Abstract
The multi-route reversible chemical scheme involves several chemical species and needs to inspect their behavior, activation energy, and the transition period before attaining equilibrium. The comparison between the transition periods of each reaction route is perceived. However, the behavior of the involved species in a multi-routes of the reaction mechanism is presented graphically. The computational procedure is adopted by using MATLAB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Form the last 3 decades, the collaboration between mathematics and chemistry were bloom. The most dominating name of pioneers in constructing the concept of stoichiometry is Lavoisier and Dalton, on the parallel bases, Cayley is the maker of matrices theory. In the 1950–1960s, the re-new computational results on the interface of mathematics and chemistry had made. In the current era, in mathematical chemistry, many severe hypotheticals are easily to fathom. There is a vast application of chemical reactions like the process of burning, rust, the formation of many useful chemicals and systems of biochemical regularly that’s why we cannot neglect the enthusiastic role of chemistry in our domestic or industrials level (Kooshkbaghi et al. 2014; Shahzad et al. 2015a; Aris 1965; Constales et al. 2016; Maxwell 1867; Boltzmann 1970). Many researchers develop their interest in the field of complex chemical reaction mechanisms and serve their efforts. By the brief knowledge of chemical mechanisms, we can get access to the complication of chemistry. Many microscopic species take part in chemical reactions (Sultan et al. 2019b, 2020; Shahzad et al. 2015b, 2016a, 2019a; Kooshkbaghi et al. 2016;). It is difficult to handle these species easily. For this, we need to model the microscopic system mathematically and then find their features (Shahzad et al. 2019b,2019c,2016b; Gorban and Karlin 2003).
The analysis of this article demonstrates the steady-state behavior of reduced species in the reaction mechanism and the transition time for the common species of both reaction routes. Although both routes are not in the same dimension, we have delivered the new idea of their unique equilibrium lies in the respective domain. Moreover, it is observed from the above discussion that the current consideration is unique, and no such analysis has been reflected before in the literature.
Chemical reactions
Firstly, we need to construct the model of the complex chemical reaction mechanism that may occur through a series of distinct steps, known as stoichiometric mechanisms. Each of these steps can be written as a chemical equation, i.e., if we have \(i{\text{th}}\) chemical species \(A_{{_{i} }}\), participating in chemical reactions then sth generic stoichiometric mechanisms can be written as:
These formal sums \(\sum\nolimits_{i = 1}^{n} {\beta_{si} \cdot A_{{_{i} }} } = (\beta_{s} ,A)\) appearing on both sides of each elementary reactions are called complexes \(\Theta_{i}\). Thus the above system (1) can be rewritten as \(\Theta_{i}^{ - } \to \Theta_{i}^{ + }\). The set of complexes for the given reaction mechanism will become \(\Theta_{i} ,...,\Theta_{q}\), while they may be different from each other or coincide in some cases, therefore, \(q\,\, < \,\,2n\).
Reaction routes
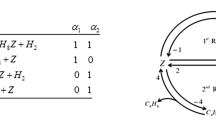
Further, the complexity of the mechanism can be reduced to study the multi-route reaction mechanism separately. Here we follow the mathematical model for multiple reaction routes mechanism (Sultan et al. 2019a) (Fig. 1).
In two-route graphs with a common step, there are three domains, but only two domains are separate, and so there are two independent reaction routes given in Fig. 2.
To deliberate the different kinetics patterns in detail, a four-step reaction mechanism is considered (Constales et al. 2016; Sultan et al. 2019a). The reaction mechanism with common step adopts two reaction routes and seven chemical components \({\text{C}}_{4} {\text{H}}_{10} ,{\text{Z}},{\text{C}}_{4} {\text{H}}_{8} {\text{Z}},{\text{H}}_{2} ,{\text{C}}_{4} {\text{H}}_{8} ,{\text{C}}_{4} {\text{H}}_{6} {\text{Z}}\,{\text{and}}\,{\text{C}}_{4} {\text{H}}_{6}\) formed by three chemical elements \(\left( {{\text{C}},{\text{H and Z}}} \right).\)
Now, we need to calculate the steady-state approximation of all chemical species for both reaction routes and compare their transition time-period of the system (2). Firstly, examine the first-route of the reaction mechanism which gives the product in the first and second steps. The initial constraints are chosen as:
Finally, the kinetic equations are the sum of the product of their stoichiometric vectors and rate equations (Shahzad and Sultan 2018).
First route
A complex chemical reaction involves
The above chemical mechanisms can be written as
Reaction rates are given by the mass action law, i.e.,
and their stoichiometric vectors are
The kinetic equations for our system (4) are given by:
Second route
The second route of the reaction mechanism is given as:
The above chemical mechanisms can be written as
The kinetic equations for the second route of reaction mechanism are:
Graphical results
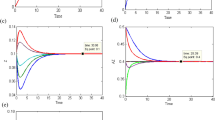
The steady-state behaviors of chemically reacting species of both routes are given in Figs. 3a–e and 4a–e, respectively at their equilibrium points. The initial trajectories that are starting from different initial points and approaching towards the equilibrium point after completing their transition time-period.
The behavior and transition time of involving species in the second route is discussed in Fig. 4a–e.
A mathematical methodology has implemented to observe the transition period of each chemical species involved in both reaction routes. It is perceived from Figs. 3a–e and 4a–e that the transition time taken by each chemical species \({\text{C}}_{4} {\text{H}}_{10}\),\({\text{C}}_{4} {\text{H}}_{8} ,{\text{Z}}\),\({\text{C}}_{4} {\text{H}}_{8} ,{\text{Z, C}}_{4} {\text{H}}_{6} {\text{, C}}_{4} {\text{H}}_{6} {\text{Z and H}}_{2}\) of both first and second route is different.
Reaction route comparison w.r.t time
Here we have addressed the comparison of common species of two different routes. It is clear from Fig. 5a–e, the first reaction rout completes its transition time-period faster than the second route.
Concluding remarks
In this article, we have measured the transition period of the same species involved in the two-route complex reaction mechanism. The behavior of the involved species is presented graphically with the following outcomes.
-
Different time-period of common species have been observed through different initials.
-
The involved species in both routes approach towards their equilibrium after passing through a different transition period.
-
This indicates the different behavior of the same species in different routes.
References
Aris R (1965) Prolegomena to the rational analysis of systems of chemical reactions. Arch Ration Mech Anal 19:81
Boltzmann L (1970) Weitere studien über das wärmegleichgewicht unter gasmolekülen. Springer, Berlin
Constales D, Yablonsky GS, D'hooge DR, Thybout JW, Marin GB (2016) Advanced data analysis and modelling in chemical engineering. Elsevier, Amsterdam
Gorban AN, Karlin IV (2003) Method of invariant manifold for chemical kinetics. Chem Eng Sci 58:4751–4768
Kooshkbaghi M, Frouzakis CE, Chiavazzo E, Boulouchos K, Karlin IV (2014) The global relaxation redistribution method for reduction of combustion kinetics. J Chem Phys 141:044102
Kooshkbaghi M, Frouzakis CE, Boulouchos K, Karlin IV (2016) Spectral quasi-equilibrium manifold for chemical kinetics. J Phys Chem A 120:3406–4313
Maxwell JC (1867) On the dynamical theory of gases. Philos Trans R Soc Lond 157:49
Shahzad M, Sultan F (2018) Complex reactions and dynamics: advanced chemical kinetics. InTech, Rijeka
Shahzad M, Rehman S, Bibi R, Wahab HA, Abdullah S, Ahmed S (2015a) Measuring the complex behavior of the SO2 oxidation reaction. Comput Ecol Softw 5:254–260
Shahzad M, Sajid M, Gulistan M, Arif H (2015b) Initially approximated quasi equilibrium manifold. J Chem Soc Pak 37:1–7
Shahzad M, Sultan F, Haq I (2016a) Computing the low dimension manifold in dissipative dynamical systems. Nucleus 53:107–113
Shahzad M, Haq I, Sultan F, Wahab A, Faizullah F, Rehman G (2016b) Slow manifolds in chemical kinetics. J Chem Soc Pak 38:5
Shahzad M, Sultan F, Haq I, Ali M, Khan WA (2019a) C-matrix and invariants in chemical kinetics: a mathematical concept. Pramana J Phys. https://doi.org/10.1007/s12043-019-1723-5
Shahzad M, Sultan F, Ali M, Khan WA, Irfan M (2019b) Slow invariant manifold assessments in multi-route reaction mechanism. J Mol Liq 284:265–270
Shahzad M, Sultan F, Shah SIA, Ali M, Khan HA, Khan WA (2019c) Physical assessments on chemically reacting species and reduction schemes for the approximation of invariant manifolds. J Mol Liq 285:237–243. https://doi.org/10.1016/j.molliq.2019.03.031
Sultan F, Khan WA, Shahzad M, Ali M, Shah SIA (2019a) Activation energy characteristics of chemically reacting species in multi-route complex reaction mechanism. Indian J Phys. https://doi.org/10.1007/s12648-019-01624-2
Sultan F, Shahzad M, Ali M, Khan WA (2019b) The reaction routes comparison with respect to slow invariant manifold and equilibrium points. AIP Adv 9:015212. https://doi.org/10.1063/1.5050265
Sultan F, Ali M, Shahzad M, Khan M (2020) Multi-route reaction mechanism and steady-state flow: a MATLAB-based analysis. Appl Nanosci. https://doi.org/10.1007/s13204-020-01410-1
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ali, M., Hamza, S., Aldila, D. et al. Evaluation of steady-state to identify the fast-slow completion-route in the multi-route reaction mechanism. Appl Nanosci 10, 3405–3410 (2020). https://doi.org/10.1007/s13204-020-01455-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-020-01455-2