Abstract
This review provides an overview of the mechanisms evolved by arbuscular mycorrhizal (AM) fungi to survive in Cu-contaminated environments. These mechanisms include avoidance strategies to restrict entry of toxic levels of Cu into their cytoplasm, intracellular complexation of the metal in the cytosol and compartmentalization strategies. Through the activity of specific metal transporters, the excess of Cu is translocated to subcellular compartments, mainly vacuoles, where it would cause less damage. At the level of the fungal colony, AM fungi have also evolved compartmentalization strategies based on the accumulation of Cu into specific fungal structures, such as extraradical spores and intraradical vesicles. In addition to the avoidance and compartmentalization strategies, AM fungi have also mechanisms to combat the Cu-generated oxidative stress or to repair the damage induced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cu is a trace element that makes up only 0.00007% of the Earth’s crust. However, copper is one of the essential micronutrients, as it is necessary for a wide range of metabolic processes in both prokaryotes and eukaryotes (Linder and Goode 1991). In biological systems Cu ions can exist in two oxidation states: Cu+ (reduced) and Cu2+ (oxidized). The reversible oxidation–reduction of Cu makes it very useful as a cofactor in electron transfer reactions. By coordinating to proteins with the assistance of a diverse spectrum of chemical ligands, including sulphur, oxygen and nitrogen, Cu confers changes in protein structure, catalytic activity and protein–protein interactions, thereby, controlling an unexpectedly diverse series of biochemical and regulatory events. Virtually all organisms require copper as a catalytic cofactor for biological processes such as respiration, iron transport, oxidative stress protection, cellular metabolism, signal transduction, and normal cell growth and development (Kim et al. 2008). However, at elevated levels it becomes toxic. Toxicity may result from the binding to sulphydryl groups in proteins, which would lead to inhibition of activity or disruption of structure, from the displacing of another essential cation resulting in deficiency effects, or from the formation of free radicals and reactive oxygen species (ROS) by autoxidation and Fenton reactions (Halliwell and Gutteridge 1989).
Given the critical role Cu plays in crucial biochemical reactions and the consequences of Cu toxicity, Cu levels in biological systems must be finely regulated. In natural environments, Cu is relatively abundant and moderately soluble (Flemming and Trevors 1989). The form taken by the plant and its bioavailability depends on environmental factors such as soil type, pH, redox potential and organic matter content (Fageria et al. 2002). Due to the variability in environmental conditions, either situations of Cu deficiency or toxicity are found in natural environments. Over the two past centuries, as a consequence of human activities such as industrial processes, pesticide application and mining, the levels of biologically accessible Cu in the environment have dramatically increased. High Cu concentrations are toxic to soil inhabitants; however, some soil microorganisms have developed adaptative mechanisms that allow them to survive and grow in environments with high Cu concentrations (Bååth 1989).
Arbuscular mycorrhizal (AM) fungi, obligate biotrophs of higher plants, constitute one of the most prominent groups of soil microorganisms (Barea 1991). AM fungi colonize the root cortex of most plant species and develop an extraradical mycelium which overgrows the soil surrounding plant roots. They expand the interface between plants and the soil environment and contribute to plant uptake of macronutrients (P and N) as well as micronutrients (Cu and Zn) (Smith and Read 2008). On the other hand, under conditions of supraoptimal levels of essential metals, or in the presence of toxic ones, AM fungi are able to alleviate metal toxicity in the plant (Leyval et al. 2002). Despite the significant role that AM fungi play in plant interactions with soil metals and the ubiquity of AM fungi in soil environments, only recently progress has been made towards understanding the cellular mechanisms used by AM fungi to control heavy metals and to avoid their toxicity.
The effects of heavy metal pollution on mycorrhizal colonization and function, the constitutive and adaptive mechanisms of arbuscular mycorrhizas to contaminated soils and the potential contribution of the AM symbiosis to heavy metal phytoremediation have been recently reviewed in depth by different authors (Leyval et al. 2002; Meharg 2003; Göhre and Paszkowski 2006; Hildebrandt et al. 2007; González-Guerrero et al. 2009). Here we do not pretend to give a recompilation of decades of intense research in this field. This paper aims to give an up-to-date glance into current knowledge of the mechanisms evolved by AM fungi to avoid uncontrolled accumulation of Cu in their cytosol and to grow in Cu contaminated environments.
Glomalin, an AM fungal protein to cope with metal pollution
Glomalin was discovered in 1996 by Wright et al. (1996) and defined as a protein secreted or released by AM fungi into the soil where it would aid in soil aggregation. Since then glomalin has been operationally defined as a soil protein fraction obtained using harsh extraction methods (citric acid buffer, autoclaving, a pH of either 7.0 or 8.0) and by its reactivity with a monoclonal antibody (MAb32B11) raised against an unknown epitope on crushed spores of Glomus intraradices. Given the uncertainty about the identity of the soil-derived material used for its quantification and the recent description of the gene sequence of the immuno-reactive AM fungal protein (Gadkar and Rillig 2006), a new nomenclature was proposed, where the term glomalin is used just for the description of the gene product, and the soil-derived proteins, so far referred to as “glomalin”, are designated glomalin-related soil proteins (GRSP) (Rillig 2004).
A role for GRSP in reducing Cu bioavailability in contaminated soils has been proposed based on their capability to sequester Cu (González-Chávez et al. 2004; Cornejo et al. 2008). In vitro experiments have shown that glomalin extracted from hyphae of Gigaspora margarita can sequester up to 28 mg Cu per g of protein (González-Chávez et al. 2004). AM fungi, thereby, may influence Cu availability at the hyphosphere and rhizosphere, which, in turn, will decrease the toxicity risk to other soil microorganisms and plants growing in these areas.
Recently, with the observation that the protein is predominantly present in the fungal mycelium (80%) rather than secreted (Driver et al. 2005) and with the description of the glomalin gene sequence as a putative heat shock protein 60 (Gadkar and Rillig 2006), a new hypothesis for glomalin function has been raised. It has been proposed that glomalin has a primary function in the living hyphae, and that effects arising in the soil are secondary consequences (Purin and Rillig 2007). Given that heat shock proteins are stress-related proteins and the strong positive correlation found between GRSP and Cu content in the soil, it is tempting to speculate that a response of the fungus to high soil Cu levels would be to over-express glomalin and that the primary role of this protein would be to protect the fungus from the damage provoked by the Cu excess by repairing the fungal proteins. Then, through the deposition of glomalin-Cu complexes, it would contribute to reduce bioavailability of the contaminant.
Immuno-localization analyses of glomalin employing the monoclonal antibody MAb32B11 revealed that the protein was mainly located at the cell wall (Purin and Rillig 2008). Location of glomalin at the cell wall would explain, at least partially, the high affinity of the fungal walls for Cu, as we revealed by using a combination of transmission electron microscopy and energy-dispersive X-ray spectroscopy (EDXS) (González-Guerrero et al. 2008).
Developmental responses of AM fungi to Cu excess
Metal contaminated soils usually contain a spatially heterogeneous distribution of metal concentrations and available nutritional resources. Assuming that AM fungi quickly adapt to changing conditions and their hyphae are particularly well suited to heterogeneous environments, an important strategy of AM fungi for acclimation and survival in Cu contaminated soils would be to avoid the contaminated areas.
By analysing the morphogenetic response of extraradical hyphae of G. intraradices monoxenically grown in association with carrot roots when confronted to several Cu concentrations, we observed that Cu induced important changes in fungal morphogenesis. These changes include loss of apical dominance, cytoplasmic protrusions, extramatrical coils or reduction of sporulation, such effects becoming more frequent at higher Cu concentrations (González-Guerrero 2005). At lower Cu concentrations, an increase in total hyphal length and branching absorbing structures number was evident; while at higher concentrations growth of the extraradical mycelium was localized and seriously limited. Similar effects have been reported for G. intraradices extraradical mycelium grown monoxenically under different stress situations, such as Cd, Pb or Zn excess, low pH or ammonium enriched media (Bago et al. 2004; Pawlowska and Charvat 2004). These morphological alterations reflect the adaptive changes of the fungus when growing under stress conditions. As stated by Gadd (2007), loss of apical dominance or negative tropisms as well as growth cessation can be viewed as a “retreat” strategy aimed at avoiding toxic-metal contaminated areas. Development of extramatrical coils might also follow a fungal strategy to ‘exclude’ the stress-promoting agent from the close proximity of hyphae thus creating a sort of stress-free havens. In this case, such aggregated mycelia could also produce high local concentrations of extracellular products, such as metal chelators, reducing metal availability. Increased hyphal elongation when grown at the lower Cu concentrations, a strategy often employed by fungi when entering toxic metal contaminated domains (Fomina et al. 2003), may be a mean of the fungus to escape local metal-enriched microenvironments and reach relatively less-contaminated pockets of the soil. These data, hence, indicate that AM fungi may be able to survive in Cu contaminated soils by using a metal avoidance strategy.
The dramatic effects produced by the highest Cu concentration on fungal development points towards an inability of the fungus to efficiently detoxify this ion when supplied at very high concentrations, which, in turn, would explain the fungicide effect of this element. Given that all these studies have been performed with a Cu-sensitive AM fungus (G. intraradices DAOM 197198) and that AM fungal isolates from contaminated substrates usually perform better under heavy metal stress that isolates from uncontaminated soils (Sudová et al. 2008), further studies using a representative set of AM fungal isolates form contaminated and uncontaminated soils are required for gaining a deeper insight into the morphogenetic response of AM fungi to Cu.
Compartimentalization strategies
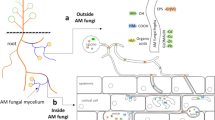
As shown in the previous sections, AM fungi have evolved avoidance mechanisms to restrict entry of toxic levels of Cu into their cytoplasm. Recently, we have provided evidence that AM fungi have also acquired a compartmentalization strategy to cope with heavy metals. Our EDXS analyses of copper ultrastructural localization in the extraradical mycelium of G. intraradices demonstrated that the metal accumulated preferentially in the fungal cell wall and in the vacuoles (González-Guerrero et al. 2008). Binding of metal ions onto cell walls has been regarded as an important passive process in both living and dead fungal biomass leading to metal immobilization (Gadd 1990; Sterflinger 2000). Actually, certain studies indicate that the fungal wall is responsible for 50% of the metal retained by AM fungi (Joner et al. 2000). The affinity of the wall for heavy metals is not surprising because of the significant metal-binding abilities of the major constituents of fungal cell walls (e.g., chitin, melanin, and, in the case of AM fungi, glomalin, as already mentioned).
Our EDXS study also revealed that once Cu enters the cytosol, it accumulates preferentially in the fungal vacuoles and that the vacuoles having the highest metal content are localized in the spores (González-Guerrero et al. 2008). Preferential accumulation of Cu in the spores has been also evidenced recently in our laboratory by the detection under the binocular microscope of some blue-green spores in the extraradical mycelium of G. intraradices when exposed to high Cu levels (Fig. 1). Given that phosphate accumulates mainly in the fungal vacuoles, the high affinity of phosphate for Cu and the emerald green colour of many Cu compounds, including copper phosphate, we hypothesize that the blue-green spores contain a high proportion of Cu-enriched vacuoles. Elemental distribution studies in mycorrhizal roots have revealed that vesicles of the intraradical mycelium might also serve as storage compartments for heavy metals (Weiersbye et al. 1999; Orlowska et al. 2008). These data indicate that in AM fungi, like in all organisms that operate an intracellular compartmentalization strategy to detoxify pollutants, the excess of Cu is translocated to subcellular compartments (vacuoles) where it can be stored away from the cytosol and to specific fungal structures (extraradical spores and intraradical vesicles) where it would cause less damage because these structures usually have more limited core metabolic functions.
Our observations under the binocular microscope of the Cu-treated mycelium also revealed that when spores appeared in clusters only one or few of them were blue (Fig. 1b). These data suggest that a fungal strategy to survive in a Cu-enriched media is to accumulate the excess of Cu in some spores, protecting in this way the rest of the fungal colony. Since a direct incorporation of metals through the spore is unlikely, our observations predict an as yet unknown mechanism of metal-sensing in the fungus that would divert metal-rich vacuoles from the hyphal cytosol to the spores. This hypothesis is supported by our EDXS observation that Cu was continuously accumulating over time in the spore vacuoles but not in the hyphal ones (González-Guerrero et al. 2008).
Molecular mechanisms of Cu homeostasis
Given the critical role Cu plays in crucial biochemical reactions, and the consequences of abnormal Cu homeostasis, it is important to understand the identity, mechanisms of action and regulation of cellular components responsible for the acquisition, distribution and detoxification of Cu. However, current knowledge on how AM fungi regulate their intracellular Cu levels in order to maintain proper Cu homeostasis is scarce. The elements integrating the molecular response of AM fungi to high Cu levels must involve, like in all organisms, Cu chelators, Cu shuttling molecules (Cu-chaperones), Cu transporters and Cu-sensing transcription factors (Kim et al. 2008).
Among the best characterized Cu chelators are the metallothioneins (MTs) (Hamer 1986; Vasak and Hasler 2000). These are short cytosolic cysteine-rich proteins which are able to tightly bind metals. Three glomeromycotan MTs have been identified to date, one in Gigaspora rosea (GrosMT1; Stommel et al. 2001), one in G. margarita (GmarMT1; Lanfranco et al. 2002) and the final one in G. intraradices (GintMT1; González-Guerrero et al. 2007b). All of them have been cloned from EST libraries in which no metal stress was applied, suggesting a high expression level even when no ligand is present. Further studies indicated that GintMT1 and GmarMT1 are able to restore Cu tolerance to MT-deficient yeasts and that their transcription is at least transiently induced by the presence of this metal. However, the lack of significant amounts of Cu present in the cytosol of Cu-treated hyphae of G. intraradices, and the inhibitory effect of prolonged Cu-exposure on GintMT1 gene expression, suggests that this is not the primary mechanism of control of cytosolic Cu levels in AM fungi, when the metal is present at supra-optimal concentrations (González-Guerrero et al. 2007b).
As mentioned above, a strategy used by AM fungi to keep cytosolic Cu concentrations low is compartmentalization of Cu excess in the fungal vacuoles. In order for the metal to accumulate in this organelle, Cu transporters are required. To the best of our knowledge, only two have been identified in AM fungi: the ABC transporter GintABC1 (González-Guerrero et al. 2007a) and a P-type Cu-ATPase (Benabdellah et al. 2007). Both genes, recently cloned in our laboratory from the AM fungus G. intraradices, are transcriptionally up-regulated by Cu and their gene products are likely localized in the fungal vacuoles. These transporters couple the hydrolysis of ATP to the efflux of Cu, either bound to a glutathione (GSH)-like molecule, in the case of GintABC1, or as a free ion provided by a Cu-chaperone in the case of the Cu-ATPase. These results point out at other levels of control of Cu homeostasis in AM fungi: the GSH homeostatic mechanism, and the role of as yet unidentified Cu-chaperones involved either in Cu delivery to Cu-ATPases or to other Cu-proteins (such as Cu, Zn-SOD or cytochromes).
Homeostasis may be also achieved by altering transport mechanisms that take up metal ions. It remains to be answered the question on how Cu gets into the fungus, although the most likely mechanism is via a member of an evolutionary conserved family of transporters called the Copper Transporter family (Ctr1), which mediates high-affinity Cu transport into cells of all eukaryotic organisms studied (Puig and Thiele 2002). However, none of these transporters has been identified yet in the Glomeromycota.
Fungal responses to Cu-induced oxidative stress
As mentioned above, Cu is a redox active metal which also exerts its damaging effects through the production of ROS via its participation in Fenton or Haber-Weiss reactions. Production of ROS by Cu in the AM fungus G. intraradices has been recently proven by Benabdellah et al. (2009) by using the ROS sensitive fluorescent dye 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA). As shown in Fig. 2, the Cu-treated mycelia exhibited a fluorescence pattern similar to that observed in the mycelia treated with the superoxide-generating agent paraquat. Furthermore, by measuring the lipid peroxidation levels of the membranes of G. intraradices when exposed to high Cu, we have also revealed that Cu induces an oxidative injury to the fungus (González-Guerrero et al. 2007b).
Intracellular production of ROS in Cu-exposed Glomus intraradices extraradical mycelia. The production of ROS was visualized by fluorescence microscopy using the ROS-sensitive probe H2DCF-DA. Control mycelia with no evidence of endogenous ROS production (a, b, c), and extraradical mycelia exposed to 500 μM paraquat (d, e, f) or 50 μM Cu (g, h, i). From Benabdellah et al. (2009) with permission
For dealing with this imbalanced redox status and to repair damage caused by the oxidative stress caused by Cu, AM fungi must enhance their ROS scavenging systems. Current knowledge on the mechanisms involved in the defence of AM fungi against oxidative stress is scarce, but they must include both non-enzymatic antioxidant systems, such as glutathione (GSH) and vitamins C, E and B6, as well as enzymatic antioxidant systems, such as catalases, superoxide dismutases, thioredoxins and glutaredoxins. To date, only a few genes encoding proteins putatively involved in ROS homeostasis have been identified and characterized in AM fungi: three superoxide dismutases (González-Guerrero 2005; Lanfranco et al. 2005; Benabdellah et al. unpublished results), ten genes putatively encoding glutathione S-tranferases (GSTs) (Waschke et al. 2006), a glutaredoxin (Benabdellah et al. 2009), a gene encoding a protein involved in vitamin B6 biosynthesis (Benabdellah et al. unpublished data) and a metallothionein (González-Guerrero et al. 2007b). Although this metallothionein, as mentioned before, was potentially involved in metal chelation, it was also shown to be involved in ROS scavenging, an activity that results from the capability of their thiolate groups to be reversibly oxidized.
Superoxide dismutases (SODs) have an antioxidant function by catalyzing the disproportionation of superoxide anion to hydrogen peroxide, and their activity requires redox active metal ions (Fridovich 1995). To date two types of SODs have been identified in AM fungi: a Cu,Zn-depending SOD, identified in the AM fungi G. margarita (GmarCuZnSOD1; Lanfranco et al. 2005) and G. intraradices (GintSOD1; González-Guerrero 2005), and a Mn-depending SOD in G. intraradices (GintSOD2; Benabdellah et al. unpublished results). Functional characterization of these genes in the Saccharomyces cerevisiae Cu,Zn-SOD (sod1) and Mn-SOD (sod2) null mutants has provided evidence about the antioxidant functions of their corresponding proteins. However, our gene expression analyses suggest that in G. intraradices, the Mn-SOD seems to be essential for defence against the superoxide generated in the mitochondrial respiratory chain, while the Cu,Zn-SOD plays a role both against externally and metabolically-generated superoxide. Up-regulation of GintSOD1 by high Cu levels indicates a role for this protein in scavenging the ROS resulting from the redox activity of this metal (González-Guerrero 2005).
While SODs belong to a group of enzymatic antioxidant systems that act directly as ROS detoxifiers, AM fungi must also posses a second group of enzymatic antioxidant systems, such as glutaredoxins and thioredoxins, which act as redox regulators of protein thiols and contribute to maintain the redox balance of the cell. Both enzymes are small oxidoreductases, conserved through evolution, which contain conserved cysteine residues in their active sites. Despite considerable functional overlap between glutaredoxins and thioredoxins, the oxidized disulphide form of thioredoxin is reduced directly by NADPH and thioredoxin reductase whereas glutaredoxins are reduced by GSH using electrons donated by NADPH (Wheeler and Grant 2004). Recently, a G. intraradices glutaredoxin (GintGRX1), belonging to the dithiol group of glutaredoxins, has been characterized (Benabdellah et al. 2009). Given that GintGRX1 reverted sensitivity to superoxide radicals of a yeast strain defective in dithiol glutaredoxins and that it is transcriptionally up-regulated by superoxide, a role for this protein in oxidative stress protection in G. intraradices was proposed. The protective effect of GintGRX1 could also be explained by its complex biochemical properties, illustrated by the fact that it is also active as GSH peroxidase and GST. Up-regulation of GintGRX1 by Cu also points towards a role for this protein in protecting the fungus against the oxidative damage induced by this ion. Although a G. intraradices thioredoxin gene has been identified in two suppressive substractive hybridization (SSH) libraries enriched in heavy metal induced transcripts (Ouziad et al. 2005; Waschke et al. 2006), the role of these proteins in AM fungi remains to be ascertained. Participation of GSTs, enzymes that catalyze the conjugation of glutathione with a variety of reactive electrophilic compounds, in the alleviation of heavy metal toxicity in AM fungi has been also proposed (Waschke et al. 2006; Hildebrant et al. 2007).
In addition to these enzymatic systems, AM fungi must also have small molecules acting as antioxidants, such as GSH and vitamins B6, C and E. Given that glutaredoxins and GSTs are induced in response to Cu and that GSH acts as redox donor for these enzymes, GSH must play a major role in repairing the oxidative damage induced by Cu in AM fungal proteins. Finally, up-regulation by superoxide and Cu of a G. intraradices gene encoding a protein involved in vitamin B6 biosynthesis (GintPDX1) suggests a potential role of vitamin B6 in the alleviation of Cu-induced oxidative stress in AM fungi (Benabdellah et al. unpublished data).
Conclusions and outlook
In spite of the important role AM fungi can play in the phytoremediation of Cu contaminated soils, little is known to date about the mechanisms involved in Cu uptake by AM fungi and the regulation of Cu homeostasis. Some advances in particular aspects of the avoidance strategies, metal compartmentalization in fungal constituents and mechanisms to cope with Cu-induced stress in AM fungi have been produced in the last few years. However, we still lack a comprehensive view of the Cu homeostatic processes. Most of the molecular mechanisms remain to be elucidated, being of special interest the transport mechanisms. A challenge for future research is to understand how Cu is safely stored and accessed within the fungus. The integration of knowledge arising from the G. intraradices whole genome sequence project with information provided by transcriptomics and advanced functional analysis of target genes from this and other AM fungi, will contribute to reveal the full range of genes potentially involved in metal tolerance and homeostasis and the potential interactions and synergies between different tolerance mechanisms in response to metal exposure. A further level of complexity will be to understand how metal tolerance, at the whole plant level, is affected by the establishment of the AM symbiosis. The mechanisms by which AM fungi effectively restrict metal translocation to the shoots and affect metal distribution among different plant organs deserve further investigation.
Isolation of indigenous, and presumably adapted AM fungi, more suitable for phytostabilization purposes than laboratory strains, can be a potential biotechnological tool for successful restoration of degraded ecosystems. Knowledge of the regulatory mechanisms that allow adapted AM fungi to tolerate the excess of Cu in a polluted environment will provide valuable information and will allow identifying the determinants of metal tolerance in these fungi. An enhanced understanding of all these processes will provide essential tools for efficient phytoremediation practices.
Abbreviations
- AM:
-
Arbuscular mycorrhiza
- EDXS:
-
Energy-dispersive X-ray spectroscopy
- GRSP:
-
Glomalin-related soil proteins
- GSH:
-
Glutathione
- GST:
-
Glutathione S-tranferase
- H2DCF-DA:
-
2′,7′-Dichlorodihydrofluorescein diacetate
- MTs:
-
Metallothioneins
- ROS:
-
Reactive oxygen species
- SODs:
-
Superoxide dismutases
References
Bååth E (1989) Effects of heavy metals in soil on microbial processes and populations (a review). Water Air Soil Pollut 47:335–379. doi:10.1007/BF00279331
Bago B, Cano C, Azcón-Aguilar C et al (2004) Differential morphogenesis of the extraradical mycelium of an arbuscular mycorrhizal fungus grown monoxenically on spatially heterogeneous culture media. Mycologia 96:452–462. doi:10.2307/3762165
Barea JM (1991) Vesicular-arbuscular mycorrhizae as modifiers of soil fertility. In: Stewart BA (ed) Advances in soil science, vol 7. Springer, New York, pp 1–40
Benabdellah K, Valderas A, Azcón-Aguilar C, et al (2007) Identification of the first Glomeromycotan P1b-ATPase. In: Abstracts of the 14th international workshop on plant membrane biology, Universidad Politécnica de Valencia, Valencia, Spain, 26–20 June 2007
Benabdellah K, Merlos MA, Azcón-Aguilar C et al (2009) GintGRX1, the first characterized glomeromycotan glutaredoxin, is a multifunctional enzyme that responds to oxidative stress. Fungal Genet Biol 46:94–103. doi:10.1016/j.fgb.2008.09.013
Cornejo P, Meiera S, Borie G et al (2008) Glomalin-related soil protein in a Mediterranean ecosystem affected by a copper smelter and its contribution to Cu and Zn sequestration. Sci Total Environ 406:154–160. doi:10.1016/j.scitotenv.2008.07.045
Driver JD, Holben WE, Rillig MC (2005) Characterization of glomalin as a hyphal wall component of arbuscular mycorrhizal fungi. Soil Biol Biochem 37:101–106. doi:10.1016/j.soilbio.2004.06.011
Fageria NK, Baligar VC, Clark RB (2002) Micronutrients in crop production. Adv Agron 77:185–268. doi:10.1016/S0065-2113(02)77015-6
Flemming CA, Trevors TJ (1989) Copper toxicity and chemistry in the environment: a review. Water Air Soil Pollut 44:143–158. doi:10.1007/BF00228784
Fomina M, Ritz K, Gadd GM (2003) Nutritional influence on the ability of fungal mycelia to penetrate toxic metal-containing domains. Mycol Res 107:861–871. doi:10.1017/S095375620300786X
Fridovich I (1995) Superoxide radical and superoxide dismutases. Annu Rev Biochem 64:97–112. doi:10.1146/annurev.bi.64.070195.000525
Gadd G (1990) Fungi and yeasts for metal accumulation. In: Ehrlich HL, Brierley C (eds) Microbial mineral recovery. McGraw-Hill, New York, pp 249–275
Gadd GM (2007) Geomycology: biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol Res 111:3–49. doi:10.1016/j.mycres.2006.12.001
Gadkar V, Rillig MC (2006) The arbuscular mycorrhizal fungal protein glomalin is a putative homolog of heat shock protein 60. FEMS Microbiol Lett 263:93–101. doi:10.1111/j.1574-6968.2006.00412.x
Göhre V, Paszkowski U (2006) Contribution of arbuscular mycorrhizal symbiosis to heavy metal phytoremediation. Planta 223:1115–1122. doi:10.1007/s00425-006-0225-0
González-Chávez MC, Carrillo-González R, Wright SF et al (2004) The role of glomalin, a protein produced by arbuscular mycorrhizal fungi, in sequestering potentially toxic elements. Environ Pollut 130:317–323. doi:10.1016/j.envpol.2004.01.004
González-Guerrero M (2005) Estudio de los mecanismos implicados en la homeostasis de metales pesados en el hongo formador de micorrizas arbusculares Glomus intraradices. Dissertation, University of Granada
González-Guerrero M, Benabdellah K, Valderas A, et al. (2007a) GintABC1 is involved in Cu and Cd homeostasis in the arbuscular mycorrhizal fungus Glomus intraradices. In: Abstracts of the 14th international workshop on plant membrane biology, Universidad Politécnica de Valencia, Valencia, Spain, 26–30 June 2007
González-Guerrero M, Cano C, Azcón-Aguilar C et al (2007b) GintMT1 encodes a functional metallothionein in Glomus intraradices that responds to oxidative stress. Mycorrhiza 17:327–335. doi:10.1007/s00572-007-0108-7
González-Guerrero M, Melville LH, Ferrol N et al (2008) Ultrastructural localization of heavy metals in the extraradical mycelium and spores of the arbuscular mycorrhizal fungus Glomus intraradices. Can J Microbiol 54:103–110. doi:10.1139/W07-119
González-Guerrero M, Benabdellah K, Ferrol N et al (2009) Mechanisms underlying heavy metal tolerance in arbuscular mycorrhizas. In: Azcón-Aguilar C, Barea JM, Gianinazzi S, Gianinazzi-Pearson V (eds) Mycorrhizas: functional processes and ecological impact. Springer, Berlin, pp 107–122
Halliwell B, Gutteridge JMC (1989) Free radicals in biology and medicine. Clanderon Press, Oxford
Hamer DH (1986) Metallothionein. Annu Rev Biochem 55:913–951
Hildebrandt U, Regvar M, Bothe H (2007) Arbuscular mycorrhiza and heavy metal tolerance. Phytochemistry 68:139–146. doi:10.1016/j.phytochem.2006.09.023
Joner EJ, Briones R, Leyval C (2000) Metal-binding capacity of arbuscular mycorrhizal mycelium. Plant Soil 226:227–234. doi:10.1023/A:1026565701391
Kim BE, Nevitt T, Thiele DJ (2008) Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol 4:176–185. doi:10.1038/nchembio.72
Lanfranco L, Bolchi A, Ros EC et al (2002) Differential expression of a metallothionein gene during the presymbiotic versus the symbiotic phase of an arbuscular mycorrhizal fungus. Plant Physiol 130:58–67. doi:10.1104/pp.003525
Lanfranco L, Novero M, Bonfante P (2005) The mycorrhizal fungus Gigaspora margarita possesses a CuZn superoxide dismutase that is up-regulated during symbiosis with legume hosts. Plant Physiol 137:1319–1330. doi:10.1104/pp.104.050435
Leyval C, Joner EJ, del Val C (2002) Potential of arbuscular mycorrhizal fungi for bioremediation. In: Gianinazzi S, Schüepp H, Barea JM, Haselwandter K et al (eds) Mycorrhizal technology in agriculture. Birkhäuser, Basel, pp 175–186
Linder MC, Goode CA (1991) Biochemistry of copper. Chapter 9. Springer, New York, pp 331–366
Meharg AA (2003) The mechanistic basis of interactions between mycorrhizal associations and toxic metal cations. Mycol Res 107:1253–1265. doi:10.1017/S0953756203008608
Orlowska E, Mesjasz-Przybylowicz J, Przybylowicz W et al (2008) Nuclear microprobe studies of elemental distribution in mycorrhizal and non-mycorrhizal roots of Ni-hyperaccumulator Berkheya coddii. XRay Spectrom 37:129–132. doi:10.1002/xrs.1034
Ouziad F, Hildebrandt U, Schmelzer E et al (2005) Differential gene expressions in arbuscular mycorrhizal-colonized tomato grown under heavy metal stress. J Plant Physiol 162:634–649. doi:10.1016/j.jplph.2004.09.014
Pawlowska TE, Charvat I (2004) Heavy-metal stress and developmental patterns of arbuscular mycorrhizal fungi. Appl Environ Microbiol 70:6643–6649. doi:10.1128/AEM.70.11.6643-6649.2004
Puig S, Thiele DJ (2002) Molecular mechanisms of copper uptake and distribution. Curr Opin Chem Biol 6:171–180. doi:10.1016/S1367-5931(02)00298-3
Purin S, Rillig MC (2007) The arbuscular mycorrhizal fungal protein glomalin: limitations, progress, and a new hypothesis for its function. Pedobiologia (Jena) 51:123–130. doi:10.1016/j.pedobi.2007.03.002
Purin S, Rillig MC (2008) Immuno-cytolocalization of glomalin in the mycelium of the arbuscular mycorrhizal fungus Glomus intraradices. Soil Biol Biochem 40:1000–1003. doi:10.1016/j.soilbio.2007.11.010
Rillig MC (2004) Arbuscular mycorrhizae, glomalin, and soil aggregation. Can J Soil Sci 84:355–363
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Elsevier, New York
Sterflinger K (2000) Fungi as geologic agents. Geomicrobiol J 17:97–124. doi:10.1080/01490450050023791
Stommel M, Mann P, Franken P (2001) EST-library construction using spore RNA of the arbuscular mycorrhizal fungus Gigaspora rosea. Mycorrhiza 10:281–285. doi:10.1007/s005720000090
Sudová R, Doubková P, Vosátka M (2008) Mycorrhizal association of Agrostis capillaris and Glomus intraradices under heavy metal stress: combination of plant clones and fungal isolates from contaminated and uncontaminated substrates. Appl Soil Ecol 40:19–29. doi:10.1016/j.apsoil.2008.02.007
Vasak M, Hasler DW (2000) Metallothioneins: new functional and structural insights. Curr Opin Chem Biol 4:177–183. doi:10.1016/S1367-5931(00)00082-X
Waschke A, Sieh D, Tamasloukht M et al (2006) Identification of heavy metal-induced genes encoding glutathione S-transferases in the arbuscular mycorrhizal fungus Glomus intraradices. Mycorrhiza 17:1–10. doi:10.1007/s00572-006-0075-4
Weiersbye IM, Straker CJ, Przybylowicz WJ (1999) Micro-PIXE mapping of elemental distribution in arbuscular mycorrhizal roots of the grass, Cynodon dactylon, from gold and uranium mine tailings. Nucl Instrum Methods Phys Res. Sec B 158:335–343
Wheeler GL, Grant CM (2004) Regulation of redox homeostasis in the yeast Saccharomyces cerevisiae. Physiol Plant 120:12–20. doi:10.1111/j.0031-9317.2004.0193.x
Wright SF, Franke-Snyder M, Morton JB et al (1996) Time-course study and partial characterization of a protein on hyphae of arbuscular mycorrhizal fungi during active colonization of roots. Plant Soil 181:193–203. doi:10.1007/BF00012053
Acknowledgments
We are grateful to the Consejería de Innovación, Ciencia y Empresa of the Andalusian Autonomic Government (Ref. P06-CVI-02263) for financial support of part of the work reported here. Karim Benabdellah was supported by an I3P contract from the Spanish Council for Scientific Research (CSIC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferrol, N., González-Guerrero, M., Valderas, A. et al. Survival strategies of arbuscular mycorrhizal fungi in Cu-polluted environments. Phytochem Rev 8, 551–559 (2009). https://doi.org/10.1007/s11101-009-9133-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-009-9133-9