Abstract
The litter-dwelling fungus Fusarium incarnatum LD-3 has been identified as a novel producer of laccase. The present work was oriented towards the optimization of various cultivation conditions for maximizing laccase production under solid substrate fermentation. The process parameters were optimized by the “one factor at a time” approach. Maximum laccsase production was obtained at pH 5.0 and at a temperature of 28 °C with 60 % moisture content using rice bran as a substrate. The laccase production was enhanced in the presence of aromatic inducer, i.e. ortho-dianisidine at a concentration of 0.5 mM. Laccase production was further increased by 52.56 % when the medium was supplemented with 2 % (v/v) alcohol. Among the various amino acids tested as a growth factor and nitrogen source, D-Serine and DL-2 Amino n-butyric acid, DL-Alanine and L-Glycine were found to be the most suitable for laccase production. The highest laccase production (1,352.64 U/g) was achieved under optimized conditions, and was 2.1-fold higher than the unoptimized conditions. Thus, the novel litter-dwelling fungal isolate Fusarium incarnatum LD-3 seems to be an efficient producer of laccase and can be further exploited for biotechnological applications. This is the first report on the optimization of cultivation conditions and inducers for laccase production from Fusarium incarnatum LD-3.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Laccase (benzenediol:oxygen oxidoreductase (E.C.1.10.3.2), is a type of copper protein belonging to the oxidoreductase family (Thurston 1994; Brenna and Bianchi 1994). This enzyme is a glycoprotein with molecular masses of 50–130 kDa and requires oxygen to oxidize phenols, polyphenols, aromatic amines, and other non-phenolic compounds. When oxidized by laccase, the reducing substrate uses a single electron and usually forms a free radical, which may undergo further laccase catalyzed oxidation or non-enzymatic reactions including hydration, disproproteonation, and polymerization. Radicals, and other small molecules called as mediators, contribute significantly to the degradation of non-phenolic compounds present in the lignin. Laccases have a broad range of industrial and biotechnological applications. Currently, laccases are used in pulp delignification, textile dye bleaching, effluent detoxification, washing powder components, removal of phenolics from cork stoppers.(Brenna and Bianchi 1994), transformation of antibiotics and steroids (Breen and Singleton 1999), and in nanobiotechnology for the development of biosensors to detect various phenoloic compounds, oxygen, or azides (Ghindilis et al. 1992). In nature, laccase is found in the diverse group of organisms such as plants, bacteria, insects, and fungi. However, most of the studies have so far been focused on the model white rot species Trametes versicolor, Pleurotus ostreatus, and a few others. There are few studies on the litter-dwelling fungi, especially ascomycetes involved in the production and distribution of phenoloxidases, laccases, and peroxidases (Steffen et al. 2000, 2003). There are several reports in the literature on the production of laccase in ascomycetes such as Gaeumannomyces graminis (Edens et al. 1999), Magnaporthe grisea (Iyer and Chattoo 2003), Ophiostoma novo-ulmi (Binz and Canevascini 1997), and Neurospora crassa (Froehner and Eriksson 1974). In addition to such plant pathogenic species, some ascomycete species of soil litter from the genera Aspergillus, Curvularia, and Penicillium have also been found to be laccase producers (Banerjee and Vohra 1991; Rodŕiguez et al. 1996; Scherer and Fischer 1998). The application of laccase in biotechnological processes requires the production of large amounts of enzyme at low cost, and hence the current focus of laccase research is oriented towards the search for an efficient production system. Culture conditions and medium composition play a major role in enzyme expression, and in natural lignin-enhanced lignin-degrading enzyme production in fungi (Piccard et al.1999). Solid Substrate Fermentation (SSF) is an important mode of fermentation where the substrate itself acts as a carbon source and occurs in the absence or near absence of free water and excretes the desired product efficiently (Pandey et al. 2000; Bellon-Maurel et al. 2003). The use of SSF as a method of laccase production can offer some apparent economic and engineering advantages, like high volume productivity, high concentration of product, lower capital investment, and lower operating costs. Another important feature of SSF is that it utilizes hetrogenous products of agriculture, mainly agricultural residues and by products of agro-based industries. Thus, this traditional method has re-ignited the interest of researchers in the production of enzymes, fine chemicals, and antibiotics (Zubeyde et al. 2003; Kumar et al. 2003; Adinarayana et al. 2003). In litter-dwelling fungi, extracellular laccase is produced in small amounts and its production is affected by many typical fermentation factors such as medium composition, carbon:nitrogen ratio, pH, temperature, aeration rate, etc. (Revankar and Lele 2006). A number of aromatic compounds related to lignin or lignin derivatives, such as ferulic acid, 2,5-xylidine, p-anisidine, veratryl alcohol, abietic acid, various phenols (catechol, 4-chlorophenol, 2,6-dimethoxy phenol, guaiacol); several derivatives of benzoic acid (benzoic, 2,6 dimethoxybenzoic, syringic, vanillic and veratric acids) are known to influence extracellular laccase production from fungi (Barbosa et al. 1996; Ikehata et al. 2004).
The present study demonstrates the potential for the special ecological group of the litter-dwelling fungus (LDF) Fusarium incarnatum LD-3 (Genbank Accession No. EU426883) belonging to the ascomycota phylum for the production of lignin modifying enzymes like laccase under solid substrate fermentation. Attempts were made to optimize the various cultivation conditions and to evaluate the effect of various inducers and amino acids by the conventional “one factor at a time” methodology.
Materials and methods
Chemicals
2,2-Azino-bis (3-ethylbenzthiozoline-6-sulphonic acid) (ABTS) was purchased from Sigma (St. Louis, MO, USA). Sabaroud dextrose agar and Malt extract agar were procured from Hi-Media (Mumbai, India). Yeast extract, peptone, and amino acid kit were procured from Hi-Media (Mumbai). Ortho-dianisidine, gallic acid, veratryl alcohol, vanillin, catechol, and guaiacol were procured from CDH (Mumbai, India). Thiamine HCl, NH4NO3, CuSO .4 5H2O, CoCl .2 6H2O, MnSO .4 H2O, ammonium ferric citrate, MgSO .4 7H2O, CaCl .2 2H2O, ZnSO .4 7H2O, and KH2PO4 were purchased from SD Fine Chemicals (Mumbai, India). Tween 80 was purchased from Merck (Mumbai, India). All other chemicals were of analytical grade procured from Qualigens (Mumbai, India). Wheat straw, sugarcane bagasse, saw dust, rice bran, and wheat bran were collected locally and used as lignocellulosic substrates.
Collection of soil sample and isolation of fungal strains
A total of 18 soil samples were collected from the different niches near the region of decomposed litter from various locations of the forest of Panchmarhi, MadhyaPradesh, India (22°28′0″N, 78°26′0″E). Soil samples 1 % (w/w) were serially diluted with the saline solution and were plated on 2 % (w/v) malt extract agar added with 0.05 % (w/v) chloromphenicol and incubated at 28 ± 2° C. Further isolation was performed on Sabaroud Dextrose Agar (SDA) containing (g/1) glucose, 20; peptone, 10; NaCl, 2.5; agar-agar, 30; penicillin G, 0.06; streptomycin sulfate, 0.0001 supplemented with filter sterilized (g/l) ortho-dianisidine 0.1, guaiacol 0.1, and ABTS 0.1. The selected fungal isolates were maintained on the 2 % (w/v) malt extract agar medium without antibiotics.
Media and culture conditions for SSF
The medium for the solid substrate fermentation was prepared by mixing 5 g of milled wheat straw and sugarcane bagasse with 50 mesh size and the same amount of oven-dried saw dust, wheat bran, and rice bran with 20 ml of medium contained (g/l) of KH2PO4, 0.2; CaCl .2 2H2O, 0.0132; MgSO .4 7H2O, 0.05; ammonium ferric citrate, 0.085; ZnSO4·7H2O, 0.0462; MnSO4·7H2O, 0.035; CoCl2·6H2O, 0.007; CuSO4·5H2O, 0.007; L-asparagine, 1.0; NH4NO3, 0.5; thiamine-HCl, 0.0025; yeast extract, 0.2; glucose, 10. The medium was supplemented with Tween-80, 0.01 % ( v/v) and buffered with 20 mM pthalate buffer pH 3.0–8.0. (Asther et al. 1988).The medium was sterilized by autoclaving at 103.4 × 103 Pa (121 °C) for 15 min. Thiamine-HCl, ortho-dianisidine, guaiacol, ABTS , aromatic inducers, and different amino acids were filter sterilized and added separately to the rest of the medium before inoculation. Lignocellulosic substrates were separately sterilized at 103.4 × 103 Pa (121 °C) for 30 min and mixed aseptically with the medium before inoculation. Each flask was inoculated with four mycelial agar plugs 8 mm in diameter (cut from the edge of an actively growing colony on malt extract agar plates) and incubated under static condition at 20–50 °C for 8 days. After 8 days of incubationm 10 ml. of acetate buffer (pH 5.0, 100 mM) was added to each flask and kept on the rotatory shaker for 30 min at 28 ± 2° C. The contents were then transferred to muslin cloth and squeezed. Liquid extract obtained was centrifuged at 8,000 g at 4 °C for 15 min and the supernatant was analyzed for enzyme activity.
Enzyme assay
Laccase activity (E.C.1.10.3.2) was determined by measuring the oxidation of 2, 2-azino-bis (3-ethylbenzthiozoline-6-sulphonic acid) (ABTS). The increase in absorbance for 3 minutes was measured spectrophotometrically (Ellico BL-198, Hyderabad, India) at 420 nm (ε = 36,000 cm−1 M−1) (Niku-Paavola et al. 1990). The reaction mixture contained 100 μl of 50 mM ABTS, 800 μl of 20 mM Na-Acetate butter (pH-5.0) and 100 μl of appropriately diluted enzyme extract. One unit of enzyme was defined as the amount of enzyme that oxidized 1 μM of substrate per minute.
Estimation of fungal biomass
Biomass was determined by weighing the dry mycelia after growth. The attached mycelia were squeezed to remove the medium, washed with the distilled water, and dried completely at 60 °C. Biomass was calculated by subtracting the initial weight of the solid substrate (5 g) measured for the abiotic (uninoculated control) from the final weight (Mazumder et al. 2009).
Data analysis
The data in subsequent sections represent arithmetic mean values of three experimental repetitions (each one was made in duplicate).
Results and discussion
Screening and isolation of fungal cultures
Forty-one fungal strains from the different soil samples were isolated and 19 were observed to be positive for the presence of laccase/phenol oxidase activity when 0.01 % (w/v) of different chromogenic indicators, like ortho-dianisidine, guaiacol, and ABTS, were incorporated into the cultivation medium (Table 1). Out of these 41 isolates, 19 showed a brown-colored zone for ortho-dianisidine and guaiacol while bluish purple-colored zone for ABTS surrounding the growth on Sabourauds dextrose agar plate. Depending upon the intensity of the color produced and the time required for the appearance of the color due to the oxidation of chromogens, fungal strains are classified as poor producer, moderate producer, good producer, and excellent producer of laccase/phenol oxidase. Among the three different chromogenic substrates tested, ABTS was found to be very sensitive, which allowed rapid screening of fungal strains producing extracellular ABTS oxidizing enzymes by means of color reaction. The isolate designated as LD-3 showing 21.55 ± 0.81 U/ml of laccase activity was found to be the best amongst the 41 isolates tested. Identification of LD-3 on the basis of morphological characteristics, like colony texture (abundant floccose and abundant powdery), colony color (beige to brownish black), pigmentation (brown to dark brown), and slow growth patterns, indicated the similarity of LD-3 with the Fusarium semitectum morphotype II. Microscopic observations like length and width of macroconidia, conidial septation, and the presence of chlamydospores and sporodochia also supported the similarity with F. semitectum morphotype II. However, when the molecular identification of LD-3 was further corroborated with studies on its 18 S rRNA, ITS 1, 5.8 S rRNA, ITS 2 and partial 28 S rRNA gene sequencing carried out by Bangalore GeNei, India, the isolate was identified as a Fusarium incarnatum LD-3 from the Fusarium incarnatum–equiseti species complex (Genbank Accession no EU426883). The boot-strapped unrooted tree was structured by the neighbor-joining method from the distance data generated by multiple alignment of the nucleotide sequence (Fig. 1).
Phylogenetic relationship of our isolate with Fusarium genera of the family Ascomycete based on 18 S rRNA, 5.8 S rRNA, and partial 28 S rRNA gene sequencing. The accession numbers for the sequences are given in parentheses. The bootstrapped unrooted tree was constructed by the neighbour-joining method from the distance data generated by multiple alignments of nucleotide sequences. The bootstrap values for major groupings of the members included in the analysis are shown on the main branches
There are few reports on the production of laccase from Fusarium sp. and litter-dwelling fungi. A comparative study on the extracellular lignolytic enzyme activity of five strains of Fusarium solani under carbon-limited medium revealed a differential production of aryl alcohol oxidase and laccase, i.e. 57 and 8.6 mU/ml, respectively (Saparrat et al. 2000). Laccase, aryl oxidase, and superoxide radicals were also detected in lignolytic cultures of Fusarium proliferatum during secondary metabolism (Rogalado et al. 1999). These reports are in agreement with our findings that Fusarium sp. possesses lignolytic activities; however, this is the first report of laccase production from Fusarium incarnatum LD-3.
Effect of different lignocellulosic substrates
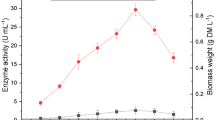
Selection of an appropriate substrate is a key factor in solid substrate fermentation which determines the success of the process. In recent years, there has been an increasing trend towards the utilization of organic waste, such as residues from the agricultural forestry and agro-based industries as raw materials to produce value-added products by the SSF technique. The most important characteristics that influence adhesive behavior of filamentous fungi to the support are hydrophobicity and surface charge. The higher hydrophobicity cause the attachment of the fungus to the carrier easily and also together with its high content in carbohydrates (Osma et al. 2007). Different lignocellulosic substrates like wheat straw, wheat bran. rice bran, sugarcane bagasse, and sawdust were screened for laccase production. The F. incarnatum LD-3 showed maximum production of laccase on the 8th day of fermentation with all different substrates used. All the five agro-industrial residues tested except sawdust supported good growth and laccase production. The growth of the organism is observed after 2–3 days of incubation, and complete colonization of fungus was observed within 8 days of incubation. Among the various substrates tested, rice bran was found to be the best substrate as it supported maximum laccase production, i.e. 1,352.64 U/g of dry substrate (Fig. 2a) with 21.88 g/l of biomass (Fig. 2b). This may be related to its composition, mainly the presence of high concentration of sugars and easy availability of polysaccharides like starch, which promote abundant growth and the presence of phenolic compounds like ferulic acid and vanillic acid reported to be inducers for laccase production (Bollag and Leonowicz 1984; Munoz et al. 1997). Vares et al. (1995) also reported higher production of laccase with Phlebia radiata under SSF using wheat straw as a substrate. Kapoor et al. (2009) reported maximum laccase production with Lentinus edodes from peanut meal-supplemented wheat straw amongst the five different organic supplements tested. Improved laccase production of 9,100 mU/mg at 13 days was observed from Fusarium proliferatum when starch-free wheat bran was used as a substrate (Hernández Fernaud et al. 2006). The present study proved the utility of rice bran as an inexpensive and easily available raw material for laccase production.
Effect of pH and temperature
pH and temperature are important parameters that determine the growth rate of fungi and significantly affect the level of laccase produced. The production of the enzyme strongly depends upon the initial pH of the culture medium as it may influence many enzymatic processes and transport of nutrients across the cytoplasmic membrane. The influence of pH was studied on the production of laccase by adjusting the initial medium pH of the Asther medium between 3.0 and 8.0 (Fig. 3). An exponential increase in the production of laccase was observed from pH 3.0 to 5.0 with a maximum production of 471.02 U/g at pH 5.0. Any further increase in the pH from 6.0 to 8.0 reduced the enzyme production. This may be attributed to unfavorable pH, which may limit the growth and production by reducing the accessibility of the substrate, and the change in the pH may also alter the 3D structure of the enzyme (Shulter and Kargi. 2000).
The effect of temperature is of considerable significance in the SSF system because during fermentation there is a general increase in the temperature of fermenting mass due to respiration (Shulter and Kargi. 2000), even though the impact of temperature is more prominent on the scale-up process where it influences fungal growth and enzyme production. To examine the effect of temperature on laccase production, F. incarnatum LD-3 was grown in the range of 20–50°C. Maximum laccase production of 1,122.30 U/g was obtained at 28 °C on the 8th day of incubation. Further increases in the temperature to 40 and 50°C were detrimental to the fungus, in turn reducing the laccase production due to the drying of the substrate. A temperature of 20 °C did not support the growth well, hence lower laccase production was observed (Fig. 4). The optimum temperature for laccase production was reported to be between 25 and 30 °C by Zadrazil et al. (1999) and Pointing et al. (2000) in Pleurotus sp., Dichomitus squalus, and Pycnoporus sanguineus, respectively. Gupte et al. (2007) also reported the same range of optimum temperature for laccase production from P. ostreatus HP-1.
Effect of moisture content
Optimization of initial moisture content in SSF is an essential parameter as it affects the substrate utilization and laccase production. Laccase produced by LDF was positively affected by increases in the moisture content (Fig. 5). With an increase in initial moisture content from 40 to 60 %, a considerable increase in the laccase production was observed. Maximum laccase production, 427.96 U/g of substrate, was obtained with 60 % moisture content. When moisture content was increased beyond 60 %, a substantial decrease in the laccase production was observed. Similar results for laccase production after 5 days of cultivation were observed using cultures of Pleurotus pulmonarius CCB-19 at 75 % initial moisture content (Farani De Souza et al. 2006). The moisture level in solid substrate fermentation varies between 30 and 85 %, and for the most of the filamentous fungi, the optimum moisture content for growth and substrate utilization is between 40 and 70 %. However, enzyme production at higher moisture levels is believed to reduce the porosity of the substrate, thus limiting oxygen transfer; hence, high moisture content restricts the growth within the whole substrate, resulting in surface growth (Raimbault. 1998).
Effect of different inducers on laccase production under optimized cultivation conditions
Mostly, the laccase of basidiomycetes is an inducible enzyme. There are some reports about constitutive laccases which are produced in media without inducers, but in the presence of inducers, laccase production increases. Different compounds, such as phenolic substrates of laccase as well as non-phenolic substrates, can act as inducers. However, there is no compound that could be a universal inducer of laccase from all fungi. The production of laccase from F. incarnatum LD-3 was tested in the presence of different aromatic compounds like 3,4,5 trihydroxybenzoic acid (gallic acid), veratryl alcohol, vanillin, catechol, 2, methoxy phenol (guaiacol), and ortho-dianisidine. Among the different inducers tested in three different concentrations, 0.5 mM ortho-dianisidine showed an increase in laccase production by 2.13-fold, i.e. from 470.98 to 1,006.16 U/g of dry substrate; however, 1.0 and 2.0 mM concentrations of ortho-dianisidine did not show any increment in the laccase production (Fig. 6). This may be due to the toxic effect of ortho-dianisidine at higher concentrations affecting both the fungal growth and the production of enzyme. There are several reports that various compounds influence laccase production from basidiomycetes. The laccase production in Trametes sp. AH28-2 has been induced by using several aromatic compounds like O-toluidine ferulic acid, guaiacol, 2,4 diaminotoluene, and 3,3 dimethoxybenzidine showing different isoenzyme patterns (Gigi et al. 1980). Niladevi and Prema (2008) also reported a 50 % increase in the laccase yield in the presence of pyrogallol and para-anisidine with Streptomyces psammoticus. These aromatic inducers may affect the transcription of laccase and hence enhance laccase production (Xiao et al. 2004). The induction sites have been found in the upstream region of lac 4 in Pleurotus sajor-caju and of lcc 1 and lcc 2 in basidiomycete 162 (Giardina et al. 2010).
The effect of different concentrations of ethanol ranging from 0.5 to 4 % (v/v) were tested on laccase production from Fusarium incarnatum LD-3. Maximum laccase production of 810.59 U/g was achieved with 2 % (v/v) alcohol. Laccase production increased by 52.56 % as compared to control (Fig. 7). The increase in laccase production in the presence of ethanol can be attributed to its dual role, i.e. gene expression and inhibition of protease activity (Juan Carlos et al. 2007). Thus, ethanol at a regulatory level is responsible for increased laccase production.
Effect of amino acids on the laccase production under optimized cultivation conditions
The effect of 15 different amino acids have been tested on laccase production. It has been found that short chain amino acids with up to four carbons, like L-glycine, D-serine, DL-alanine, and DL-2 amino n-butyric acid, showed higher laccase yield with yield indexes of 1.54, 1.92, 1.9, 1.67, respectively (Table 2). Dhawan and Kuhad (2002) reported maximum laccase production by Cyathus bulleri in the presence of methionine, tryptophan, glycine, valine, leucine, and threonine inducing a discrete increment in its production. Sharma et al. (2005) when studying the effect of amino acids, such as glycine, tryptophan, and methionine, increased the laccase production by Ganoderma sp. Kk-02 by 3.5-fold. Levin et al. (2010) obtained better laccase production with aspartic acid, asparagine, and glutamic acid in Trametes trogii, Trametes villosa, and Coriolus versicolor var. antarcticus. The positioning of –CH3 and –NH2 groups on the linear carbon or aromatic carbon chain might have contributed to the differential inductive effects of different amino acids on laccase production. The results obtained are in agreement for the effect of amino acids on laccase production.
Conclusion
To the best of our knowledge, there have been no previous reports on laccase production and its induction from the litter-dwelling fungi Fusarium incarnatum LD-3. Despite lower production of ligninolytic enzymes, LDF are found to be better degraders of lignin and lignin-related compounds. Since typical white rot fungi do not exhibit satisfactory growth, colonization, and ligninolytic enzyme production under competitive environments like soil litter, LDF represents a promising alternative to white rot fungi. Hence, parametric optimization to identify better cultivation conditions and production conditions for the selected strain to elevate the productivity of target enzyme for a variety of biotechnological applications has been attempted. This study indicates the use of different lignocellulosic substrates for the synthesis of the target enzyme. The present investigation confirms and evaluates the use of rice bran as an inexpensive and easily available substrate for the production of laccase. The laccase production ability of F. incarnatum LD-3 can be enhanced further by supplementing the basal media with various inducers like ortho-dianisidine, ethanol, and short chain amino acids. Optimization studies have thus led to increasing laccase production by 2.1-fold compared to control conditions. The substrates and inducers are cheap and safe and can be suggested for higher laccase production. However, owing to the rate-limiting steps involved in the biosynthesis of such lignin-modifying systems, strain improvement strategies should be adopted to achieve further increments in the productivity.
References
Adinarayana K, Prabhakar T, Srinivasulu V, Anitha Rao M, Jhansi Laxmi P, Ellaiah P (2003) Optimization of process parameters for cephalosporin C production under solid state fermentation from Acremonium chrysigenum. Process Biochem 39:171–177
Asther M, Lesage L, Drapron R, Corrieu G, Odier E (1988) Phospholipid and fatty acid enhancement of Phanerochaete chrysosporium INA-12 in relation to ligninase production. Appl Microbiol Biotechnol 27:393–398
Banerjee UC, Vohra RM (1991) Production of laccase by Curvularia sp. Folia Microbiol 36:343–346
Barbosa AM, Dekker RFH, Hardy GE (1996) Veratryl alcohol as an inducer of laccase by an ascomycete, Bortyosphaeria sp., when screened on polymeric dye Ploy R-478. Letters Appl Microbiol 23:393–398
Bellon-Maurel V, Orliac O, Christen P (2003) Sensors and measures in solid state fermentation: a review. Process Biochem 38:881–896
Binz T, Canevascini G (1997) Purification and partial characterization of the extracellular laccase from Ophiostoma novo-ulmi. Curr Microbiol 35:278–281
Bollag JM, Leonowicz A (1984) Comparative studies of extracellular fungal laccases. Appl Environ Microbiol 48:849–854
Breen A, Singleton FL (1999) Fungi in lignocellulose breakdown and biopulping. Curr Opin Biotechnol 10:252–258
Brenna O, Bianchi E (1994) Immobilized laccase for phenolic removal in must and wine. Biotechnol Lett 24:35–40
Dhawan S, Kuhad RC (2002) Effect of aminoacids and vitamins on laccase production by the bird’s nest fungus Cyathus bulleri. Bioresour Technol 84:35–38
Edens WA, Goins TQ, Dooley D, Henson JM (1999) Purification and characterization of a secreted laccase of Gaeumannomyces graminis var. tritici. Appl Environ Microbiol 65:3071–3074
Farani De Souza D, Tychanowicz GK, Marques De Souza CG, Peralta RM (2006) Coproduction of ligninolytic enzymes by Pleurotus pulmonarius on wheat bran solid state cultures. J Basic Microbiol 46:126–134
Froehner SC, Eriksson KE (1974) Purification and properties of Neurospora crassa laccase. J Bacteriol 120:458–465
Ghindilis AL, Garvrilova VP, Yaropolov AI (1992) Laccase based bioreactor for the determination of catechols in tea. Biosens Bioelectron 7:127–131
Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G (2010) Laccase: a never ending story. Cell Mol Life Sci 67:369–385
Gigi OI, Marbach, Mayer AM (1980) Induction of laccase formation in Botrytis. Phytochemistry 19:2273–2275
Gupte A, Gupte S, Patel H (2007) Ligninolytic enzyme production under solid substrate fermentation by white rot fungi. J Sci Ind Res 66:611–614
Hernández Fernaud JR, Marina A, González K, Vázquez J, Flacón MA (2006) Production, partial characterization and mass spectrometric studies of the extracellular laccase activity from Fusarium proliferatum. Appl Microbiol Biotechnol 70:212–221
Ikehata K, Buchanan DL, Smith DW (2004) Recent developments in the production of extracellular fungal peroxidases and laccases for waste treatment. J Environ Engin Sci 3:1–19
Iyer G, Chattoo BB (2003) Purification and characterization of laccase from the rice blast fungus Magnoporthe grisea. FEMS Microbiol Lett 227:121–126
Juan Carlos M, Richard A, Anne L, Jean-Claude S, Laurence C (2007) Role of ethanol on growth laccase production and protease activity in Pycnoporus cinnabarinus ss3. Enzyme Microb Technol 41:162–168
Kapoor S, Khanna PK, Katyal P (2009) Effect of supplementation of wheat straw on growth and lignocellulolytic Enzyme Potential of Lentinus edodes. World J Agric Sci 5:328–331
Kumar D, Jain VK, Shankar G, Srivastava A (2003) Citric acid production by solid state fermentation using sugarcane bagasse. Process Biochem 38:1731–1738
Levin L, Melignani E, Ramos AM (2010) Effect of nitrogen sources and vitamins on ligninolytic enzyme production by some white-rot fungi. Dye decolorization by selected culture filtrates. Bioresour Technol 101:4554–4563
Mazumder S, Basu SK, Mukherjee M (2009) Laccase production in solid-state and submerged fermentation by Pleurotus ostreatus. Eng Life Sci 9:45–52
Munoz C, Guillen F, Martinez AT, Martinez MJ (1997) Induction and characterization of laccase in the lininolytic fungus Pleurotus eryngii. Curr Microbiol 34:1–5
Niku-Paavola ML, Karhunen E, Kentelinen A, Viikari L, Lundell T, Hatakka A (1990) The effect of culture conditions on the production of lignin modifying enzymes by the white rot fungus Phlebia radiata. J Biotechnol 13:211–221
Niladevi KN, Prema P (2008) Effect of inducers and process parameters on laccase production by Streptomyces psammoticus and its application in dye decolourization. Bioresource Technol 11:4583–4589
Osma JF, Herrera JLT, Couto SR (2007) Banana skin: a novel waste for laccase production by Trametes pubescens under solid-state conditions: application to synthetic dye decolouration. Dyes Pigments 75:32–37
Pandey A, Carlos RS, David M (2000) New developments in solid state fermentation: I-bioprocesses and products. Process Biochem 35:1153–1169
Piccard MA, Vandertol H, Roman R, Vanquez-Duhalt R (1999) High production of ligninolytic enzymes from white rot fungiin cereal bran liquid medium. Can J Microbiol 45:627–631
Pointing SB, Jones EGB, Vrijmoed LLP (2000) Optimization of laccase production by Pycnoporus sanguineus in submerged liquid culture. Mycologia 92:139–144
Raimbault M (1998) General and microbiological aspects of solid substrate fermentation. Electron J Biotechnol 1:174–188
Revankar MS, Lele SS (2006) Enhanced production of laccase using new isolate of white rot fungus WR-1. Process Biochem 41:581–588
Rodŕiguez A, Falcón MA, Carnicero F, Perestelo G, Fuente da le G, Trojanowaski J (1996) Laccase activity of Penicillium chrysogenum in relation to lignin degradation. Appl Microbiol Biotechnol 45:399–403
Rogalado V, Parestelo F, Rodriguez A, Carnicero A, Sosa FJ, De la Fuente G et al (1999) Activated oxygen species and two extracellular enzymes: laccase and aryl alcohol oxidase, novel for the lignin degrading fungus Fusarium proliferatum. Appl Microbiol Biotechnol 51:388–390
Saparrat MCN, Martinez MJ, TournierHA CMN, Arambarri AM (2000) Production of lignolytic enzymes by Fusarium solani strains isolated from different substrata. World J Microbiol Biotechnol 16:799–803
Scherer M, Fischer R (1998) Purification and characterization of laccase II of Aspergillus nidulans. Arch Microbiol 170:78–84
Sharma KK, Kapoor M, Kuhad RC (2005) In vivo enzymatic digestion, in vitro xylanase digestion, metabolic analogues, surfactants and polyethylene glycol ameliorate laccase production from Ganoderma sp. Kk-02. Lett Appl Microbiol 41:24–31
Shulter ML, Kargi F (2000) Bioprocess Engineering Basic Concept. Parentice Hall, New Delhi
Steffen KT, Hofrichter M, Hatakka A (2000) Mineralization of C14 labelled synthetic lignin and ligninolytic enzyme activities of litter decomposing basidiomycetous fungi. Appl Microbiol Bioeng 54:736–744
Steffen KT, Hofrichter M, Hatakka A (2003) Degradation of benzo{a}pyrene by the litter decomposing basidiomycete Stropharia corrolina: role of manganese peroxidase. Appl Environ Microbiol 69:3957–3964
Thurston CF (1994) The structure and function of fungal laccases. Microbiology 40:19–26
Vares T, Kalsi M, Hatakka A (1995) Lignin peroxidase, manganese peroxidase and other ligninolytic enzymes produced by Phlebia radiate during solid state fermentation on wheat straw. Appl Environ Microbiol 61:3515–3520
Xiao YZ, Chen Q, Hang J, Shi YY, Wu J, Hong YZ, Wang YP (2004) Selective induction,purification and characterization of laccase isozyme from the basidiomycete Trametes sp. AH28-2. Mycologia 96:26–35
Zadrazil F, Gonser A, Lang E (1999) Influence of incubation temperature on the secretion of extracellular lignolytic enzymes of Pleurotus and Dichomitus squalus into soil. Proceedings of the Conference on Enzymes in Environment, Granada, Spain
Zubeyde B, Fikret U, Cetin A (2003) Solid state fermentation for production of α –amylase by a thermotolerant Bacillus subtilis from hot spring water. Process Biochem 38:1665–1668
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chhaya, U., Gupte, A. Effect of different cultivation conditions and inducers on the production of laccase by the litter-dwelling fungal isolate Fusarium incarnatum LD-3 under solid substrate fermentation. Ann Microbiol 63, 215–223 (2013). https://doi.org/10.1007/s13213-012-0464-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-012-0464-1