Abstract
Fungal laccase is a robust enzyme with broad specificity and applicability in industrial processes. The successful use of enzymes requires large scale production within a short time. As laccase production is highly dependent on medium components and cultural conditions, the optimization of the same is essential for enhancement of its production efficiency. The objectives of present study were to screen litter dwelling fungi for their laccase production and optimize the culture conditions for hyper-production of laccase. A total of 58 fungal isolates were procured from 25 litter samples of plant origin collected from the Western Ghats of Karnataka, India. Among these, five including Mucor circinelloides GL1, Fusarium oxysporum GL2, F. oxysporum GL3, F. verticillioides GL5 and Ceriporiopsis sp. PA1 were selected for optimization studies based on their laccase producing ability. Maximum production was noticed in the optimized culture media and conditions compared to minimal media. Enhanced laccase production was observed by incubating them in optimized media at 28 °C for 8 days at 150 rpm. The laccase enzyme was purified from M. circinelloides GL1 using series of purification steps. The purified enzyme was a monomeric protein band with an apparent molecular weight of about 40 kDa. The present study reported that an indigenous litter dwelling fungus M. circinelloides GL1 found to be efficacious laccase producer in comparison to other isolates tested in this study. Therefore, it can further be utilized as a better biocatalyst in pertinent biotechnological applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Laccase (EC 1.10.3.2) is a blue multicopper phenol-oxidase that catalyzes the oxidation of several aromatic and inorganic compounds in lignin (mainly phenolic compounds) resulting in the reduction of oxygen (O2) to water (H2O) (Majcherczyk et al. 1998; Bhamare et al. 2018). It has a wide array of substrates which can be used for the industrial applications and bioremediation of pollutants. The capability of laccase in degradation of lignocellulosic materials is improved by the addition of natural or synthetic compounds, such as 3-hydroxyanthranilic acid (3HA) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), respectively, which act as the redox mediators. Laccase enzyme generates phenoxy free radicals in presence of redox mediators leading to cleavage of the polymers (Murugesan et al. 2010; Arregui et al. 2019). It has been intensely studied for the degradation of diverse recalcitrant compounds such as lignin-related structures, chlorophenols, polycyclic aromatic hydrocarbons, phenols, azo dyes and organophosphorus compounds (Abadulla et al. 2000; Saratale et al. 2009). In global enzyme market, it is valued at $2965.6 million in 2020 and is expected to reach about $2850 million by the end of 2026 with growth at the compounded annual growth rate (CAGR) of – 0.66% from 2021 to 2026 (Global Laccase Market Research Report 2020). The fungal laccase is used in several industries for the delignification and production of bioethanol and other value-added products, and bioremediation of chemical pollutants (Upadhyay et al. 2016; Bhamare et al. 2018).
Laccase activity is mainly studied in several wood-rotting fungi which are reported as its leading producers (Valášková and Baldrian 2006; Arora and Sharma 2010; Brijwani et al. 2010). Even though, fungal wood decomposers are very well known to produce laccase, most of them show rather low rates of growth, colonization and laccase producing ability under the competitive environmental conditions. In this context, litter dwelling fungi that inhabit the particular natural environment of soil-litter layers are representing a promising alternative to wood decomposing fungi (Chhaya and Gupte 2013). Laccase production is reported in some litter dwelling fungi and exploited in the pertinent biotechnological applications especially in lignin degradation (Chhaya and Gupte 2010; Chhaya and Gupte 2013). The application of fungal laccase in biotechnological industries requires its production in huge amount at relatively low cost. Therefore, the present research work is concerned towards the exploration of an efficient production system of laccase. Since, the secretion of laccase by fungi depends on the particular strains and their culture growing conditions, a greater number of fungi need to be screened for their ability to degrade recalcitrant xenobiotic compounds (Kiiskinen et al. 2004; Zouari-Mechichi et al. 2006).
The optimization of fermentation process in various industrial purposes is the decisive target of research to reduce enzyme production cost (Gassara et al. 2011). Fungal laccase production is influenced by culture media composition (Periasamy and Palvannan 2010; Chhaya and Gupte 2013), fermentation factors (Couto et al. 2002) and the presence of inducers and surfactants (Patel et al. 2009; Mahmoud et al. 2013). Hence, in the present study, we focused on screening of litter dwelling fungi for their laccase producing ability and optimize their culture growth conditions for hyper-production of laccase. Besides, after successful screening of fungi, the laccase enzyme was purified from Mucor circinelloides GL1 and characterized to understand its properties toward its possible utility and practical applications.
Materials and methods
Collection of litter samples
A total of twenty-five different litter samples of plant origin were collected from different locations of Kodagu district in the Western Ghats of Karnataka, India, from October, 2015 to June, 2016. Each plant litter sample was collected from three randomly selected sampling plots (5 × 5 m2) containing different plantations in each location. First, the soil attached on the surface of samples was removed and then, the samples were cut into small pieces. The pieces were placed directly in the clean plastic bags marked with information including the nature of sample, type of sample, dominant vegetation, sample numbers, isolation sites, replicates number, etc. The collected samples were immediately transfered to the laboratory and stored in the refrigerator (at 4 °C) and used for the isolation of fungi.
Isolation of litter dwelling fungi
To isolate the litter dwelling fungi, 1 g of litter sample was serially diluted in sterile distilled water (up to 10−7) and spread plated onto the Petri plates containing sterile malt-extract agar (MEA) medium amended with 0.5% tannic acid used as the laccase detection system (Kiiskinen et al. 2004). The plates were then incubated in an incubator for 3–5 days at 28 ± 2 °C. The plates were regularly observed for the formation of complete browning zone around fungal growth (Bavendamm 1928). Through visual observation, the fungal strains which formed a prominent reddish-brown coloured zone around the colony in MEA plates were considered as a positive for the oxidation of tannic acid. Among the strains, those which showed the prominent zone were isolated and used for further studies.
Screening of litter dwelling fungi for laccase activity
Laccase production was performed by adding a mycelial plug (5 mm of diameter) of 7-days-old culture of each strain to Erlenmeyer flask (250 ml) containing 100 ml of sterilized malt-extract broth (MEB) amended with 0.01% of guaiacol as a substrate. The flasks were then incubated in a rotary shaker (at 150 rpm) for 12 day at 28 ± 2 °C. At regular intervals after incubation, the fungal mycelium was separated by filtrating through a Whatman No. 1 filter paper. The culture filtrate was harvested by centrifugation at 10,000 rpm at 4 °C for 30 min. The supernatant thus obtained was subjected to membrane filtration (0.45 µm) and then used for laccase assay. Laccase activity was assayed by examining the oxidation of guaiacol according to the method described by Arora and Sandhu (1985) and expressed as International Units (IU) ml−1 of culture filtrate.
Identification of laccase producing fungi
Morphological identification of selected laccase-producing fungi was performed by macroscopic and microscopic analyses (Dugan 2006). The colony morphological, conidial, fruiting bodies and culture characters of fungi were recorded to assess their identity up to the genus level after 10–15 days of incubation. The amplification and sequencing of fungal 18S ribosomal RNA gene were carried out to identify up to species level. The nucleotide sequences were compared with the reference 18S ribosomal RNA gene sequence data from fungal strains already published on the NCBI database using BLAST search algorithm (https://blast.ncbi.nlm.nih.gov/Blast.cgi) as described by Altschul et al. (1997). The sequences have been deposited to the GenBank nucleotide collection and obtained the accession numbers. The phylogenetic tree was constructed using Molecular Evolutionary Genetics Analysis (MEGA) X software by the neighbor-joining (NJ) method with Kimura 2-parameter model (Kumar et al. 2008).
Optimization of culture growth conditions for hyper-production of laccase
In the laboratory, the culture growth conditions for hyper-production of laccase by selected fungal isolates were optimized concerning: different types of substrates, incubation time, incubation temperature, pH of the media, source of carbon and nitrogen, types of inducers and surfactants.
Optimization of substrate
Kraft lignin from Sigma-Aldrich and three different substrates (such as wheat bran, rice bran and coffee bran) at 5 g l−1 were used to check the suitable substrate for maximum laccase production. The fungal inoculum (a mycelial plug of 5 mm diameter) was added to the flasks (250 ml) containing 100 ml of minimal medium amended with one of the solid substrates mentioned above. The minimal medium was composed of glucose (10 g l−1), peptone (2 g l−1), KH2PO4 (3 g l−1), MgSO4 (0.5 g l−1), Vitamin B1 (0.02 g l−1), NaCl (0.1 g l−1), CaCl2 (0.01 g l−1) at pH 8.5 and supplemented with different substrates. The non-inoculated media added with each substrate were incubated in parallel and used as the negative controls. The flasks were then incubated at 28 ± 2 °C for 12 day in orbital shaking condition (at 150 rpm). At the end of incubation, the broth cultures were harvested and treated as above before laccase activity determination. The lignin (5 g l−1) turned out to be the best substrate for maximum laccase production and thus, it was used for further optimization studies.
Effect of incubation time, temperature and pH of the media
The optimum incubation time was estimated by growing the fungi in the flasks containing minimal medium supplemented with lignin along with non-inoculated media (as a negative control). The flasks were then incubated for 12 d at 28 ± 2 °C in shaking (at 150 rpm) and stationary conditions separately. The cultures were harvested for every 2 d of intervals up to 12 d of incubation as explained above.
Optimum temperature was estimated by incubating the fungal inoculated and non-inoculated media at various temperatures (20, 28, 35 and 45 °C) for 8 d at 150 rpm. At the end of incubation, the cultures were harvested as explained above.
Optimum pH of the media was also estimated by growing the fungi in the flasks containing media with varied pH (4–10) adjusted using 0.1 N HCl and 0.1 N NaOH. The non-inoculated media with varied pH were taken as the negative controls. The flasks were then incubated for 8 d at 28 °C with shaking at 150 rpm. After incubation period, the cultures were harvested as described above.
Effect of sources of carbon and nitrogen
Different carbon sources (such as glucose, lactose, maltose and soluble starch) and nitrogen sources (such as peptone, malt extract, yeast extract, ammonium nitrate, ammonium sulfate and sodium nitrate) were used as co-substrates at 10 g l−1 each (based on the preliminary studies, Supplementary Table 1) to investigate their effects on laccase production. Each fungal isolate was inoculated on to lignin-containing minimal media (pH 6) supplemented with different sources of carbon and nitrogen. The media amended with different sources of carbon and nitrogen without fungal inoculation were taken as the negative controls for the respective media. The flasks were then incubated for 8 days at 28 °C in shaking at 150 rpm. At the end of incubation, the cultures were harvested as described above.
Effect of inducers
Different inducers (such as veratryl alcohol, manganese sulfate, guaiacol and copper sulfate) at 0.03 g l−1 each (based on the preliminary studies, Supplementary Table 1) were used to investigate their effects on laccase production. Each fungal strain was inoculated on to the lignin-containing minimal media (pH 6) supplemented with different inducers, glucose (carbon source) and peptone (nitrogen source). The media supplemented with different inducers, glucose and peptone without fungal inoculation were used as the negative controls for the respective media. The flasks were then incubated in shaking condition (at 150 rpm) at 28 °C. The enzyme was extracted as described above after 8 d of incubation.
Effect of surfactants
Different surfactants (such as Tween 20, Tween 40, Tween 80 and sodium dodecyl sulfate (SDS)) at 0.15 g l−1 each (based on the preliminary studies, Supplementary Table 1) were used to investigate their effects on laccase production. Each fungal isolate was inoculated on to the lignin-containing minimal media (pH 6) supplemented with different surfactants, glucose (carbon source), peptone (nitrogen source) and copper sulfate (inducer). The media supplemented with different surfactants, glucose, peptone and copper sulfate without fungal inoculation were taken as the negative controls for the respective media. The flasks were then incubated for 8 d at 28 °C in shaking condition. The enzyme was extracted at the end of incubation period as explained above.
Optimized culture media and conditions for hyper-production of laccase
The optimized medium comprised of 5 g l−1 lignin, 10 g l−1 glucose, 10 g l−1 peptone, 3 g l−1 KH2PO4, 0.5 g l−1 MgSO4, 0.02 g l−1 Vitamin B1, 0.1 g l−1 NaCl, 0.01 g l−1 CaCl2, 0.03 g l−1 copper sulfate and 0.15 g l−1 Tween 80. The pH of medium was adjusted to 6 using 0.1 N HCl. The fungi were grown in the optimized conditions (at 28 °C and 150 rpm) for the production of laccase. The optimized medium incubated without fungal inoculation was used as a negative control. The enzyme was extracted for every 2 day of intervals up to 12 day of incubation as described above.
Partial purification of laccase enzyme from M. circinelloides GL1
M. circinelloides GL1 was grown in the optimized media and conditions mentioned above. The enzyme was extracted at the end of 8 d as explained above. The crude extract was used to purify the enzyme and to determine its activity and molecular weight.
Ammonium sulfate precipitation and dialysis
Crude extract extracted from 8 d old M. circinelloides GL1 culture was added with ammonium sulfate up to 80% saturation, left overnight at low temperature, and then centrifuged (10,000 × g) at 4 °C for 10 min. The pellet obtained was dissolved in minimum required sodium acetate buffer (100 mM, pH 5) and dialyzed overnight (cut-off, 12–14 kDa).
Gel filtration chromatography (GFC)
The enzyme was purified with the gel filtration chromatography (GFC) on the DEAE Sephadex G–75 column (1.5 cm × 30 cm). The column was equilibrated first and eluted at a flow rate of 0.2 ml min−1 with sodium acetate buffer (100 mM, pH 5). The protein content (Bradford 1976) and laccase activity of each fraction were determined.
Ion exchange chromatography
The fractions with highest laccase activity were pooled and subjected to ion exchange chromatography on the DEAE Sephadex A–50 column (1.5 cm × 30 cm) with 25 ml bed volume already equilibrated with same buffer used in GFC. Continuous linear salt gradients from 0.1 to 1 M NaCl were applied and the fractions were collected at above mentioned flow rate. The protein content and laccase activity of each fraction were evaluated. The fractions with highest laccase activity were pooled together, concentrated and then stored in refrigerator (at 4 °C) for further analysis.
Determination of molecular weight
The dialyzed sample and purified enzyme were exposed to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 12% gel. The SDS-PAGE was carried out to find out the purity and approximate molecular weight of the enzyme as per the modified method of Laemmli (1970). The protein bands were observed by silver staining (Blum et al. 1987). Molecular weight was found out by comparing with the low molecular weight protein marker; phosphorylase b (94 kDa), bovine serum albumin (67 kDa), chicken egg white ovalbumin (43 kDa), bovine erythrocyte carbonic anhydrase (30 kDa), soybean trypsin inhibitor (20.1 kDa) and α-lactalbumin (14.4 kDa).
Characterization of laccase enzyme
Effect of temperature
Optimum temperature for the maximum activity of purified enzyme was evaluated by carrying out the enzymatic assays at different temperatures from 10 to 70 °C. Specific activity was investigated by pre-incubating the purified enzyme in sodium acetate buffer (100 mM, pH 5) with 5 mM guaiacol as a substrate at different temperatures ranging from 10 to 70 °C with regular interval of 5 °C for a period of 1 h. After incubation, percentage of residual activity was determined after considering the maximum specific activity found at 25 °C as 100% activity.
Effect of pH
Optimum pH for the maximum activity of purified enzyme was evaluated by carrying out the enzymatic assays at different pH levels (2.5–11). The different levels of pH were adjusted using the following 100 mM buffer solutions with 5 mM guaiacol: glycine HCl buffer solution (pH 2.5–3), sodium acetate buffer solution (pH 3.5–6), sodium phosphate buffer solution (pH 6.5–8), Tris–HCl buffer solution (pH 8.5–9) and sodium carbonate-sodium bicarbonate buffer solution (pH 9.5–11). The effect of pH on specific activity was evaluated by incubating the purified enzyme in respective buffer solutions containing 5 mM guaiacol with different pH levels at 25 °C for 1 h. After incubation, percentage of residual activity was determined after considering the maximum specific activity found in sodium acetate buffer (100 mM, pH 5) as 100% activity.
Effect of metal ions
The effect of different metal ions (such as 1 mM of MgSO4, MnCl2, FeCl3, CaCl2 and BaCl2) on the activity of purified enzyme was evaluated by adding them into sodium acetate buffer (100 mM, pH 5) prior to addition of 5 mM guaiacol. The specific activity was estimated after the period of 1 h incubation of the purified enzyme in the buffer containing the metal ions at 25 °C (Forootanfar et al. 2011). The percent relative activity was determined after considering the specific activity without any metal ion as 100% activity.
Statistical analysis
Each experiment was carried out with triplicates and repeated three times. The experimental data were analyzed statistically by subjecting to analysis of variance (ANOVA) (arcsine transformation and analysis of variance) using IBM SPSS Statistics, version 23 (Wagner 2016). Significant differences observed between the treatments mean were determined by HSD (highest significant difference) using Tukey’s test level at P ≤ 0.05.
Results
Isolation of litter dwelling fungi
A total of 58 fungal isolates were obtained from 25 diverse litter samples collected from different localities of Kodagu district in the Western Ghats of Karnataka, India. The fungal strains were isolated on the basis of appearance of reddish brown coloured zone around their colony growth on MEA plates supplemented with tannic acid.
Screening of litter dwelling fungi for laccase activity
Among 58 fungal isolates, maximum production of laccase (1.7 IU ml−1) was exhibited by the isolate GL1 which was considered as 100% of production. Apart from this, other four potential isolates such as GL2, GL3, GL5 and PA1 which selected for further optimization studies showed laccase activity of 1.62, 1.34, 1.67 and 0.61 IU ml−1, respectivley (Fig. 1).
Identification of laccase producing fungi
The selected laccase producing fungal isolates were identified up to genus level by the morphological characters of the respective colonies in pure cultures (Data not shown). In addition, the outcome of the BLAST and phylogenetic analyses (combined with morphology of colonies) revealed that the isolate GL1 corresponds to M. circinelloides, isolates GL2, GL3 and GL5 to Fusarium sp. and isolate PA1 to Ceriporiopsis sp. (Table 1, Fig. 2). The nucleotide sequences were deposited in GenBank database and the accession numbers are mentioned in Table 1. Thus, the five potential isolates M. circinelloides GL1, F. oxysporum GL2, F. oxysporum GL3, F. verticillioides GL5 and Ceriporiopsis sp. PA1 were used to optimize the fungal culture conditions for hyper-production of laccase.
Optimization of substrate
The selected fungal isolates exhibited an laccase production in all substrates used. However, the highest laccase activity was observed at 5 g l−1 of kraft lignin supplemented media in the fungal treatments(Fig. 3). M. circinelloides GL1 significantly showed the maximum laccase activity of 1.72 IU ml−1 and followed by F. verticillioides GL5 (1.67 IU ml−1) in lignin supplemented medium when compared to other isolates. Hence, lignin (5 g l−1) was considered to be an appropriate substrate for maximum production of laccase.
Optimization of a suitable substrate for laccase production by litter dwelling fungi. Laccase activity was performed using the cultures harvested at the end of 12 d of incubation. Each value is the mean of three replicates (n = 3) and vertical bars indicate the standard error (SE ±). Mean values followed by the same letter(s) written within the same substrate are not different significantly (at p ≤ 0.05) according to Tukey’s HSD test
Effect of incubation time
Significant differences in laccase activity were examined irrespective of fungal isolate at different incubation time intervals from 0 to 12 d in both shaking and stationary conditions (Fig. 4). A significantly lowered laccase production was observed in stationary cultures as compared to shaken ones. Regardless of the fungal isolate and agitation conditions, the production increased with increasing incubation time up to 8 d from inoculation, as shown in Fig. 4.
Optimization of incubation time for laccase production by litter dwelling fungi in both shaking and stationary conditions. Laccase activity was performed using the cultures harvested at every 2 d of intervals up to 12 d of incubation. Each value is the mean of three replicates (n = 3) and vertical bars indicate the standard error (SE ±). Mean values followed by the same letter(s) written within the same time are not different significantly (at p ≤ 0.05) according to Tukey’s HSD test
Maximum laccase production was observed by M. circinelloides GL1 (6.4 IU ml−1) followed by F. verticillioides GL5 (4.73 IU ml−1) in shaking condition. Whereas, in case of stationary conditions, maximum activity (3.5 IU ml−1) was showed at 8 d after inoculation with M. circinelloides GL1, followed by F. verticillioides GL5 (3.3 IU ml−1). Production significantly decreased after that. Since, maximum production of laccase at 8 d of incubation in shaking condition (150 rpm), it was considered to be optimum incubation time and condition for laccase production. Therefore, these conditions were used for further studies.
Effect of incubation temperature
Varied laccase production by the fungal isolates was observed at a wide temperature range from 28 to 45 °C. However, maximum activity was found out at 28 °C under shaking conditions (Fig. 5a). The laccase activity was found to decrease below and above 28 °C of incubation wherein the maximum laccase activity of 6.7 IU ml−1 was showed by M. circinelloides GL1 followed by F. verticillioides GL5 (4.96 IU ml−1). Therefore, the incubation temperature of 28 °C was observed and taken to be optimum for maximum laccase production.
Optimization of incubation temperature (a) and pH of the media (b) for laccase production by litter dwelling fungi. Each value is the mean of three replicates (n = 3) and vertical bars indicate the standard error (SE ±). Mean values followed by the same letter(s) written within the same temperature and pH are not different significantly (at p ≤ 0.05) according to Tukey’s HSD test
Effect of pH of the media
Varied laccase production by the fungal isolates was observed at a wide range of pH of media (4–10). Maximum laccase production by the fungal isolates was observed at pH 6 under shaking conditions. However, laccase activity was enhanced with the increase in pH of media from 4 to 6 and decreased after that (Fig. 5b). Maximum activity (7.0 IU ml−1) was showed at pH 6 by M. circinelloides GL1 followed by F. verticillioides GL5 (5.13 IU ml−1).
Effect of carbon source
All four carbon sources used as co-substrates significantly increased laccase production irrespective of fungal isolates. However, the results revealed that the highest laccase activity was recorded in glucose supplemented media (Fig. 6a). The glucose supplementation into lignin-containing media resulted in the maximum activity (9.0 IU ml−1) by M. circinelloides GL1 followed by F. verticillioides GL5 (6.93 IU ml−1). Hence, glucose (10 g l−1) was considered to be a suitable carbon source for maximum laccase production.
Optimization of incubation temperature (a) and pH of the media (b) for laccase production by litter dwelling fungi. Each value is the mean of three replicates (n = 3) and vertical bars indicate the standard error (SE ±). Mean values followed by the same letter(s) written within the same carbon and nitrogen source are not different significantly (at p ≤ 0.05) according to Tukey’s HSD test
Effect of nitrogen source
All six nitrogen sources used improved laccase production irrespective of fungal isolates. However, maximum activity was offered by supplementation of peptone (Fig. 6b). The peptone supplementation into lignin-containing media significantly offered the maximum activity (10.56 IU ml−1) by M. circinelloides GL1 followed by F. verticillioides GL5 (7.4 IU ml−1). Hence, peptone (10 g l−1) was considered to be a suitable nitrogen source for maximum laccase production.
Effect of inducers
The different inducers tested significantly enhanced laccase production irrespective of fungal isolates. However, the maximum activity was observed by supplementation of copper sulfate (Fig. 7a). The supplementation of copper sulfate into lignin-containing media significantly resulted in maximum activity (13.4 IU ml−1) by M. circinelloides GL1 followed by F. verticillioides GL5 (10.46 IU ml−1). Hence, copper sulfate (0.03 g l−1) was considered to be a suitable inducer for maximum laccase production.
Effect of inducers (A) and surfactants (B) on laccase production with litter dwelling fungi. Each value is the mean of three replicates (n = 3) and vertical bars indicate the standard error (SE ±). Mean values followed by the same letter(s) written within the same inducers and surfactants are not different significantly (at p ≤ 0.05) according to Tukey’s HSD test
Effect of surfactants
Maximum laccase activity was recorded in Tween 80 supplemented media by all of the fungal isolates (Fig. 7b). Supplementation of Tween 80 into lignin-containing media showed maximum activity (16.4 IU ml−1) by M. circinelloides GL1 followed by F. verticillioides GL5 (13.4 IU ml−1). Hence, Tween 80 (0.15 g l−1) was considered to be an appropriate surfactant for maximum laccase production.
Optimized culture conditions for hyper-production of laccase
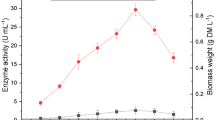
Significant improvement was observed in laccase production by selected fungal isolates grown in the optimized production media and conditions when compared to minimal media (Fig. 8). There was a significant increase in laccase production optimized culture conditions with an increase in incubation time and the maximum production was found after 8 d after inoculation in shaking conditions. Laccase production decreased after that with increasing time of incubation. Maximum activity (16.46 IU ml−1) was exhibited by M. circinelloides GL1 followed by F. verticillioides GL5 (13.69 IU ml−1) after 8 d of incubation. There after production decreased significantly.
Production of laccase by litter dwelling fungi in the optimized liquid culture media and conditions. Composition of optimized media: 5 g l−1 lignin, 10 g l−1 glucose, 10 g l−1 peptone, 3 g l−1 KH2PO4, 0.5 g l−1 MgSO4, 0.02 g l−1 Vitamin B1, 0.1 g l−1 NaCl, 0.01 g l−1 CaCl2, 0.03 g l−1 copper sulfate and 0.15 g l−1 Tween 80, pH 6. Incubation conditions: temperature 28 °C with shaking at 150 rpm for 8 d. Each value is the mean of three replicates (n = 3) and vertical bars indicate the standard error (SE ±). Mean values followed by the same letter(s) written within the same conditions are not different significantly (at p ≤ 0.05) according to Tukey’s HSD test
Partial purification of laccase enzyme from M. circinelloides GL1
The laccase enzyme from M. circinelloides GL1 was purified to 37.6-folds with the yield of 18.8% (Supplementary Table 2), using a series of purification steps mentioned earlier. The purified enzyme was detected as a monomeric protein band on SDS-PAGE with an apparent molecular weight of about 40 kDa when compared to known standard protein markers (Supplementary Fig. 1).
Characterization of laccase enzyme
Effect of temperature
Purified enzyme was active for the oxidation of guaiacol in a wide range of temperature from 10 to 70 °C. Maximum activity (404 IU ml−1) was detected at the optimum temperature of 25 °C which was considered as 100% activity. The data suggested that the specific activity was increased sharply from 10 to 25 °C, but it declined gradually after that. We also found that the retained 64.5% residual activity was observed at 10 °C and increased to reach 100% activity at 25 °C (Supplementary Fig. 2a). After that it decreased gradually with increasing temperature and reached 46.2% residual activity at 70 °C.
Effect of pH
Purified enzyme was active for the oxidation of guaiacol in wide ranges of pH from 2.5 to 11. Maximum activity (425 IU ml−1) was detected at optimum pH 5 which was considered as 100% activity. Above pH 5, the activity gradually decreased with increasing pH. The retained 60.3% residual activity was observed at pH 2.5 and increased to reach 100% at pH 5 (Supplementary Fig. 2b). After that it decreased gradually with increasing pH and reached 1.4% residual activity at pH 11.
Effect of metal ions
Study on the effect of metal ions on the activity of purified enzyme showed that the specific activity of 426 IU ml−1 was observed in incubating enzyme in buffer without any metal ion which was considered as 100% activity. At 1 mM concentration, BaCl2 and MnCl2 had considerable stimulatory effect with 260.5% and 235.9% of relative activity, respectively, compared to 100% activity in treatment without metal ion (Supplementary Table 3). However, the metal ion, MgSO4 (24.1%) showed strongest inhibition followed by CaCl2 (39.3%), but FeCl3 (81.3%) was slightly inhibitory compared to treatment without metal ion.
Discussion
Plant litter constitutes an environmentally realistic material which represents the largest resource of organic carbon in the diverse forest soils (Tuomi et al. 2011; Filser et al. 2016). Understanding the plant litter decomposition process by microbes is a complex phenomenon that includes organic matter mineralization and transformation impacting the global anthropogenic carbon fluxes (Prescott 2010; Voříšková and Baldrian 2013). Typically, the fungi account for more than 90% of total soil microbial biomass linked with the decaying plant litter (Komínková et al. 2000). Interestingly, laccases are ubiquitous enzymes that are associated with lignin-degrading ability and play an important role in the developmental cycle of various fungi such as in sporulation, production of pigments, formation of fruiting bodies and in plant pathogenesis (Kunamneni et al. 2007; Arregui et al. 2019; Góralczyk-Bińkowska et al. 2020). Recently, laccases have drawn tremendous attention of scientists due to their potential industrial uses (Xavier et al. 2007; Arora and Sharma 2010; Singh and Gupta 2020).
Primary selection of potential fungi exhibiting relatively greater laccase producing ability is one of the fundamental criteria in the application of bioremediation. In this context, after screening, five potential isolates were selected for the optimization studies based on their laccase producing ability. Secondly, optimization of growing conditions for the fungal isolates was also done to achieve the maximum enzyme production. The fungal laccase production is influenced by numerous distinguishing fermentation factors such as medium composition, incubation time, temperature, pH of media, type and concentrations of carbon and nitrogen, and type of inducers and surfactants (Couto et al. 2002; Zhu et al. 2016).
In this study, we optimized fungal culture conditions for their enhanced production of laccase. Kraft lignin (5 g l−1) was confirmed to be the most appropriate substrate for the maximum laccase production compared to others. Banakar and Thippeswamy (2014) isolated extracellular ligninolytic fungi using lignin (1%) as a substrate and reported it as an efficient substrate to achieve maximum laccase production. Maximum laccase production was observed at 28 °C in shaking conditions after 8 d of inoculation. Šnajdr and Baldrian (2007) determined that the highest laccase production being recorded at 25–30 °C by Pleurotus ostreatus. A remarkable observation was that the fungi could decompose lignin at optimum culture pH of 4.0 to 4.5 with suppression at pH > 5.5 and < 3.5 (Kirk et al. 1978). Fungal growth and their laccase production are highly associated with the nutrients readily available to them (Viswanath et al. 2014). In the present study glucose and peptone were noticed to be the most proficient source of carbon and nitrogen, respectively, for laccase production. The results are similar to the findings of Kanwal and Reddy (2011) who reported glucose and peptone as the best sources of carbon and nitrogen, respectively, supporting maximum growth of Morchella crassipes and relatively higher laccase production.
Several studies on the induction of fungal laccase production have reported a diverse group of inducers (Patel et al. 2009; Mahmoud et al. 2013; Gomaa and Momtaz 2015; Wang et al. 2019). In present study also attempt was made to enhance the production of laccase by incorporation of inducers into the growth media. The addition of low concentration of copper sulfate enhances laccase activity by interacting with the fungal cells and thus, results in the formation of hydrogen peroxide (H2O2) as copper oxidative stress response (Banakar and Thippeswamy 2014; Gomaa and Momtaz 2015; Damián-Robles et al. 2017). The copper ions (Cu+2) catalyze the production of free oxygen radicals and form copper-dioxygen complexes which are considered as a plausible mechanism of Fenton-like reactions (Urbański and Beręsewicz 2000). Similarly, the use of non-toxic surfactants can also induce fungal growth and improve the bioavailability of poorly soluble substrates thereby increasing laccase production (Teodoro et al. 2018). Our findings showed that Tween 80 was an effective surfactant for laccase activity. The result was in concurrence with Teodoro et al. (2018) who reported the induction of laccase production by Pleurotus sajor-caju after incorporation of Tween 80 to the culture medium.
Significant laccase production by the selected fungal isolates was noticed in the optimized culture media when compared to minimal media. Beside, M. circinelloides GL1 was reported here as a potential laccase producing strain. Prasad et al. (2005) showed an increased laccase yield of 803.3 U from 538.8 U (which corresponds to laccase expression of 32.9% improvement) by P. ostreatus 1804 strain in optimized submerged cultural conditions. In the present study, the enzyme purified from M. circinelloides GL1 has a molecular weight of about 40 kDa which was closest match of the extracellular laccase purified from Pleurotus sp. (Liu et al. 2009; More et al. 2011). The data illustrated that 100% residual activity reached at 25 °C with maximum specific activity (404 IU ml−1) for the oxidation of guaiacol. Hu et al. (2014) have reported the optimum temperature for the activity of laccase purified from Leptographium qinlingensis for guaiacol oxidation was 45 °C, wherein the maximum activity found was 5640 IU l−1 and the residual activity was still more than 90% at 25 °C even though the enzyme was incubated with guaiacol for 2 h. Variations in the effect of temperatures on laccase activity might be associated with a number of disulfide bonds in protein, thermal dissociation and origin of the fungal strain (Díaz et al. 2018).
The enzymatic reaction mechanism depends on pH-dependent enzymatic redox potential change of associated substrate. The buffer pH influences the ionization states of substrate and consequently disturbs the ability of laccase (Patel et al. 2014). The purified laccase oxidized guaiacol at pH 5.0 in the reaction mixture which was considered as optimum. The result was consistent with the findings of Junghanns et al. (2009) who found that laccase purified from Phoma sp. (UHH 5-1-03) showed maximum specific activity at optimum pH of 5.0 for guaiacol oxidation. At higher pH, a progressive decrease in the rate of guaiacol oxidation may be due to the ionization of critical amino acids (either Asp or Glu) and enzyme inactivity (Salony et al. 2006; Patel et al. 2014). The residual activity reached 100% for the oxidation of guaiacol at optimum pH 5.0 at 25 °C. Hu et al. (2014) have also revealed that the maximum activity (6305 IU l−1) of laccase purified from L. qinlingensis for guaiacol oxidation was achieved at pH 4.4 and the residual activity (5302 IU l−1) reached the maximum activity of 84% even if kept at same pH for 10 h. Our study also showed that the metal ions such as 1 mM of BaCl2 and MnCl2 significantly stimulated the relative activity. Jeon and Lim (2017) have reported that the laccase purified from Marasmius scorodonius was found very stable in presence of several metal ions at low concentration (1 mM).
Conclusion
Fungal laccase being an ideal biocatalyst can easily replace toxic chemical catalysts and has great potential in industrial applications with less negative environmental impacts. In this study, the fungal culture conditions were optimized for attaining the optimum production of laccase. In addition, the present study highlights that an indigenous litter dwelling fungus M. circinelloides GL1 as a potential laccase producer and it can be used as a potential biocatalyst towards a propitious future in pertinent biotechnological applications.
References
Abadulla E, Tzanov T, Costa S, Robra KH, Cavaco-Paulo A, Gübitz GM (2000) Decolorization and detoxification of textile dyes with a laccase from Trametes hirsuta. Appl Environ Microbiol 66(8):3357–3362. https://doi.org/10.1128/AEM.66.8.3357-3362.2000
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25(17):3389–3402. https://doi.org/10.1093/nar/25.17.3389
Arora DS, Sandhu DK (1985) Laccase production and wood degradation by a white-rot fungus Daedalea flavida. Enzyme Microb Technol 7(8):405–408. https://doi.org/10.1016/0141-0229(85)90131-0
Arora DS, Sharma RK (2010) Ligninolytic fungal laccases and their biotechnological applications. Appl Biochem Biotechnol 160(6):1760–1788. https://doi.org/10.1007/s12010-009-8676-y
Arregui L, Ayala M, Gómez-Gil X, Gutiérrez-Soto G, Hernández-Luna CE, de los Santos MH, Levin L, Rojo-Domínguez A, Romero-Martínez D, Saparrat MCN, Trujillo-Roldán MA, Valdez-Cruz NA (2019) Laccases: structure, function, and potential application in water bioremediation. Microb Cell Fact 18:200. https://doi.org/10.1186/s12934-019-1248-0
Banakar SP, Thippeswamy B (2014) Isolation and partial purification of fungal ligninolytic enzymes from the forest soil fungi isolated from Bhadra Wildlife Sanctuary. Front Biol 9(4):291–299. https://doi.org/10.1007/s11515-014-1319-x
Bavendamm W (1928) Originalabhandlungen. Über das Vorkommen und den Nachweis von Oxydasen bei Holzzerstörenden Pilzen. Z Pflanzenkr Pflanzenschutz 38:257–276
Bhamare HM, Jadhav HP, Sayyed RZ (2018) Statistical optimization for enhanced production of extracellular laccase from Aspergillus sp. HB_RZ4 isolated from bark scrapping. Environ Sustain 1:159–166. https://doi.org/10.1007/s42398-018-0015-1
Blum H, Beier H, Gross HJ (1987) Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8(2):93–99. https://doi.org/10.1002/elps.1150080203
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brijwani K, Rigdon A, Vadlani PV (2010) Fungal laccases: production, function, and applications in food processing. Enzyme Res 2010:1–10. https://doi.org/10.4061/2010/149748
Chhaya U, Gupte A (2010) Optimization of media components for laccase production by litter dwelling fungal isolate Fusarium incarnatum LD-3. J Basic Microbiol 50(1):43–51. https://doi.org/10.1002/jobm.200900203
Chhaya U, Gupte A (2013) Effect of different cultivation conditions and inducers on the production of laccase by the litter-dwelling fungal isolate Fusarium incarnatum LD-3 under solid substrate fermentation. Ann Microbiol 63(1):215–223. https://doi.org/10.1007/s13213-012-0464-1
Couto SR, Gundı́n M, Lorenzo M, Sanromán MÁ (2002) Screening of supports and inducers for laccase production by Trametes versicolor in semi-solid-state conditions. Process Biochem 38(2):249–255. https://doi.org/10.1016/S0032-9592(02)00087-0
Damián-Robles RM, Castro-Montoya AJ, Saucedo-Luna J, Vázquez-Garcidueñas MS, Arredondo-Santoyo M, Vázquez-Marrufo G (2017) Characterization of ligninolytic enzyme production in white-rot wild fungal strains suitable for kraft pulp bleaching. 3 Biotech 7(5):319. https://doi.org/10.1007/s13205-017-0968-2
Díaz R, Díaz-Godínez G, Anducho-Reyes MA, Mercado-Flores Y, Herrera-Zúñiga LD (2018) In silico design of laccase thermostable mutants from Lacc 6 of Pleurotus ostreatus. Front Microbiol 9:2743. https://doi.org/10.3389/fmicb.2018.02743
Dugan FM (2006) The identification of fungi: an illustrated introduction with keys, glossary, and guide to literature. The American Phytopathological Society, US Department of Agriculture, Agricultural Research Service, Washington State University, Pullman, APS Press, USA
Filser J, Faber JH, Tiunov AV, Brussaard L, Frouz J, Deyn GD, Uvarov AV, Berg MP, Lavelle P, Loreau M, Wall DH, Querner P, Eijsackers H, Jiménez JJ (2016) Soil fauna: key to new carbon models. Soil 2(4):565–582. https://doi.org/10.5194/soil-2-565-2016
Forootanfar H, Faramarzi MA, Shahverdi AR, Yazdi MT (2011) Purification and biochemical characterization of extracellular laccase from the ascomycete Paraconiothyrium variabile. Bioresour Technol 102(2):1808–1814. https://doi.org/10.1016/j.biortech.2010.09.043
Gassara F, Brar SK, Tyagi RD, John RP, Verma M, Valero JR (2011) Parameter optimization for production of ligninolytic enzymes using agro-industrial wastes by response surface method. Biotechnol Bioprocess Eng 16(2):343–351. https://doi.org/10.1007/s12257-010-0264-z
Global Laccase Market Research Report (2020) https://www.360marketupdates.com/enquiry/request-sample/14846118. Published 09 Jan 2020
Gomaa OM, Momtaz OA (2015) Copper induction and differential expression of laccase in Aspergillus flavus. Braz J Microbiol 46(1):285–292. https://doi.org/10.1590/S1517-838246120120118
Góralczyk-Bińkowska A, Jasińska A, Długoński A, Płociński P, Długoński J (2020) Laccase activity of the ascomycete fungus Nectriella pironii and innovative strategies for its production on leaf litter of an urban park. PLoS ONE 15(5):e0233553. https://doi.org/10.1371/journal.pone.0231453
Hu X, Wang C, Wang L, Zhang R, Chen H (2014) Influence of temperature, pH and metal ions on guaiacol oxidation of purified laccase from Leptographium qinlingensis. World J Microbiol Biotechnol 30(4):1285–1290. https://doi.org/10.1007/s11274-013-1554-3
Jeon SJ, Lim SJ (2017) Purification and characterization of the laccase involved in dye decolorization by the white-rot fungus Marasmius scorodonius. J Microbiol Biotechn 27(6):1120–1127. https://doi.org/10.4014/jmb.1701.01004
Junghanns C, Pecyna MJ, Böhm D, Jehmlich N, Martin C, von Bergen M, Schauer F, Hofrichter M, Schlosser D (2009) Biochemical and molecular genetic characterisation of a novel laccase produced by the aquatic ascomycete Phoma sp UHH 5-1-03. Appl Microbiol Biotechnol 84(6):1095–1105. https://doi.org/10.1007/s00253-009-2028-2
Kanwal HK, Reddy MS (2011) Effect of carbon, nitrogen sources and inducers on ligninolytic enzyme production by Morchella crassipes. World J Microbiol Biotechnol 27(3):687–691. https://doi.org/10.1007/s11274-010-0507-3
Kiiskinen LL, Rättö M, Kruus K (2004) Screening for novel laccase-producing microbes. J Appl Microbiol 97(3):640–646. https://doi.org/10.1111/j.1365-2672.2004.02348.x
Kirk TK, Schultz E, Connors WJ, Lorenz LF, Zeikus JG (1978) Influence of culture parameters on lignin metabolism by Phanerochaete chrysosporium. Arch Microbiol 117(3):277–285. https://doi.org/10.1007/BF00738547
Komínková D, Kuehn KA, Büsing N, Steiner D, Gessner MO (2000) Microbial biomass, growth, and respiration associated with submerged litter of Phragmites australis decomposing in a littoral reed stand of a large lake. Aquat Microb Ecol 22(3):271–282. https://doi.org/10.3354/ame022271
Kumar S, Nei M, Dudley J, Tamura K (2008) MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform 9(4):299–306. https://doi.org/10.1093/bib/bbn017
Kunamneni A, Ballesteros A, Plou FJ, Alcalde M (2007) Fungal laccase–a versatile enzyme for biotechnological applications. In: Méndez-Vilas A (ed) Communicating current research and educational topics and trends in applied microbiology, vol 1, FORMATEX: Badajoz, Spain, pp 233–245
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680. https://doi.org/10.1038/227680a0
Liu L, Lin Z, Zheng T, Lin L, Zheng C, Lin Z, Wang S, Wang Z (2009) Fermentation optimization and characterization of the laccase from Pleurotus ostreatus strain 10969. Enzyme Microb Technol 44(6–7):426–433. https://doi.org/10.1016/j.enzmictec.2009.02.008
Mahmoud MG, Rifaat HM, El Sayed OH, El Beih FM, Selim MS (2013) Effect of inducers and process parameters on laccase production by locally isolated marine Streptomyces lydicus from Red Sea, Egypt. Int J Chemtech Res 5(1):15–23
Majcherczyk A, Johannes C, Hüttermann A (1998) Oxidation of polycyclic aromatic hydrocarbons (PAH) by laccase of Trametes versicolor. Enzyme Microb Technol 22(5):335–341. https://doi.org/10.1016/S0141-0229(97)00199-3
More SS, Renuka PS, Pruthvi K, Swetha M, Malini S, Veena SM (2011) Isolation, purification, and characterization of fungal laccase from Pleurotus sp. Enzyme Res 2011:1–7. https://doi.org/10.4061/2011/248735
Murugesan K, Chang YY, Kim YM, Jeon JR, Kim EJ, Chang YS (2010) Enhanced transformation of triclosan by laccase in the presence of redox mediators. Water Res 44(1):298–308. https://doi.org/10.1016/j.watres.2009.09.058
Patel H, Gupte A, Gupte S (2009) Effect of different culture conditions and inducers on production of laccase by a basidiomycete fungal isolate Pleurotus ostreatus HP-1 under solid state fermentation. BioResources 4(1):268–284
Patel H, Gupte S, Gahlout M, Gupte A (2014) Purification and characterization of an extracellular laccase from solid-state culture of Pleurotus ostreatus HP-1. 3 Biotech 4(1):77–84. https://doi.org/10.1007/s13205-013-0129-1
Periasamy R, Palvannan T (2010) Optimization of laccase production by Pleurotus ostreatus IMI 395545 using the Taguchi DOE methodology. J Basic Microbiol 50(6):548–556. https://doi.org/10.1002/jobm.201000095
Prasad KK, Mohan SV, Rao RS, Pati BR, Sarma PN (2005) Laccase production by Pleurotus ostreatus 1804: optimization of submerged culture conditions by Taguchi DOE methodology. Biochem Eng J 24(1):17–26. https://doi.org/10.1016/j.bej.2005.01.019
Prescott CE (2010) Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 101:133–149. https://doi.org/10.1007/s10533-010-9439-0
Salony Mishra S, Bisaria VS (2006) Production and characterization of laccase from Cyathus bulleri and its use in decolourization of recalcitrant textile dyes. Appl Microbiol Biotechnol 71(5):646–653. https://doi.org/10.1007/s00253-005-0206-4
Saratale RG, Saratale GD, Chang JS, Govindwar SP (2009) Decolorization and biodegradation of textile dye Navy blue HER by Trichosporon beigelii NCIM-3326. J Hazard Mater 166(2–3):1421–1428. https://doi.org/10.1016/j.jhazmat.2008.12.068
Singh D, Gupta N (2020) Microbial laccase: a robust enzyme and its industrial applications. Biologia 75:1183–1193. https://doi.org/10.2478/s11756-019-00414-9
Šnajdr J, Baldrian P (2007) Temperature affects the production, activity and stability of ligninolytic enzymes in Pleurotus ostreatus and Trametes versicolor. Folia Microbiol 52(5):498–502. https://doi.org/10.1007/BF02932110
Teodoro TS, Oliveira FD, Poffo C, Braga LP, Arbigaus A, Rampinelli JR, Wisbeck E, Bonatti-Chaves M, Furlan SA (2018) The influence of Tween 80 on laccase production by Pleurotus sajor-caju and the efficiency of crude enzyme broth in the removal of bisphenol-A. Arq Inst Biol 85:1–10. https://doi.org/10.1590/1808-1657001022017
Tuomi M, Rasinmäki J, Repo A, Vanhala P, Liski J (2011) Soil carbon model Yasso07 graphical user interface. Environ Model Softw 26(11):1358–1362. https://doi.org/10.1016/j.envsoft.2011.05.009
Upadhyay P, Shrivastava R, Agrawal PK (2016) Bioprospecting and biotechnological applications of fungal laccase. 3 Biotech 6(1):15. https://doi.org/10.1007/s13205-015-0316-3
Urbaski NK, Bersewicz A (2000) Generation of ·OH initiated by interaction of Fe2+ and Cu+ with dioxygen comparison with the Fenton chemistry. Acta Biochim Pol 47(4):951–962. https://doi.org/10.18388/abp.2000_3950
Valášková V, Baldrian P (2006) Estimation of bound and free fractions of lignocellulose-degrading enzymes of wood-rotting fungi Pleurotus ostreatus, Trametes versicolor and Piptoporus betulinus. Res Microbiol 157(2):119–124. https://doi.org/10.1016/j.resmic.2005.06.004
Viswanath B, Rajesh B, Janardhan A, Kumar AP, Narasimha G (2014) Fungal laccases and their applications in bioremediation. Enzyme Res 2014:1–21. https://doi.org/10.1155/2014/163242
Voříšková J, Baldrian P (2013) Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J 7(3):477. https://doi.org/10.1038/ismej.2012.116
Wagner WE III (2016) Using IBM® SPSS® statistics for research methods and social science statistics. Sage Publications Inc., USA
Wang F, Xu L, Zhao L, Ding Z, Ma H, Terry N (2019) Fungal laccase production from lignocellulosic agricultural wastes by solid-state fermentation: a review. Microorganisms 7(12):665. https://doi.org/10.3390/microorganisms7120665
Xavier RB, Maria A, Mora Tavares AP, Ferreira R, Amado F (2007) Trametes versicolor growth and laccase induction with by-products of pulp and paper industry. Electron J Biotechn 10(3):444–451. https://doi.org/10.2225/vol10-issue3-fulltext-1
Zhu C, Bao G, Huang S (2016) Optimization of laccase production in the white-rot fungus Pleurotus ostreatus (ACCC 52857) induced through yeast extract and copper. Biotechnol Biotechnol Equip 30(2):270–276. https://doi.org/10.1080/13102818.2015.1135081
Zouari-Mechichi H, Mechichi T, Dhouib A, Sayadi S, Martinez AT, Martinez MJ (2006) Laccase purification and characterization from Trametes trogii isolated in Tunisia: decolorization of textile dyes by the purified enzyme. Enzyme Microb Technol 39(1):141–148. https://doi.org/10.1016/j.enzmictec.2005.11.027
Acknowledgements
The authors are thankful to Mangalore University and University of Mysore, Karnataka, India for providing the laboratory facilities.
Funding
This research work was supported by the University Grants Commission (UGC), New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Geethanjali, P.A., Gowtham, H.G. & Jayashankar, M. Optimization of culture conditions for hyper-production of laccase from an indigenous litter dwelling fungus Mucor circinelloides GL1. Environmental Sustainability 3, 481–495 (2020). https://doi.org/10.1007/s42398-020-00137-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42398-020-00137-7