of 0.925, indicating that the standardized data matrix adequately represented the original data. The estimated kinetic parameters analyzed by means of PCA showed the first discriminant function (PC 1) as the most important, accounting for 47 % of the explained variance, while 21.3 % was explained by the second function. However, kinetic parameters exhibited a wide variability evidencing the heterogeneity of LAB population associated with the studied fermented sausages. The biplot graph led to the identification of a well-defined group involving eight LAB isolates from the four analyzed SSF (A, B, C and D) presenting the best kinetic properties. The estimated parameters of LAB isolates selected from the PCA were summarized in Table 3.
Genetic identification
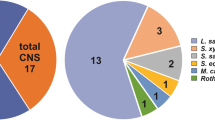
Taxonomical identification was performed by comparing the 16S rRNA gene sequences of isolates with sequences present in the GenBank database. Selected LAB isolates 434, 441, 442, 448, 470, 479, 487 were identified as Lactobacillus sakei, while isolate 464 was identified as Lactobacillus farciminis. In addition, CNC isolates were assigned to Staphylococcus vitulinus (one isolate), Staphylococcus xylosus (two isolates), and Staphylococcus hominis (one isolate).
Discussion
The physicochemical characterization of naturally fermented sausages from northeastern Argentina revealed a great variability among batches from the same SSF. There appeared to be no correlation between pH, moisture, and NaCl values within these products as expected for dry- or semi-dry fermented sausages. Since final NaCl and moisture levels in sausages are highly dependent on the conditions and extent of the ripening period, the differences found in batches from the same SSF highlight the lack of standardization of fermentation and ripening processes; conditions are highly variable in artisanal plants where ripening rooms with controlled temperature, relative humidity, and air speed are not common. According to Vignolo et al. (2010a) and based on final pH (>5.0) and moisture (>35 %) found in this study, most of the analyzed sausages may be described as belonging to semi-dry products. Semi-dry fermented sausages are typical from central and northern European countries, which coincides with the origin of immigrated people established in northeast Argentina.
Despite the different sausage provenances, LAB became the dominant population in all samples owing to the good adaptation of LAB to the meat environment as well as the fast growth rate displayed during fermentation and ripening of sausages. This is in agreement with data reported for other fermented sausages (Fontana et al. 2005; Silvestri et al. 2007; Cocolin et al. 2009; Federici et al. 2014; Ducic et al. 2014). Concerning the CNC counts, they were found to be variable, in some batches being higher than those found in other fermented sausages in which numbers were lower than 106 CFU g−1 (Fontana et al. 2005; Kozačinski et al. 2008), while in other batches they were similar to those reported by Polka et al. (2015), García Fontán et al. (2007), and Comi et al. (2005). The lower counts for CNC in fermented sausages reflect their poor competiveness in the presence of actively growing acidogenic LAB (Leroy et al. 2006). Yeast and mold numbers showed to be in the range of those found at the end of ripening in other naturally fermented Argentinian sausages (Fontana et al. 2005), though counts were variable depending on the moisture content of samples. On the other hand, the presence of low Enterobacteriaceae counts and the absence of sulfite-reducing bacteria, Staphylococcus aureus, Salmonella sp., and Listeria sp. confirm the strong competitive effect of LAB on the rest of the endogenous microbiota as is observed in other traditional fermentations (Aymerich et al. 2003; Comi et al. 2005; Drosinos et al. 2007; Polka et al. 2015). These results underline the good hygienic quality of the meat, spices, and natural casings used for sausage production, as well as the appropriate manufacturing practices applied in the different SSF as no pathogens were detected.
In view of selecting LAB and CNC with optimal characteristics to be used as starter culture, presumptive LAB (57) and CNC (30) isolates were subjected to physiological, technological, and safety characterization (Table 2). LAB and CNC isolates exhibited physiological traits characteristic for each bacterial group; however, differences for several physiological properties such as growth at 45 °C, pH 3.5 and in the presence of 10 % NaCl for LAB and growth at pH 4.5 for CNC isolates were found, these traits being considered strain dependent (Sanchez et al. 2000). Nevertheless, LAB and CNC isolates grew at temperatures usually used for meat fermentation (15 and 20 °C), from pH 4.5 to 5.5 and in the presence of 4.0 to 8.0 % NaCl, in agreement to those reported for isolates from naturally fermented Greek and Italian sausages (Papamanoli et al. 2003; Casaburi et al. 2005).
The growth and acidification rate under conditions prevailing during sausage fermentation are limiting factors affecting the persistence and competitiveness of the starter culture over the entire process. In this study, although kinetic parameters associated with BAL showed a great variability, eight LAB isolates presenting the best kinetic characteristics from the four SSF were discriminated by applying PCA (Fig. 1). Similar growth and acidification parameters were reported for L. sakei isolates from different stages of dry sausages fermentation (Ammor et al. 2005).
Enzyme profiling is an important factor for the selection of isolates as starter cultures. It was observed in this study that CNC and, to a lesser extent, LAB isolates displayed the ability to reduce nitrate. Nitrate-reductase activity is a widespread feature among CNC; it has been detected in staphylococci isolates including Staphylococcus equorum, S. xylosus, and S. carnosus (Gøtterup et al. 2008; Cachaldora et al. 2013). Staphylococcus vitulinis and S. hominis isolated from Spanish dry cured meat products also showed nitrate-reductase activity (Landeta et al. 2013a).
Proteolytic and lipolytic activities are important technological properties of LAB and CNC in fermented meat products because of their influence on texture and flavor development (Drosinos et al. 2007). The degradation of sarcoplasmic proteins by 35 presumptive LAB in this study is in agreement with the role of meat-borne LAB exo-peptidases previously described (Sanz et al. 2002). As regards CNC, only 10 % of these isolates showed proteolytic activity. Landeta et al. (2013a) also found a low percentage of CNC isolates having proteolytic activity (15 %). On the contrary, Cachaldora et al. (2013) reported that 89.5 % of CNC isolates were able to hydrolyze sarcoplasmic proteins. These differences could be attributed to the strain-specific character of this property and the methodology used to evaluate it. Among the LAB and CNC isolates evaluated for lipolytic activity, only ten presumptive CNC were able to degrade triolein, underlining the relative abundance of lipolytic enzymes amongst staphylococci as well as the weak lipolytic system of LAB as reported by Drosinos et al. (2007). Results found in this study are not easily comparable against results from other authors working with fermented meat products since substrates used to evaluate proteolytic and lipolytic properties differ from each other. To overcome this drawback, and consequently harmonize data to be comparable, Landeta et al. (2013a) suggested that meat substrates (muscle proteins, sarcoplasm, or pork fat) could be more suitable for the detection of these activities on potential meat starters than other substrates currently used (powdered milk, gelatin, tributyrin, or Tween 80 and Tween 20).
Concerning safety features, antimicrobial activity against Staphylococcus aureus and Listeria was detected for both LAB and CNC presumptive isolates while a compound anti-Pseudomonas was only produced by several CNC isolates. Although LAB are well known as bacteriocin producers, the non-protein nature of the produced antagonistic substances leads to suggest lactic acid as responsible for the antimicrobial effect, which correlates with the high sensitivity of S. aureus to acid environment. On the other hand, no staphylococci isolated from fermented sausages have been described as bacteriocinogenic despite the fact that a few species from other sources have already been described (Nascimento et al. 2005).
The presence of biogenic amines is a relevant food issue in meat products. Therefore, a screening for amino acid descarboxylase activity is recommended before selecting LAB and CNC as appropriate starter cultures for this food industry (Landeta et al. 2013a, 2013b). None of the LAB isolates examined in this study possessed decarboxylase activity. These results are in agreement with those reported by Landeta et al. (2013b) for non-enterococcal LAB. Twenty-five presumptive CNC isolates were found as amine positive; available data are consistent with the fact that biogenic amine production is more common amongst staphylococci than LAB isolates (Ansorena et al. 2002; Drosinos et al. 2007). The biogenic amines produced by CNC isolates in this study are in coincidence with those reported for different traditional fermented sausages (Cachaldora et al. 2013; Landeta et al. 2013a).
The high prevalence of antibioresistance in commercial starter cultures (Kastner et al. 2006) raised the demand for new starters lacking antibiotic resistance genes. Results from this study showed that from five CNC isolates only one exhibited tetracycline resistance while the eight selected LAB isolates displayed resistance to at least three antibiotics (ciprofloxacin, gentamicin and vancomycin). As reported by Talon and Leroy (2011), although being variable between isolates, the most frequent resistances found in CNC of food origin were those to ampicillin, erythromycin, penicillin, lincomycin and tetracycline. Concerning LAB isolates, results are in agreement with the high natural intrinsic resistance to ciprofloxacin, gentamicin and streptomycin reported (Talon and Leroy 2011; van Reenen and Dicks 2011). Particularly, Lactobacillus sakei and L. curvatus from slightly fermented sausages were reported to be resistant to vancomycin and gentamicin (Aymerich et al. 2006). However and, as adopted by EFSA (2005), intrinsic resistance and resistance due to mutation of chromosomal genes present low risk of horizontal dissemination and such isolates should be acceptable for consumption.
On the basis of physiological and technological characterization as well as safety considerations, selected LAB (eight) and CNC (four) isolates were identified by sequencing the 16S rRNA gene revealing LAB isolates 434, 441, 442, 448, 470, 479, and 487 as belonging to Lactobacillus sakei and isolate 464 as L. farciminis species. On the other hand, two CNC isolates were identified as Staphylococcus xylosus while the remaining selected isolates corresponded to S. vitulinus and S. hominis. These results are in agreement with those reported for other traditional fermented sausages such as southern European and Argentinian products, in which L. sakei and L. curvatus were shown to be the main LAB species strongly present, while S. xylosus was found representing CNC (Vignolo et al. 2010a). Since the main bacterial species were almost the same among the different products, the great adaptation of these microorganisms to the stringent environment of fermented meat ecological niches must be highlighted. These well-adapted biotypes are promising isolates for the development of autochthonous starter cultures that will allow sausages to be produced with both high hygienic and sensory quality (Casaburi et al. 2007; Talon et al. 2008).
Conclusions
Physicochemical and microbiological parameters found for naturally fermented sausages from northeastern Argentina were within the range of those observed for other traditional fermented products from several countries; however, a great variability among batches from the same small-scale facility was found. Whilst the hygienic quality of these products was acceptable, the heterogeneity shown by technological features lead to lack of uniformity of the final products. The selection and identification of eight LAB isolates, seven Lactobacillus sakei and one L. farciminis, as well as four CNC isolates, Staphylococcus xylosus (two isolates) and S. vitulinus and S. hominis, constitutes the first stage in the designing process of starter culture endogenous to small-scale facilities. The use of autochthonous starter cultures is an effective tool for limiting the formation of unsafe compounds in traditional sausage while preserving their typical sensory properties and keeping traditions alive.
References
Altschul S, Madden T, Schüffer A, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST a new generation of protein database search programs. Nucleic Acid Res 25:3389–3402
Ammor S, Dufour E, Zagorec M, Chaillou S, Chevallier I (2005) Characterization and selection of Lactobacillus sakei strains isolated from traditional dry sausage for their potential use as starter cultures. Food Microbiol 22:529–538
Andrews W, Jacobson A, Hammack T (2007) Bacteriological Analytical Manual. Chapter 5: Salmonella. U.S. FDA Editions http://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm070149.htm. Accessed 2 April 2012
Ansorena D, Montel MC, Rokka M, Talon R, Eerola S, Rizzo A, Raemaekers M, Demeyer D (2002) Analysis of biogenic amines in northern and southern European sausages and rol of flora in amine production. Meat Sci 61(2):141–147
AOAC (1995) Official methods of analysis, 14th edn. Association of Official Analytical Chemists, Washington
Aymerich T, Martín B, Garriga M, Hugas M (2003) Microbial quality and direct PCR identification of lactic acid bacteria and non-pathogenic staphylococci from artisanal low-acid sausages. Appl Environ Microbiol 69(8):4583–4594
Aymerich T, Martín B, Garriga M, Vidal-Carou M, Bover-Cid S, Hugas M (2006) Safety properties and molecular strain typing of lactic acid bacteria from slightly fermented sausages. J Appl Microbiol 100(1):40–49
Babić I, Markov M, Kovačević D, Trontel A, Slavica A, Đugum J, Čvek D, Svetec IK, Posavec S, Frece J (2011) Identification and characterization of potential autochthonous starter cultures from a Croatian “brand” product “Slavonski kulen. Meat Sci 88:517–524
Bover-Cid S, Holzapfel W (1999) Improved screening procedure for biogenic amine production by lactic acid bacteria. Int J Food Microbiol 53:33–41
Cachaldora A, Fonseca S, Franco I, Carballo J (2013) Technological and safety characteristics of Staphylococcaceae isolated from Spanish traditional dry-cured sausages. Food Microbiol 33:61–68
Casaburi A, Blaiotta G, Mauriello G, Pepe O, Villani F (2005) Technological activities of Staphylococcus carnosus and Staphylococcus simulans strains isolated from fermented sausages. Meat Sci 71:643–650
Casaburi A, Aristoy MC, Cavella S, Di Monaco R, Ercolini D, Toldrá F, Villani F (2007) Biochemical and sensory characteristics of traditional fermented sausages of Vallo di Diano (Southern Italy) as affected by use of starter cultures. Meat Sci 76:295–307
Casaburi A, Di Monaco R, Cavella S, Toldrá F, Ercolini D, Villani F (2008) Proteolytic and lipolytic starter cultures and their effect on traditional fermented sausages ripening and sensory traits. Food Microbiol 25:335–347
Castro M, Palavecino N, Herman C, Garro O, Campos C (2011) Lactic acid bacteria isolated from artisanal dry sausages: characterization of antibacterial compounds and study of the factors affecting bacteriocin production. Meat Sci 87:321–329
Charteris W, Kelly P, Morelli L, Collins J (1998) Antibiotic susceptibility of potentially probiotic Lactobacillus species. J Food Prot 61:1636–1643
CLSI. Clinical and Laboratory Standards Institute (2010) Performance standards for antimicrobial susceptibility testing. Twentieth informational suplement. Document M100-S20 (ISBN 1-56238-716-2). West Valley Road, Suite 1400, Wayne
Cocolin L, Dolci P, Rantsiou K, Urso R, Cantoni C, Comi G (2009) Lactic acid bacteria ecology of three traditional fermented sausages produced in the North of Italy as determined by molecular methods. Meat Sci 82:125–132
Comi G, Urso R, Iacumin L, Rantsiou K, Cattaneo P, Cantoni C, Cocolin L (2005) Characterisation of naturally fermented sausages produced in the North East of Italy. Meat Sci 69:381–392
Drake M, Small C, Spence K, Swanson B (1996) Rapid detection and identification of Lactobacillus spp. in dairy products by using the polymerase chain reaction. J Food Prot 59:1031–1036
Drosinos EH, Paramithiotis S, Kolovos G, Tsikouras I, Metaxopoulos I (2007) Phenotypic and technological diversity of lactic acid bacteria and staphylococci isolated from traditionally fermented sausages in Southern Greece. Food Microbiol 24:260–270
Ducic M, Blagojevic B, Markov S, Velicanski A, Buncic S (2014) General patterns of background microbiota and selected bacterial pathogens during production of fermented sausages in Serbia. Food Control 43:231–237
EFSA (2005) Opinion of the scientific panel on additives and products or substances used in animal feed on the updating of the criteria used in the assessment of bacteria for resistance to antibiotics of human or veterinary importance. EFSA J 223:1–12
Fadda SG, Vignolo GM (2007) Central and South American products. In: Toldrá F (ed) Handbook of fermented meat and poultry. Blackwell Publishing, London, pp 387–392
Federici S, Ciarrocchi F, Campana R, Ciandrini E, Blasi G, Baffone W (2014) Identification and functional traits of lactic acid bacteria isolated from Ciauscolo salami produced in Central Italy. Meat Sci 98:575–584
Fontana C, Cocconcelli P, Vignolo G (2005) Monitoring the population dynamics during fermentation of artisanal argentinian sausages. Int J Food Microbiol 103:131–142
García Fontán M, Lorenzo J, Martinez S, Franco I, Carballo J (2007) Microbiological characteristics of Botillo, a Spanish traditional pork sausage. LWT Food Sci Technol 40:1610–1622
Gøtterup J, Olsen K, Knøchel S, Tjener K, Stahnke L, Møller J (2008) Colour formation in fermented sausages by meat-associated staphylococci with different nitrite- and nitrate-reductase activities. Meat Sci 78:492–501
Gounadaki A, Skandamis P, Drosinos E, Nychas G (2008) Microbial ecology of food contact surfaces and products of small-scale facilities producing traditional sausages. Food Microbiol 25:313–323
Hammes W (2012) Metabolism of nitrate in fermented meats: the characteristic feature of a specific group of fermented foods. Food Microbiol 29:151–156
Hitchins A, Jinneman K (2011) Bacteriological Analytical Manual. Chapter 10: Listeria monocytogenes. U.S. FDA Editions. http://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm071400.htm. Accessed 2 April 2012
Kastner S, Perreten V, Bleuler H, Hugenschmidt G, Lacroix C, Meile L (2006) Antibiotic susceptibility patterns and resistance genes of starter cultures and probiotic bacteria used in food. Syst Appl Microbiol 29:145–155
Kozačinski L, Drosinos E, Čaklovica F, Cocolin L, Gasparik-Reichardt J, Veskovič S (2008) Investigation of microbial association of traditionally fermented sausages. Food Technol Biotechnol 46:93–106
Kullen M, Sanozky-Dawes R, Klaenhammer T (1999) Use of the DNA sequence of a variable (V1) region of the 16S gene for rapid and accurate identification of bacteria in the Lactobacillus acidophilus complex. NCBI, Databank. Accession number AF133264
Landeta G, Curiel J, Carrascosa A, Muñoz R, de las Rivas B (2013a) Characterization of coagulase-negative staphylococci isolated from Spanish dry cured meat products. Meat Sci 93:387–396
Landeta G, Curiel J, Carrascosa A, Muñoz R, de las Rivas B (2013b) Technological and safety properties of lactic acid bacteria isolated from Spanish dry-cured sausages. Meat Sci 95:272–280
Lebert I, Leroy S, Talon R (2007) Microorganisms in traditional fermented meats. In: Toldrá F, Nip WK, Sebranek JG, Stahnke LH, Expedito T, Silvera FT, Talon R, Hui YH (eds) Handbook of fermented meat and poultry. Blackwell Publishing, Ames, pp 113–124
Leroy F, Verluyten J, De Vuyst L (2006) Functional meat starter cultures for improved sausage fermentation. Int J Food Microbiol 106:270–285
Løvseth A, Loncarevic S, Verdal K (2004) Modified multiplex PCR method for detection of pirogenic exotoxin genes in staphylococcal isolate. J Clin Microbiol 42(8):3869–3872
Martín B, Garriga M, Hugas M, Bover-Cid S, Veciana-Nogués M, Aymerich T (2006) Molecular, technological and safety characterization of Gram-positive catalase-positive cocci from slightly fermented sausages. Int J Food Microbiol 107:148–158
Nascimento J, Giambiagi-deMarval M, de Oliveira S, Ceotto H, dos Santos K, Freire Bastos M (2005) Genomic fingerprinting of bacteriocin-producer strains of Staphylococcus aureus. Res Microbiol 156:837–842
Papamanoli E, Tzanetakis N, Litopoulou-Tzanetaki P, Kotzekidou P (2003) Characterization of lactic acid bacteria isolated from a Greek dry-fermented sausage in respect of their technological and probiotic properties. Meat Sci 65:859–867
Polka J, Rebecchi A, Pisacane V, Morelli L, Puglisi E (2015) Bacterial diversity in typical Italian salami at different ripening stages as revealed by high-throughput sequencing of 16S rRNA amplicons. Food Microbiol 46:342–356
Pospiech A, Neumann B (1995) A versatile quick-prep of genomic DNA from Gram-positive bacteria. Trends Genet 11:217–218
Ravyts F, Steen L, Goemaere O, Paelinck H, De Vuyst L, Leroy F (2010) The application of staphylococci with flavour-generating potential is affected by acidification in fermented dry sausages. Food Microbiol 27:945–954
Sanchez I, Palop L, Ballesteros C (2000) Biochemical characterization of lactic acid bacteria isolated from spontaneous fermentation of ‘Almagro’ eggplants. Int J Food Microbiol 59:9–17
Sanz Y, Sentandreu M, Toldrá F (2002) Role of muscle and bacterial exopeptidases in meat fermentation. In: Toldrá F (ed) Research advances in the quality of meat and meat products. Research Singpost, Trivandrum, pp 143–155
Silvestri G, Santarelli S, Aquilanti L, Beccaceci A, Osimani A, Tonucci F, Clementi F (2007) Investigation of the microbial ecology of Ciauscolo, a traditional Italian salami, by culture-dependent techniques and PCR-DGGE. Meat Sci 77:413–423
Sperber W, Swan J (1976) Hot-loop test for the determination of carbon dioxide production from glucose by lactic acid bacteria. Appl Environ Microbiol 31:990–991
Suzzi G, Gardini F (2003) Biogenic amines in dry fermented sausages: a review. Int J Food Microbiol 88:41–54
Talon R, Leroy S (2011) Diversity and safety hazards of bacteria involved in meat fermentations. Meat Sci 89:303–309
Talon R, Leroy S, Lebert I, Giammarinaro P, Chacornac JP, Latorre-Moratalla ML, Vidal-Carou MC, Zanardi E, Conter M, Lebecque A (2008) Safety improvement and preservation of typical sensory qualities of traditional dry fermented sausages using autochthonous starter cultures. Int J Food Microbiol 15:227–234
van Reenen C, Dicks L (2011) Horizontal gene transfer amongst probiotic lactic acid bacteria and other intestinal microbiota: what are the possibilities? A review. Arch Microbiol 193:157–168
Vignolo GM, Fontana C, Cocconcelli PS (2010a) New approaches for the study of lactic acid bacteria biodiversity: a focus on meat ecosystems. In: Mozzi F, Raya R, Vignolo G (eds) Biotecnology of lactic acid bacteria. Novel applications. Blackwell Publishing, Iowa, pp 251–271
Vignolo G, Saavedra L, Sesma F, Raya R (2010b) Food bioprotection: lactic acid bacteria as natural preservatives. In: Bhat R, Alias AK, Paliyath G (eds) Progress in food preservation. Blackwell Publishing, Iowa, pp 379–398
Acknowledgments
We acknowledge the financial support from Consejo Nacional de Investigaciones Científicas y Técnicas de la República Argentina (CONICET), Agencia Nacional de Investigaciones Científicas y Técnicas de la República Argentina (ANPCyT, PICT 2008 1371), and Universidad Nacional del Chaco Austral. We are thankful to Mirtha Dupraz for protein quantification in the proteolytic activity screening.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palavecino Prpich, N.Z., Castro, M.P., Cayré, M.E. et al. Autochthonous starter culture selection to keep traditions in the manufacture of dry sausages alive. Ann Microbiol 65, 1709–1719 (2015). https://doi.org/10.1007/s13213-014-1010-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-014-1010-0