Abstract
Agricultural and industrial activity generates high concentrations of organic and inorganic pollutants, many of which are incorporated into the trophic chain, affecting ecosystems. There are several strategies for the remediation of polluted areas; we discuss one of them in the present review that shows the successful evidence of the use of arbuscular mycorrhizal symbiosis in phytoextraction (the removal of contaminants from soil and water sources with mycorrhizal plants), and in the process of phytostabilization (the reduction of the mobility of heavy metals in soil by mycorrhizal roots, absorption onto roots, or precipitation within the root zone). Mechanisms of action of arbuscular mycorrhizal fungi (AMF) including, altered uptake and distribution of heavy metals, improvement in the mineral nutrition and water availability, protection against oxidative stress and increment in the physical stability of the soil by producing glomalin has been discussed with reference to heavy metals (HMs) and persistent oxidative pollutants (POPs). We report plant species associated with species of mycorrhizal fungi as strategy for phytostabilizing heavy metals and reducing biotranslocation to the aerial parts of plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Anthropogenic activities (e.g., mining, pesticides, smelting, electroplating, sludge waste, industrial discharge, burning of fossil fuel) have dramatically accelerated the process of environmental contamination by the discharge of hazardous wastes into soil and water (Sodango et al. 2018; Riaz et al. 2020). This situation has caused air and soil pollution, acid precipitation, soil degradation, salinity, increasing UV-B radiation and climate change (Schutzendubel and Polle 2002). Agricultural wastes include a wide range of organic materials (often containing pesticides), animal wastes, and timber by-products (Setyorini et al. 2002). Agricultural soils are a major environmental reservoir for antibiotic residues. Antibiotics are commonly used in livestock farming and much of it eventually ends up in manure, which is subsequently applied to agricultural land (Cao et al. 2018). Mining and smelting of metalliferous ores combined with combustion of fossil fuels have dramatically increased the global deposition of heavy metals (HMs) over the past two centuries (Agarwal et al. 2017). Cadmium (Cd) is added to agricultural systems through atmospheric deposition, application of sewage sludges and manures, irrigation water, and in fertilizers and soil amendments (Grant and Sheppard 2008). The excessive accumulation of heavy metals in agricultural soils results in a decrease in the soil quality and crop growth (Babadi et al. 2019). The latent for toxicity, carcinogenicity, and bioaccumulation in living systems are also a concern (Tchounwou et al. 2014).
Anthropogenic soil pollution by organic and inorganic compounds is a global problem. Such compounds include HMs, fuels, hazardous waste, explosives, and petroleum products. The most significant inorganic pollutants are HMs that include group of metals and metalloids that have relatively high density and are toxic even at ppb (parts per billions) levels (Csuros and Csuros 2002; Ali and Khan 2017). HMs are considered hazardous due to three reasons: persistence, bioaccumulation, and toxicity (Ali et al. 2019). Bioaccumulation is the process whereby the accumulation of toxic substances in living beings increases in concentration following a rise in the trophic level: the higher the trophic level is, the stronger the concentration of HMs is as well (Aprile and De Bellis 2020). Regarding their roles in biological systems, HMs are classified as essential and nonessential (Ali et al. 2019). Some examples of metals categorized as essential copper (Cu), iron (Fe), manganese (Mn) and zinc (Zn) and non-essential include arsenic (As), cadmium (Cd), chromium (Cr), cobalt (Co), lead (Pb), mercury (Hg), nickel (Ni) and vanadium (V) (Aprile and De Bellis 2020). The metals are toxic at higher concentrations because they induce oxidative stress (reactive oxygen species ROS) through the formation of free radicals, which inhibits most cellular processes at various levels of metabolism (Appenroth 2010; Sytar et al. 2013). HMs are also considered as trace elements because of their presence in trace concentrations (ppb range to less than 10 ppm) in various environmental matrices (Tchounwou et al. 2014), but they can also be toxic at relatively low concentrations (Ross 1975). The HMs are persistent in the environment. They accumulate in living organisms and are transferred from one trophic level to another in the food chains (Ali et al. 2019;).
The organic pollutants added as a result of anthropognic activities, commonly called persistent organic pollutants (POPs), are resistant to environmental degradation process and are affecting health of ecosystems and humans (reviewed by Lenoir et al. 2016a; Oyetibo et al. 2017). POPs can persist in the body fat of humans and animals for decades, and can cause cancer, birth defects, learning disabilities, and immunological, endocrinal, behavioral, neurological, and reproductive problems (Lenoir et al. 2016a). The United States Environmental Protection Agency lists POPs in the soil, such as polycyclic aromatic hydrocarbons (PAHs), as priority pollutants and having carcinogenic and mutagenic properties make them a cause of global concern (Gao et al. 2010).
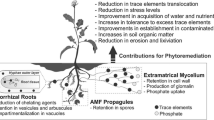
In order to remove these toxic compounds from polluted soils, different technologies and methods have been developed, most of which include the physical elimination of soil into landfills or extraction through physical or chemical means (Oyetibo et al. 2017). Even though these techniques are fast, their economic and environmental cost and potential detrimental impact on the physical, chemical, and biological properties of the soil make them less desirable and feasible (Glick 2010). As an alternative to these methods, researchers have developed phytoremediation approaches that include the use of plants for the elimination or neutralization of a variety of compounds. Phytobioremediation is the process of using plants and soil microbes for removing and cleaning chemical pollutants from soil, both organic and inorganic pollutants (Dua et al. 2002). Phytoremediation could be classified as: phytoextraction, phytodegradation, rhizodegradation, phytostabilization, and phytovolatilization (Miransari 2011). Phytoextraction and phytostabilization are the most researched processes of phytoremediation. In the process of phytoextraction, plants concentrate the HMs in their aerial parts by removing them from soil, while the process of phytostabilization HMs are not removed from the environment but immobilizes them in plant roots (Abdelhameed and Metwally 2019) (Fig. 1). Phytostabilization is an alternative strategy that reduces the mobility and bioavailability of heavy metals in soil, thereby preventing their migration into groundwater or entry into the food chain (Chen et al. 2018b).
Scheme summarizing the phytostabilization and phytoextraction strategies for bioremediation of soils contaminated with heavy metals using plants with arbuscular mycorrhizal symbiosis: 1) phytostabilization, absorption of HMs to the roots with AMF, to be deposited within the cell wall or accumulated within the vacuoles of AMF and or roots. 2) Phytoextraction: HMs are transferred from the roots to the host shoots via xylem and phloem, to be accumulated in the aerial parts (leaf vacuoles)
Plant root-fungal symbioses (mycorrhizas) have recently been projected to have a role in phytoremediation of anthropogenic soil pollution (Dhalaria et al. 2020; Janeeshma and Puthur 2020; Riaz et al. 2020). Mycorrhizas are ubiquitous and comprise two main groups: ectomycorrhizas, formed mainly by forest trees; and, arbuscular mycorrhizas (AM), formed mainly by herbaceous plants. The fungi derive carbon and lipids from the plant and transfer mineral nutrients, mainly phosphorus (P) and nitrogen (N) to the plant (Smith and Read 1997). They also help in alleviation of HM stress in soil, improvement of soil structure, protection of roots from plant pathogens and interaction with other soil microbes (Miransari 2011; Gupta et al. 2019; Gupta and Abbott 2020). The arbuscular mycorrhizal fungi (AMF) belong to the subphylum Glomeromycotina which is composed of approximately 330 fungi species (Schüßler et al. 2001, Spatafora et al. 2016; Tedersoo et al. 2018, Goto and Jobim 2020, Gupta and Abbott 2020; Wijayawardene et al. 2020). These fungi form mutualistic and obligate symbiotic associations with around 80% of vascular plants and particularly important components because, they can significantly increase the efficiency of agro-ecosystems (Wang and Qiu 2006; Brundrett and Tedersoo 2018; Solís-Ramos and Andrade-Torres 2020). AMF can alter productivity, by acting as biofertilizers, bioprotectors or biodegraders (Xavier and Boyetchko 2002; Gupta et al. 2018; Chen et al. 2018a).
In the present review, we have explored the role of arbuscular mycorrhizal symbiosis in phytoremediation of anthropogenic soil pollution. The review summarizes the current knowledge regarding AMF assisted remediation of HMs and POPs and some of the strategies used by mycorrhizal fungi to cope with stressful environments. Moreover, this review provides the specific information on application of different AMF species along with the mechanism involved in both phytoaccumulation and phytoextraction of these pollutants.
2 Applications of AMF in phytoremediation
Notwithstanding the role of AMF in plant-soil-microbe interactions and plant nutrition, there were fewer studies focusing on the potential of bioremediation. One possible reason is that the initial studies on bioremediation were focused on the use of plant families reported as non-mycorrhizal, such as Brassicaceae and Caryophyllaceae (Abdul 2006). The plant families Chenopodiaceae, Cruciferaceae, Plumbaginaceae, Juncaceae, Juncaginaceae, Amaranthaceae, and some members of the Fabaceae, do not form symbiosis with AMF (Smith and Read 1997; Brundrett and Tedersoo 2020). However, Thlaspi praecox (Brassicaceae) was discovered as metal hyperaccumulating plants species colonized by AMF. The first report of AMF colonization of Zn, Cd and Pb hyperaccumulating Thlaspi praecox Wulfen (Brassicaceae) under greenhouse conditions, which was favored by a high demand of nutrients (for example, during the reproductive period) (Vogel-Mikus et al. 2006). The roots colonization of Thlaspi praecox (Brassicacear) in the polluted soils was characterized by the presence of AMF typical structures of Glomus species (Pongrac et al. 2009). Changes in Zn, Cd and Pb uptake strategies strongly suggest AM colonization may be one of the tolerance strategies of plant establishment of T. praecox on polluted sites (Vogel-Mikus et al. 2006).
Research approaches have mostly focused on the diversity and tolerance of AMF in soils polluted with HMs, trying to understand the fundamental basis of the adaptation and tolerance of AMF to HMs in the soil in order to facilitate their soil microorganisms for restoration and bioremediation programs (Leyval et al. 2002). AMF can contribute to phytoremediation in two ways: First. they can either accumulate and sequester toxic metal ions themselves, thus protecting their host from the pollutant (phytoaccumulation) or they can deliver HMs to the host just like essential mineral nutrients such as Cu and Zn, resulting in heavy metal accumulation in the host (phtyostabilization) (Chen et al. 2018a). The situation is applied for plant production in polluted sites, with minimal toxic effect on the crop. In the second case, however, harvested plants are destroyed to reduce the heavy metal load of the site.
Arbuscular mycorrhizae exhibit different tolerance levels depending on HM type and concentration. For example, Acaulospora laevis is sensitive to Cu and particularly to Cd and Glomus caledonium is more tolerant to these two HMs under the same sand culture experimental conditions. This study suggests that G. caledonium can be a promising mycorrhizal fungus for the bioremediation of soils polluted with these HMs (Liao et al. 2003). Mycorrhizal fungi are capable of increasing the growth and fitness of plants in soils containing Cd. The addition of AMF to polluted agricultural soils is also a viable option if the fungi decrease or do not increase the amount of Cd accumulated in the parts of plants for human consumption (Hancock et al. 2012). Another study demonstrated that AMF (Glomus macrocarpum, Paraglomus occultum and Glomus sp.) have beneficial effects on plant growth and alleviation of pollutants in Acacia mangium, Sorghum bicolor, and Urochloa brizantha in soils polluted with Zn, Cu, Pb, and Cd, even though there were no differences in HMs concentration between shoots of plants with and without mycorrhizas (Pedroso et al. 2018). Nevertheless, AMF is a complex system and the inconsistent results regarding the effect of AMF on HMs uptake are a consequence of a wide range of factors, such as metal concentration and species (Andrade et al. 2010), competition between metals, physical-chemical soil characteristics, plant-microorganism association type, plant growth conditions, and root density (Lebeau et al. 2008), mycorrhizal fungus species, plant tolerance to contaminants and bioavailability of heavy metals (Yang et al. 2015). It is important to highlight that the results vary between treated and untreated soils (pasteurized/sterilized) (Joner and Leyval 2001).
There has been a diverse influence of the pollutants on these fungi. For example, Whitfield et al. (2004) mentioned that HMs concentration only influences vesicle (lipid storage structures) abundance, which were higher in polluted sites, and probably reflects a difference in the fungal species mix colonizing the roots, where Glomus was the predominant species. However, Del Val et al. (1999) showed that AMF spore number and species richness depend on the level of soil pollution and, host plant species selectively influence AMF population size and diversity. Furthermore, Orłowska et al. (2012) observed a lower amount of mycelium in strains isolated from sites polluted with As, than in those from non-polluted sites inoculated into plants of Plantago lanceolata. One study showed that G. mosseae had the highest extracellular HMs absorption of Cd and it was higher than Ca and Zn (Joner et al. 2000).
Mycorrhization benefits revegetation processes in polluted areas due to a better establishment of plants in these areas (Pedroso et al. 2018). According to the results by Hassan (2005), cotton plants are good candidates for revegetation and phytostabilization of HMs in polluted soils, since AMF use an exclusion strategy in which the deposition of metals within the mycelium and cortical cells of the roots of AMF prevent the translocation of metals from roots to shoots. The application of amendments allows the increase of P, which, at the same time, can increase biomass as well as growth parameters and, thus, detoxify the potential effects of metals by the dilution, precipitation, or absorption of metals on phosphate granules; in that way, limiting their entrance to root cells. The results of the study of Gu et al. (2017), indicated that AMF inoculation has a species-specific effect: each plant species showed variation in biomass production and metal accumulation. For example, among the plants studied Perennial Ryegrass (Lolium perenne), Tall Fescue (Festuca arundinacea), Showy Stonecrop (Hylotelephium spectabile), and Purple Heart (Tradescantia pallida), H. spectabile showed the greatest growth response to mycorrhizal inoculation and the lowest concentrations of Pb, Zn, Cu, and Cd in both shoots and roots. A relevant aspect to be considered in the design of bioremediation programs together with the selection of endemic metallophytes and AM fungal strains, is the selection of species that can produce glomalin at high quantities (Cornejo et al. 2017).
The success of AMF for phytoremediation of POPs has showed a wide range of components and their mixtures, such as aliphatic hydrocarbons, fuel oils and other petroleum hydrocarbon mixtures, polycyclic aromatic hydrocarbons (PAHs), explosives, pesticides, and chlorinated organic compounds (Joner and Leyval 2003a). The hyphae and extraradical mycelium of AMF can play an important role in the uptake and translocation of phenanthrene (PHE) and pyrene (PYR) in plants, which suggests their potential use for the remediation of soils polluted with polycyclic aromatic hydrocarbons (PAHs) (Gao et al. 2010; Gao et al. 2011). AMF inoculated into plants significantly contribute to the degradation of petroleum hydrocarbon (Joner and Leyval 2003b; Volante et al. 2005; Verdin et al. 2006; Alarcón et al. 2008; Wu et al. 2009; Hernández-Ortega et al. 2012). There is evidence that AMF can reduce the presence of aromatic hydrocarbons (benzene, toluene, ethylbenzene, and xylene, BTEX) in artificially polluted soils. It is interesting to see that the effects vary with AMF species and BTEX nature (Volante et al. 2005). One study documented that AMF colonizing and establishing in the rhizosphere of Eleocharis obtusa and Panicum capillare grown at high petroleum hydrocarbon levels, where twenty-one taxa were identified, encompassing the major families within Glomeromycota. This suggests that AMF can be potentially important microbial candidates in the bioremediation of oil-contaminated soils (De la Providencia et al. 2015). It has also been observed that the application of mycorrhizal fungi combined with surfactants has a potential biotechnological use in the decontamination of soils with organic pollutants (Wu et al. 2008). This was demonstrated by Wu et al. (2008), who found that the colonization of alfalfa roots by AMF (Glomus etunicatum) and the application of Triton X-100 favor the accumulation of DDD (1,1-dichloro-2,2-bis (p chlorophenyl) ethane) in the roots and decrease it in the shoots.
3 Mechanism of AMF mediated phytoremediation
The general role of AMF symbiosis in phytoremediation involves several processes including, enhanced uptake through an enhanced microbial activity in soils with low HMs concentrations, metal-binding contributing to plant biomass and tolerance to HMs stress in soils with high HMs concentrations, absorption by extraradical hyphae or spores and chelation in fungal cells or through chelating molecules (Rivera-Becerril et al. 2005; Audet and Charest 2007; Riaz et al. 2020; Dhalaria et al. 2020). AMF can facilitate the movement of HMs to plant roots through various mechanisms, such as: deposition in the cellular wall or fungal vacuoles, sequestration by siderophores that can deposit HMs in root apoplasm or in the soil, metallothioneins or phytochelatins can result in the deposition of HMs in fungal or plant cells, and allocation of HMs from the cytoplasm by metal transporters in the plasmalemma or tonoplast of both symbionts (Miransari 2011).
Some examples are included below to illustrate the mechanisms through which AMF immobilize heavy metals in soil or roots and thus, demonstrate the suitability of AMF for phytostabilization applications (Ambrosini et al. 2015).
-
1.
AMF influence the uptake and distribution of metals in host plants - For example, in roots of Lotus japonicus inoculated with AMF species Rhizophagus irregularis, it was observed that the arbuscules and intercellular hyphae accumulated large amounts of Cd, followed by the vesicles, while plant cells did not. This distribution pattern suggested that after the extraradical hyphae uptake and translocate Cd to intraradical hyphae, this toxic metal was mainly retained in the fungal structure, particularly in the arbuscules, and did not seem to be delivered to plant cells (Chen et al. 2018b). The tolerance mediated by AMF can occur by metal exclusion mechanisms, where fungal structures, such as the extraradical mycelium, can play an important role (Ambrosini et al. 2015). For example, AMF can immobilize uranium (U) in soil by absorption and, potentially, by the formation of complexes with AMF glycoproteins and intracellular polyphosphates. Even though AMF can transfer U to their hosts and consequently, participate directly in U accumulation by the plants, it is also clear that most of the U translocated by AMF towards their intraradical mycelium remain within AMF structures, thus restricting roots to shoots translocation of U (Dupré de Boulois et al. 2008). In mycorrhized coffee plants, it has been observed that if the concentration of Cu in the soil is between 50 and 100 mg-kg, the metal is mostly retained in the roots, which acts as a barrier for translocation to the shoots (Andrade et al. 2010).
The AMF promotes the absorption of P through the roots and may cause the formation of less mobile metal-phosphate compounds in plants, reducing the translocation of trace elements from the roots to the shoots. This was observed in the wine plants, in the presence of Cu and inoculated with six AMF species (Dentiscutata heterogama, Gigaspora gigantea, Acaulospora morrowiae, A. colombiana, Rhizophagus clarus, R. irregularis). Where R. clarus and R. irregularis showed a high colonization in the wine roots and improved the P absorption and roots growth in soils with high levels of Cu (Ambrosini et al. 2015). Different levels of Cd in the soil have an important effect on the behavior of mycorrhiza fungi, and these fungi could increase or decrease the uptake of Cd by plants and regulate accumulation in the plant tissues. In a study related to inoculate sorghum with Claroideoglomus etunicatum under stress for Cd, the results revealed the key role of AMF in translocation of Cd in the rhizobox and also, in precise control of Cd concentration of plant tissues (increment or decrease of them depending on Cd composition and Cd availability). The metal is probably stored in roots, in fungal hyphae and mycelium, and its transmission and toxic effects to shoots are largely prevented. AMF action enhance both, plant tolerance and phytostabilization of Cd contaminated soil (Babadi et al. 2019).
HMs can be deposited in root cell walls or accumulated within root cells, forming complexes with organic molecules such as polyphosphates, amino acids, metallothioneins, or phytochelatins (Gupta and Goldsbrough 1991; Andrade et al. 2010). One study showed that AMF isolated from a HM-tolerant plant (Viola calaminaria) have a significant effect on HM accumulation in plant roots in a non-toxic form;apparently, by restricting the transfer of metals to shoots (Tonin et al. 2001). HM can be stored in cellular compartments, including spores and vesicles. Following this storage process, the metabolic rate is reduced and the effect of HMs on plant metabolism is decreased, having a beneficial effect on the plant and AMF growth, for example Cu (Ambrosini et al. 2015). Rhizophagus irregularis accumulates Cu in vesicles, improving the tolerance of Tagetes erecta L., even when accumulation increases in the roots, which suggests that this system has a potential use as phytostabilizer of Cu in polluted soils (Castillo et al. 2011).
-
2.
The plants inoculated with AMF reduce the HMs toxicity- For example, in mycorrhizal coffee plants, it was observed that Cu and Zn in high concentrations cause a decrease in the shoots and roots growth, indicating the high phytotoxicity at these concentration (Andrade et al. 2010). Nevertheless, Cd tolerance with AMF inoculation is ascribed to augmented accumulation of stress metabolites such as sugar, proteins, proline, and glycine betaine, eventually leading to increased growth (Sharma et al. 2016; Janousková et al. 2006; Abdelhameed and Metwally 2019).
-
3.
The inoculation with AMF significantly increases the antioxidant enzyme activity - For example, this was evident in trigonella plants (Trigonella foenumgraecum L.) inoculated with AMFs (Glomus monosporum, G. clarum, Gigaspora nigra and Acaulospora laevis), where the damage to the plant caused by the stress provoked by the metal was reduced due to the increase in the antioxidant enzymes activity (Abdelhameed and Metwally 2019). This was suggested to be a tolerance strategy of mycorrhizal trigonella plants against Cd stress (Abdelhameed and Metwally 2019). This agrees with a study where Cassia italica Mill plants under Cd stress, inoculated with the AMF mixture (Funneliformis mosseae syn. Glomus mosseae, Rhizophagus intraradices syn. Rhizophagus irregularis and Claroideoglomus etunicatum syn. Glomus etunicatum), show an increment of the chlorophyll and protein content and additionally, reduced the Cd uptake (Hashem et al. 2016). The inoculated plants with AMF under stress by the metal, reduced the peroxidation of membranes, that may be caused due to the possible role of AMF in phosphate uptake and antioxidant activity. The negative impact mitigation of the stress caused by the metal, due to the increased activity of antioxidants mediate quick scavenging of reactive oxygen species and hence, result in membrane protection, mitigating the negative impact. (Hashem et al. 2016). Mycorrhizal red kidney plants accumulated relatively high metal concentrations (Zn, Cu, Pb and Cd) in shoots more than in their roots. This is attributed to the reduced heavy metal toxicity effects in AMF red kidney plants to antioxidative protection through detoxification of heavy metals, chelation through metal-binding proteins (peptides) and dilution through increased plant growth induced by AMF (Glomus mosseae) (Hassan 2005).
-
4.
The AMF increase the production of GRSP- Glomalin related soil protein (GRSP) can join some metals. GRSP, an insoluble glycoprotein produced in high quantities by AMF external hyphae, is an important component of the organic matter complex in the soil and plays different roles, like in carbon fixation and cycle, aggregate soil stability, prevent water loss and alleviate in toxic or harsh conditions (Vodnik et al. 2008; Malekzadeh et al. 2016; Gao et al. 2019). This was seen in the study related to inoculated sorghum with Claroideoglomus etunicatum under stress for Cd. The results showed that the glomalin production increased, suggesting a role of glomalin in response to soil stresses (Babadi et al. 2019). Also, in Oenothera picensis inoculated with Claroideoglomus claroideum it was determined the high capability of union of Cu for Bradford-reactive soil protein whose fraction includes the glomalin produced by AMF.
The principal suggested function of GRSP production is to protect the living hyphae and AMF itself, and the effects in the soil are secondary, so, it is a stress induced protein (Cornejo et al. 2008; Ferrol et al. 2009; Malekzadeh et al. 2016; Gao et al. 2019). In the “secondary” roles GRSP can sequestrate different heavy metals (González-Chávez et al. 2004; Cornejo et al. 2008; Vodnik et al. 2008; Ferrol et al. 2009; Gil-Cardeza et al. 2014; Wu et al. 2014; Singh 2015; Malekzadeh et al. 2016; Ghasemi et al. 2017; Ferreira et al. 2018; Wang et al. 2020a; Wang et al. 2020b) and toxics like phenanthrene (Gao et al. 2017; Chen et al. 2019; Chen et al. 2020), and it contributes to reduce the bioavailability of the toxics. It has different affinities for bonding to HM, depending on factors like metal chemistry and content. It seems that GRSP is more abundant in high concentrations of the toxic (Vodnik et al. 2008; Wu et al. 2014; Malekzadeh et al. 2016; Ferreira et al. 2018; Wang et al. 2020a). The binding mechanisms of the toxics to the GRSP are not well elucidated. González-Chávez et al. (2004) suggests that the binding of Copper was caused by, electrostatic sorption or strong complex formations. Recently, it has been demonstrated that for the bonding of certain heavy metals, ion exchange is the principal mechanism, so functional groups like carbonyl, hydroxyl, amide and carboxyl may participate in this process (Wang et al. 2020a; Wang et al. 2020b).
GRSP is part of the mechanism that AMF could use on alleviation in remediation processes. Elucidating more information of this protein could be considered to maximize the potential of the applications, because of environmental processes and conditions, and also, the AMF species can affect the production or peak intensity of GRSP (Singh 2012, 2015; Wu et al. 2014; Wang et al. 2020a; Wang et al. 2020b). Diversity of AMF and glomalin content can be considered good indicators of rehabilitation of soils contaminated with Zn, Cu, Pb, and Cd (Leal et al. 2016). GRSP production should be considered in biostabilization of polluted soils since it participates in the sequestration of different PTEs (potentially toxic elements) (González-Chávez et al. 2004; Rilling and Steinberg 2002). AMF protect plants against stress caused by the HMs pollution when it accumulates high concentrations in the radical system and decreases the translocation to the aerial parts (Tonin et al. 2001).
-
5.
Stimulating the growth of hyperaccumulators – The plants that have the capacity to tolerate high levels of HMs present in the soil and after that, and accumulate it in their tissues, are known as metallophytes or hyperaccumulators. Tolerance is the capacity of plants or microorganisms to live and adapt to elevated heavy metal concentrations in soil (Dietz et al. 1999). These hyperaccumulators absorb heavy metals, translocate them through tonoplast and accumulate in vacuoles, in that way, they protect cell metabolism from metal toxicity (Maiti et al. 2004). The use of plants, with hyperaccumulating ability or in association with soil microbes including the symbiotic fungi, arbuscular mycorrhiza, are among the most common biological methods of treating heavy metals in soil (Miransari 2011). Once metals enter the hyphae of AMF, they can be immobilized or transferred to the root, and, in the root, they can be sequestered or translocated to the shoot (Leyval et al. 1997).
In a study focused on Cannabis sativa (var. Carmagnola) associated with Glomus mosseae, in a soil polluted artificially with Cr, Cd and Ni, it was reported a significantly higher concentration of Ni in the plants leaves and stems. So, this association G. mosseae-C. sativa stimulated the hyperaccumulating plant species, enhancing the root to shoot metal translocation to sequester the exceeding toxic metals in the shoot cell vacuoles by means of molecules such as metallothioneins and phytochelatins (Citterio et al. 2005). Eucalyptus globulus is suitable to grow and rehabilitate heavy-metal-polluted soils (Arriagada et al. 2004, 2007). In a study, it was shown that the synergy action of AMF (Glomus mosseae or with Glomus deserticola), with a saprophyte fungus (Fusarium concolor and Trichoderma koningii), allowed a higher Cd and Pb growth and absorption in trees stems and leaves of E. globulus (Arriagada et al. 2007). The AM fungi seems to contribute to the redistribution of Cd inside the plant. In fact, it was higher accumulation of Cd in the stem that in the leaves of eucalyptus colonized with G. deserticola, where the harmful effects on the development of the plant are minimal (Arriagada et al. 2004), This redistribution of heavy metals in the less metabolically active part of the plant might explain why AMF increased the content of heavy metals and enhanced the growth of eucalyptus (Arriagada et al. 2007) (Table 1).
-
6.
Mycorrhizal fungi change the structure of the microbial community and the physical and chemical properties of rhizosphere soils – For example Ogar et al. (2015) and Ma et al. (2019) evaluated the impact of microbial inoculation on phytoremediation. In case of nickel (Ni)-contaminated saline soils using Helianthus annuus together with salt resistant plant beneficial bacterium, Pseudomonas libanensis TR1 and AMF Claroideoglomus claroideum showed bioaugmentation. The results of this study showed that the bioaugmentation using other microbial strains in addition to AMF may be a preferred strategy for improving phytoremediation of metal-polluted saline soils. (Ma et al. 2019).
4 Species of AMF in phytorremediation
Different species of AMF are useful for phytoremediation and their efficiency depend on plant species; however, few AMF are widely used and studied (Table 1). Research has focused mainly on the effects of AMF on HMs, but there are also species that have been used for other kind of pollutants. Studies analyzing species such as Glomus mosseae, G. intraradices, Funneliformis mosseae, or Rhizophagus irregularis are the most common. Some of the most studied heavy metals in the presence of AMF are Cd, Pb, Cr, and Ni. In the case of these metals, the symbiosis provides benefits in the alleviation of different plants by using species like G. mosseae (Jamal et al. 2002; Janousková et al. 2006; Azcón et al. 2009; Ruscitti et al. 2011; Garg and Aggarwal 2012; Garg and Bhandari 2012), G. intraradices (Turnau and Mesjasz-Przybylowicz 2003; Malcová et al. 2003; Janousková et al. 2006; Sudová and Vosátka 2007; Andrade et al. 2008; Ruscitti et al. 2011; Liu et al. 2018; Zhang et al. 2019a), G. aggregatum (Singh et al. 2019; Zhang et al. 2019a), R. fasciculatus (Singh et al. 2019), R. intraradices (Yang et al. 2015; Jiang et al. 2016; Singh et al. 2019), F. mosseae (Yang et al. 2015; Singh et al. 2019; Zhan et al. 2019), and Diversispora spurcum; this last species has been used for Pb, Cd, and Zn (Zhan et al. 2019). For Pb and Cd, species used have been G. etunicatum (Souza et al. 2012; Zhan et al. 2019) and R. irregularis (Zhang et al. 2019b; Wang et al. 2020). For the presence of Cd or Cr, AMFs such as G. deserticola (Mohammad and Mittra 2013; Singh et al. 2014) have been used, and G. geosporum, G. claroideum (Janousková et al. 2006), and G. versiforme (Jiang et al. 2016) have been used only for Cd.
There are also studies on phytoremediation involving other HMs, such as: Zn, Cu, As, and Lanthanum (La). For these HMs, some investigations have used common species or species that also participate in other common HMs remediation, like the ones mentioned above. Glomus mosseae is also used in the presence of Zn and Cu (Jamal et al. 2002; Chen et al. 2007; Azcón et al. 2009). Rhizophagus irregularis is used for Cu or La (Toler et al. 2005; Chen and Zhao 2007) and, for La, other less used species are Acaulospora laevis or Gigaspora margarita (Chen and Zhao 2007). Rhizophagus irregularis has also been used for Cu (Wu et al. 2020). In presence of Zn and As, G. mosseae (Jamal et al. 2002; Azcón et al. 2009) and G. deserticola (Arriagada et al. 2009; Arriagada et al. 2010; Mohammad and Mittra 2013) could have alleviation benefits. For compounds such as TiO2 nanoparticles or iron–cyanide (Fe-CN), F. mosseae (Xu et al. 2019) and R. irregularis (Sut et al. 2016), respectively, can be used for alleviation in plants.
Glomus versiforme has a more beneficial role than Glomus mosseae in promoting plant growth, nutrient absorption, C: N:P stoichiometric adjustment, and alleviation of rare earth element (REE) and HM toxicity in plants. Corn and sorghum show opposite tendencies in REE uptake in response to AMF colonization. Results suggest that the effect of AMF on REE uptake could be related to plant species, AMF isolate, and REE type and concentration in mine residues. Results indicated that AMF could increase the ability of plants to restore ecosystems polluted with the chemical complex of REE in mine residues or with heavy metals (Guo et al. 2013).
AMF are not only used in HM phytoremediation, since they may have benefits for plant alleviation in the presence of oil or PAHs. For PAHs, Lu and Lu (2015) used G. caledonium. For products like Phenanthrene or Pyrene, G. mosseae (Gao et al. 2011) has been used, and G. intraradices has been used for Phenanthrene (Zhou et al. 2013). In addition, Calonne-Salmon et al. (2018) observed that R. irregularis could alleviate the studied host plant in the presence of Benzo[a]pyrene. Rhizophagus irregularis alleviated mixed petroleum (Xun et al. 2015) or Phenol (Ibáñez et al. 2011), and G. clarum can be used for crude oil (Nkereuwem et al. 2020). There are reports of AM resulting in plant alleviation and enhanced removal of less studied human pollutants used in agriculture or veterinary. For herbicides, Dong et al. (2016) observed that F. mosseae may have benefits in the presence of chloro-s-triazine or atrazine. For antibiotics widely used in veterinary, such as Oxytetracycline (OTC), phytoremediation with R. intraradices could enhance the process (Cao et al. 2015).
5 Conclusions and perspectives
The pollution caused by anthropogenic activities is a severe global problem. We can apply physical and chemical remediation methods. However, they have a high economic and environmental cost. Phytoremediation is a better option, using plants and microorganisms to remediate contaminated sites. By specifically using AMF cosmopolitan organisms that require a host to complete their life cycle, both, the fungus and the plants receive benefits from this interaction and we obtain better results. Different reports show that use of AMF improves plant tolerance to HMs and POPs pollution, as AMF influence the uptake and distribution of HMs in host plants. They also immobilize the contaminant at the root level, transport it in smaller amounts to the aerial parts (phytostabilization) or efficiently translocate in to the aerial parts of hyperaccumulating plants (phytoextraction).
The application of AMF in the phytoremediation allows: 1) to improve mineral nutrition and water availability, 2) to protect against oxidative stress, 3) to increase soil physical stability, 4) to increase plant tolerance to soil stress, 5) to increase concentration in chlorophyll pigments, amino acids, carbohydrates, total sugars and essential elements such as P and N, 6) that glomalin production protects hyphae and AMF from stress caused by contaminants, 7) that mycorrhizal interaction favors that contaminants can accumulate in AMF structures (spores, extraradical and intraradical hyphae, vesicles, arbuscules), or in the plant (in root cell, shoots leaves or stems), where metabolic activity is reduced and harmful effects to the plant are low.
Depending on the type of contaminant, an appropriate selection of plant species and AMF is required to enhance the phytoremediation process. Selection of a hyperaccumulator plant, but with higher biomass (e.g., forest species) inoculated with AMF species could be considered to aid the phytoextraction process of soil contaminants.
The identification and isolation of indigenous and tolerant AMF strains can have implications for the future of phytoremediation of contaminated soils. Some studies have also documented the application of saprophytic fungi in synergy with the AMF to take advantage of pollution stress tolerance (bioaugmentation). In the case of application of AMF for HMs uptake with plants, better results are obtained by using a consortium of fungi adapted to metal containing soils rather than individual fungal species. However, it is necessary a deeper study and compare the diversity of AMF in HM contaminated and non-contaminated soils when associated with HM tolerant and non tolerant plants. Consequently, we will acquire more knowledge about these symbiotic relationships, which promise to be a safe, clean, sustainable, and economical management strategy that enhances plant growth and facilitates the remediation of heavy metals in contaminated soils (Shahabivand et al. 2012).
Modern biotechnology and gene editing promise a major breakthrough in bioremediation, including the improvement of AMF strains for such complex subjects as HMs. However, there is a need to continue studying indigenous organisms and record natural biodiversity to learn more about the wide range of possibilities that already exist, and to conduct further studies on the application of AMFs in bioremediation strategies. It is also important to continue researching on the basic principles and molecular mechanisms that allow us to understand how contaminants are taken up and how they act at the cellular and tissue level in both AMF and plants, as well as to identify genes involved that are attractive for breeding programs. This will allow us to increase the tolerance and efficiency of plants and fungi to obtain new soil bioremediation strategies.
References
Abdelhameed RE, Metwally RA (2019) Alleviation of cadmium stress by arbuscular mycorrhizal symbiosis. Int J Phytoremediation 21(7):663–671. https://doi.org/10.1080/15226514.2018.1556584
Abdul K (2006) Mycorrhizoremediation-an enhanced form of phytoremediation. J Zhejiang Univ Sci B 7(7):503–514. https://doi.org/10.1631/jzus.2006.B0503
Agarwal A, Singh J, Sing AP (2017) Review paper Arbuscular Mycorrhizal fungi and its role sequestration of heavy metal. Biosci Trends 10(21):4068–4077
Alarcón A, Davies F Jr, Autenrieth RL, Zuberer DA (2008) Arbuscular mycorrhiza and petroleum-degrading microorganisms enhance phytoremediation of petroleum-contaminated soil. Int J Phytorem 10:251–263. https://doi.org/10.1080/15226510802096002
Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity and bioaccumulation. Hindawi J Chem 14:1–14. https://doi.org/10.1155/2019/6730305
Ali H, Khan E (2017) What are heavy metals? Long-standing controversy over the scientific use of the term ‘heavy metals’–proposal of a comprehensive definition. Toxicol Environ Chem 100(1):1–2. https://doi.org/10.1080/02772248.2017.1413652
Ambrosini VG, Voges JG, Canton L, Couto RR, Ferreira PAA, Comin JJ, Bastos de Melo GW, Brunetto G, Soares FCR (2015) Effect of arbuscular mycorrhizal fungi on young vines in copper-contaminated soil. Braz J Microbiol 46(4):1045–1052. https://doi.org/10.1590/S1517-838246420140622
Andrade SAL, Silveira APD, Mazzafera P (2010) Arbuscular mycorrhiza alters metal uptake and the physiological response of Coffea arabica seedlings to increasing Zn and Cu concentrations in soils. Sci Total Environ 408:5381–5391. https://doi.org/10.1016/j.scitotenv.2010.07.064
Andrade SAL, Da Silveira APD, Jorge RA, De Abreu MF (2008) Cadmium accumulation in sunflower plants influenced by arbuscular mycorrhiza. Int J Phytoremediation 10(1):1–13. https://doi.org/10.1080/1522651070182700
Appenroth KJ (2010) Definition of “heavy metals” and their role in biological systems. In: Sherameti I, Varma A (eds) Soil heavy metals. Springer, pp 19–29. https://doi.org/10.1007/978-3-642-02436-8_2
Aprile A, De Bellis L (2020) Editorial for special issue: heavy metals accumulation, toxicity, and detoxification in plants. Int J Mol Sci 21(11):4103. https://doi.org/10.3390/ijms21114103
Arriagada C, Pereira G, García-Romera I, Ocampo JA (2010) Improved zinc tolerance in Eucalyptus globulus inoculated with Glomus deserticola and Trametes versicolor or Coriolopsis rigida. Soil Biol Biochem 42(1):118–124. https://doi.org/10.1016/j.soilbio.2009.10.011
Arriagada C, Aranda E, Sampedro I, García-Romera I, Ocampo JA (2009) Contribution of the saprobic fungi Trametes versicolor and Trichoderma harzianum and the arbuscular mycorrhizal fungi Glomus deserticola and G. claroideum to arsenic tolerance of Eucalyptus globulus. Bioresour Technol 100(24):6250–6257. https://doi.org/10.1016/j.biortech.2009.07.010
Arriagada CA, Herrera MA, Ocampo JA (2007) Beneficial effect of saprobe and arbuscular mycorrhizal fungi on growth of Eucalyptus globulus co-cultured with Glycine max in soil contaminated with heavy metals. J Environ Manag 84:93–99. https://doi.org/10.1016/j.jenvman.2006.05.005
Arriagada CA, Herrera MA, García-Romera I, Ocampo JA (2004) Tolerance to cd of soybean (Glycine max) and eucalyptus (Eucalyptus globulus) inoculated with arbuscular mycorrhizal and saprobe fungi. Symbiosis 36:285–299
Audet P, Charest C (2007) Dynamics of arbuscular mycorrhizal symbiosis in heavy metal phytoremediation: meta-analytical and conceptual perspectives. Environ Pollut 147:609–614. https://doi.org/10.1016/j.envpol.2006.10.006
Azcón R, Perálvarez MC, Biró B, Roldán A, Ruíz-Lozano JM (2009) Antioxidant activities and metal acquisition in mycorrhizal plants growing in a heavy-metal multicontaminated soil amended with treated lignocellulosic agrowaste. Appl Soil Ecol 41(2):168–177. https://doi.org/10.1016/j.apsoil.2008.10.008
Babadi M, Zalaghi R, Taghavi M (2019) A non-toxic polymer enhances sorghum-mycorrhiza symbiosis for bioremediation of cd. Mycorrhiza. 29:375–387. https://doi.org/10.1007/s00572-019-00902-5
Brundrett MC, Tedersoo L (2020) Resolving the mycorrhizal status of important northern hemisphere trees. Plant Soil 454:3–34. https://doi.org/10.1007/s11104-020-04627-9
Brundrett MC, Tedersoo L (2018) Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol 220:1108–1115. https://doi.org/10.1111/nph.14976
Calonne-Salmon M, Plouznikoff K, Declerck S (2018) The arbuscular mycorrhizal fungus Rhizophagus irregularis MUCL 41833 increases the phosphorus uptake and biomass of Medicago truncatula, a benzo[a]pyrene-tolerant plant species. Mycorrhiza 28:761–771. https://doi.org/10.1007/s00572-018-0861-9
Cao J, Wang C, Dou Z, Liu M, Ji D (2018) Hyphospheric impacts of earthworms and arbuscular mycorrhizal fungus on soil bacterial community to promote oxytetracycline degradation. J Hazard Mater 341:346–354. https://doi.org/10.1016/j.jhazmat.2017.07.038
Cao J, Ji D, Wang C (2015) Interaction between earthworms and arbuscular mycorrhizal fungi on the degradation of oxytetracycline in soils. Soil Biol Biochem 90:283–292. https://doi.org/10.1016/j.soilbio.2015.08.020
Castillo OS, Dasgupta-Schubert N, Alvarado CJ, Zaragoza EM, Villegas HJ (2011) The effect of the symbiosis between Tagetes erecta L. (marigold) and Glomus intraradices in the uptake of copper (II) and its implications for phytoremediation. N Biotechnol 29(1):156–164. https://doi.org/10.1016/j.nbt.2011.05.009
Cheng H, Wang J, Tu C, Lin S, Xing D, Hill P, Chadwick D, Jones DL (2021) Arbuscular mycorrhizal fungi and biochar influence simazine decomposition and leaching. Glob Change Biol Bioenergy 13:708–718. https://doi.org/10.1111/gcbb.12802
Chen S, Zhou Z, Tsang DCW, Wang J, Odinga ES, Gao Y (2020) Glomalin-related soil protein reduces the sorption of polycyclic aromatic hydrocarbons by soils. Chemosphere 260:105093. https://doi.org/10.1016/j.chemosphere.2020.127603
Chen S, Sheng X, Qin C, Waigi MG, Gao Y (2019) Glomalin-related soil protein enhances the sorption of polycyclic aromatic hydrocarbons on cation-modified montmorillonite. Environ Int 132:105093. https://doi.org/10.1016/j.envint.2019.105093
Chen M, Arato M, Borghi L, Nouri E, Reinhardt D (2018a) Beneficial services of arbuscular mycorrhizal fungi–from ecology to application. Front Plant Sci 9:1270. https://doi.org/10.3389/fpls.2018.01270
Chen BD, Nayuki K, Kuga Y, Zhang X, Wu S, Ohtomo R (2018b) Uptake and Intraradical immobilization of cadmium by Arbuscular Mycorrhizal Fungi as revealed by stable isotope tracer and synchrotron radiation μX-ray fluorescence analysis. Microbes Environ 33(3):257–263. https://doi.org/10.1264/jsme2.ME18010
Chen XH, Zhao B (2007) Arbuscular mycorrhizal fungi mediated uptake of lanthanum in Chinese milk vetch (Astragalus sinicus L.). Chemosphere 68(8):1548–1555. https://doi.org/10.1016/j.chemosphere.2007.02.068
Chen BD, Zhu YG, Duan J, Xiao XY, Smith SE (2007) Effects of the arbuscular mycorrhizal fungus Glomus mosseae on growth and metal uptake by four plant species in copper mine tailings. Environ Pollut 147(2):374–380. https://doi.org/10.1016/j.envpol.2006.04.027
Chhabra ML, Jalali BL (2013) Impact of pesticides-mycorrhia interaction on growth and development of wheat. J Biopestic 6(2):129–132
Citterio S, Prato N, Pietro Fumagalli P, Aina R, Massa N, Santagostino A, Sgorbati S, Berta G (2005) The arbuscular mycorrhizal fungus Glomus mosseae induces growth and metal accumulation changes in Cannabis sativa L. Chemosphere 59:21–29. https://doi.org/10.1016/j.chemosphere.2004.10.009
Cornejo P, Meier S, García S, Ferrol N, Durán P, Borie F, Seguel A (2017) Contribution of inoculation with arbuscular mycorrhizal fungi to the bioremediation of a copper polluted soil using Oenothera picensis. Journal of soil science and plant nutrition. J Soil Sci Plant Nutr 17(1):14–21. https://doi.org/10.4067/S0718-95162016005000070
Cornejo P, Meier S, Borie G, Rillig MC, Borie F (2008) Glomalin-related soil protein in a Mediterranean ecosystem affected by a copper smelter and its contribution to cu and Zn sequestration. Sci Total Environ 406(1–2):154–160
Csuros M, Csuros C (2002) Environmental sampling and analysis for metals, Lewis publishers, Boca Raton, FL, USA. 1st Edition CRC Press. https://doi.org/10.1201/9781420032345
De la Providencia IE, Stefani FOP, Labridy M, St-Arnaud M, Hijri M (2015) Arbuscular mycorrhizal fungal diversity associated with Eleocharis obtusa and Panicum capillare growing in an extreme petroleum hydrocarbon-polluted sedimentation basin. FEMS Microbiol Lett 362(12):fnv081. https://doi.org/10.1093/femsle/fnv081
Del Val C, Barea JM, Azcón-Aguilar C (1999) Diversity of arbuscular mycorrhizal fungus populations in heavy metal contaminated soils. Appl Environ Microbiol 99:718–723. https://doi.org/10.1128/AEM.65.2.718-723.1999
Dhalaria R, Kumar D, Kumar H, Nepovimova E, Kuča K, Torequl Islam M, Verma R (2020) Arbuscular mycorrhizal fungi as potential agents in ameliorating heavy metal stress in plants. Agronomy 10(6):815. https://doi.org/10.3390/agronomy10060815
Dietz KJ, Baier M, Kramer U (1999) Free radicals and reactive oxygen species as mediators of heavy metal toxicity in plants. In: Prasad H (ed) Heavy metal stress in plants. From Molecules to Ecosystem. Springer, Berlin, pp 73–97. https://doi.org/10.1007/978-3-662-07745-0_4
Dong J, Wang L, Ma F, Yang J, Qi S, Zhao T (2016) The effect of Funnelliformis mosseae inoculation on the phytoremediation of atrazine by the aquatic plant Canna indica L. var. flava Roxb. RSC Adv 6(27):22538–22549. https://doi.org/10.1039/c5ra23583a
Dua M, Singh A, Sethunathan N, Jhri AK (2002) Biotechnology and bioremediation: successes and limitations. Appl Microbiol Biotechnol 59:143–152. https://doi.org/10.1007/s00253-002-1024-6
Dupré de Boulois H, Joner EJ, Leyval C, Jakobsen I, Chen BD, Roos P, Thiry Y, Rufyikiri G, Delvaux B, Declerck S (2008) Impact of arbuscular mycorrhizal fungi on uranium accumulation by plants. J Environ Radioact 99(5):775–784. https://doi.org/10.1016/j.jenvrad.2007.10.009
Fan X, Song F (2018) Responses of nonenzymatic antioxidants to atrazine in arbuscular mycorrhizal roots of Medicago sativa L. Mycorrhiza 28:567–571. https://doi.org/10.1007/s00572-018-0848-6
Ferreira PA, Ceretta CA, Tiecher T, Facco DB, Garlet LP, Soares CRFS et al (2018) Rhizophagus Clarus and phosphorus in Crotalaria juncea: growth, Glomalin content and acid phosphatase activity in a copper-contaminated soil. Rev Bras Cienc Solo 42:e0170245. https://doi.org/10.1590/18069657rbcs20170245
Ferrol N, González-Guerrero M, Valderas A, Benabdellah K, Azcón-Aguilar C (2009) Survival strategies of arbuscular mycorrhizal fungi in cu-polluted environments. Phytochem Rev 8:551–559. https://doi.org/10.1007/s11101-009-9133-9
Gao WQ, Wang P, Wu QS (2019) Functions and application of glomalin-related soil proteins: a review. Sains Malaysiana 48(1):111–119. https://doi.org/10.17576/jsm-2019-4801-13
Gao Y, Zhou Z, Ling W, Hu X, Chen S (2017) Glomalin-related soil protein enhances the availability of polycyclic aromatic hydrocarbons in soil. Soil Biol Biochem 107:129–132. https://doi.org/10.1016/j.soilbio.2017.01.002
Gao Y, Li Q, Ling W, Zhu X (2011) Arbuscular mycorrhizal phytoremediation of soils contaminated with phenanthrene and pyrene. J Hazard Mater 185:703–709. https://doi.org/10.1016/j.jhazmat.2010.09.076
Gao Y, Cheng Z, Ling W, Huang J (2010) Arbuscular mycorrhizal fungal hyphae contribute to the uptake of polycyclic aromatic hydrocarbons by plant roots. Bioresour Technol 101:6895–6901
Garg N, Aggarwal N (2012) Effect of mycorrhizal inoculations on heavy metal uptake and stress alleviation of Cajanus cajan (L.) Millsp. Genotypes grown in cadmium and lead contaminated soils. Plant Growth Regul 66:9–26. https://doi.org/10.1007/s10725-011-9624-8
Garg N, Bhandari P (2012) International journal of phytoremediation influence of cadmium stress and Arbuscular Mycorrhizal Fungi on nodule senescence in Cajanus Cajan (L.) MILLSP. Int J Phytoremediation 14(1):62–74. https://doi.org/10.1080/15226514.2011.573822
Ghasemi NS, Fallah S, Pokhrel LR, Rostamnejadi A (2017) Natural amelioration of zinc oxide nanoparticle toxicity in fenugreek (Trigonella foenum-gracum) by arbuscular mycorrhizal (Glomus intraradices) secretion of glomalin. Plant Physiol Biochem 112:227–238. https://doi.org/10.1016/j.plaphy.2017.01.001
Gil-Cardeza ML, Ferri A, Cornejo P, Gomez E (2014) Distribution of chromium species in a Cr-polluted soil: presence of Cr(III) in glomalin related protein fraction. Sci Total Environ 493:828–833. https://doi.org/10.1016/j.scitotenv.2014.06.080
Glick BR (2010) Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 28:367–374. https://doi.org/10.1016/j.biotechadv.2010.02.001
González-Chávez MC, Carrillo-González R, Wright SF, Nichols KA (2004) The role of glomalin, a protein produced by arbuscular mycorrhizal fungi, in sequestering potentially toxic elements. Environ Pollut 130:317–323. https://doi.org/10.1016/j.envpol.2004.01.004
Goto BT, Jobim K. Laboratório de Biologia de Micorrizas. Disponible en: < http://glomeromycota.wixsite.com/lbmicorrizas >. Acesso em: 10/03/2020
Grant CA, Sheppard SC (2008) Fertilizer impacts on cadmium availability in agricultural soils and crops. Hum Ecol risk assess: an international Journal,14(2):210-228. 14:210–228. https://doi.org/10.1080/10807030801934895
Gu HH, Zhou Z, Gao YQ, Yuan XT, Ai YJ, Zhang JY, Li FP (2017) The influences of arbuscular mycorrhizal fungus on phytostabilization of lead/zinc tailings using four plant species. Intl J Phytoremediation 19(8):739–745. https://doi.org/10.1080/15226514.2017.1284751
Guo W, Zhao R, Zhao W, Fu R, Guo J, Bi N, Zhang J (2013) Effects of arbuscular mycorrhizal fungi on maize (Zea mays L.) and sorghum (Sorghum bicolor L. Moench) grown in rare earth elements of mine tailings. Appl. Soil Ecol 72:85–92. https://doi.org/10.1016/j.apsoil.2013.06.001
Gupta MM, Abbott LK (2020) Exploring economic assessment of the arbuscular mycorrhizal symbiosis. Symbiosis. https://doi.org/10.1007/s13199-020-00738
Gupta MM, Chourasiya D, Sharma MP (2019) Diversity of arbuscular mycorrhizal fungi in relation to sustainable plant production systems. In: Microbial diversity in ecosystem sustainability and biotechnological applications. Springer, Singapore, pp 167–186. https://doi.org/10.1007/978-981-13-8487-5_7
Gupta MM, Aggarwal A, Asha (2018) From mycorrhizosphere to rhizo-sphere microbiome: the paradigm shift. In: Giri B, Prasad R, Varma A (eds) Root Biology Springer, Cham, pp. 487–500. doi: https://doi.org/10.1007/978-3-319-75910-4_20
Gupta SC, Goldsbrough PB (1991) Phytochelatin accumulation and cadmium tolerance in selected tomato cell lines. Plant Physiol 97:306–312
Hancock LM, Ernst CL, Charneskie R, Ruane LG (2012) Effects of cadmium and mycorrhizal fungi on growth, fitness, and cadmium accumulation in flax (Linum usitatissimum; Linaceae). Am J Bot 99:1445–1452. https://doi.org/10.3732/ajb.1100497
Hashem A, Abd-Allah EF, Alqarawi AA, Egamberdieva D (2016) Bioremediation of adverse impact of cadmium toxicity on Cassia italica mill by arbuscular mycorrhizal fungi. Saudi J Biol Sci 23(1):39–47. https://doi.org/10.1016/j.sjbs.2015.11.007
Hassan GR (2005) Contribution of arbuscular mycorrhizal fungus to red kidney and wheat plants tolerance grown in heavy metal-polluted soil. Afr J Biotech 4(4):332–345
Hernández-Ortega HA, Alarcón A, Ferrera-Cerrato R, Zavaleta-Mancera HA, López-Delgado HA, Mendoza-López MR (2012) Arbuscular mycorrhizal fungi on growth, nutrient status, and total antioxidant activity of Melilotus albus during phytoremediation of a diesel-contaminated substrate. J Environ Manag 95:S319–S324. https://doi.org/10.1016/j.jenvman
Huang H, Zhang S, Shan X-Q, Chen BD, Zhu YG, Bell JNB (2007) Effect of arbuscular mycorrhizal fungus (Glomus caledonium) on the accumulation and metabolism of atrazine in maize (Zea mays L.) and atrazine dissipation in soil. Environ. Pollut., 146(2), 452–457. https://doi.org/10.1016/j.envpol.2006.07.001
Ibáñez SG, Medina MI, Agostini E (2011) Phenol tolerance, changes of antioxidative enzymes and cellular damage in transgenic tobacco hairy roots colonized by arbuscular mycorrhizal fungi. Chemosphere 83(5):700–705. https://doi.org/10.1016/j.chemosphere.2011.02.021
Jamal A, Ayub N, Usman M, Khan AG (2002) Arbuscular mycorrhizal fungi enhance zinc and nickel uptake from contaminated soil by soybean and lentil. Int J Phytoremediation 4(3):205–221. https://doi.org/10.1080/15226510208500083
Janeeshma E, Puthur JT (2020) Direct and indirect influence of arbuscular mycorrhizae on enhancing metal tolerance of plants. Arch microbiol 202(1):1–6. https://doi.org/10.1007/s00203-019-01730-z
Janousková M, Pavliková D, Vosátka M (2006) Potential contribution of arbuscular mycorrhiza to cadmium immobilisation in soil. Chemosphere 65:1959–1965. https://doi.org/10.1016/j.chemosphere.2006.07.007
Jiang QY, Zhuo F, Long SH, Zhao HD, Yang DJ, Ye ZH, Li SS, Jing YX (2016) Can arbuscular mycorrhizal fungi reduce cd uptake and alleviate cd toxicity of Lonicera japonica grown in cd-added soils? Sci Rep 6:21805. https://doi.org/10.1038/srep21805
Joner E, Leyval C (2003a) Phytoremediation of organic pollutants using mycorrhizal plants: a new aspect of rhizosphere interactions. Agronomie 23(5):495–502. https://doi.org/10.1051/agro:2003021
Joner EJ, Leyval C (2003b) Rhizospheric degradation of Phenanthrene is a funtion proximity to roots. Plant Soil 257:143–150. https://doi.org/10.1023/A:1026278424871
Joner EJ, Leyval C (2001) Influence of arbuscular mycorrhiza on clover and ryegrass grown together in a soil spiked with polycyclic aromatic hydrocarbons. Mycorrhiza 10:155–159. https://doi.org/10.1007/s005720000071
Joner EJ, Briones R, Leyval C (2000) Metal-binding capacity of arbuscular mycorrhizal mycelium. Plant Soil 226:227–234. https://doi.org/10.1023/A:1026565701391
Leal LP, Varón-López M, de Gonçalves OPI, Valentim dos Santos J, Fonsêca SSCR, Siqueira JO, de Souza MFM (2016) Enrichment of arbuscular mycorrhizal fungi in a contaminated soil after rehabilitation. Braz J Microbiol 47(4):853–862. https://doi.org/10.1016/j.bjm.2016.06.001
Lebeau T, Braud A, Jézequel K (2008) Performance of bioaugmentation.Assisted phytoextraction applied to metal contaminated soils: a review. Environ Pollut 153:497–522. https://doi.org/10.1016/j.envpol.2007.09.015
Lenoir IA, Lounés-Hadj S, Fontaine J (2016a) Arbuscular mycorrhizal fungal-asssited phytoremediation of soil contaminated with persistente organic pollutants: a review. Eur J Soil Sci, September 67:624–640. https://doi.org/10.1111/ejss.12375
Lenoir IA, Lounés-Hadj SA, Laruelle F, Dalpé Y, Fontaine J (2016b) Arbuscular mycorrhizal wheat inoculation promotes alkane and polycyclic aromatic hydrocarbon biodegradation: microcosm experiment on aged-contaminated soil. Environ Pollut 213:549–560. https://doi.org/10.1016/j.envpol.2016.02.056
Leyval C, Poner EJ, del Val C, Haselwandter K (2002) Potential or arbuscular mycorrhizal fungi for bioremediation. In: Gianinazzi S, Schüepp H, Barea JM, Haselwandter K (eds) Mycorrhizal technology in agriculture. Birkhäuser, Base, pp 175–186. https://doi.org/10.1007/978-3-0348-8117-3_14
Leyval C, Turnau K, Haselwandter K (1997) Effect of heavy metal pollution on mycorrhizal colonisation and function, physiological, ecological and applied aspects. Mycorrhiza 7:139–153. https://doi.org/10.1007/s005720050174
Liao JP, Lin XG, Cao ZH, Shi YQ, Wong MH (2003) Interactions between arbuscular mycorrhizae and heavy metals under sand culture experiment. Chemosphere 50:847–853. https://doi.org/10.1016/S0045-6535(02)00229-1
Liu L, Li J, Yue F, Yan X, Wang F, Bloszies S, Wang Y (2018) Effects of arbuscular mycorrhizal inoculation and biochar amendment on maize growth, cadmium uptake and soil cadmium speciation in cd-contaminated soil. Chemosphere 194:495–503. https://doi.org/10.1016/j.chemosphere.2017.12.025
Lu YF, Lu M (2015) Remediation of PAH-contaminated soil by the combination of tall fescue, arbuscular mycorrhizal fungus and epigenic earthworms. J Hazard Mater 285:535–541. https://doi.org/10.1016/j.jhazmat.2014.07.021
Lu YF, Lu M, Peng F, Wan Y, Liao MH (2014) Remediation of polychlorinated biphenyl-contaminated soil by using a combination of ryegrass, arbuscular mycorrhizal fungi and earthworms. Chemosphere 106:44–50. https://doi.org/10.1016/j.chemosphere.2013.12.089
Ma Y, Rajkumar M, Oliveira RS, Zhang C, Freitas H (2019) Potential of plant beneficial bacteria and arbuscular mycorrhizal fungi in phytoremediation of metal-contaminated saline soils. J Hazard Mater 379:120813. https://doi.org/10.1016/j.jhazmat.2019.120813
Maiti RK, Hernández JL, González A, López D (2004) Plant based Biorremediation and mechanisms of heavy metal tolerance of plants: a review. Proc Indian natn Sci Acad B70(1):1–12
Malcová R, Vosátka M, Gryndler M (2003) Effects of inoculation with Glomus intraradices on lead uptake by Zea mays L. and Agrostis capillaris L. Appl Soil Ecol 23(1):55–67. https://doi.org/10.1016/S0929-1393(02)00160-9
Malekzadeh E, Aliasgharzad N, Majidi J, Abdolalizadeh J, Aghebati-Maleki L (2016) Contribution of glomalin to Pb sequestration by arbuscular mycorrhizal fungus in a sand culture system with clover plant. Eur J Soil Biol 74:45–51. https://doi.org/10.1016/j.ejsobi.2016.03.003
Miransari M (2011) Hyperaccumulators, arbuscular mycorrhizal fungi and stress of heavy metals. Biotechnol Adv 29(6):645–653. https://doi.org/10.1016/j.biotechadv.2011.04.006
Mohammad A, Mittra B (2013) Effects of inoculation with stress-adapted arbuscular mycorrhizal fungus Glomus deserticola on growth of Solanum melogena L. and Sorghum sudanese staph. Seedlings under salinity and heavy metal stress conditions. Arch. Agron. Soil Sci. 59(2):173–183. https://doi.org/10.1080/03650340.2011.610029
Nkereuwem ME, Fagbola O, Okon IE, Adeleye AO, Nzamouhe M (2020) Bioremediation potential of mycorrhiza fungi in crude oil contaminated soil planted with Costus lucanusianus. Amazon J Plant Res 4(1):441–455. https://doi.org/10.26545/ajpr.2020.b00053x
Ogar A, Sobczyk Ł, Turnau K (2015) Effect of combined microbes on plant tolerance to Zn–Pb contaminations. Environ Sci Pollut Res 22(23):19142–19156. https://doi.org/10.1007/s11356-015-5094-2
Orłowska E, Godzik B, Turnau K (2012) Effect of different arbuscular mycorrhizal fungal isolates on growth and arsenic accumulation in Plantago lanceolate L. Environ Poll 168:121–130. https://doi.org/10.1016/j.envpol.2012.04.026
Oyetibo GO, Miyauchi K, Huang Y, Chien MF, Ilori MO, Amund OO, Endo G (2017) Biotechnological remedies for the estuarine environment polluted with heavy metals and persistent organic pollutants. Intl Biodeterioration Biodegradation 119:614–625. https://doi.org/10.1016/j.ibiod.2016.10.005
Pedroso D, Barbosa MV, dos Santos JV, Pinto FA, Siqueira JO, Carneiro MAC (2018) Arbuscular Mycorrhizal Fungi favor the initial growth of Acacia mangium, Sorghum bicolor, and Urochloa brizantha in soil contaminated with Zn, cu, Pb, and cd. Bull Environ Contam Toxicol 101(3):386–391. https://doi.org/10.1007/s00128-018-2405-6
Pongrac P, Soniak S, Vogel-Mikus K, Kump P, Neemer M, Regvar M (2009) Roots of metal hyperaccumulating population of Thlaspipraecox (Brassicacear) harbour arbuscular mycorrhizal and other fungi under experimental conditions. Int J Phytoremediation 11:4,347–4,359. https://doi.org/10.1080/15226510802565527
Qin H, Brookes PCXJ, Feng Y (2014) Bacterial degradation of Aroclor 1242 in the mycorrhizosphere soils of zucchini (Cucurbita pepo L.) inoculated with arbuscular mycorrhizal fungi. Environ Sci Pollut Res 21:12790–12799. https://doi.org/10.1007/s11356-014-3231-y
Riaz M, Kamran M, Fang Y, Wang Q, Cao H, Yang G, Deng L, Wang Y, Zhou Y, Anastopoulos I, Wang X (2020) Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: a critical review. J Hazard Mater 402:123919. https://doi.org/10.1016/j.jhazmat.2020.123919
Rilling MC, Steinberg PD (2002) Glomalin production by an arbuscular mycorrhizal fungus: a mechanism of habitat modification? Short communication. Soil Biol Biochem. 34:1371–1374. https://doi.org/10.1016/S0038-0717(02)00060-3
Rivera-Becerril F, van Tuinen D, Martin-Laurent F, Metwally A, Dietz KJ, Gianinazzi S (2005) Gianinazzi-Pearson V (2005) molecular changes in Pisum sativum L. roots during arbuscular mycorrhizal buffering of cadmium stress. Mycorrhiza 16:51–60.77. https://doi.org/10.1007/s00572-005-0016-7
Ross IS (1975) Some effects of heavy metals on fungal cells. Trans Br Mycol Soc 64(2):175–193. https://doi.org/10.1016/S0007-1536(75)80101-X
Ruscitti M, Arango M, Ronco M, Beltrano J (2011) Inoculation with mycorrhizal fungi modifies praline metabolism and increases chromium tolerance in pepper plants (Capsicum annuum L.). Brazilian. J Plant Physiol 23(1):15–25. https://doi.org/10.1590/S1677-04202011000100004
Sainz MJ, González-Penalta B, Vilariño A (2006) Effects of hexachlorocyclohexane on rhizosphere fungal propagules and root colonization by arbuscular mycorrhizal fungi in Plantago lanceolata. European Eur J Soil Sci 57(1):83–90. https://doi.org/10.1111/j.1365-2389.2005.00775.x
Schutzendubel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 53(372):1351–1365. https://doi.org/10.1093/jexbot/53.372.1351
Schüßler A, Schwarzott D, Walker C (2001) A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol Res 105:1413–1421. https://doi.org/10.1017/S0953756201005196
Setyorini D, Prihatini T, Kurnia U (2002) Pollution of soil by agricultural and industrial waste. Food and Fertelizer Technology Center, Indonesia
Shahabivand S, Maivan HZ, Goltapeh EM, Sharifi M, Aliloo AA (2012) The effects of root endophyte and arbuscular mycorrhizal fungi on growth and cadmium accumulation in wheat under cadmium toxicity. Plant Physiol Biochem 60:53–58. https://doi.org/10.1016/j.plaphy.2012.07.018
Sharma V, Parmar P, Kumari N (2016) Differential cadmium stress tolerance in wheat genotypes under mycorrhizal association. J Plant Nutr 39(14):2025–2036. https://doi.org/10.1080/01904167.2016.1170851
Singh G, Pankaj U, Chand S, Kumar RV (2019) Arbuscular Mycorrhizal Fungi-assisted Phytoextraction of toxic metals by Zea mays L. from tannery sludge. Soil Sediment Contam 28(8):729–746. https://doi.org/10.1080/15320383.2019.165738
Singh J, Kumar M, Vyas A (2014) Healthy response from chromium survived Pteridophytic plant-Ampelopteris prolifera with the interaction of Mycorrhizal fungus Glomus deserticola. Intl J Phytoremediation 16(5):524–535. https://doi.org/10.1080/15226514.2013.798619
Singh PK (2015) In vitro cu-sequestration by Glomalin from Acaulospora spinosa Walker and Trappe. Natl Acad Sci Lett 38:183–185. https://doi.org/10.1007/s40009-014-0309-5
Singh PK (2012) Role of Glomalin related soil protein produced by Arbuscular Mycorrhizal Fungi : a review. J Agric Res Sci 2(3):119–125
Smith SE, Read DJ (1997) Mycorrhizal Symbiosis, 2nd edn. Academic Press, San Diego
Sodango TH, Li X, Sha J, Bao Z (2018) Review of the spatial distribution, source and extent of heavy metal pollution of soil in China: impacts and mitigation approaches. J Health Pollut 8(17):53–70. https://doi.org/10.5696/2156-9614-8.17.53
Solís-Ramos LY, Andrade-Torres A (2020) Arbuscular Mycorrhizal Fungi in tropical ecosystems towards its management? Agri res & Tech: Open Access J. 24(4):556279. https://doi.org/10.19080/ARTOAJ.2020.24.556279
Souza AL, Andrade SAL, Souza CS, Schiavinato AM (2012) Arbuscular mycorrhiza confers Pb tolerance in Calopogonium mucunoides. Acta Physiol Plant 34:523–531. https://doi.org/10.1007/s11738-011-0849-y
Spatafora JW, Chang Y, Benny GL, Lazarus K, Smith ME, Berbee ML, Bonito G, Corradi N, Grigoriev I, Gryganskyi A, James TY, O’Donnell K, Roberson RW, Taylor TN, Uehling J, Vilgalys R, White MM, Stajich JE (2016) A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 108:1028–1046. https://doi.org/10.3852/16-042
Sudová R, Vosátka M (2007) Differences in the effects of three arbuscular mycorrhizal fungal strains on P and Pb accumulation by maize plants. Plant Soil 296:77–83. https://doi.org/10.1007/s11104-007-9291-8
Sut M, Boldt-Burisch K, Raab T (2016) Possible evidence for contribution of arbuscular mycorrhizal fungi (AMF) in phytoremediation of iron–cyanide (Fe–CN) complexes. Ecotoxicology 25(6):1260–1269. https://doi.org/10.1007/s10646-016-1678-y
Sytar O, Kumar A, Latowski D, Kuczynska P, Strzalka K, Prasad MNV (2013) Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanism in plants. Acta Physiol Plant 35:985–999. https://doi.org/10.1007/s11738-012-1169-6
Tang M, Chen H, Huang JC, Tian ZQ (2009) AM fungi effects on the growth and physiology of Zea mays seedlings under diesel stress. Soil Biol Biochem 41(5):936–940. https://doi.org/10.1016/j.soilbio.2008.11.007
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2014) Heavy metals toxicity and the environment, A. Luch (ed.), molecular, clinical and environmental toxicology, 133-166. doi: https://doi.org/10.1007/978-3-7643-8340-4_6
Tedersoo L, Sánchez-Ramírez S, Koljalg U, Bahram M, Döring M, Schigel D, May T, Ryberg M, Abarenkov K (2018) High-level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Divers 90:135–159
Teng Y, Luo Y, Sun X, Tu C, Xu L, Liu W, Li Z, Christie P (2010) Influence of arbuscular mycorrhiza and Rhizobium on phytoremediation by alfalfa of an agricultural soil contaminated with weathered PCBs: a field study. Int J Phytoremediation 12(5):516–533. https://doi.org/10.1080/15226510903353120
Toler HD, Morton JB, Cumming JR (2005) Growth and metal accumulation of mycorrhizal sorghum exposed to elevated copper and zinc. Water Air Soil Pollut 164:155–172. https://doi.org/10.1007/s11270-005-2718-z
Tonin C, Vandenkoornhuyse P, Joner EJ, Straczek J, Leyval C (2001) Assessment of arbuscular mycorrhizal fungi diversity in the rhizosphere of Viola calaminaria and effect of these fungi on heavy metal uptake by clover. Mycorrhiza 10:161–168. https://doi.org/10.1007/s005720000072
Turnau K, Mesjasz-Przybylowicz J (2003) Arbuscular mycorrhiza of Berkheya coddii and other Ni-hyperaccumulating members of Asteraceae from ultramafic soils in South Africa. Mycorrhiza 13:185–190. https://doi.org/10.1007/s00572-002-0213-6
Verdin A, Sahraoui ALH, Fontaine J, Grandmoungin-Ferjani A, Durand R (2006) Effects of anthracene on development of an arbuscular mycorrhizal fungus and contribution of the symbiotic association to pollutant dissipation. Mycorrhiza 16:397–405
Vodnik D, Grčman H, Maček I, van Elteren JT, Kovačevič M (2008) The contribution of glomalin-related soil protein to Pb and Zn sequestration in polluted soil. Sci Total Environ 392(1):130–136. https://doi.org/10.1016/j.scitotenv.2007.11.016
Vogel-Mikus K, Pongrac P, Kump P, Necemer M, Regvar M (2006) Colonisation of a Zn, cd and Pb hyperaccumulator Thlaspi praecox Wulfen with indigenous arbuscular mycorrhizal fungal mixture induces changes in heavy metal and nutrient uptake. Environ Pollut 139:362–371. https://doi.org/10.1016/j.envpol.2005.05.005
Volante A, Lingua G, Cesaro P, Cresta A, Puppo M, Ariati L, Berta G (2005) Influence of three species of arbuscular mycorrhizal fungi on the persistence of aromatic hydrocarbons in contaminated substrates. Mycorrhiza 16:43–50. https://doi.org/10.1007/s00572-005-0012-y
Wang B, Qiu YL (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16(5):299–363. https://doi.org/10.1007/s00572-005-0033-6
Wang G, Wang L, Ma F, You Y, Wang Y, Yang D (2020) Integration of earthworms and arbuscular mycorrhizal fungi into phytoremediation of cadmium-contaminated soil by Solanum nigrum L. J Hazard Mater 389:121873. https://doi.org/10.1016/j.jhazmat.2019.121873
Wang Q, Chen J, Chen S, Qian L, Yuan B, Tian Y, Wang Y, Liu J, Yan C, Lu H (2020a) Terrestrial-derived soil protein in coastal water: metal sequestration mechanism and ecological function. J Hazard Mater 386:121655. https://doi.org/10.1016/j.jhazmat.2019.121655
Wang Q, Lu H, Chen J, Jiang Y, Williams MA, Wu S, Li J, Liu J, Yang G, Yan C (2020b) Interactions of soil metals with glomalin-related soil protein as soil pollution bioindicators in mangrove wetland ecosystems. Sci Total Environ 709:105093. https://doi.org/10.1016/j.scitotenv.2019.136051
Wang S, Zhang S, Huang H, Christie P (2011) Behavior of decabromodiphenyl ether (BDE-209) in soil: effects of rhizosphere and mycorrhizal colonization of ryegrass roots. Environ Pollut 159(3):749–753. https://doi.org/10.1016/j.envpol.2010.11.035
Whitfield L, Richards AJ, Rimmer DL (2004) Relationships between soil heavy metal concentration and mycorrhizal colonization in Thymus polytrichus in northern England. Mycorrhiza. 14:55–62
Wijayawardene et al (2020) Outline of Fungi and fungi-like taxa. Micosphere 11:1060–1456
Wu JT, Wang L, Zhao L, Huang XC, Ma F (2020) Arbuscular mycorrhizal fungi effect growth and photosynthesis of Phragmites australis (Cav.) Trin ex. Steudel under copper stress. Plant Biol 22(1):62–69. https://doi.org/10.1111/plb.13039
Wu N, Huang H, Zhang S, Zhu YG, Christie P, Zhang Y (2009) Phenanthrene uptake by Medicago sativa L. under the influence of an arbuscular mycorrhizal fungus. Environ Pollut 157:1613–1618. https://doi.org/10.1016/j.envpol.2008.12.022
Wu N, Shuzhen Zhang S, Huang H, Shan X, Christie P, Wang Y (2008) DDT uptake by arbuscular mycorrhizal alfalfa and depletion in soil as influenced by soil application of a non-ionic surfactant. Environ Pollut 151:569–575. https://doi.org/10.1016/j.envpol.2007.04.005
Wu Z, McGrouther K, Huang J, Wu P, Wu W, Wang H (2014) Decomposition and the contribution of glomalin-related soil protein (GRSP) in heavy metal sequestration: field experiment. Soil Biol Biochem 68:283–290. https://doi.org/10.1016/j.soilbio.2013.10.010
Xavier IJ, Boyetchko SM (2002) Arbuscular Mycorrhizal Fungi as biostimulants and bioprotectants of crops. In: Khachatourians, G.G., Arora, D.K. (Eds.), app. Mycol. And Biotechnol. Vol. 2: agriculture and food production. Elsevier, Amsterdam, p.311-330. https://doi.org/10.1016/S1874-5334(02)80015-6
Xu Z, Wu Y, Xiao Z, Ban Y, Belvett N (2019) Positive effects of Funneliformis mosseae inoculation on reed seedlings under water and TiO2 nanoparticles stresses. World J Microbiol Biotechnol 35:81. https://doi.org/10.1007/s11274-019-2656-3
Xun F, Xie B, Liu S, Guo C (2015) Effect of plant growth-promoting bacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) inoculation on oats in saline-alkali soil contaminated by petroleum to enhance phytoremediation. Environ Sci Pollut Res 22:598–608. https://doi.org/10.1007/s11356-014-3396-4
Yang Y, Han X, Liang Y, Ghosh A, Chen J, Tang M (2015) The combined effects of arbuscular mycorrhizal fungi (AMF) and lead (Pb) stress on Pb accumulation, plant growth parameters, photosynthesis, and antioxidant enzymes in robinia pseudoacacia L. PLoS One 10(12):e0145726. https://doi.org/10.1371/journal.pone.0145726
Yu XZ, Wu SC, Wu FY, Wong M (2011) Enhanced dissipation of PAHs from soil using mycorrhizal ryegrass and PAH-degrading bacteria. J Hazard Mater 186(2–3):1206–1217. https://doi.org/10.1016/j.jhazmat.2010.11.116
Zhan F, Li B, Jiang M, Li T, He Y, Li Y, Wang Y (2019) Effects of arbuscular mycorrhizal fungi on the growth and heavy metal accumulation of bermudagrass [Cynodon dactylon (L.) Pers.] grown in a lead–zinc mine wasteland. Int J Phytoremediation 21(9):849–856. https://doi.org/10.1080/15226514.2019.1577353
Zhang F, Liu M, Li Y, Che Y, Xiao Y (2019a) Effects of arbuscular mycorrhizal fungi, biochar and cadmium on the yield and element uptake of Medicago sativa. Sci Total Environ 655:1150–1158. https://doi.org/10.1016/j.scitotenv.2018.11.317
Zhang X, Zhang H, Lou X, Tang M (2019b) Mycorrhizal and non-mycorrhizal Medicago truncatula roots exhibit differentially regulated NADPH oxidase and antioxidant response under Pb stress. Environ Exp Bot 164:10–19. https://doi.org/10.1016/j.envexpbot.2019.04.01
Zhang XH, Zhu YG, Lin AJ, Chen BD, Smith SE, Smith FA (2006) Arbuscular mycorrhizal fungi can alleviate the adverse effects of chlorothalonil on Oryza sativa L. Chemosphere 64(10):1627–1632. https://doi.org/10.1016/j.chemosphere.2006.01.034
Zhou X, Zhou J, Xiang X, Cébron A, Béguiristain T, Leyval C (2013) Impact of four plant species and Arbuscular Mycorrhizal (AM) Fungi on polycyclic aromatic hydrocarbon (PAH) dissipation in spiked soil. Pol J Environ Stud 22(4):1239–1245
Hu B, Hu S, Chen Z, Vymazal J (2020) Employ of arbuscular mycorrhizal fungi for pharmaceuticals ibuprofen and diclofenac removal in mesocosm-scale constructed wetlands. J. Hazard. Mater., 409: 124524. https://doi.org/10.1016/j.jhazmat.2020.124524.
Acknowledgments
Thanks to the Vice-Rectory of Research (University of Costa Rica) for research funding (project No. C0057). To Jenny Muñoz Valverde for feeding the database of some scientific articles. Thanks to Helena Ajuria and Nidia González Lara for the English language proofreading and José Salazar Ferrer for his assistance with the figure. Authors are grateful to anonymous referees and the Editor Manju M. Gupta for critical reading and improvement of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Authors declare that there is no conflict of interest of any kind.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Solís-Ramos, L.Y., Coto-López, C. & Andrade-Torres, A. Role of arbuscular mycorrhizal symbiosis in remediation of anthropogenic soil pollution. Symbiosis 84, 321–336 (2021). https://doi.org/10.1007/s13199-021-00774-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-021-00774-4