Abstract
Paenibacillus polymyxa rhizobacteria associate with a wide range of plants and promote plant growth and development. These bacteria produce exopolysaccharides, which are very important for P. polymyxa adaptation to changing environmental conditions. In this study, five P. polymyxa strains differing in exopolysaccharide yield and properties (CCM 1465, CCM 1460, CCM 1459T, 88A, and 92) were investigated for motility in a liquid and a semisolid nutrient medium with either glucose or sucrose as the carbon source. In the liquid medium, all strains except CCM 1460 were motile and swam by peritrichous flagella. After being stab inoculated into the semisolid (0.4% agar) medium, all strains except CCM 1460 switched to collective swarming and formed concentric macrocolonies with different diameters, depending on the strain and the carbon source used. On the semisolid medium containing the vital dye Congo red, P. polymyxa adsorbed the dye, forming stained colonies. No changes in colony morphology were observed. At 37.5 μg ml−1 of Congo red, swarming was strongly suppressed: the swarming ring diameters of strains 92, CCM 1459T, 88A, and CCM 1465 ranged from 12.4 to 17% (glucose) and from 9.5 to 16.0% (sucrose) of the colony diameters obtained from bacterial growth without the dye. The results suggest that the speed of collective migration of P. polymyxa on agarized media may be affected, among other factors, by the yield and physicochemical properties of the bacterial exopolysaccharides. Further, the results suggest that Congo red influences the collective migration speed of P. polymyxa by forming a complex with the bacteria’s carbohydrate polymers – an event that alters bacterial surface structure and affects intercellular interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Paenibacillus polymyxa is a nonpathogenic, endospore-forming, gram-positive bacterium that occurs in diverse climatic zones and ecological niches, including water, soil, rhizosphere, plant roots and tissues, and marine sediments (Ravi et al. 2007; Anand and Chanway 2013; Timmusk et al. 2015). It is widely used in agriculture, industry, and environmental remediation because of its multiple functions (Acosta et al. 2005; Lal and Tabacchioni 2009; Yegorenkova 2013; Puri et al. 2015). P. polymyxa belongs to plant-growth-promoting rhizobacteria (PGPR), which benefit plants by fixing nitrogen; by producing phytohormones, antibiotics, and exopolysaccharides (EPSs); by inducing systemic resistance; and by improving soil structure (Bezzate et al. 2000; Haggag 2010; Yegorenkova 2013; Lei et al. 2015). The acidic and neutral EPSs produced actively by P. polymyxa have unique properties and diverse spheres of application (Matora et al. 1992; Lal and Tabacchioni 2009; Yegorenkova 2013; Liang and Wang 2015). In addition, these biopolymers play a key role in plant–bacterial interactions because they are involved in bacterial colonization of plant roots and in biofilm formation (Timmusk et al. 2005; Haggag 2010; Yegorenkova et al. 2011). A key structural component of biofilms, which has received close attention in the past decade, is the extracellular polymeric substance called the exopolysaccharide matrix (Smirnova et al. 2010). The abilities of P. polymyxa to colonize roots and form biofilms are essential for increasing plant resistance to biotic and abiotic stresses (Haggag 2010; Timmusk et al. 2015). Most P. polymyxa are motile, using peritrichous flagella (He et al. 2007).
Motility is vital to bacterial physiology, and bacteria living in different environments should have suitable movement systems (Merino et al. 2006). Bacteria are capable of several types of motility, including flagellar swimming and swarming and type IV pili-mediated twitching (Smirnova et al. 2010). For example, the migration to plant roots of the N2-fixing rhizobacterium Azospirillum brasilense, a model object for studying plant–microbe associations, occurs by swimming and is restricted by soil moisture (Bashan 1986). The adhesion of A. brasilense to plant roots is mediated by a polar flagellum (Croes et al. 1993), but lateral flagella enable cells to move along the root and are thought to be important for long-term colonization (Moens et al. 1995). This bacterium can express both a constitutive polar flagellum for swimming and an inducible lateral flagella system for swarming in viscous media or on surfaces. Because flagella synthesis and motility carry a high metabolic cost, the expression of the lateral flagella is highly regulated by a number of environmental factors and regulators (Merino et al. 2006).
The motility of P. polymyxa can be strongly promoted by root-exuded organic acids such as malic and citric acids, which may have a substantial role in root colonization (Ling et al. 2011). Park et al. (2008) reported that swarming of P. polymyxa Е681 on a 1.0% agar nutrient medium was induced by citrinin, a secondary metabolite of Penicillium citrinum KCTC6549. They concluded that the finding of the new function of citrinin contributes substantially to the understanding of how bacteria and fungi interact in nature. There is a whole set of transporter–cargo interactions between motile and nonmotile microflora. Thus, two very different rhizosphere dwellers, the nonmotile fungus Aspergillus fumigatus and the swarming bacterium Paenibacillus vortex, can facilitate each other’s dispersal (Ingham et al. 2011).
Motile bacteria can reversibly shift from free swimming of individual cells to collective motility (Henrichsen 1972). The mechanisms responsible for variations in collective motility depend not only on the work of motility organelles but also on intercellular interactions (Harshey 2003), which are determined by bacterial surface structure. Although the role of EPSs in bacterial swarming has been studied, the results for different strains have turned out to be contradictory. Faster-swarming mutants of Sinorhizobium meliloti RMB7201 synthesized less EPS, and all EPS-I-overproducing mutants had swarming defects (Wei and Bauer 1999). In contrast, EPS-defective mutants of Bacillus subtilis were unable to swarm (Nagorska et al. 2010), as was EPS-II-defective S. meliloti strain Rm8530 (Gao et al. 2012).
The motility of bacteria is affected by structural changes in their surface (Harshey 2003). Adsorption of the sulfonated azo dye Congo red, which modifies the cell surface, to cells of several A. brasilense strains and A. brasilense Sp245 mutants may suppress swarming and may make the bacteria able to consistently spread in semisolid media and form microcolonies (Shelud’ko et al. 2006). The extracellular lipopolysaccharide–protein complexes and the polysaccharides of Azospirillum can bind to Congo red, suggesting that these bacterial products are involved in cell adsorption of the dye (Skvortsov and Ignatov 1998). Arnold and Shimkets (1988), studying the effect of Congo red on cell–cell interactions in Myxococcus xanthus, suggested that the binding of the dye to the M. xanthus receptor had a manifold effect, inhibiting cell agglutination, collective motility, and fruiting body formation. As reported by Ingham and Ben-Jacob (2008), the addition of 100 μg ml−1 of Congo red delayed the onset of P. vortex swarming on a solid (1.5% agar) medium by 6 to 8 h, and higher concentrations (400 μg ml−1) completely suppressed swarming (> 72 h) while not impairing growth. Light microscopy observation showed that the P. vortex cells grown with Congo red were nonmotile, had an elongated, curved shape, and lacked flagella (Ingham and Ben-Jacob 2008).
Lauriano et al. (2004) showed that exopolysaccharide expression in Vibrio cholerae is regulated via a flagellum-dependent pathway. They identified the sodium-driven flagellar motor as an important component of the EPS signaling cascade, because mutations in the motor abolished EPS production, biofilm formation, and vps gene transcription. Janesch et al. (2013) demonstrated that the S-layer homology (SLH) domain-containing protein SlhA is involved in swarming and biofilm formation in Paenibacillus alvei CCM 2051T. They proposed that SlhA possibly has a major (and still unknown) role in signal transduction that leads to EPS expression, and they speculated that a complex relationship exists between motility, EPS production, and biofilm formation.
Studying the motility of the PGPR Paenibacillus is essential for understanding the mechanisms by which the bacteria adapt to heterogeneous habitats. It is not yet known how environmental factors affect motility and flagella synthesis. Our prior work has shown that the EPSs of P. polymyxa play a substantial part in plant–bacterial interactions, specifically in colonization of wheat seedling roots and in biofilm formation (Yegorenkova et al. 2011; Yegorenkova 2013), and that EPS composition and properties depend on the bacterial growth conditions (Yegorenkova et al. 2008). Using ELISA with rabbit polyclonal antibodies against isolated EPSs of P. polymyxa, we detected specific EPS determinants of P. polymyxa in its biofilm (Yegorenkova et al. 2011).
This study was undertaken to evaluate how the exoglycans of P. polymyxa affect its swimming and swarming – processes that can be important for interactions of the bacteria with their plant partners. To this end, we investigated the motility of five P. polymyxa strains differing in exopolysaccharide yield and properties (CCM 1465, CCM 1460, CCM 1459T, 88A, and 92) in a liquid and a semisolid nutrient medium with either glucose or sucrose as the carbon source. We isolated P. polymyxa EPSs and characterized them by physicochemical methods. We analyzed how changes in EPS composition and properties resulting from cell growth with a different carbon source affect P. polymyxa motility. Finally, we examined the behavior of P. polymyxa in a liquid and a semisolid medium in the presence of the vital dye Congo red, which can complex with bacterial polysaccharide components.
2 Materials and methods
2.1 Strains and growth conditions
The soil bacteria Paenibacillus polymyxa CCM 1465 (Rhodes et al. 1987) and CCM 1460 (Nakamura 1987) and the type strain P. polymyxa CCM 1459T (Smith et al. 1964; Nakamura 1987) were obtained from the Czech Collection of Microorganisms (Brno, Czech Republic). P. polymyxa 88A (IBPPM 384) was from the IBPPM RAS Collection of Rhizosphere Microorganisms (http://collection.ibppm.ru/). This strain was generated from P. polymyxa type strain CCM 1459T by short-term treatment with intense microwave radiation at 2375 MHz (Matora et al. 1992; Porozhnyakova et al. 2004) and is deposited as B-4556 in the Russian National Collection of Industrial Microorganisms (VKPM), Moscow, Russian Federation. P. polymyxa 92 (VNIISHM 92) was isolated from wheat roots by Dr. Y.M. Voznyakovskaya (All-Russia Research Institute for Agricultural Microbiology, Russian Academy of Agricultural Sciences, Pushkin-8, St. Petersburg, Russian Federation). Bacteria were grown with rotary shaking (220 rpm) at 30 oС in a liquid nutrient medium of the following composition (g l−1): yeast extract, 4; Na2HPO4, 1.1; KH2PO4, 0.5; MgSO4 · 7H2O, 0.2; (NH4)2SO4, 0.1; CaCO3, 0.2; glucose or sucrose, 30; distilled water, up to 1 l (pH 7.2–7.5). When appropriate, 37.5 μg ml−1 of the vital dye Congo red was added to the growth medium. The number of colony-forming units (CFU) was determined by plating serial tenfold dilutions of P. polymyxa liquid cultures onto plates with an agarized nutrient medium.
2.2 Assay for bacterial motility in liquid medium
Cultures were grown with 3% glucose or sucrose. P. polymyxa motility was observed in a drop of liquid with a Jenaval phase-contrast microscope (Carl Zeiss, Jena, Germany) and was video recorded with a Sony DCR-TRV900E digital camera (Sony, Tokyo, Japan) at a recording rate of 25 shots s−1. The video files, in .avi format, were stored on a personal computer. The swimming speed of 50–100 individual cells was measured with a computer program designed by V.A. Krestinenko, as follows (Schelud’ko et al. 2009): a cell was chosen with the mouse cursor, and the initial cell position was fixed by clicking the right mouse button and changing the shot. Then, the cell position was marked in rectangular coordinates frame by frame. The movement trajectory was drawn in each frame to calculate the traversed distance and the average speed. The trajectory was stored as a graphics file; the traversed distance and the average speed, as a text file. The results were statistically processed.
2.3 Assay for bacterial motility on semisolid medium
The semisolid medium contained 0.4% Bacto agar and 3% glucose or sucrose. The molten medium was cooled to 42 °C, poured into petri plates to a depth of 3 to 5 mm, and allowed to harden. Bacteria were stab inoculated with a loop from a solid medium plate containing the appropriate carbon source. The petri plates, without being overturned, were placed in a thermostat and incubated at 30 oС. The speed of bacterial collective motility was measured by the diameter of the macrocolonies formed by cells migrating away from the inoculation stab. The morphology of the zones of cell spreading was observed with the unaided eye and with phase-contrast microscopy (Leica LMD7000; Leica Microsystems, Germany). Bacterial collective motility was also examined in the presence of Congo red (37.5 μg ml−1), added to the molten and cooled (to 42 °C) semisolid (0.4% Bacto agar) medium.
2.4 EPS isolation and analysis
The total EPS of P. polymyxa was isolated after the bacteria were grown in the liquid nutrient medium containing 3% glucose or sucrose. The culture liquid was diluted two to threefold with distilled water to decrease viscosity. The cells were separated by centrifugation at 15000 g for 30 min, the supernatant liquid was concentrated to the original volume of the culture liquid by rotary vacuum evaporation (40 °C), and the EPS was precipitated with 3 volumes of acetone. The precipitate was separated by centrifugation at 3000 g for 20 min, washed repeatedly with acetone, and lyophilized in a BENCHTOP 2 K lyophilizer (VirTis, NY, USA). The EPS content in the culture liquid was analyzed by the weight method. Total carbohydrates in the resultant EPS samples were measured spectrophotometrically by a reaction with phenol and sulfuric acid, and protein was estimated by a modified Bradford assay (Zakharova and Kosenko 1982) and was expressed as percent of the weight of arbitrarily dry EPS.
The kinematic viscosity of the culture liquid and of 0.1% aqueous EPS solutions was measured with an Ostwald capillary viscometer (Type VPZh-2; inside capillary diameter, 0.73 or 0.99 mm) thermostatted in a 20 °C water bath (accuracy, ± 1 °C). For measurement of the intrinsic viscosity of aqueous polysaccharide solutions, the EPSs isolated from the culture liquid were suspended in distilled water and 0.1% solutions were made.
Infrared (IR) spectra of the EPSs were recorded on an Infralum FT-801 Fourier transform IR spectrometer (LUMEX, Russia). Samples were run in KBr pellets prepared at a pressure of 7 tons cm−2. Measurements were made in the frequency range 4000–500 cm−1.
Neutral monosaccharides, uronic acids, and amino sugars were identified by thin-layer chromatography (Zakharova and Kosenko 1982). Neutral monosaccharides were also analyzed by the content of alditol acetate derivatives of the hydrolyzate monosaccharides (Slonecker 1972). The monosaccharides were identified by gas–liquid chromatography (GLC) on a Hewlett-Packard 5890 chromatograph equipped with an Ultra 2 capillary column (Hewlett-Packard, USA) and on a GC-2010 chromatograph (Shimadzu, Japan) fitted with an Equity-1 column (Supelco, USA). Uronic acids were also measured colorimetrically by the method of Dische (1962).
2.5 Light microscopy
Light microscopy of bacteria was done with a Biolar PI polarizing interference microscope (Poland; objective lenses, 10×, 20×, and 40×; ocular lens, 12×).
2.6 Statistics
Data were processed with Excel 2007 software (Microsoft Corp., USA). Results are expressed as mean ± standard error of the mean (SEM) and are given with 95% confidence limits. Means were compared between treatments by Student’s t-test, and differences were considered significant at p < 0.05.
3 Results and discussion
3.1 P. polymyxa motility in liquid and semisolid media
The motility of P. polymyxa strains CCM 1465, CCM 1460, CCM 1459T, 88A, and 92 was investigated in the liquid and semisolid media containing either glucose or sucrose as the carbon source. Attention was given to P. polymyxa’s behavior in the semisolid medium, because these conditions make it possible to simulate the physicochemical properties of mucigel – a gellike substance that surrounds bacteria on plant roots and is composed of plant and microbial polysaccharides (Skvortsov 1994). Agarized media allow detection in bacteria of the capacity for different kinds of collective motility, both flagella-mediated and conditioned by other mechanisms (Henrichsen 1972).
All liquid-grown strains except CCM 1460 were motile, having trajectories characteristic of peritrichously flagellated bacteria (Berg and Brown 1972) – short “runs” with temporary stops followed by “twiddles” or “tumbles.” The direction and duration of motion along a new trajectory chosen after a stop was not associated with the previous direction (Fig. 1). Type strain CCM 1459T was the fastest of the P. polymyxa strains tested (Table 1). The carbon source in the medium had little effect on the character and speed of cell motion, except for strain 92, which showed a statistically significant difference in motion speed when grown with glucose and sucrose (Table 1).
On a solid (1.8% agar) nutrient medium, all strains grew along the streak lines as convex, mucoid, colorless colonies. After being stab inoculated into the 0.4% agar semisolid medium, all strains except CCM 1460 switched to collective swarming and produced concentric macrocolonies (Fig. 2). After 48 h, the macrocolony diameter ranged from 33.1 to 48.4 mm with glucose and from 44.4 to 55.4 mm with sucrose (Table 1, Fig. 3). The size of the semisolid-grown colonies differed among the strains (Fig. 3). Overall, strains CCM 1459T, 88A, and 92 formed larger “swarming rings” when grown on the semisolid medium with sucrose for 48 h, with statistically significant differences (Table 1). The sessile strain CCM 1460 almost did not migrate away from the inoculation stab, and the spreading of CCM 1465 on the semisolid medium with glucose was almost the same as on the semisolid medium with sucrose.
The differences in the speed (or effectiveness) of spreading of P. polymyxa on the semisolid medium could not be linked only to differences in the locomotion speed of individual cells. Most of the strains gave larger-diameter macrocolonies on sucrose than on glucose, but, as noted above, the carbon source in the medium was insignificant to the speed of cell motion. Strain CCM 1459T was moving faster than the other bacteria, with the differences being statistically significant, but in some cases, its macrocolonies were of comparable size to those produced by the other strains (Table 1).
Some authors believe that the formation of complex, multicellular structures involves surface-active substances and extracellular polymers, as well as the work of motility organelles (Kuner and Kaiser 1982; Di Franco et al. 2002). In several bacteria, including Pseudomonas solanacearum (Brumbley and Denny 1990) and S. meliloti (Wei and Bauer 1999), differences in the speed of cell spreading on semisolid media have been associated with the yield of produced EPS (the spreading speed and EPS yield are inversely correlated). From our data, we cannot unequivocally conclude that EPS yields were solely responsible for P. polymyxa macrocolony size. P. polymyxa CCM 1459T produced 12.9 g l−1 of EPS when grown in the liquid medium with sucrose (EPSSUC) and 7.3 g l−1 of EPS when grown with glucose (EPSGL) (Glukhova et al. 1986), and mutant strain 88A, which secretes a highly viscous EPS termed polymyxan, produced 10 g l−1 of EPSSUC and 11.4 g l−1 of EPSGL (Porozhnyakova et al. 2004). Yet, both strains formed larger-sized macrocolonies when grown with sucrose. Note that strain 88A is a mutant of P. polymyxa CCM 1459T that was generated by short-term treatment with intense microwave radiation (Matora et al. 1992).
We speculate that the speed of collective migration depends, alongside other factors, on the viscosity of the bacteria’s surroundings, which may be determined by the composition and properties of the polymers produced by the bacteria. The culture liquid viscosity for strains CCM 1459T and 88A grown with glucose was greater than that for these strains grown with sucrose (Glukhova et al. 1986; Matora et al. 1992), perhaps owing to the higher own viscosity of their EPSGL. For instance, the viscosity of a 1% aqueous solution of EPS88A produced on sucrose is four- to sevenfold lower than that obtained from growth on glucose across the range of shear rates (Matora et al. 1992). Therefore, the colony size of CCM 1459T and 88A on the semisolid medium, as well as the culture-liquid and EPS viscosities, depended on the source of carbon (Fig. 3). In addition, the viscosity of an aqueous solution of EPS88A (and especially EPSGL) was much higher than that for the other strains tested and the swarming rings were the smallest.
When batch cultivated in the liquid medium for 3 days, P. polymyxa CCM 1465 accumulated as much as 6.0 and 11.8 g l−1 of EPSSUC versus 1.2 and 1.4 g l−1 of EPSGL within 24 and 48 h, respectively (Fig. 4a). The culture liquid viscosities obtained from cell growth on glucose and sucrose were approximately the same at both the 24 h and the 48 h time points (Fig. 4b). No differences were observed in the size of the macrocolonies formed by strain CCM 1465 on the semisolid medium with the different carbon sources (Fig. 3). When glucose was replaced by sucrose, the culture liquid viscosity did not increase as much as EPS yield did. A more intense synthesis of EPS is not always related to a greater kinematic viscosity of the culture liquid: the yield of EPS may be low but the kinematic viscosity may be fairly high. This fact could be explained as follows: a change in the carbon source produces a change in the ratio between the acidic and the neutral fraction of the EPS being synthesized, thereby affecting the viscosity of the culture liquid and of aqueous EPS solutions. The EPS of strain CCM 1465 is a heterogeneous polysaccharide in which the acidic component predominates when the bacteria are grown with glucose; this predominance correlates with the greater viscosity of their aqueous EPS solutions (Yegorenkova et al. 2008).
Strain 92 had similar culture liquid viscosities on glucose and sucrose (Fig. 4b) but statistically significant differences in swarming speed when grown on the semisolid medium with these carbon sources (Table 1). Importantly, the viscosity of a 0.1% aqueous solution of EPSSUC was twofold lower than that for EPSGL (1.3 and 2.8 mm2 s−1, respectively). In EPSGL, the predominant fraction is acidic, and rheological measurements indicate that solutions of acidic polysaccharides are the most viscous and that the contribution of neutral polysaccharides to the rheological characteristics of total polysaccharide preparations is minor. It is worth recalling that when grown in the liquid medium with the different carbon sources, strain 92 had a statistically significant difference in motion speed, which could also have influenced collective motility (Table 1).
It can be inferred that the collective migration speed of P. polymyxa on agar-supplemented media may be affected, among other factors, by the viscosity of the bacteria’s surroundings, which in turn depends on the production and physicochemical properties of the bacterial EPSs.
In some cases, semisolid-grown P. polymyxa CCM 1465 formed “supercolonies” (Fig. 2a1) that resembled colonies produced by P. vortex (Ingham and Ben-Jacob 2008), a bacterium so named because of the vigorous rotation of its entire colonies and of areas within colonies (Ben-Jacob et al. 1997). The P. polymyxa CCM 1465 colonies were concentric and consisted of protuberances formed by cells migrating away from the inoculation stab (Fig. 2a1). By 48 to 72 h of growth on the semisolid medium, some of the other P. polymyxa strains tested by us had sometimes formed protuberances as well, which extended from the colony edge. One can speculate that such protuberances are formed by a subpopulation of faster-swarming P. polymyxa cells.
3.2 Effect of Congo red on P. polymyxa collective motility
To find whether P. polymyxa could adsorb Congo red, we plated them on a solid medium with 37.5 μg ml−1 of the dye by following the procedure described by Bastarrachea et al. (1988). On 1.8% agar with Congo red, all strains formed stained colonies. Bacterial production of extracellular polysaccharides on a solid medium and in liquid flocculating cultures was shown by Bastarrachea et al. (1988) with Congo red and by Michiels et al. (1990) with calcofluor. β-Linked polysaccharides are known to interact in solution with calcofluor and Congo red, bringing about changes in solubility and in the dyes’ absorption spectra (Wood 1980).
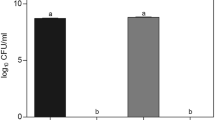
By 24 h of growth in the liquid medium supplied with 37.5 μg ml−1 of Congo red, all strains had attained approximately the same cell concentrations as in the medium without the dye. The average numbers of P. polymyxa cells were (2.8–3.3) × 108 CFU with the dye and (3.0–3.6) × 108 CFU without it. Observations with light microscopy showed that when grown with Congo red, cells of strains CCM 1459T, CCM 1465, 88A, and 92 remained motile but were moving somewhat slower. All strains, including the sessile strain CCM 1460, had much greater cell lengths than when grown without the dye (16.6–32.9 μm with Congo red versus 7.7–9.9 μm without it). By 24 h of cell growth with Congo red, the culture liquid had become viscous, indicating that substantial amounts of EPS had accumulated in the medium.
On the 0.4% agar semisolid medium containing Congo red (37.5 μg ml−1), all strains adsorbed the dye, forming stained colonies (Fig. 5). No changes in colony morphology were observed. Earlier, Shelud’ko et al. (2006) showed that in semisolid media, A. brasilense bacteria migrated, producing swarming rings, and that at 37.5 μg ml−1 of Congo red, they changed their behavior radically, beginning to spread and form microcolonies. In this study, the presence of 37.5 μg ml−1 of Congo red strongly suppressed P. polymyxa swarming: after 48 h, the swarming ring diameters for strains 92, CCM 1459T, 88A, and CCM 1465 ranged from 12.4 to 17% (glucose) and from 9.5 to 16.0% (sucrose) of the colony diameters of the strains grown without the dye (Table 1, Fig. 5). Possibly, Congo red influences the collective migration speed of P. polymyxa by forming a complex with the bacteria’s carbohydrate polymers – an event that alters bacterial surface structure and affects intercellular interactions. This assumption is in line with previous findings (Arnold and Shimkets 1988; Shelud’ko et al. 2006).
For comparison, in a semisolid medium with Congo red, A. brasilense Sp245 had a colony size approximately 50% smaller than that of the colony grown without the dye. Our analysis of Shelud’ko et al.’s (2006) studies of the motility of several A. brasilense strains indicated that Congo red inhibited the collective migration of semisolid-grown P. polymyxa more strongly than it inhibited the migration of Azospirillum. This may be due to P. polymyxa being able to accumulate much larger quantities of extracellular polysaccharide-containing polymers, with which Congo red can complex, as compared with Azospirillum. Specifically, the yields of polysaccharide-containing polymers of A. brasilense (strains Sp7 and Sp245) from the capsules and culture liquids were 0.038–0.062 and 0.072–0.095 mg ml−1, respectively; their protein content ranged from 8 to 33%, whereas their carbohydrate content ranged from 19.5 to 60% (Konnova et al. 1994).
The binding of Congo red to polysaccharides depends on the type of linkages between the monosaccharide units, the degree of substitution of the polysaccharide chains, and the homogeneity of the materials analyzed (Wood 1980). As shown by us earlier, the EPS synthesized by P. polymyxa CCM 1465 on glucose differs greatly from that synthesized on sucrose in molecular mass, composition, and rheological and antigenic properties (Yegorenkova et al. 2008). In this study, the structure of the P. polymyxa EPSs was investigated by IR spectroscopy, a method used widely to deduce the structures of polysaccharide molecules, detect functional groups and their mutual arrangement, determine linkage types, and establish the identity of compounds. The IR spectra of the P. polymyxa EPSs examined by us were very similar, displaying absorption bands characteristic of polysaccharides. As an example, we present the IR spectra of the EPS of P. polymyxa 92 grown with glucose (Fig. 6a) and sucrose (Fig. 6b). The absorption peaks indicated that the EPS molecules contained hydroxyl, NH, СН, СН2, С = О, and С–О–С groups, as well as both α- and β-glycosidic linkages (Fig. 6a, b, Table 2). At 3409–3413 cm−1, the IR spectra showed very broad and fairly intense bands that were characteristic of stretching vibrations of the v(OH) groups. At 1613–1636 cm−1, bands of bending vibrations of δ(ОН) were observed (Zhbankov 1972; Hineno 1977). The band at 2922–2923 cm−1 was due to stretching vibrations of v(СН). The absorption band at 1737 cm−1, corresponding to stretching vibrations of the v(C = О) groups in acids and esters, possibly indicated uronic acids (Zhbankov 1972). The small peaks at 1520–1560 cm−1 (amide II) corresponded to bending vibrations of δ(NH). The spectra showed absorption bands characteristic of polysaccharides, viz., broad bands of the ν(С–О–С) glycosidic linkages at 1070–1076 cm−1 (Wu et al. 2009). The absorption peaks at 800–805 cm−1 could have been due to bending vibrations of the equatorial С–Н groups of the pyranose rings. The region of 500–800 cm−1 contained weak absorption bands and was not interpreted.
Although having similar total profiles and similar positions of the major characteristic bands, EPSGL and EPSSUC had some differences, indicating that the EPS structures had specific peculiarities (Fig. 6a, b). The spectral region of 700–1000 cm−1 was characterized by absorption bands of low and medium intensity. In this region lie the absorption bands of sugars that are associated with their stereochemical peculiarities, and it is this region that makes structural differences between polysaccharides most clearly visible. Specifically, the EPSSUC spectra had absorption bands at 920 cm−1, characteristic, according to some authors (Scherba and Babitskaya 2008), of the α-glycosidic bond (Fig. 6b). Previous systematic studies of a large number of sugar spectra (Zhbankov 1972) found that derivatives of three β-hexapyranoses (glucose, mannose, and galactose) have an absorption band at 890 cm−1. In this study, the EPSGL spectra had more intense absorption bands at 885–890 cm−1, possibly indicating that β-glycosidic linkages were predominant (Fig. 6a).
Colorimetrically, it was found that the P. polymyxa EPSs contained 72–85% carbohydrates and 1.4–2.2% protein (% of EPS weight). GLC of polyol acetates after complete acidic hydrolysis of the P. polymyxa EPSs showed that the polymers contained mostly mannose, glucose, galactose, and uronic acids in various ratios (Table 3). The preparations obtained from growth on sucrose had the same monosaccharide constituents but much less galactose and uronic acids than those obtained from growth on glucose. Overall, the results of our analysis of the EPS chemical composition agree well with literature data for other P. polymyxa strains (Liang and Wang 2015).
Although the chemical nature of the Congo red receptor is unknown, an extracellular polysaccharide (made up predominantly of galactosamine and N-acetyl-d-galactosamine) that binds Congo red was isolated from the bacterium Flexibacter columnaris (formerly Chondrococcus columnaris; Johnson and Chilton 1966). In myxobacteria, the major Congo red receptor is presumably localized to fibrils (polysaccharidic filaments associated with a multimeric protein; Arnold and Shimkets 1988). Smirnova et al. (2010) found by cytochemical studies that the matrix of biofilms developed by Salmonella typhimurium includes acidic mucopolysaccharides, detected by alcian blue staining, and cellulose, stained with Congo red. Considering that the P. polymyxa strains examined by us produced, during growth, substantial quantities of heterogeneous EPSs containing about 72–85% carbohydrates, glucose as a major monosaccharide, and β-glycosidic linkages, we speculate that Congo red may form complexes with these EPSs, altering bacterial surface structure and interfering with bacterial swarming.
In summary, our results suggest that the EPSs of the plant-associative bacteria P. polymyxa are involved in their collective migration. The speed of collective migration may be affected by EPS yield, composition, and properties, which together determine the viscosity of the bacteria’s surroundings. As shown in this and previous work (Yegorenkova et al. 2008), P. polymyxa grown on different carbohydrates synthesize exoglycans that differ in composition and properties. The vital dye Congo red inhibits P. polymyxa collective motility possibly through binding to the carbohydrate polymers on the bacterial surface.
This study has provided novel data showing that the swarming motility of P. polymyxa depends, alongside other factors, on the physicochemical properties of the bacterial EPSs. The major source of carbon and energy for rhizosphere microorganisms is provided by the exudate from roots, whose composition may have a substantial effect on bacterial metabolism, specifically for bacterial polysaccharides and their properties. Our data expand current views on the mechanisms enabling bacteria to better adapt to diverse ecological niches, including associations with plants.
References
Acosta MP, Valdman E, Leite SGF, Battaglini F, Ruzal SM (2005) Biosorption of copper by Paenibacillus polymyxa cells and their exopolysaccharide. World J Microbiol Biotechnol 21:1157–1163
Anand R, Chanway C (2013) Detection of GFP-labeled Paenibacillus polymyxa in autofluorescing pine seedling tissues. Biol Fertil Soils 49:111–118
Arnold JW, Shimkets LJ (1988) Inhibition of cell-cell interactions in Myxococcus xanthus by congo red. J Bacteriol 170:5765–5770
Bashan Y (1986) Migration of the rhizosphere bacteria Azospirillum brasilense and Pseudomonas fluorescens towards wheat roots in the soil. J Gen Microbiol 132:3407–3414
Bastarrachea F, Zamudio M, Rivas R (1988) Non-encapsulated mutants of Azospirillum brasilense and Azospirillum lipoferum. Can J Microbiol 34:24–29
Ben-Jacob E, Cohen I, Czirok A, Vicsek T, Gutnick DL (1997) Chemomodulation of cellular movement: collective formation of vortices by swarming bacteria and colonial development. Physica A 238:181–197
Berg HC, Brown DA (1972) Chemotaxis in Escherichia coli analyzed by three-dimensional tracking. Nature 239:500–504
Bezzate S, Aymerich S, Chambert R, Czarnes S, Berge O, Heulin T (2000) Disruption of the Paenibacillus polymyxa levansucrase gene impairs its ability to aggregate soil in the wheat rhizosphere. Environ Microbiol 2:333–342
Brumbley SM, Denny TP (1990) Cloning of wild-type Pseudomonas solanacearum phcA, a gene that when mutated alters expression of multiple traits that contribute to virulence. J Bacteriol 172:5677–5685
Croes CL, Moens S, van Bastelaere E, Vanderleyden J, Michiels KW (1993) The polar flagellum mediates Azospirillum brasilense adsorption to wheat roots. J Gen Microbiol 139:2261–2269
Di Franco C, Beccari E, Santini T, Pisneschi G, Tecce G (2002) Colony shape as a genetic trait in the pattern-forming Bacillus mycoides. BMC Microbiol 2:33
Dische Z (1962) Color reactions of hexuronic acids. Methods Carbohydr Chem 1:497–501
Gao M, Coggin A, Yagnik K, Teplitski M (2012) Role of specific quorum-sensing signals in the regulation of exopolysaccharide II production within Sinorhizobium meliloti spreading colonies. PLoS ONE 7:e42611. doi:10.1371/journal.pone.0042611
Glukhova EV, Yarotsky SV, Deryabin VV, Shenderov BA, Ignatov VV (1986) Extracellular polysaccharides of Bacillus polymyxa and its mutant strain. Antibiotiki i meditsinskaya biotekhnologiya 31:669–674 [in Russian]
Haggag WM (2010) The role of biofilm exopolysaccharides on biocontrol of plant disease. In: Elnashar M (ed) Biopolymers, vol 14. Rijeka, Sciyo, pp 271–284
Harshey RM (2003) Bacterial motility on a surface: many ways to a common goal. Annu Rev Microbiol 57:249–273
He Z, Kisla D, Zhang L, Yuan C, Green-Church KB, Yousef AE (2007) Isolation and identification of a Paenibacillus polymyxa strain that coproduces a novel lantibiotic and polymyxin. Appl Environ Microbiol 73:168–178
Henrichsen J (1972) Bacterial surface translocation: a survey and a classification. Bacteriol Rev 36:478–503
Hineno M (1977) Infrared spectra and normal vibration of β-D-glucopyranose. Carbohydr Res 56:219–227
Ingham CJ, Ben-Jacob E (2008) Swarming and complex pattern formation in Paenibacillus vortex studied by imaging and tracking cells. BMC Microbiol 8:36
Ingham CJ, Kalisman O, Finkelshtein A, Ben-Jacob E (2011) Mutually facilitated dispersal between the nonmotile fungus Aspergillus fumigatus and the swarming bacterium Paenibacillus vortex. Proc Natl Acad Sci U S A 108:19731–19736
Janesch B, Koerdt A, Messner P, Schäffer C (2013) The S-layer homology domain-containing protein SlhA from Paenibacillus alvei CCM 2051T is important for swarming and biofilm formation. PLoS One 8:e76566. doi:10.1371/journal.pone.0076566
Johnson JL, Chilton WS (1966) Galactosamine glycan of Chondrococcus columnaris. Science 152:1247–1248
Konnova SA, Makarov OE, Skvortsov IM, Ignatov VV (1994) Isolation, fractionation and some properties of polysaccharides produced in a bound form by Azospirillum brasilense and their possible involvement in Azospirillum–wheat root interactions. FEMS Microbiol Lett 118:93–99
Kuner JM, Kaiser D (1982) Fruiting body morphogenesis in submerged cultures of Myxococcus xanthus. J Bacteriol 151:458–461
Lal S, Tabacchioni S (2009) Ecology and biotechnological potential of Paenibacillus polymyxa: a minireview. Indian J Microbiol 49:2–10
Lauriano CM, Ghosh C, Correa NE, Klose KE (2004) The sodium-driven flagellar motor controls exopolysaccharide expression in Vibrio cholerae. J Bacteriol 186:4864–4874
Lei M, Lu P, Jin L, Wang Y, Qin J, Xu X, Zhang L, Wang Y (2015) Complete genome sequence of Paenibacillus polymyxa CF05, a strain of plant growth-promoting rhizobacterium with elicitation of induced systemic resistance. Genome Announc 3:e00198–e00115. doi:10.1128/genomeA.00198-15
Liang T-W, Wang S-L (2015) Recent advances in exopolysaccharides from Paenibacillus spp.: production, isolation, structure, and bioactivities. Mar Drugs 13:1847–1863
Ling N, Raza W, Ma J, Huang Q, Shen Q (2011) Identification and role of organic acids in watermelon root exudates for recruiting Paenibacillus polymyxa SQR-21 in the rhizosphere. Eur J Soil Biol 47:374–379
Matora AV, Ignatova EN, Zhemerichkin DA, Egorenkova IV, Shipin OV, Panasenko VI, Arsenieva IY, Barkovsky AL (1992) Bacterial polysaccharide polymyxan 88A. Main characteristics and possible applications. Prikl Biokhim Mikrobiol 28:731–737 [in Russian]
Merino S, Shaw JG, Tomas JM (2006) Bacterial lateral flagella: an inducible flagella system. FEMS Microbiol Lett 263:127–135
Michiels K, Verreth C, Vanderleyden J (1990) Azospirillum lipoferum and Azospirillum brasilense surface polysaccharide mutants that are affected in flocculation. J Appl Bacteriol 69:705–711
Moens S, Michiels K, Keijer V, Leuven FV, Vanderleyden J (1995) Cloning, sequencing, and phenotypic analysis of laf1, encoding the flagellin of the lateral flagella of Azospirillum brasilense Sp7. J Bacteriol 177:5419–5426
Nagorska K, Ostrowski A, Hinc K, Holland IB, Obuchowski M (2010) Importance of eps genes from Bacillus subtilis in biofilm formation and swarming. J Appl Genet 51:369–381. doi:10.1007/BF03208867
Nakamura LK (1987) Bacillus polymyxa (Prazmowski) Mace 1889 deoxyribonucleic acid relatedness and base composition. Int J Syst Bacteriol 37:391–397
Park S-Y, Kim R, Ryu C-M, Choi S-K, Lee C-H, Kim J-G, Park S-H (2008) Citrinin, a mycotoxin from Penicillium citrinum, plays a role in inducing motility of Paenibacillus polymyxa. FEMS Microbiol Ecol 65:229–237
Porozhnyakova [Yegorenkova] IV, Matora AV, Shipin OV, Ignatova EN, Ignatov VV, Panasenko VI, Bystrov ММ (2004) Shtamm bakteriy Bacillus polymyxa – produtsent polisakharidov (A polysaccharide-producing strain of Bacillus polymyxa bacteria). Russian Federation Patent No 1826520 RF (IPC C12N1/20, C12P19/04) Publication date: 27 November 2004 [in Russian]
Puri A, Padda K, Chanway C (2015) Can a diazotrophic endophyte originally isolated from lodgepole pine colonize an agricultural crop (corn) and promote its growth? Soil Biol Biochem 89:210–216
Ravi AV, Musthafa KS, Jegathammbal G, Kathiresan K, Pandian SK (2007) Screening and evaluation of probiotics as a biocontrol agent against pathogenic Vibrios in marine aquaculture. Lett Appl Microbiol 45:219–223
Rhodes C, Strasser J, Friedberg F (1987) Sequence of an active fragment of B. polymyxa beta amylase. Nucleic Acids Res 15:3934
Schelud’ko AV, Makrushin KV, Tugarova AV, Krestinenko VA, Panasenko VI, Antonyuk LP, Katsy EI (2009) Changes in motility of the rhizobacterium Azospirillum brasilense in the presence of plant lectins. Microbiol Res 164:149–156
Scherba VV, Babitskaya VG (2008) Polysaccharides of xylotrophic basidiomycetes. Appl Biochem Microbiol 44:78–83
Shelud’ko AV, Borisov IV, Krestinenko VA, Panasenko VI, Katsy EI (2006) Effect of Congo red on the motility of the bacterium Azospirillum brasilense. Microbiology (Moscow) 75:48–54
Skvortsov IM (1994) Mutsigel’ i sliz’ poverkhnosti kornei rasteniy (the mucigel and slime of the plant root surface). Usp Sovr Biol 114:372–384 [in Russian]
Skvortsov IM, Ignatov VV (1998) Extracellular polysaccharides and polysaccharide-containing biopolymers from Azospirillum species: properties and the possible role in interaction with plant roots. FEMS Microbiol Lett 165:223–229
Slonecker JH (1972) Gas–liquid chromatography of alditol acetates. Methods Carbohydr Chem 6:20–24
Smirnova TA, Didenko LV, Romanova YM, Azizbekyan RR (2010) Structural and functional characteristics of bacterial biofilms. Microbiology (Moscow) 79:413–423
Smith N, Gibson T, Gordon RE, Sneath P (1964) Type cultures and proposed neotype cultures of some species in the genus Bacillus. J Gen Microbiol 34:269–272
Timmusk S, Grantcharova N, Wagner EGH (2005) Paenibacillus polymyxa invades plant roots and forms biofilms. Appl Environ Microbiol 71:7292–7300
Timmusk S, Kim S-B, Nevo E, Abd El Daim I, Ek B, Bergquist J, Behers L (2015) Sfp-type PPTase inactivation promotes bacterial biofilm formation and ability to enhance wheat drought tolerance. Front Microbiol 6:387. doi:10.3389/fmicb.2015.00387
Wei X, Bauer WD (1999) Tn5-induced and spontaneous switching of Sinorhizobium meliloti to faster-swarming behavior. Appl Environ Microbiol 65:1228–1235
Wood PJ (1980) Specificity in the interaction of direct dyes with polysaccharides. Carbohydr Res 85:271–287
Wu M, Wu Y, Zhou J, Pan Y (2009) Structural characterisation of a watersoluble polysaccharide with high branches from the leaves of Taxus chinensis Var. mairei. Food Chem 113:1020–1024
Yegorenkova IV (2013) Exopolysaccharides of Paenibacillus polymyxa rhizobacteria in plant–bacterial interactions. In: Maheshwari DK, Saraf M, Aeron A (eds) Bacteria in agrobiology: crop productivity, vol 7. Springer-Verlag, Berlin, pp 401–437
Yegorenkova IV, Tregubova KV, Matora LY, Burygin GL, Ignatov VV (2008) Composition and immunochemical characteristics of exopolysaccharides from the rhizobacterium Paenibacillus polymyxa 1465. Microbiology (Moscow) 77:553–558
Yegorenkova IV, Tregubova KV, Matora LY, Burygin GL, Ignatov VV (2011) Biofilm formation by Paenibacillus polymyxa strains differing in the production and rheological properties of their exopolysaccharides. Curr Microbiol 62:1554–1559
Zakharova IM, Kosenko LV (1982) Metody izucheniya mikrobnykh polisakharidov (Methods in the study of microbial polysaccharides). Naukova dumka, Kiev [in Russian]
Zhbankov RG (1972) Infrakrasnye spektry i struktura uglevodov (Infrared spectra and carbohydrate structure). Nauka i Tekhnika, Minsk [in Russian]
Acknowledgments
Our thanks go to Mr. Dmitry N. Tychinin (this institute) for translating the original manuscript into English.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yegorenkova, I.V., Tregubova, K.V. & Schelud’ko, A.V. Motility in liquid and semisolid media of Paenibacillus polymyxa associative rhizobacteria differing in exopolysaccharide yield and properties. Symbiosis 74, 31–42 (2018). https://doi.org/10.1007/s13199-017-0492-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-017-0492-5