Abstract
Paenibacillus polymyxa (formerly known as Bacillus polymyxa), a nonpathogenic endospore-forming rhizobacterium, is one of the most industrially interesting facultative anaerobes. It is an active producer of various biologically active substances, including exopolysaccharides, which possess a range of unique properties. This chapter outlines the present state of research on P. polymyxa bacteria, their ecology and biotechnological potential, and the mechanisms of their stimulatory effect on plants. Special emphasis is placed on the structural–functional characterization of P. polymyxa exoglycans and on their important role in the establishment of plant–microbe associations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Root Hair
- Extracellular Polymeric Substance
- Paenibacillus Polymyxa
- Polyglutamic Acid
- Root Hair Deformation
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
17.1 Introduction
Among the myriads of bacteria thriving in the plant rhizosphere, some spore-forming plant-growth-promoting rhizobacteria (PGPR), in particular gram-positive bacilli and streptomycetes, have attracted special attention because of their advantages over non-spore formers in product formulation and stable maintenance in soil (Emmert and Handelsman 1999). Among these, the genus Paenibacillus (species of a genus previously included in the genus Bacillus, Ash et al. 1993; Trüper 2005) comprises more than 130 species with the type species Paenibacillus polymyxa.
The nitrogen-fixing soil rhizobacteria P. polymyxa promote the growth and development of a wide range of plants through the establishment of effective associative relationships. This has been associated with the capacities of these microorganisms for nitrogen fixation, phosphate mobilization, and production of phytohormones, antibiotics (Mannanov and Sattarova 2001), and a wide range of lytic enzymes, as well as with their high adaptability to living conditions (Lebuhn et al. 1997; Da Mota et al. 2002; Lal and Tabacchioni 2009). It has been proven experimentally that in association with plants, P. polymyxa can increase plant resistance to biotic and abiotic stresses (Timmusk and Wagner 1999; Khan et al. 2008; McSpadden Gardener 2004; Selim et al. 2005; Timmusk et al. 2005). Some investigators believe that in this process, a major role is played by P. polymyxa’s capacities for effective colonization and biofilm formation (Haggag and Timmusk 2008; Timmusk et al. 2009b; Haggag 2010). Certain strains not only colonize the surface of roots (Bent et al. 2002) but also penetrate the root interior (Shishido et al. 1999).
P. polymyxa is capable of producing acidic and neutral exopolysaccharides (EPSs; Matora et al. 1992; Hebbar et al. 1992; Lee et al. 1997; Jung et al. 2007), which have unparalleled properties. This explains the diversity of spheres of possible application of these polymers. In addition, P. polymyxa exoglycans have been assigned an important role in the formation of plant–microbe associations (Hebbar et al. 1992; Bezzate et al. 2000; Timmusk et al. 2005; Haggag 2007). P. polymyxa is widely used as the major component of complex bacterial fertilizers, enriching the environment with excreted polysaccharides (PSs), whose effect on humans and animals is not quite known yet.
The surface localization of the extracellular PSs confers on them the properties of mediators in the interaction of P. polymyxa with other micro- and macroorganisms. In addition, by forming a dense layer on the bacterial surface, EPSs may shield other cellular structures underneath them and may also determine bacterial immunological properties. Several investigators (Jung et al. 2007; Chang et al. 2009, 2010) have shown that the EPSs of P. polymyxa are biologically active substances (BASs) with an immunomodulatory action.
Despite the intensity of research on these bacterial PSs and the considerable progress made toward elucidating their physiological role, the properties and the chemical structure of a wide range of EPSs remain to be fully clarified. A thorough study of these biopolymers will allow uncovering the functional linkages between the structure of exoglycans and their biological role, which may facilitate a deeper understanding of the molecular foundations of intercellular, interspecies, and interorganismal interactions.
17.2 Ecology and Biotechnological Potential of P. polymyxa
P. polymyxa has attracted considerable interest because of its great biotechnological potential in different industrial processes and in sustainable agriculture.
17.2.1 Morphological and Physiological Peculiarities
The genus Paenibacillus was created by Ash et al. (1993) to accommodate the former “group 3” of the genus Bacillus. Paenibacillus species are facultatively anaerobic, endospore-forming, neutrophilic, periflagellated heterotrophic, and low G+C gram-positive bacilli (Euzéby 2011). In Latin, paene means almost, and therefore the Paenibacillus is almost a Bacillus. Comparative 16S rRNA sequence analyses revealed that rRNA group 3 bacilli represent a phylogenetically distinct group, exhibit high intragroup sequence relatedness, and are only remotely related to B. subtilis—the type species of the genus Bacillus. The taxon contains various species such as B. alvei, B. amylolyticus, B. azotofixans, B. gordonae, B. larvae, B. macerans, B. macquariensis, B. pabuli, B. polymyxa, B. pulvifaciens, and B. validus (Ash et al. 1991).
Phenotypically, species of this group react weakly with Gram’s stain and even young cultures appear gram negative. They differentiate into ellipsoidal spores, which distinctly swell the mother cell. The combination of morphology and physiology is sufficient to distinguish rRNA group 3 bacilli from all other mesophilic species of Bacillus with the exception of B. circulans, B. lautus, B. lentimorbus, and B. popilliae. The latter four species are, however, phylogenetically only remotely related to B. polymyxa; and its relatives and the described rRNA group 3 specific gene probe provide an unequivocal method for distinguishing these taxa (Ash et al. 1993). Among the 51,713 Firmicutes sequences listed in Ribosomal Database Project II, the family Paenibacillaceae comprises 1,057 16S rRNA sequences with 74 as P. polymyxa (as on January 2008) (Lal and Tabacchioni 2009). Strains of P. polymyxa (the type species of the genus) were found to be capable of suppressing several plant diseases and promoting plant growth (Benedict and Langlykke 1947).
Kim et al. (2010) presented the complete genome sequence of P. polymyxa E681. Its 5.4-Mb genome encodes functions specialized to the plant-associated lifestyle and characteristics that are beneficial to plants, such as the production of a plant growth hormone, antibiotics, and hydrolytic enzymes. The complete genome sequence of an important plant-growth-promoting rhizobacterium, P. polymyxa SC2, was reported by Ma et al. (2011), who found multiple sets of functional genes in the genome.
P. polymyxa occurs widely in water, soil, and the rhizosphere (Von der Weid et al. 2000; Guemouri-Athmani et al. 2000; Cheong et al. 2005). P. polymyxa spores cause sporangium deformation and have thick walls with a star-shaped section. These can remain in a dormant state for long periods, being resistant to heat, drying, radiation, and toxic chemicals (Comas-Riu and Vives-Rego 2002).
17.2.2 Practical Use
Ever since it became known that P. polymyxa can elaborate certain antibiotics, this microorganism has continued to generate increased interest owing to the promise for use that it shows. The high activity of N2 fixation and phosphate mobilization in Paenibacillus was a prerequisite to the use of these bacteria as a biofertilizer component (Kozyrovskaya et al. 2005). By now, technologies have been developed for the manufacture and use of P. polymyxa-based biopreparations [biopolitsid (BSP) and polimiksobakterin], which have found wide application in Ukrainian agriculture. BSP is based on B. polymyxa strain P, which is antagonistic to a wide range of phytopathogenic fungi, including such widespread crop pathogens as Bipolaris sorokiniana, Fusarium avenaceum Sacc., F. graminearum, Trichothecium roseum, Ascochyta pisi Lib., Cercosporella herpotrichoides Fron., Colletotrichum gloeosporioides Penz., Phomopsis leptostromiformis Bubak, Rhizoctonia violaceae Tul., and Sclerotinia sclerotiorum Lib. De Bar (http://www.ecobiology.com.ua). These preparations are polyfunctional in that they promote transformation of plant-unavailable P-containing mineral and organic compounds, defense against plant pathogens (through the formation of antibiotics), effective use of biological N, and enhancement of soil fertility. Similarly, in India, a consortium, named an Indian Agricultural Research Institute (IARI) microphos culture, was developed that contains two very efficient phosphate-solubilizing bacteria (Pseudomonas striata and B. polymyxa) and three phosphate-solubilizing fungi (Aspergillus awamori, A. niger, and Penicillium digitatum) (Gaur 1990).
Some investigators believe that Paenibacillus bacteria stimulate the growth and development of a wide range of plants (cereals, conifers, legumes, etc.) (Bent et al. 2002; Timmusk et al. 2005), improve the germinability of cultivated plants (Gupta et al. 2000), and are able to degrade pesticides and insecticides. P. polymyxa exhibits clear antagonistic activity against soilborne fungal and oomycetic pathogens (Dijksterhuis et al. 1999; Timmusk 2003; Ryu et al. 2006; Choi et al. 2008; Haggag 2007) (Table 17.1).
In recent years, investigators’ attention has also been turned to nonagriculture-related possibilities of using P. polymyxa. These include biosorption of metals (copper) from polluted soils (Piuri et al. 1998; Philip et al. 2000; Acosta et al. 2005; Chu and Kim 2006); degradation of toxic substances, based on P. polymyxa’s ability to degrade phenanthrene and chlorobenzene (Daane et al. 2001; Vogt et al. 2004); wastewater purification, owing to the ability of these bacteria to decompose organic waste (Chockalingam et al. 2003); biosynthesis of a range of BASs (Mavingui and Heulin 1994; Jung et al. 2007); production of enzymes (Budi et al. 2000; Alvarez et al. 2006), e.g., inulinase, which is used in the manufacture of glucose–fructose syrups (Zherebtsov et al. 2003); cellulose degradation (in combination with cellulolytic bacteria) (Gorska et al. 2001); and large-scale production of medicinal antibiotics (Nakajima et al. 1972; Girardin et al. 2002; Zengguo et al. 2007; Tupinambá et al. 2008). The bacterium displays inhibitory activity against human and animal pathogenic microorganisms (Rosado and Seldin 1993; Seldin et al. 1999; Alvarez et al. 2006; Ravi et al. 2007) (Table 17.1).
There are data on the utility of P. polymyxa for the building industry and for mining operations, owing to the ability of Paenibacillus to adhere to minerals and degrade them (Patra and Natarajan 2004, 2006). Furthermore, predictions have been made that the use of the latest achievements of biotechnology may lead to some fermentation processes becoming competitive with the preparation of the same products (ethanol, butanol, butanediol) from petroleum. Because of its nonpathogenicity, genetic stability, and ability to ferment variously composed polysaccharides of plant raw material, P. polymyxa has been assigned to the group of potentially industrial microorganisms, 2,3-butanediol producers (Ui et al. 1983; Nakashimada et al. 1998; Syu 2001) (Table 17.1).
Thus, despite the existing limited information on the genomes of P. polymyxa, the past few decades have seen a growing interest in these bacteria owing to their great biotechnological potential in various industrial processes and in agriculture (Lal and Tabacchioni 2009).
17.2.3 Plant Growth Promotion
P. polymyxa occurs widely in various climatic zones and is found in chernozemic, brownearth, serozemic (gray), krasnozemic (red), and sod-podzolic soils. P. polymyxa strains have been isolated from the rhizosphere of a variety of crops such as wheat (Triticum aestivum), barley (Hordeum gramineae; Lindberg et al. 1985), white clover (Trifolium repens), perennial ryegrass (Lolium perenne), crested wheatgrass (Agropyron cristatum; Holl et al. 1988), lodgepole pine (Pinus contorta latifolia; Holl and Chanway 1992), douglas fir (Pseudotsuga menziesii; Shishido et al. 1996), green bean (Phaseolus vulgaris; Petersen et al. 1996), and garlic (Allium sativum; Kajimura and Kaneda 1996). In the wheat root zone, these bacteria may predominate over other N2-fixing anaerobes, and they have a leading role in the accumulation of N in soils (Döbereiner 1977).
P. polymyxa is grouped with PGPR (Timmusk and Wagner 1999; Haggag 2007). Extensive results are available on the effect of P. polymyxa inoculation on the yields of major cereal crops, including wheat, barley, rice, sorghum, millet, and maize (Chanway 1995; Maes and Baeyen 2003). Data from growth chamber experiments have been published concerning yields and N assimilation in winter wheat inoculated with various rhizobacteria. It was shown that P. polymyxa inoculation promotes an increase in grain yield (De Freitas 2000). A considerable effect on the growth and yield of wheat and maize was found with a certain plant–bacterial combination and was absent with another combination, demonstrating the existence of an interrelation between the plant genotype and the bacterial strain (Renni and Thomas 1987; Chanway et al. 1988; Da Mota et al. 2002). The most N accumulation was observed when a wheat cultivar was inoculated with P. polymyxa isolated from its rhizosphere (Renni and Thomas 1987). Several authors have reported a large positive effect from the introduction of P. polymyxa strains into the plant rhizosphere, considering such parameters as plant viability and weight, the concentration of chlorophyll in the leaf mesophyll, the state of the root, and the formation of root hairs. Seed treatment with P. polymyxa resulted in better seed germinability and in faster seedling growth (Maes and Baeyen 2003).
Despite the numerous studies of plant interactions with associative N2-fixing and growth-promoting bacteria, there have so far been no reliable predictions of plant response to inoculation. This response, however, may vary from positive or neutral to negative; and P. polymyxa may have adverse effects on plants. For example, when roots of Arabidopsis thaliana were soaked for 24 h in cultures of P. polymyxa strains B2, B3, and B4 in L medium, plants responded with 30 % growth reduction and a stunted root system, compared to uninoculated plants. These effects were observed in a gnotobiotic system and in soil, pointing to a mild pathogenic effect (Timmusk and Wagner 1999; Timmusk 2003). Consequently, under these conditions, P. polymyxa can be considered a deleterious rhizobacterium (DRB). Furthermore, inoculation with P. polymyxa strain L6-16R promoted growth of lodgepole pine in one location, inhibited it in a second site, and had no discernible effect in a third site (Chanway and Holl 1994).
The inconsistency of results from P. polymyxa inoculations gave impetus to new research on the use of combined inoculation of bacilli and other microorganisms. Combined inoculation of plants with associative bacteria of different genera is one of the most advanced technologies in agriculture. Skvortsova et al. (1998), using cereal grasses, studied the effect of inoculation with two-component cultures composed of B. polymyxa and various Pseudomonas strains on N2 fixation, denitrification, and heterotrophic nitrification. Inoculation with such cultures not only produced substantial increases in yields but also significantly enhanced crop N content. More specifically, yields increased up to 25–77 %. Combined inoculation with Azospirillum brasilense, Azotobacter chroococcum, B. polymyxa, and Enterobacter cloacae was found to have positive effects on the yield, dry weight, and total nitrogen of winter wheat (De Freitas 2000); and P. polymyxa inoculation, alone or in combination with Rhizobium, enhanced the growth of lentil and protected the plant against Meloidogyne javanica nematodes (Siddiqui et al. 2007).
For a long time, the beneficial effect of associative rhizosphere bacteria has been attributed largely to fixation of molecular N (a parallel drawn with symbiotic N2 fixation). In P. polymyxa, however, N2 fixation makes only a partial contribution to the stimulation of plant growth (Chanway and Holl 1991). Apart from the improvement of N nutrition, these bacteria have other mechanisms responsible for the positive effect on plants (Costacurta and Vanderleyden 1995). Several authors have reported that P. polymyxa produces hormones of the cytokinin group (Timmusk et al. 1999) and auxins, specifically indole-3-acetic acid (IAA) (Holl et al. 1988; Lebuhn et al. 1997). Treatment with auxins accelerated bacterial colonization of roots and promoted the formation of paranodules (Narula et al. 2006). Lebuhn et al. (1997) examined the actual and potential abilities to form indolic and phenolic compounds on different media in P. polymyxa isolated at different distances from the roots of wheat. They observed a gradual decrease in the potential for IAA production by the strains isolated from nonrhizosphere soil, as compared with those from the rhizosphere and the rhizoplane. These metabolic differences indicate that near plant roots, P. polymyxa subpopulations undergo selection for genetic and physiological parameters.
Most studies of biological variability within the P. polymyxa species have pointed out the influence of various factors on the degree of bacterial genetic polymorphism. Specifically, a study of the effect of plant development stages on a population of P. polymyxa in the maize rhizosphere demonstrated that the population observed in the middle stage of plant growth (30–60 days after planting) was more homogeneous than that in the initial stage (10 days) or after 90 days of maize growth (Von der Weid et al. 2000). Long-term cultivation of wheat on Algerian soils (>70 years) was reported to change the rhizosphere population of P. polymyxa, increasing its size, decreasing bacterial diversity, choosing the dominant genotype, and enhancing N2 fixation (Guemouri-Athmani et al. 2000).
The mechanism of P. polymyxa’s stimulatory effect on plants is not quite clear yet. It is believed that the effectiveness of plant–P. polymyxa associations is determined by such bacterial characteristics as N2-fixation ability (Lindberg et al. 1985); production of phytohormones (Holl et al. 1988; Lebuhn et al. 1997; Timmusk et al. 1999), antibiotics (Rosado and Seldin 1993), hydrolytic enzymes (Nielsen and Sorensen 1997), and exopolysaccharides (Hebbar et al. 1992; Bezzate et al. 2000; Timmusk et al. 2005; Haggag 2007; Yegorenkova et al. 2010); and improvement of the mineral nutrition and aquatic balance of inoculated plants through phosphate mobilization (Singh and Singh 1993) and soil structure amelioration (Bezzate et al. 2000; Czames et al. 2000).
The significance of the above mechanisms of P. polymyxa’s effect on plants is different under different conditions. Apart from climatic factors, a large role is played by the species and strain characteristics of the bacteria used and by the species and cultivar peculiarities of the plants used. It is believed that growth-promoting factors should be considered collectively, because trying to emphasize the role of any one may lead to a substantial understatement of the effect of each of them (Bashan and Holguin 1997).
17.3 Role of EPSs in the Formation of Plant–Bacterial Associations
Microbial EPSs are the primary or secondary metabolites produced by a variety of microorganisms. These EPSs have been widely used within bioindustries, because the production cost of microbial EPSs is lower than that of algal or plant polysaccharides (Kumar et al. 2007). Additionally, microbial EPSs are nontoxic, biodegradable, and environmentally benign (Shoda and Sugano 2005). P. polymyxa elaborates a broad range of neutral and acidic exopolysaccharides (Matora et al. 1992; Hebbar et al. 1992; Lee et al. 1997; Haggag 2007; Jung et al. 2007), which have diverse structures and physical–chemical properties. Most of them are of low or no toxicity. The EPSs of P. polymyxa are BASs with immunotropic activity (Jung et al. 2007). They have been assumed to be essential for the development of plant–microbial associations (Bezzate et al. 2000; Timmusk et al. 2005; Haggag 2007).
17.3.1 Physical–Chemical Characterization, Properties, and Use of P. polymyxa EPSs
P. polymyxa can synthesize neutral polysaccharides [levan (Iman and Abd-Allah 1974; Han 1989), mannan (Ball and Adams 1959), and glucan (Jung et al. 2007)], acidic polysaccharides, or heteropolysaccharides (Ninomiya et al. 1968a; Glukhova et al. 1986; Matora et al. 1992; Yegorenkova et al. 2008).
Among other bacteria, P. polymyxa stands out as one of the most active levan producers. The levans are 2,6-bonded, sometimes branched, regular polymers with a repeating unit (Han 1989, 1990). The levan of P. polymyxa can be used to suppress allotransplant rejection; to prolong the action of pharmaceutics; to act as an immunomodulator or a plasma substitute; to increase soil water capacity; to improve plant seed germinability (Iman and Abd-Allah 1974); to prepare pure fructose (Tkachenko and Sevryugina 1989); and to encapsulate substances in the manufacture of cosmetics and in paper and fabric printing (Han 1990). P. polymyxa JB115 was isolated from Korean soil as a glucan producer for the development of animal feed additives. As shown by IR, 1Н NMR, and 13С NMR spectroscopy, the JB115 glucan is a linear glucan that has β-(1→3) and β-(1→6) structure. High-molecular-weight glucan (above 100 kDa) can be used as an animal feed additive for immune enhancement and as a potential antitumor agent for livestock (Jung et al. 2007; Chang et al. 2009).
The heteropolysaccharides of P. polymyxa have diverse compositions, structures, and properties. The bacteria B. polymyxa 458, isolated in Japan, synthesize a highly viscous, nontoxic EPS that is composed of residues of glucose (Glc), mannose (Man), and glucuronic acid (GlcA) at a 7:7:2 molar ratio (Mitsuda et al. 1981). Heating and cooling EPS solutions (>0.7 %) gives rise to agar-like gels, but their strength is slightly inferior to that of gels of the same concentration. P. polymyxa strain S-4 produces an acidic EPS that is composed of β-d-Glc, d-Man, d-galactose (Gal), d-GlcA, and d-ManA at a 3:3:1:2:1 ratio. In the main chain of this polymer, Glc and Gal are (1→3)-bonded, Man and Gal are (1→4)-bonded, and GlcA and Man are (1→3)-bonded; the side chains contain Glc, Man, and ManA, which are mostly (1→4)-bonded. The EPS has an “antisclerotic” action by decreasing the concentration of cholesterol in blood and the liver (Fukui et al. 1985).
When grown on saccharose, P. polymyxa 271 (FERM P-1824), isolated from Japanese soil, synthesizes two EPSs—an acidic one and a neutral one (Ninomiya et al. 1968a). When grown on glucose, it produces only the acidic polymer, which is composed of d-Glc, d-Man, d-Gal, and d-GlcA at a 3:3:1:2 ratio (Ninomiya et al. 1968b). The neutral EPS is made up of Glc, Man, Gal, and fructose (Fru). The molecular mass (M m) of the acidic EPS is greater than 1 MDa, and the EPS forms highly viscous aqueous solutions (Ninomiya and Kizaki 1969). An important peculiarity of this EPS is that it forms a stable viscoelastic gel with 40 % ethanol, with the polymer concentration being 2 %. This polymer has both a technical and a pharmacological action. The EPS and its cationic forms lower the concentrations of lipids and cholesterol in blood and the liver, and they reduce the atherogenic index, reducing the probability of atherosclerosis and myocardial infarction (Tanaka et al. 1982).
P. polymyxa mutant strain 1459В excretes two EPSs, one being acidic, viscous, and of a high M m and the other being weakly viscous, neutral, and of a lower M m (Glukhova et al. 1986). The ratio between the EPSs depends on the source of carbon in the medium. The neutral EPS is a levan, and the acidic EPS is made up of Glc, Gal, Man, and GlcA residues and trace amounts of arabinose and xylose (Xyl) (Glukhova et al. 1986). Solutions of 1459В EPS are compatible with high concentrations of mono- and bivalent cations and Al3+ at pH 3–11. The acidic EPS forms thermolabile elastic gels. It was suggested that the levan of P. polymyxa 1459В be used for the preparation of pure fructose, as an immunomodulator, and as a blood substitute, and that the acidic EPS be used for increasing oil reservoir recovery, preparing drill fluids, and regulating the rheological properties of freshwater and mineral water solutions.
A new highly viscous EPS, named polymyxan, was described by Matora et al. (1992). It is synthesized by the producer strain P. polymyxa 88A, obtained by short-term treatment with intense microwave radiation at a frequency of 2,375 MHz. Polymyxan consists of an acidic, highly viscous PS (M m of 1–10 MDa, composition of 35 % Glc, 36 % Man, 7 % Gal, and 21 % GlcA) and a neutral, low-viscous PS (M m of 100–300 kDa), which is a glucomannan with equal contents of both monosaccharides and with trace amounts of uronic acids. The structure of this EPS is believed by the authors to be irregular. Data were presented on the use of polymyxan in bread making and also as a polymeric agent for the preparation of drill fluids and the conservation of wells (Matora et al. 1992).
Hebbar et al. (1992) established that P. polymyxa АТСС 842 and АТСС 21551 synthesize EPSs composed of Glc, Gal, Man, uronic acids, pyruvate, acetate, and succinate. The EPSs of batch-cultivated P. polymyxa 1465 were found to contain neutral and acidic fractions and to be heterogeneous PSs represented by a complex of macromolecules with M ms ranging from 7 × 104 to 2 × 106 Da (Yegorenkova et al. 2008). When the bacteria were grown on glucose, the acidic component predominated, which correlated with the higher viscosity of aqueous solutions of the EPSs. The exoglycans were found to contain Glc, Man, Gal, and uronic acids. Rabbit polyclonal antibodies were developed to an isolated EPS of P. polymyxa 1465, and the presence of common EPS antigenic determinants within the species P. polymyxa was shown (Yegorenkova et al. 2008).
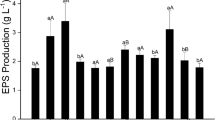
P. polymyxa strain P13 was described as an EPS producer by Acosta et al. (2005). Those authors found that 100 ml of a stationary-phase P13 culture formed 27 (±4) mg (±SD) and 15 (±4) mg (±SD) EPS in BHI medium containing 1 M NaCl and in control BHI medium, respectively. This strain exhibited a significant capacity for biosorption of Cu(II) originating from several industries. EPS production was associated with hyperosmotic stress caused by high salt content (1 M NaCl), which led to a significant increase in the biosorption capacity of whole cells (Acosta et al. 2005) (Table 17.1). The adsorption of P. polymyxa cells or their EPSs on the surface of several minerals has been reported as a method to selectively separate metal ions from a binary mixture such as sphalerite and galena, galena and pyrite, suggesting their use in biomineral processing by means of microbial flotation and flocculation (Deo and Natarajan 1998; Patra and Natarajan 2004, 2006).
Analysis of the literature data shows that the process of exoglycan biosynthesis and their monosaccharide composition are highly labile—the yield of EPSs, their composition, and their physical–chemical properties depend on several factors (Matora et al. 1992; Lee et al. 1997; Yegorenkova et al. 2008). Sutherland (1972, 1994) examined the interrelation between the structure of polysaccharides and their physical characteristics and functions. There is need for accumulation of data on the chemical structure of these important macromolecules before any justified inferences about the functions of concrete glycopolymers can be made.
The richness of the microbial world determines the diversity of the structures and physical–chemical and biological properties of EPSs, which dictates the possibility of their wide use. Microbial EPSs can be used as an alternative to the traditionally applied synthetic or natural polymers and can also be considered to be new polymers (Sutherland 1986). PSs have already found application in several fields, including environmental management (soil cleanup from petroleum residues), the petroleum industry (enhancement of the effectiveness of petroleum production), metallurgy (involvement in the extraction, processing, and beneficiation of ore) (Santhiya et al. 2002; Acosta et al. 2005), agriculture (enhancement of crop capacity and soil fertilization), food production (emulsifiers, biofilms, and thickeners) (Matora et al. 1992; Moon et al. 2006), the cosmetic industry (emulsions), and medicine (blood plasma substitutes, drug carriers, and drug components) (Zanchetta et al. 2003). This series will be extended as polysaccharides and their active producers come to be better understood.
Here, I present briefly a selection of data on the EPSs of P. polymyxa, because a more detailed consideration would fall outside the scope of the problem being dealt with.
17.3.2 The Capacity of P. polymyxa for Plant Root Colonization and Root Hair Deformation
Effective colonization of plant roots by associative bacteria and the maintenance of population size at an ecologically significant level play an important role in plant growth promotion, regardless of the mechanism of action (production of metabolites and of antibiotics against phytopathogens, stimulation with nutrients, or induction of plant resistance) (Timmusk et al. 2005).
17.3.2.1 Attachment to Roots
Notwithstanding the fact that biological control has been used for decades, its use has not been consistent, possibly because its nature and action have not been understood fully (Gamalero et al. 2003). The plant root is not a passive target for soil organisms (Timmusk et al. 2005); therefore, it became necessary to accumulate experimental data concerning the mechanisms responsible for the formation of plant–bacterial associations. The methods used for the study of root colonization by growth-promoting bacteria have been covered in sufficient detail in a review by Gamalero et al. (2003). The endophytic colonization of seedling roots by P. polymyxa has been studied with fir (Shishido et al. 1999), pine (Bent et al. 2002), and Arabidopsis (Timmusk et al. 2005). Visualization of P. polymyxa through FITC-labeled antibodies has demonstrated that this bacterium can colonize the surface of roots (Bent et al. 2002) and can penetrate the root interior (Shishido et al. 1999). P. polymyxa was found accumulating in the intercellular spaces outside the vascular cylinder. According to the data of several authors, there was no dispersal at a systemic level, because the bacteria were found to be absent from aerial tissue (Timmusk et al. 2005).
Timmusk et al. (2005), using fluorescence stereomicroscopy, examined the localization of P. polymyxa strains B1 and B2 and the formation of biofilms on plant roots in model experiments and in soil systems. They found that colonization begins at the root tip, where the bacteria form microcolonies composed of cells and a semitransparent matrix. Subsequently (within 2 h), the microcolonies spread over the surface and aggregate, forming biofilms. Root invasion was observed after 5 h of contact, and in a longer period of time, the differentiation zone of the root was colonized. It seems logical that the rhizobacteria predominated at the sites at which the amount of nutrients was greatest and at which nutrient inflow was associated with young plant tissues. Root exudates, secretions, and/or lysates accumulate in such root regions as the tip, the root hairs, and the epithelial cracks at the sites of recent lateral-root formation (Timmusk et al. 2005). The greatest density of the bacteria occurred on the surface of young root tissues, which possibly has to do with the greater intensity of the physiological processes occurring in them (Bent et al. 2002). Timmusk et al. (2005) demonstrated that P. polymyxa colonizes the root regions targeted by phytopathogens, thereby keeping these bacteria from accessing the plant and fulfilling a protective function. The polysaccharides produced by P. polymyxa are highly complex, and only few organisms may possess the specific enzymatic machinery for their degradation, e.g., P. polymyxa itself (Bezzate et al. 1994). For investigating bacterial interactions in natural systems, real-time PCR for the rapid detection of biofilm-forming bacteria was also developed (Timmusk et al. 2009a).
According to the data of many investigators, the complex process of plant–bacterial interaction begins in mucigel, which covers the plant root hairs in large quantities. The interaction of Paenibacillus lectins with the carbohydrate moiety of the wheat-root exocomponent fraction changed the enzyme activity of the lectins, as did the interaction with carbohydrate preparations (Karpunina et al. 2003). The authors believe that in the contact of bacteria with plants, a large role is initially played by the adhesive properties of bacterial lectins, which are realized through lectin interactions with the specific sugars present in mucigel.
The formation of N2-fixing systems calls for a physical and functional interaction between bacterial and plant cells, in which, along with adhesion, a great role is played by enzymatic processes. P. polymyxa has complex specific relationships with its plant host at a molecular–genetic level, altering the expression profile for the host’s genes (Timmusk and Wagner 1999). There have been reports of stimulation of the activities of chitinase and β-1,3-d-glucanase (Haggag 2007; Algam et al. 2010) and of glucose-6-phosphate dehydrogenase, glutathione reductase, and glutathione S-transferase (Cakmakci et al. 2007) in P. polymyxa-inoculated plants. It is known that increased chitinase and β-1,3-d-glucanase activities in plants correlate with resistance to phytopathogens (Timmusk and Wagner 1999). Numerous publications attest that besides plant hydrolytic enzymes, the degradation (hydrolysis) of the plant cell wall involves the work of the hydrolytic enzymes of certain soil bacteria (Ljunggren and Fåhraeus 1961; Hubbell et al. 1978; Tien et al. 1981). From the totality of experimental data obtained, some authors speculate that the penetration of N2-fixing bacteria into plant root tissues is facilitated by Rhizobium agglutinins and Paenibacillus lectins, as well as by the enzymatic activity of rhizobial and bacillar cells (Karpunina et al. 2003).
Cell-associated extracellular rhizobial PSs, including lipopolysaccharides (LPSs), the acidic capsular polysaccharides (CPSs), and EPSs, have also been considered as potential symbiotic factors. York et al. (1996) proposed that EPSs may be involved in cell attachment to plants and in plant infection, ensure protection against plant defense responses, act as signal molecules, and function similarly to flavonoids and lipochitooligosaccharides in the formation of symbiosis. A good example is the oversaturation of the genome of the alfalfa rhizobium Sinorhizobium meliloti with genes of EPS synthesis (Kahn et al. 2004). Owing to this, deletion mutants in the exo clusters can preserve their symbiotic properties, which are very important, as normal synthesis of EPSs is necessary for the development of nodules (Provorov et al. 2008). Many investigators (Michiels et al. 1991; Yegorenkova et al. 2001) believe that the EPSs of associative bacteria of the genus Azospirillum are also involved in the realization of contact between bacterial and plant cells. It has repeatedly been shown that azospirilla on plant roots or root hairs were observed as aggregates surrounded by mucigel or fibril-like material (Bashan et al. 1986; Okon and Kapulnik 1986).
In the root environment, i.e., the rhizosphere, bacterial EPSs contribute to soil aggregation by cementing particles together (Chenu 1995). Inoculation of plants with EPS-producing rhizobacteria, such as Pantoea agglomerans (Amellal et al. 1998), Rhizobium sp. YAS34 (Alami et al. 2000; Santaella et al. 2008), and Rhizobium sp. KYGT207 (Kaci et al. 2005), modifies the aggregation of root-adhering soil and eventually improves plant growth.
As said earlier, P. polymyxa can synthesize various EPSs, which are believed to play a large role in cell adhesion to diverse substrates (Deo et al. 2001; Vijayalakshmi and Raichur 2002; Sharma and Rao 2003) and in the formation of plant–microbe associations (Hebbar et al. 1992; Bezzate et al. 2000; Timmusk et al. 2005).
Gouzou et al. (1993) showed that inoculation of wheat with a rhizosphere strain of P. polymyxa increased the mass of soil adhering to the roots by 57 %. Comparison of aggregate size distributions suggested a more porous structure for the inoculated rhizosphere soil than for the uninoculated soil. Bezzate et al. (2000) tested the role of levan, a fructosyl polymer produced by strain CF43, in the aggregation of soil adhering to wheat roots. Inoculation of wheat roots with P. polymyxa CF43 increased the mass of root-adhering soil. The P. polymyxa gene homologous to the B. subtilis sacB gene encoding levansucrase was cloned and sequenced. The corresponding gene product synthesized a high-molecular-weight levan. In contrast, inoculation with P. polymyxa mutant strain SB03 had no effect on the mass of root-adhering soil, compared with the noninoculated treatment. P. polymyxa SB03 is a mutant whose gene encoding the enzyme for levan synthesis, sacB, was inactivated. Thus, the results strongly suggest that levan synthesis by strain CF43 is the main mechanism involved in the improvement of the structure of root-adhering soil (Bezzate et al. 2000). Soil structure determines the total volume of soil pores and their size distribution, geometry, and connectivity. The resulting properties of the soil and rhizosphere, such as aeration, resistance to root penetration, water reserves, and therefore water and solute movement, are essential parameters that control plant growth. The stability of soil structure is, therefore, one of the basic determinants of the quality of soil and the rhizosphere, if not of ecosystem stability (Santaella et al. 2008). Consequently, inoculation by P. polymyxa can play an important role in water retention and nutrient transfer in the rhizosphere by increasing porosity.
The effect of two EPS-producing strains of P. polymyxa (B5 and B6) on the control of crown rot disease caused by Aspergillus niger on peanut was investigated (Haggag 2007; Haggag and Timmusk 2008). Both strains were inhibitory to A. niger, but strain B5 proved to be more active. Bacterial growth and protein and biopolymer production were evaluated. Carbohydrate analysis using various color reactions, infrared spectroscopy, and high-performance liquid chromatography revealed that the biopolymer is a homopolysaccharide consisting of various sugars, including Glc, Gal, Man, and Xyl. It was found that P. polymyxa B5 produces high levels of sugars compared to the other strain used (Haggag 2007). The ability of P. polymyxa to colonize the peanut rhizosphere was evaluated for 60 days (greenhouse) and 140 days (field experiment). The colonization efficiency of B5 was significantly higher than was that of B6 during the first 30 and 60 days (in the greenhouse and in the field experiment, respectively). In both experiments, the author observed a substantial increase in the number of peanut nodules and in plant growth and performance when seeds were treated with B5, as compared to treatment with B6 or to the control (untreated plants) (Haggag 2007).
Enzyme-linked immunosorbent assay (ELISA) with rabbit polyclonal antibodies developed to an isolated EPS of P. polymyxa 1465 was used to evaluate the colonization of wheat-seedling roots by this bacterium (Yegorenkova et al. 2010). The assay conditions were optimized for detection of the P. polymyxa EPS determinants forming part of the samples used (homogenates of inoculated roots). The dynamics of the immunoenzymatic revealing of specific polysaccharidic antigenic determinants in the samples’ composition correlated with an increase in P. polymyxa numbers on the roots found by estimation of colony-forming units (Fig. 17.1). The dynamics of P. polymyxa attachment was similar to that found for other rhizosphere bacteria: the number of attached cells increased with an extension of the incubation time, and the cell number on the roots stabilized by 18–24 h of contact (Michiels et al. 1991; Zamudio and Bastarrachea 1994; Yegorenkova et al. 2001).
ELISA determination of the number of specific bacterial antigenic determinants in homogenates of wheat roots inoculated with P. polymyxa 1465 (a), as compared with the results of CFU counting (b) (taken from Yegorenkova et al. 2010)
17.3.2.2 Morphological Changes in Root Hairs
The deformation of root hairs is one of the earliest responses of plants to the presence of bacteria in their environment. Some investigators (Gaskins and Hubbell 1979; Baldani et al. 1983) believe that deformation may serve as a quantitative measure of plant responsiveness to inoculation, i.e., it characterizes the activity of a given strain toward the plant. The morphological changes in roots that are induced by soil bacteria have been studied in sufficient detail for the legume–Rhizobium symbiosis (Halverson and Stacey 1986) and the plant–Azospirillum association (Okon and Kapulnik 1986). Several types of root hair deformations have been recorded, including branches of equal lengths, branches of different lengths, and other deformations (curlings, swellings, wavy hairs, etc.). Symmetrical “tuning fork” branches have been observed mostly when homologous strains were used.
The compounds inducing such changes in roots are different in nature, including both high- and low-molecular-weight components. These compounds have been best studied for legume–rhizobial systems. It is known that Rhizobium EPSs can stimulate morphological changes in legume root hairs that are similar to the changes produced by whole bacterial cells (Halverson and Stacey 1986). The effect of low-molecular-weight compounds on the deformation of root hairs has been well documented (Patriquin et al. 1983). For Azospirillum bacteria, the inducers of morphological changes in root hairs have been found to include both low- and high-molecular-weight compounds. Patriquin et al. (1983) reported the induction of deformations by the supernatant liquid of A. brasilense Sp245. Several authors have described the influence on root morphology of the phytohormones produced by azospirilla (Tien et al. 1979) and also of the PS-containing complexes localized in the capsular material and excreted into the environment during bacterial growth (Konnova et al. 1995). It was established that the LPSs of azospirilla also induced deformations and that the changes in the LPS composition of the A. brasilense Sp245 outer membrane as a result of omegon insertion into the 120 MDa plasmid decreased the biological activity of the mutant strains toward wheat-seedling roots (Fedonenko et al. 2001). Boyko et al. (2011) concluded that the activity of the LPSs of serogroup 1 azospirilla toward wheat root hair morphology is determined by the fatty acid ratio and the length of the O chains and that in azospirilla whose O-specific PSs are branched heteropolysaccharides, LPS activity also depends on the character of the side chain substituents.
The molecular mechanism of root hair deformation is fairly complex and almost unknown. Possibly, it includes a chain of reactions, one of which may be the interaction of the root surface receptors with the PS-containing complexes (or their components) on the bacterial surface (Konnova et al. 1995). In the opinion of Patriquin et al. (1983), the deformations may result from altered synthesis in the root hair walls or from stabilization of the cell walls during their growth.
There have been very few publications addressed to the study of the morphology of plant roots inoculated with P. polymyxa. When legume plants were inoculated with the cocultures Rhizobium etli and P. polymyxa, the latter indirectly (through the plant) promoted an increase in the population size of R. etli (Petersen et al. 1996). The authors observed an increase in the length of lateral roots and in the number of nodules in the plants. A similar effect was found upon dual inoculation of legumes with Azospirillum and Rhizobium (Andreyeva et al. 1993). This was explained by Azospirillum stimulation of nodule formation, nodule functioning, and possibly plant metabolism. The phytohormones produced by Azospirillum facilitate epidermal–cellular differentiation in root hairs, increasing the number of potential sites for rhizobial infection (Yahalom et al. 1991).
The ability of EPS preparations from several P. polymyxa strains to induce root hair deformations was studied with seedlings of wheat (cv. Saratovskaya 29). Fåhraeus’s glass-slide technique was used to test the EPSs of several P. polymyxa strains, including 1465, 1460, 1459, 88A, and 92. It was demonstrated that the isolated EPSs can induce deformations with different intensities, which may count in favor of the assumption that the exoglycans of P. polymyxa have an active role in the formation of plant–microbe associations (Yegorenkova et al. 2013).
Among aerobic spore-forming bacteria of the genus Bacillus, a B. subtilis strain, IB-22, was revealed that excels at cytokinin production. In the culture liquid of this strain, a novel form of biologically active cytokinins was found for the first time: a complex formed between hormones and PSs (Arkhipova 1999). It was suggested that slow dissociation of cytokinins from this complex could have ensured the prolonged and nontoxic action on plants.
17.3.3 EPSs in Biofilms
In natural ecosystems and at industrial and healthcare facilities, microorganisms exist not as free-living cells suspended in their environment (plankton) but mainly as an organized community attached to various biotic and abiotic surfaces. Such communities are called biofilms (Davey and O’Toole 2000). A biofilm is characterized by cells that are attached to a surface or to one another, are enclosed in a matrix of extracellular polymeric substances synthesized by them, and demonstrate a phenotypic change manifested as a change in the growth parameters and in the expression of specific genes (Donlan and Costerton 2002). The development of biofilm communities is a major strategy used by bacteria for survival in the external milieu. In biofilms, bacteria are stuck to each other by complex intercellular linkages and are functionally similar to multicellular organisms (Ilyina et al. 2004). The EPSs of the contributing microbial flora provide a major part of the dry matter of biofilms, flocs, and related structures. These polymers also play major roles in determining the physical properties and structures of the microbial agglomerations (Sutherland 2001).
17.3.3.1 Biofilm Formation
A bacterial biofilm is formed as a result of complex coordinated interactions of microorganisms with a surface. There are complete reviews in the literature covering biofilm biology and genetics (Watnick and Kolter 2000; Ilyina et al. 2004; Branda et al. 2005; Costerton 2007; Lloyd et al. 2007; Moons et al. 2009; Plakunov et al. 2010; Smirnova et al. 2010). Several investigators have considered the ecological implications of biofilm formation by associative bacteria (Morris and Monier 2003; Danhorn and Fuqua 2007; Eberl et al. 2007; Haggag 2010). Most generally, the sequence of events is as follows.
Biofilm formation begins with an initial, reversible attachment, when planktonic bacteria make contact with the substrate and become temporarily fixed, with some cells being able to detach. In this process, an important role is played by flagella. This stage involves the work of nonspecific physicochemical forces of interaction between the molecules and structures on the surfaces of the microorganism and the solid substrate (Van der Waals, hydrophobic, electrostatic, and London dispersion forces) (Van Loosdrecht 1988). The next developmental stages are the irreversible attachment to the surface; the formation of microcolonies, with aggregation of already attached cells; the formation of macrocolonies; and, finally, the aging of the macrocolonies with the formation of biofilms. The biofilm development cycle is completed when the bacteria resume their planktonic lifestyle (Ilyina et al. 2004). The start of biofilm formation may be signaled by osmolarity, the pH of the medium, soil content of metals, oxygen supply, temperature, and other factors (Davey and O’Toole 2000; Karatan and Watnick 2009).
There is evidence that in biofilms, cells differentiate according to function—the motile, matrix-producing, sporulating cells are located at a distance away from one another and are present in different parts of a biofilm (Vlamakis et al. 2008). Biofilm formation is a complex process that requires the activity of a multitude of genes responsible for both general functions (such as motility, metabolism, and maintenance of cell structure) and special functions, which ensure biofilm formation. In the process of biofilm aging, a multitude of genes are differentially expressed, the work of which requires a regulatory system that would ensure control of expression (Voloshin et al. 2005). In the past decade, there has been an explosion of studies that have led to the discovery of a wide variety of “communication molecules,” which are secreted to the medium and induce specific changes in bacterial metabolism when a definite critical concentration of producer cells is reached. This principle, named quorum sensing (QS), is effected at the cost of various chemical compounds, including low-molecular-weight substances [secondary metabolic products, peptides, lipids, and secreted proteins (Voloshin and Kaprelyants 2004)]. For broader coverage, readers are referred to Faure et al. (2009), Dickschat (2010), and Thoendel and Horswill (2010).
17.3.3.2 Methods of Biofilm Visualization
In recent years, methods have been developed to prepare biofilms in artificial systems, thereby creating controlled conditions for biofilm study. Biofilms are estimated in microtitration plates by spectrophotometric counting of a specific stain bound by the cells in the biofilm (Ferrieres and Clarke 2003). Biofilm formation is also modeled in flow-through chambers and test tubes and on the surface of cover slips and other objects. For studies of living cells and for observations of cells in motion, a phase-contrast microscope and an interference microscope are employed (Smirnova et al. 2010). Stained preparations are examined by using stains specific for the matrix as the major biofilm component. These include the vital dye Congo red, which binds to cellulose and the curli pili in the process of staining Salmonella biofilm (Römling et al. 1998); fuchsin (Yi et al. 2004); and another cellulose indicator, the fluorescent vital dye calcofluor (Solano et al. 2002). For light-optical observations, investigators use ruthenium red (RR) and alcian blue (AB), which interact with acidic mucopolysaccharides (Smirnova et al. 2009).
Biofilms on nontransparent materials are visualized by epifluorescence microscopy. In the Luft method of EPS visualization by transmission electron microscopy (TEM) of biofilms, RR interacts with osmium tetroxide, which forms part of the fixative (Luft 1971). The external PSs of a range of bacteria have been demonstrated with RR and AB (Karlyshev et al. 2001; Hunter and Beveridge 2005). Beveridge (2006) suggested the use of cryoTEM to explore the native hydrated structures of the biofilm.
With accumulation of data on the occurrence and role of biofilms in natural processes, industry, and medicine, the need arose to look for new research methods. With the help of confocal laser scanning microscopy (CLSM), it became possible to directly observe native films. More specifically, the use of CLSM and luminescent dyes allows one to distinguish, within a biofilm, the bacteria selectively stained with propidium iodide and matrix PSs that bind to the FITC-conjugated lectin ConA (Kania et al. 2007). For analysis of biofilm composition, investigators employ fluorochrome-conjugated lectins of different specificities. For example, visualization of P. polymyxa through FITC-labeled antibodies has demonstrated that this bacterium can colonize the surface of roots (Bent et al. 2002) and can penetrate the root interior (Shishido et al. 1999). Timmusk et al. (2005), using fluorescence stereomicroscopy, examined the localization of P. polymyxa and the formation of biofilms on plant roots in a model and a soil system.
For cell visualization, a labeling method is currently used in which a DNA sequence is inserted into the bacterial chromosome through the agency of a plasmid vector. The DNA sequence codes for a fluorescent label, e.g., green fluorescence protein (GFP). It is possible to conduct a direct real-time observation in vivo of GFR expression in individual cells of cell populations (Zogaj et al. 2001; Santaella et al. 2008).
Recently, atomic force microscopy (AFM) has been applied to studying the components of the metabolites of biofilm-forming bacteria (Hinterdorfer and Dufrêne 2006). By AFM, Jonas et al. (2007) examined the colonies and biofilms of Salmonella strains that synthesize cellulose and the surface protein BapA and that form curli pili. For example, AFM imaging and force measurement studies have been performed on surface PSs of Lactobacillus sp. Lecithin-modified tips were used to examine individual PS molecules on the surface of biofilms (Francius et al. 2008). For understanding their function in biofilms, PSs were characterized by single-molecule force spectroscopy (Sletmoen et al. 2003). Glucans of Streptococcus mutans biofilms were characterized, and their possible role in biofilm formation was explored (Cross et al. 2007). The study was conducted with various mutants with an impaired ability to synthesize glucans. The technique also provides the possibility for microbial surface molecular recognition by using specific binding such as antibody–antigen interaction.
17.3.3.3 Extracellular Matrix
Usually, cells in biofilms are embedded in an extrapolymeric matrix that ensures biofilm stability and safety from external stresses (Costerton et al. 1995; Smirnova et al. 2010). The matrix is formed from a mixture of components, including EPSs, proteins, nucleic acids (Voloshin et al. 2005), glycosyl phosphate-containing biopolymers (e.g., teichoic acids), glycoproteins, and (in certain bacteria, e.g., bacilli) polyglutamic acid and other biopolymers (Branda et al. 2006; Safronova and Botvinko 1998). A key structural component of biofilms, which has received close attention in the past decade, is the extracellular polymeric substance called the exopolysaccharide matrix (Sutherland 2001; Verhoef et al. 2005; Smirnova et al. 2010). In different bacterial species, this matrix differs in physical properties and chemical composition; as a rule, however, it is an anionic polymer. The EPS of the matrix consists mostly of homo- and heteropolysaccharides. The EPS is composed of uronic (mainly glucuronic) acids and amino sugars. By now, the EPS composition of several bacteria has been identified (Cunha et al. 2004; Da Re and Ghigo 2006; Hentzer et al. 2001; Ledeboer and Jones 2005).
For example, it has been shown that Pseudomonas aeruginosa forms alginate, a copolymer of mannuronic and glucuronic acids (Hentzer et al. 2001). It is an unbranched PS, a property distinguishing it from polymers such as xanthan and dextran. However, several recent reports have shown that other PSs contribute to biofilms formed by nonmucoid P. aeruginosa strains, which are believed to be the first to colonize cystic fibrosis patients. A recent example is the expression of the psl operon, which is required in order to maintain the biofilm structure after attachment. Overproduction of the Psl PS led to enhanced cell surface and intercellular adhesion of P. aeruginosa, which translated into significant changes in the architecture of the biofilm (Ma et al. 2006).
Branda et al. (2006) discussed the role of PS, proteins, and the extracellular polymer polyglutamic acid as components of the B. subtilis matrix. Using microscopical methods, they showed that mutants in the eps and tasA genes form a weak unstructured film and that double mutants in these genes do not form a film at all. This attests to the need for the presence of both PS and protein in the matrix.
More specifically, Smirnova et al. (2009) found by cytochemical studies that the matrix of biofilms developed by Salmonella typhimurium includes acidic mucopolysaccharides, revealed by alcian blue staining, and cellulose, stained with Congo red. Sheludko et al. (2008) compared the thickness and antigenic properties of biofilms produced by A. brasilense Sp245 and its mutants deficient in the synthesis of LPSs and calcofluor-binding PSs (CBPSs) at the interface between water and hydrophilic or hydrophobic solid surfaces. They found that the mutants deficient in acidic LpsI synthesis produce thicker biofilms on hydrophilic surfaces. Biofilms produced on hydrophobic surfaces by bacteria that are unable to synthesize CBPSs are less pronounced. Defects in CBPS production in Azospirillum mutants with impaired flagellar motility can cause adverse effects on the cell ability to attach to hydrophobic and hydrophilic surfaces. The loss of the neutral LpsII antigen by the mutants capable of producing CBPSs does not affect their behavior on hydrophobic surfaces, which is probably due to the compensatory increase in the total PS production. The fundamental change in the Lps structure correlates with the activation of biofilm formation by the relevant mutants on hydrophilic and hydrophobic surfaces. The effectiveness of biofilm formation by A. brasilense Sp7 and its variants was analyzed by Petrova et al. (2010). Those authors reported that spontaneous changes in plasmid composition had a negative effect on biofilm formation by A. brasilense on hydrophobic and (more rarely) hydrophilic abiotic surfaces. The derivatives of Sp7 that had lost p115 and harbored an altered pRhico were less active in colonizing plant roots during the first hours of interaction.
Yegorenkova et al. (2010) evaluated the ability of several strains of the rhizobacterium P. polymyxa, differing in the yield and rheological properties of their EPSs, to form biofilms on abiotic surfaces. Of these strains, P. polymyxa 1465, giving the highest yield of EPSs and the highest kinematic viscosity of the culture liquid and of aqueous PS solutions, proved to be the most active in forming biofilms on hydrophobic and hydrophilic surfaces (Fig. 17.2). Enzyme-linked immunosorbent assay (ELISA) with rabbit polyclonal antibodies developed to isolated EPSs of P. polymyxa 1465 and 92 was used to detect P. polymyxa’s polysaccharidic determinants in the composition of the biofilm materials. According to the data of Timmusk et al. (2005), the EPSs of P. polymyxa participate in biofilm formation on the roots of Arabidopsis thaliana.
Evaluation of the ability of P. polymyxa 92, 1460, and 1465 to form biofilms on hydrophilic and hydrophobic surfaces by using crystal violet staining. А 570 is the absorbance of samples in polystyrene plates, and А 590 is the absorbance of samples in glass test tubes (taken from Yegorenkova et al. 2011)
Santaella et al. (2008) focused on the function of an EPS produced by Rhizobium sp. YAS34 in the colonization and biofilm formation on nonlegume plant roots (Arabidopsis thaliana and Brassica napus). Using random transposon mutagenesis, they isolated an EPS-deficient mutant of strain YAS34 impaired in a glycosyltransferase gene (gta). The wild-type and mutant strains were tagged with a plasmid-borne GFP, and for the first time, the EPS produced by the wild-type strain was seen in the rhizosphere by using selective carbohydrate probing with a fluorescent lectin and confocal laser scanning microscopy. The authors observed for the first time that Rhizobium forms biofilms on the roots of nonlegumes, independently of the EPS synthesis. When produced by wild-type strain YAS34, EPS is targeted at specific parts of the plant root system. Nutrient fluctuations, root exudates, and the bacterial growth phase can account for such a production pattern. The EPS synthesis in Rhizobium sp. YAS34 is not essential for biofilm formation on roots, but it is critical to colonization of the basal part of the root system and to increasing of the stability of root-adhering soil. The authors believe that in the interactions of Rhizobium sp. YAS34 with the nonlegume plants, microbial EPS is implicated in the root–soil interface, root colonization, but not in biofilm formation.
Growth and EPS production may be more prolific under attached conditions for some bacteria (Hughes 1997), and attachment to solid surfaces may stimulate PS synthesis, as suggested by Vandevivere and Kirchman (1993). Also, Allison and Sutherland (1987) demonstrated that two strains of freshwater bacteria synthesized significant amounts of EPS only after attachment, indicating that the polymers were not needed for initial adhesion to inert surfaces. These results may again all stem from stress responses. It is possible that even relatively small quantities of preformed EPS from the planktonic cells assisted in adhesion either to the solid surface or to the conditioning film on it. A recent report on colanic acid synthesis in K12 confirmed these results and also indicated that the PS is required for the formation of the biofilm structure rather than for initial attachment (Danese et al. 2000).
When biofilms or flocs are established, the PS components of microbial origin may exhibit phenotypic differences from planktonic bacteria of the same species. However, it is more likely that the microorganisms secrete EPSs identical in composition and probably also in physical properties with those formed by the same bacteria when grown in planktonic culture. Another possibility is that the polymers formed may be of identical composition to those formed by the free-living bacteria, but, owing to minor structural differences such as the degree of acylation or the molecular mass, they differ in their physical properties. These differences may result in altered viscosity or gel-forming capacity (Sutherland 2001). Costerton et al. (1981) used antibodies against polymers synthesized planktonically to reveal interaction with material in a biofilm matrix. This indicated that at least some of the biofilm EPSs had the same or very similar composition as the planktonic products. Further confirmation of the close similarity or identity of biofilm and planktonic PSs was obtained with highly specific, phage-induced polysaccharases (Hughes et al. 1998). On the other hand, if only very small amounts of one polymer are produced and are impossible to separate from large quantities of a second PS, this might explain the apparent complexity of composition reported for some materials obtained from biofilm isolates (Sutherland 2001).
Possibly, EPSs play various roles (depending on the environmental conditions) in the structure and functions of biofilm communities (Ilyina et al. 2004). Production of EPSs is generally important in biofilm formation, and likewise, it can affect the interaction of microbes with roots and root appendages (Bianciotto et al. 2001). EPSs protect the biofilm against a range of unfavorable environmental effects (UV radiation, changes in the pH of the medium, osmotic shock, and drying), adsorb xenobiotics, promote the mechanisms of nutrient accumulation, and ensure tolerance for antimicrobial agents by limiting the penetration of these agents from the surrounding milieu (Smirnova et al. 2010). For example, water retention varies with the type of PSs, but EPS water retention capacity may exceed 70 g of water per g of PS (Zhang et al. 1998; Vu et al. 2009). Further detail on the EPSs of biofilms and on their structure and functions is available in a review by Sutherland (2001).
The role of the matrix in the formation of polymicrobial biofilms was established (Smirnova et al. 2010). In mixed bacterial populations, the formation of coaggregates is commonly observed, which occurs owing to the cells being stuck together through EPS. On the one hand, coaggregation is conducive to the formation of mixed biofilms, bringing together various microorganisms on the basis of synergism, and on the other hand, it may “cleanse” the surroundings of pathogenic bacteria during their interaction with antagonistic bacteria (Smirnova et al. 2010).
17.3.3.4 Role of Biofilm in the Biocontrol of Plant Diseases
The use of microorganisms for plant disease control is an attractive alternative to the use of synthetic chemical substances. There is a vast literature describing various mechanisms of the biocontrol ability of bacteria, e.g., siderophore production, secretion of hydrolytic enzymes, antibiosis, ISR, and certain others (Timmusk et al. 1999, 2005; Weller et al. 2002; Timmusk 2003; Perneel et al. 2007; Rezzonico et al. 2007; Tran et al. 2007). The biocontrol ability of bacilli has been adequately covered by Kumar et al. (2011). In this section, I will only touch on the possible role of biofilm in the biocontrol of plant diseases.
Bacterial biofilms formed on the roots of plants can protect colonization sites and act as scavengers of nutrients in the rhizosphere, thereby decreasing the availability of root exudate nutrients for stimulation of plant pathogens and subsequent root colonization by them. The mechanism initially reported by Thomashow’s group (Weller and Thomashow 1994) has gained less attention, most likely because of difficulties in studying natural systems. However, biofilms can have the potential to be successful in fighting similar root-colonizing pathogens under natural conditions (Timmusk et al. 2009b).
One beneficial rhizobacterium is Bacillus subtilis, which is ubiquitous in soil. It can promote plant growth, protect plants against fungal pathogen attack, and play a role in the degradation of organic polymers in soil (Emmert and Handelsman 1999). Recently, it has been reported that B. subtilis forms adhering biofilms on inert surfaces under the control of a variety of transcription factors (Stanley et al. 2003). Bais et al. (2004), using an infection model, demonstrated the biocontrol ability of a wild-type B. subtilis strain, 6051, against P. syringae. Arabidopsis root surfaces treated with B. subtilis were analyzed by CLSM to reveal a three-dimensional B. subtilis biofilm.
Owing to its broad host range and its ability to form endospores and synthesize various types of antibiotics, P. polymyxa has the potential of being a commercially useful biocontrol agent. P. polymyxa was found to be successful in controlling Botrytis cinerea, the causal agent of gray mold, in strawberries (Helbig 2001); Fusarium oxysporum and Pythium spp., the causal agents of seedling blight, wilt, and root rot of cucumber and watermelon (Dijksterhuis et al. 1999; Yang et al. 2004); sesame damping-off (Ryu et al. 2006); and diseases of Arabidopsis caused by Phytophthora palmivora and Pythium aphanidermatum (Timmusk and Wagner 1999). This can occur by direct antagonism, when protective bacteria and attacking organisms are in close proximity, in which case disease suppression is expected to be restricted to soilborne pathogens. On the other hand, PGPR may stimulate systemic defenses, inducing sustained changes in the plant, which increase its tolerance to further infection by foliar or root pathogens (Timmusk 2003).
So far, most research on the biocontrol activity of P. polymyxa has centered on the elaboration of antibiotics by this bacterium. Haggag and Timmusk (2008) investigated the role of biofilm-forming P. polymyxa strains in controlling crown root rot disease (A. niger) and highlighted the importance of efficient rhizosphere colonization and biofilm formation in biocontrol. Two plant-growth-promoting P. polymyxa strains were isolated from the peanut rhizosphere (from A. niger-suppressive soils). The strains were tested under greenhouse and field conditions for inhibition of the crown root rot pathogen of the peanut, as well as for biofilm formation in the peanut rhizosphere. The strains’ colonization and biofilm formation were further studied on roots of the model plant A. thaliana and with solid surface assays. Their crown root rot inhibition performance was studied in field and pot experiments. The strains’ ability to form biofilms in gnotobiotic and soil systems was studied by SEM. It was noted that both strains produced similar amounts of antagonistic substances and were able to suppress the pathogen but that the superior biofilm former offered significantly better protection against crown rot.
Oomycetic pathogens are responsible for one of the most destructive groups of diseases. They are present in almost all cultivated soils and attack the root system, particularly in warm and humid environments. Despite the decades of biological control research, no commercially successful methods for combating diseases caused by Pythium and Phytophthora have yet appeared. Timmusk (2003) observed a significant yet inconsistent reduction in Pythium root rot under natural conditions, when the plants were preinoculated by P. polymyxa biocontrol strains. Subsequently, Timmusk et al. (2009b) presented experiments with an A. thaliana model system, in which they studied the antagonistic properties of P. polymyxa strains toward the oomycete plant pathogens P. palmivora and P. aphanidermatum. The experiments were conducted on agar plates, in liquid media, and in soil. It was shown that P. polymyxa strains significantly reduced P. aphanidermatum and P. palmivora colonization in liquid assays. Most plants that had been treated with P. polymyxa survived the P. aphanidermatum inoculations in soil assays. In the authors’ opinion, the antagonistic abilities of both systems correlated well with mycoidal substance production and not with the production of antagonistic substances from the biocontrol bacteria. Possibly, the P. polymyxa biofilm formed on the roots coincides with the colonization sites of several pathogens and thereby functions as a protective layer to prevent access by the pathogens (Timmusk et al. 2005). The protective layer might also contribute to plant-enhanced drought tolerance (Timmusk and Wagner 1999).
Thus, given the information available to date, one can confidently speak of the substantial role of the biofilm in plant defense against pathogens.
Once the pathways to biofilm development are more fully understood, the management of P. polymyxa biofilm formation in resident populations of cropping systems could become possible (Battin et al. 2007). This will be a step to ensuring their reproducible performance in natural environments.
17.4 Concluding Remarks
In this chapter, I have analyzed what is currently known about the EPSs of P. polymyxa rhizobacteria, with an emphasis on their role in plant–bacterial interactions. In the past few years, great strides have been made toward elucidating the physiological role of microbial EPSs, and the ecological significance of these polymers has been proven. The involvement of exoglycans in the formation of symbiotic communities and their large role in preserving population viability under extreme conditions are beyond question. PSs constitute an extremely important category of biopolymers with a wide range of biological functions, first and foremost receptor functions, which ensure the interaction of cells with one another and with members of other species. However, for a fuller understanding of the physiological significance of these polymers, it is necessary to elucidate the possibility of a change in their functions that is adequate to external influence.
One of the keys to studying complex biological systems is the development of accurate and realistic models for natural communities in laboratory settings and the application of state-of-the-art research methods that adequately reflect the processes occurring under natural conditions. In the rhizosphere, P. polymyxa bacteria operate together with plant roots as communities with increased levels of complexity and plasticity, allowing this system to adapt to the environmental conditions. Within the framework of the concept of symbiogenetics, being developed currently (Tikhonovich and Provorov 2003), symbioses are regarded as biological complexes arising from the functioning (and sometimes the structural integration) of the gene systems of unallied organisms. Owing to the work of the newly formed “supraorganismal” genome, the partners implement new programs of development and adaptation, which are unavailable to free-living organisms (Provorov et al. 2008).
The negative effects of some P. polymyxa strains (e.g., root invasion) appear to be mostly strain specific and negligible, as compared with beneficial effects (e.g., growth promotion and pathogen control). Interaction of a bacterial strain with a host plant as a PGPR or a DRB is strongly dependent on the prevalent rhizosphere environment conditions. In any case, the role of P. polymyxa in the rhizosphere microbial community requires further studies, also because there is every reason to believe that gaining a greater understanding of these processes will facilitate in the long run the efforts to wean off the dependence on agricultural chemicals (Raza et al. 2008).
References
Acosta MP, Valdman E, Leite SGF, Battaglini F, Ruzal SM (2005) Biosorption of copper by Paenibacillus polymyxa cells and their exopolysaccharide. World J Microbiol Biotechnol 21:1157–1163
Alami Y, Achouak W, Marol C, Heulin T (2000) Rhizosphere soil aggregation and plant growth promotion of sunflowers by an exopolysaccharide-producing Rhizobium sp. strain isolated from sunflower roots. Appl Environ Microbiol 66:3393–3398
Algam SAE, Xie G, Li B, Yu S, Su T, Larsen J (2010) Effects of Paenibacillus strains and chitosan on plant growth promotion and control of Ralstonia wilt in tomato. J Plant Pathol 92:593–600
Allison DG, Sutherland IW (1987) The role of exopolysaccharides in adhesion of freshwater bacteria. J Gen Microbiol 133:1319–1327
Alvarez VM, von der Weid I, Seldin L, Santos ALS (2006) Influence of growth conditions on the production of extracellular proteolytic enzymes in Paenibacillus peoriae NRRL BD-62 and Paenibacillus polymyxa SCE2. Lett Appl Microbiol 43:625–630
Amellal N, Burtin G, Bartoli F, Heulin T (1998) Colonization of wheat roots by an exopolysaccharide-producing Pantoea agglomerans strain and its effect on rhizosphere soil aggregation. Appl Environ Microbiol 64:3740–3747
Andreyeva IN, Red’kina TV, Izmailov SF (1993) Role of indole-3-acetic acid in the stimulatory effect of Azospirillum brasilense on the legume–Rhizobium symbiosis. Fiziol Rast (Moscow) 40:902–907 (in Russian)
Arkhipova TN (1999) Study of the cytokinins produced by rhizosphere microorganisms. Extended abstract of Cand. Sci. dissertation, Ufa (in Russian)
Ash C, Farrow JAE, Wallbanks S, Collins MD (1991) Phylogenetic heterogeneity of the genus Bacillus revealed by comparative analysis of small subunit – ribosomal RNA sequences. Lett Appl Microbiol 13:202–206
Ash C, Priest FG, Collins MD (1993) Molecular identification of rRNA group 3 bacilli (Ash, Farrow, Wallbanks and Collins) using a PCR probe test. Proposal for the creation of a new genus Paenibacillus. Antonie Van Leeuwenhoek 64:253–260
Bais HP, Fall R, Vivanco JM (2004) Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol 134:307–319
Baldani VLD, Baldani JI, Dobereiner J (1983) Effects of Azospirillum inoculation on root infection and nitrogen incorporation in wheat. Can J Microbiol 29:924–929
Ball DH, Adams GA (1959) A mannan produced by Bacillus polymyxa. Can J Chem 37:1012–1017
Bashan Y, Holguin G (1997) Azospirillum-plant relationships: environmental and physiological advances (1990–1996). Can J Microbiol 43:103–121
Bashan Y, Levanony H, Klein E (1986) Evidence for a weak active external adsorption of Azospirillum brasilense Cd to wheat roots. J Gen Microbiol 132:3069–3073
Battin TJ, Sloan WT, Kjelleberg S, Daims H, Head IM, Curtis TP, Eberl L (2007) Microbial landscapes: new paths to biofilm research. Nat Rev Microbiol 5:76–81
Benedict RG, Langlykke AF (1947) Antibiotic activity of Bacillus polymyxa. J Bacteriol 54:24–25
Bent E, Breuil C, Enebak S, Chanway CP (2002) Surface colonization of lodgepole pine (Pinus contorta var. latifolia) roots by Pseudomonas fluorescens and Paenibacillus polymyxa under gnotobiotic conditions. Plant Soil 241:187–196
Beveridge TJ (2006) Visualizing bacterial cell walls and biofilms. Microbe 1:279–284
Bezzate S, Steinmetz M, Aymerich S (1994) Cloning, sequencing, and disruption of a levanase gene of Bacillus polymyxa CF43. J Bacteriol 176:2177–2183
Bezzate S, Aymerich S, Chambert R, Czarnes S, Berge O, Heulin T (2000) Disruption of the Paenibacillus polymyxa levansucrase gene impairs its ability to aggregate soil in the wheat rhizosphere. Environ Microbiol 2:333–342
Bianciotto V, Andreotti S, Balestrini R, Bonfante P, Perotto S (2001) Mucoid mutants of the biocontrol strain Pseudomonas fluorescens CHA0 show increased ability in biofilm formation on mycorrhizal and nonmycorrhizal carrot roots. Mol Plant Microbe Interact 14:255–260
Boyko AS, Konnova SA, Fedonenko YP, Zdorovenko EL, Smol’kina ON, Kachala VV, Ignatov VV (2011) Structural and functional peculiarities of the lipopolysaccharide of Azospirillum brasilense SR55 isolated from the roots of Triticum durum. Microbiol Res 166:585–593
Branda SS, Vik S, Friedman L, Kolter R (2005) Biofilms: the matrix revisited. Trends Microbiol 13:20–26
Branda SS, Chu F, Kearns DB, Losick R, Kolter R (2006) A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol 59:1229–1238
Budi SW, van Tuinen D, Arnould C, Dumas-Gaudot E, Gianinazzi-Pearson V, Gianinazzi S (2000) Hydrolytic enzyme activity of Paenibacillus sp. strain B2 and effects of the antagonistic bacterium on cell integrity of two soil-borne pathogenic fungi. Appl Soil Ecol 15:191–199
Cakmakci R, Erat M, Erdogan U, Donmez MF (2007) The influence of plant growth–promoting rhizobacteria on growth and enzyme activities in wheat and spinach plants. J Plant Nutr Soil Sci 170:288–295
Chang ZQ, Lee JS, Hwang MH, Hong JH, Jung HK, Lee SP, Park SC (2009) A novel β-glucan produced by Paenibacillus polymyxa JB115 induces nitric oxide production in RAW264.7 macrophages. J Vet Sci 10:165–167
Chang ZQ, Lee JS, Gebru E, Hong JH, Jung HK, Jo WS, Park SC (2010) Mechanism of macrophage activation induced by β-glucan produced from Paenibacillus polymyxa JB115. Biochem Biophys Res Commun 391:1358–1362
Chanway CP (1995) Differential response of western hemlock from low and high elevations to inoculation with plant growth-promoting Bacillus polymyxa. Soil Biol Biochem 27:767–775
Chanway CP, Holl FB (1991) Biomass increase and associative nitrogen fixation of mycorrhizal Pinus contorta Dougl. seedlings inoculated with a plant growth promoting Bacillus strain. Can J Bot 69:507–511
Chanway CP, Holl FB (1994) Growth of outplanted lodgepole pine seedlings, one year after inoculation with plant growth promoting rhizobacteria. For Sci 40:238–246
Chanway CP, Holl FB, Turkington R (1988) Genotypic coadaptation in plant growth promotion of forage species by Bacillus polymyxa. Plant Soil 106:281–284
Chenu C (1995) Extracellular polysaccharides: an interface between microorganisms and soil constituents. In: Huang PM, Berthelin J, Bollag JM, McGill WB, Page AL (eds) Environmental impact of soil components interactions. CRC Lewis, Boca Raton, NY, pp 217–233
Cheong H, Park S-Y, Ryu C-M, Kim JF, Park S-H, Chang SP (2005) Diversity of root-associated Paenibacillus spp in winter crops from the southern part of Korea. J Microbiol Biotechnol 15:1286–1298
Chockalingam E, Subramanian S, Natarajan KA (2003) Studies on biodegradation of organic flotation collectors using Bacillus polymyxa. Hydrometallurgy 71:249–256
Choi SK, Park SY, Kim R, Lee CH, Kim JF, Park SH (2008) Identification and functional analysis of the fusaricidin biosynthetic gene of Paenibacillus polymyxa E681. Biochem Biophys Res Commun 365:89–95
Chu KH, Kim EY (2006) Predictive modelling of competitive biosorption equilibrium data. Biotechnol Bioprocess Eng 11:67–71
Comas-Riu J, Vives-Rego J (2002) Cytometric monitoring of growth, sporogenesis and spore cell sorting in Paenibacillus polymyxa (formerly Bacillus polymyxa). J Appl Microbiol 92:475–481
Costacurta A, Vanderleyden J (1995) Synthesis of phytohormones by plant-associated bacteria. Crit Rev Microbiol 21:1–18
Costerton JW (2007) The biofilm primer. Springer, Berlin
Costerton JW, Irvin RT, Cheng KJ (1981) The bacterial glycocalyx in nature and disease. Annu Rev Microbiol 35:399–424
Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM (1995) Microbial biofilms. Annu Rev Microbiol 49:711–745
Cross SE, Kreth J, Zhu L, Sullivan R, Shi W, Qi F, Gimzewski JK (2007) Nanomechanical properties of glucans and associated cell-surface adhesion of Streptococcus mutans probed by atomic force microscopy under in situ conditions. Microbiology 153:3124–3132
Cunha MV, Sousa SA, Leitão JH, Moreira LM, Videira PA, Sá-Correia I (2004) Studies on the involvement of the exopolysaccharide produced by cystic fibrosis-associated isolates of the Burkholderia cepacia complex in biofilm formation and in persistence of respiratory infections. J Clin Microbiol 42:3052–3058
Czames S, Hallett PD, Bengough AG, Young IM (2000) Root- and microbial-derived mucilages affect soil structure and water transport. Eur J Soil Sci 51:435–443
Da Mota F, Nobrega A, Marriel IE, Paiva E, Seldin L (2002) Genetic diversity of Paenibacillus polymyxa populations isolated from rhizosphere of four cultivars of maize (Zea mays) planted in Cerrado soil. Appl Soil Ecol 20:119–132
Da Re S, Ghigo J-M (2006) A CsgD-independent pathway for cellulose production and biofilm formation in Escherichia coli. J Bacteriol 188:3073–3087
Daane LL, Harjono I, Zylstra GJ, Haggblom MM (2001) Isolation and characterization of polycyclic aromatic hydrocarbon-degrading bacteria associated with the rhizosphere of salt marsh plants. Appl Environ Microbiol 67:2683–2691
Danese PN, Pratt LA, Kolter R (2000) Exopolysaccharide production is required for development of Escherichia coli K12 biofilm architecture. J Bacteriol 182:3593–3596
Danhorn T, Fuqua C (2007) Biofilm formation by plant-associated bacteria. Annu Rev Microbiol 61:401–422
Davey ME, O’Toole GA (2000) Microbial biofilm: from ecology to molecular genetics. Microbiol Mol Biol Rev 64:847–867
De Freitas JR (2000) Yield and assimilation of winter wheat (Triticum aestivum L., var. Norstar) inoculated with rhizobacteria. Pedobiologia 44:97–104
Deo N, Natarajan KA (1998) Studies on interaction of Paenibacillus polymyxa with iron ore minerals in relation to beneficiation. Int J Miner Process 55:41–60
Deo N, Natarajan KA, Somasundaran P (2001) Mechanisms of adhesion of Paenibacillus polymyxa onto hematite, corundum and quartz. Int J Miner Process 62:27–39
Dickschat JS (2010) Quorum sensing and bacterial biofilms. Nat Prod Rep 27:343–369
Dijksterhuis J, Sanders M, Gorris LGM, Smid EJ (1999) Antibiosis plays a role in the context of direct interaction during antagonism of Paenibacillus polymyxa towards Fusarium oxysporum. J Appl Microbiol 86:13–21
Döbereiner J (1977) Physiological aspects of N2 fixation in grass-bacteria association. In: Newton W, Postgate JR, Rodriguez-Barrueco C (eds) Recent developments in nitrogen fixation. Academic, London, pp 518–538
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193
Eberl L, von Bodman SB, Fuqua C (2007) Biofilms on plant surfaces. In: Kjelleberg S, Givskov M (eds) The Biofilm mode of life: mechanisms and adaptations, vol 12. Horizon Bioscience, Norfolk, pp 215–234
Emmert EAB, Handelsman J (1999) Biocontrol of plant disease: a Gram-positive perspective. FEMS Microbiol Lett 171:1–9
Euzéby JP (2011) List of prokaryotic names with standing in nomenclature – genus Paenibacillus. http://www.bacterio.cict.fr/p/paenibacillus. Accessed 20 Dec 2011
Faure D, Vereecke D, Leveau JH (2009) Molecular communication in the rhizosphere. Plant Soil 321:279–303
Fedonenko YP, Yegorenkova IV, Konnova SA, Ignatov VV (2001) Involvement of the lipopolysaccharides of Azospirilla in the interaction with wheat seedling roots. Microbiology (Moscow, Russ Fed) 70:329–334
Ferrieres L, Clarke DJ (2003) The RcsC sensor kinase is required for normal biofilm formation in Escherichia coli K12 and controls the expression of a regulon in response to growth on a solid surface. Mol Microbiol 50:1665–1682
Francius G, Lebeer S, Alsteens D, Wildling L, Gruber HJ, Hols P, De Keersmaecker S, Vanderleyden J, Dufrene YF (2008) Detection, localization, and conformational analysis of single polysaccharide molecules on live bacteria. ACS Nano 2:1921–1929
Fukui H, Tanaka M, Misaki A (1985) Structure of a physiologically active polysaccharide produced by Bacillus polymyxa S-4. Agric Biol Chem 49:2343–2349
Gamalero E, Lingua G, Berta G, Lemanceau P (2003) Methods for studying root colonization by introduced beneficial bacteria. Agronomie 23:407–418
Gaskins MH, Hubbell DH (1979) Response of non-leguminous plants to root inoculation with free-living diazotrophic bacteria. In: Harley JL, Russel RS (eds) The soil–root interface. Academic, New York, pp 175–182
Gaur AC (1990) Phosphate solubilizing microorganisms as biofertilizers. Omega Scientific, New Delhi, p 176
Girardin H, Albagnas C, Dargaignaratz C, Nguyen-The C, Carlin F (2002) Antimicrobial activity of foodborne Paenibacillus and Bacillus sp against Clostridium botulinum. J Food Prot 65:806–813
Glukhova EV, Yarotsky SV, Deryabin VV, Shenderov BA, Ignatov VV (1986) Extracellular polysaccharides of Bacillus polymyxa and its mutant strain. Antibiot Med Biotek 31:669–674 (in Russian)
Gong X-Y, Luan Z-K, Pei Y-S, Wang S-G (2003) Culture conditions for flocculant production by Paenibacillus polymyxa BY-28. J Environ Sci Health A 38:657–669
Gorska E, Tudek B, Russel S (2001) Degradation of cellulose by nitrogen-fixing strain of Bacillus polymyxa. Acta Microbiol Pol 50:129–137
Gouzou L, Burtin G, Philippy R, Bartoli F, Heulin T (1993) Effect of inoculation with Bacillus polymyxa on soil aggregation in the wheat rhizosphere: preliminary examination. Geoderma 56:479–491
Guemouri-Athmani S, Berge O, Bourrain M, Mavingui P, Thiery JM, Bhatnagar T, Heulin T (2000) Diversity of Paenibacillus polymyxa populations in the rhizosphere of wheat (Triticum durum) in Algerian soils. Eur J Soil Biol 36:149–159
Gupta A, Gopal M, Tilak KV (2000) Mechanism of plant growth promotion by rhizobacteria. Indian J Exp Biol 38:856–862
Haggag WM (2007) Colonization of exopolysaccharide-producing Paenibacillus polymyxa on peanut roots for enhancing resistance against crown rot disease. Afr J Biotechnol 6:1568–1577
Haggag WM (2010) The role of biofilm exopolysaccharides on biocontrol of plant disease. In: Elnashar M (ed) Biopolymers, vol 14. Sciyo, Rijeka, pp 271–284
Haggag WM, Timmusk S (2008) Colonization of peanut roots by biofilm forming Paenibacillus polymyxa initiates biocontrol against crown rot disease. J Appl Microbiol 104:961–969
Halverson LJ, Stacey G (1986) Signal exchange in plant-microbe interactions. Microbiol Rev 50:193–225
Han YW (1989) Levan production by Bacillus polymyxa. J Indian Microbiol 4:447–452
Han YW (1990) Microbial levan. Adv Appl Microbiol 35:171–194
He Z, Kisla D, Zhang L, Yuan C, Green-Church KB, Yousef AE (2007) Isolation and identification of a Paenibacillus polymyxa strain that coproduces a novel lantibiotic and polymyxin. Appl Environ Microbiol 73:168–178
Hebbar KP, Gueniot B, Heyraud A, Colin-Morel P, Heulin T, Balandreau J, Rinaudo M (1992) Characterization of exopolysaccharides produced by rhizobacteria. Appl Microbiol Biotechnol 38:248–253
Helbig J (2001) Biological control of Botrytis cinerea Pers. ex Fr. in strawberry by Paenibacillus polymyxa (Isolate 18191). J Phytopathol 149:265–273
Hentzer M, Teitzel GM, Balzer GJ, Heydorn A, Molin S, Givskov M, Parsek MR (2001) Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J Bacteriol 183:5395–5401
Hinterdorfer P, Dufrêne YF (2006) Detection and localization of single molecular recognition events using atomic force microscopy. Nat Methods 3:347–355
Holl FB, Chanway CP (1992) Rhizosphere colonization and seedling growth promotion of lodgepole pine by Bacillus polymyxa. Can J Microbiol 38:303–308
Holl FB, Chanway CP, Turkington R, Radley RA (1988) Response of crested wheatgrass (Agropyron cristatum L.), perennial ryegrass (Lolium perenne L.) and white clover (Trifolium repens L.) to inoculation with Bacillus polymyxa. Soil Biol Biochem 20:19–24
http://www.ecobiology.com.ua. Cited 20 Dec 2011
Hubbell DH, Morales VM, Umali-Garcia M (1978) Proteolytic enzymes in Rhizobium. Appl Environ Microbiol 31:210–213
Hughes KA (1997) Bacterial biofilms and their exopolysaccharides. PhD thesis, Edinburgh University, Edinburgh
Hughes KA, Sutherland IW, Clark J, Jones MV (1998) Bacteriophage and associated polysaccharide depolymerases – novel tools for study of bacterial biofilms. J Appl Microbiol 85:583–590
Hunter RC, Beveridge TJ (2005) High-resolution visualization of Pseudomonas aeruginosa PAO1 biofilms by freeze-substitution transmission electron microscopy. J Bacteriol 187:7619–7630
Ilyina TS, Romanova YM, Gintsburg AL (2004) Biofilms as a mode of existence of bacteria in external environment and host body: the phenomenon, genetic control, and regulation systems of development. Russ J Genet 40:1189–1198
Iman GM, Abd-Allah NM (1974) Fructosan, a new soil conditishing polysaccharide isolated from the metabolites of Bacillus polymyxa AS-1 and its clinical application. Egypt J Bot 17:19–26
Jonas K, Tomenius H, Kader A, Staffan N, Römling U, Belova LM, Melefore O (2007) Roles of curli, cellulose and Bap A in Salmonella biofilm morphology studied by atomic force microscopy. BMC Microbiol 7:1–13
Jung H-K, Hong J-H, Park S-C, Park B-K, Nam D-H, Kim S-D (2007) Production and physicochemical characterization of β-glucan produced by Paenibacillus polymyxa JB115. Biotechnol Bioprocess Eng 12:713–719
Kaci Y, Heyraud A, Barakat M, Heulin T (2005) Isolation and identification of an EPS-producing Rhizobium strain from arid soil (Algeria): characterization of its EPS and the effect of inoculation on wheat rhizosphere soil structure. Res Microbiol 156:522–531
Kahn ML, Schroeder BK, House BL, Mortimer MM, Yurgel SN, Maloney SC, Warren KL, Fisher RF, Barnett MJ, Toman C, Long SR (2004) Foraging for meaning: postgenome approaches to Sinorhizobium meliloti. In: Lugtenberg B, Tikhonovich I, Provorov N (eds) Biology of molecular plant–microbe interactions, vol 4. IS-MPMI, St. Paul, MN, pp 416–422
Kajimura Y, Kaneda M (1996) Fusaricidin A, a new depsipeptide antibiotic produced by Bacillus polymyxa KT-8. Taxonomy, fermentation, isolation, structure elucidation, and biological activity. J Antibiot 49:129–135
Kania RE, Lamers GEM, Vonk MJ, Huy PTB, Hiemstra PS, Bloemberg GV, Grote JJ (2007) Demonstration of bacterial cells and glycocalyx in biofilms on human tonsils. Arch Otolaryngol Head Neck Surg 133:115–121
Karatan E, Watnick P (2009) Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol Mol Biol Rev 73:310–347
Karlyshev AV, McCrossan MV, Wren BW (2001) Demonstration of polysaccharide capsule in Campylobacter jejuni using electron microscopy. Infect Immun 69:5921–5924
Karpunina LV, Melnikova UY, Konnova SA (2003) Biological role of lectins from the nitrogen-fixing Paenibacillus polymyxa strains 1460 during bacterial–plant-root interactions. Curr Microbiol 47:376–378
Khan Z, Kim SG, Jeon YH, Khan HU, Son SH, Kim YH (2008) A plant growth promoting rhizobacterium, Paenibacillus polymyxa strain GBR-1, suppresses root-knot nematode. Bioresour Technol 99:3016–3023
Kim JF, Jeong H, Park SY, Kim SB, Park YK, Choi SK, Ryu CM, Hur CG, Ghim SY, Oh TK, Kim JJ, Park CS, Park SH (2010) Genome sequence of the polymyxin-producing plant-probiotic rhizobacterium Paenibacillus polymyxa E681. J Bacteriol 192:6103–6104
Konnova SA, Skvortsov IM, Makarov OE, Prokhorova RN, Rogova TA, Ignatov VV (1995) Polysaccharide complexes secreted by Azospirillum brasilense and their possible role in the interaction of bacteria with wheat roots. Mikrobiologiya 64:762–768 (in Russian)
Kozyrovskaya NA, Negrutskaya VV, Koval’chuk MV, Voznyuk TN (2005) Paenibacillus sp. is a promising bacterium for the creation of a technology for manufacturing bacteria-based preparations for plants. Biopolim Kletka 21:312–318 (in Russian)
Kumar AS, Mody K, Jha B (2007) Bacterial exopolysaccharides – a perception. J Basic Microbiol 47:103–117
Kumar A, Prakash A, Johri BN (2011) Bacillus as PGPR in crop ecosystem. In: Maheshwari DK (ed) Bacteria in agrobiology: crop ecosystems, vol 2. Springer, Berlin, pp 37–59
Lal S, Tabacchioni S (2009) Ecology and biotechnological potential of Paenibacillus polymyxa: a minireview. Indian J Microbiol 49:2–10
Lebuhn M, Heulin T, Hartmann A (1997) Production of auxin and other indolic and phenolic compounds by Paenibacillus polymyxa strains isolated from different proximity to plant roots. FEMS Microbiol Ecol 22:325–334
Ledeboer NA, Jones BD (2005) Exopolysaccharide sugars contribute to biofilm formation by Salmonella enterica serovar typhimurium on Hep-2 cells and chicken intestinal epithelium. J Bacteriol 187:3214–3226
Lee IY, Seo WT, Kim GJ, Kim MK, Ahn SG, Kwon GS, Park YH (1997) Optimization of fermentation conditions for production of exopolysaccharide by Bacillus polymyxa. Bioprocess Eng 16:71–75
Lindberg T, Granhall U, Tomenius K (1985) Infectivity and acetylene reduction of diazotrophic rhizosphere bacteria in wheat (Triticum aestivum) seedlings under gnotobiotic conditions. Biol Fertil Soils 1:123–129
Ljunggren H, Fåhraeus G (1961) The role of polygalacturonase in root-hair invasion by nodule bacteria. J Gen Microbiol 26:521–528
Lloyd DH, Viac J, Werling D, Reme CA, Gatto H (2007) Role of sugars in surface microbe-host interactions and immune reaction modulation. Vet Dermatol 18:197–204
Lu F, Sun L, Lu Z, Bie X, Fang Y, Liu S (2007) Isolation and identification of an endophytic strain EJS-3 producing novel fibrinolytic enzymes. Curr Microbiol 54:435–439
Luft JH (1971) Ruthenium red and violet. 1. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec 171:347–368
Ma L, Jackson KD, Landry RM, Parsek MR, Wozniak DJ (2006) Analysis of Pseudomonas aeruginosa conditional Psl variant reveals roles for the Psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J Bacteriol 188:8213–8221
Ma M, Wang C, Ding Y, Li L, Shen D, Jiang X, Guan D, Cao F, Chen H, Feng R, Wang X, Ge Y, Yao L, Bing X, Yang X, Li J, Du B (2011) Complete genome sequence of Paenibacillus polymyxa SC2, a strain of plant growth-promoting rhizobacterium with broad-spectrum antimicrobial activity. J Bacteriol 193:311–312
Maes M, Baeyen S (2003) Experiences and perspectives for the use of a Paenibacillus strain as a plant protectant. Commun Agric Appl Biol Sci 68:457–462
Mannanov RN, Sattarova RK (2001) Antibiotics produced by Bacillus bacteria. Chem Nat Compd 37:117–123
Matora AV, Ignatova EN, Zhemerichkin DA, Yegorenkova IV, Shipin OV, Panasenko VI, Arsenieva LY, Barkovsky AL (1992) Bacterial polysaccharide polymyxan 88A. Main characteristics and possible applications. Prikl Biokhim Mikrobiol 28:731–737 (in Russian)
Mavingui P, Heulin T (1994) In vitro chitinase and antifungal activity of a soil, rhizosphere and rhizoplane population of Bacillus polymyxa. Soil Biol Biochem 26:801–803
McSpadden Gardener BB (2004) Ecology of Bacillus and Paenibacillus species in agricultural systems. Phytopathology 94:1252–1258
Michiels KW, Croes CL, Vanderleyden J (1991) Two different modes of attachment of Azospirillum brasilense Sp7 to wheat roots. J Gen Microbiol 137:2241–2246
Mitsuda S, Miyata N, Hirota T, Kikuchi T (1981) High-viscosity polysaccharide produced by Bacillus polymyxa. Hakkokogaku 59:303–309
Moon SH, Park JM, Chun HY, Kim SJ (2006) Comparisons of physical properties of bacterial cellulose produced in different culture conditions using saccharified food wastes. Biotechnol Bioprocess Eng 11:26–31
Moons P, Michiels CW, Aertsen A (2009) Bacterial interactions in biofilms. Crit Rev Microbiol 35:157–168
Morris CE, Monier J-M (2003) The ecological significance of biofilm formation by plant-associated bacteria. Annu Rev Phytopathol 41:429–453
Nakajima N, Chihara S, Koyama V (1972) A new antibiotic, gatavalin. J Antibiot 25:243–252
Nakashimada Y, Kanai K, Nishio N (1998) Optimization of dilution rate, pH and oxygen supply on optical purity of 2, 3-butanediol produced by Paenibacillus polymyxa in chemostat culture. Biotechnol Lett 20:1133–1138
Narula N, Deubel A, Gans W, Behl RK, Merbach W (2006) Paranodules and colonization of wheat roots by phytohormone producing bacteria in soil. Plant Soil Environ 52:119–129
Nielsen P, Sorensen J (1997) Multi-target and medium-independent fungal antagonism by hydrolytic enzymes in Paenibacillus polymyxa and Bacillus pumilus strains from barley rhizosphere. FEMS Microbiol Ecol 22:183–192
Ninomiya E, Kizaki T (1969) Bacterial polysaccharide produced from Bacillus polymyxa № 271. Angew Makromol Chem 6:179–185
Ninomiya E, Kizaki T, Harada K (1968a) High viscous polysaccharide produced by spore-forming bacterium. Part I. Identification of product. J Agric Chem Soc Jpn 42:178–184
Ninomiya E, Kizaki T, Harada K (1968b) High viscous polysaccharide produced by spore-forming bacterium. Part II. Physical properties and consistent sugar ratio. J Agric Chem Soc Jpn 42:431–434
Okon Y, Kapulnik Y (1986) Development and function of Azospirillum-inoculated roots. Plant Soil 90:3–16
Patra P, Natarajan KA (2004) Microbially induced flotation and flocculation of pyrite and sphalerite. Coll Surf B 36:91–99
Patra P, Natarajan KA (2006) Surface chemical studies on selective separation of pyrite and galena in the presence of bacterial cells and metabolic products of Paenibacillus polymyxa. J Colloid Interface Sci 298:720–729
Patriquin DG, Döbereiner J, Jain DK (1983) Sites and processes of association between diazotrophs and grasses. Сan J Microbiol 29:900–915
Perneel M, Heyrman J, Adiobo A, De Maeyer K, Raaijmakers JM, De Vos P, Hofte M (2007) Characterization of CMR5c and CMR12a, novel fluorescent Pseudomonas strains from the cocoyam rhizosphere with biocontrol activity. J Appl Microbiol 103:1007–1020
Petersen DJ, Srinivasan M, Chanway CP (1996) Bacillus polymyxa stimulates increased Rhizobium etli populations and nodulation when co-resident in the rhizosphere of Phaseolus vulgaris. FEMS Microbiol Lett 142:271–276
Petrova LP, Shelud’ko AV, Katsy EI (2010) Plasmid rearrangements and alterations in Azospirillum brasilense biofilm formation. Microbiology (Moscow, Russ Fed) 79:120–123
Philip L, Iyengar L, Venkobachar C (2000) Site of interaction of copper on Bacillus polymyxa. Water Air Soil Pollut 119:11–21
Piuri M, Sanchez-Rivas C, Ruzal SM (1998) A novel antimicrobial activity of a Paenibacillus polymyxa strain isolated from regional fermented sausages. Lett Appl Microbiol 27:9–13
Plakunov VK, Strelkova EA, Zhurina MV (2010) Persistence and adaptive mutagenesis in biofilms. Microbiology 79:424–434
Provorov NA, Vorobyov NI, Andronov EE (2008) Macro- and microevolution of bacteria in symbiotic systems. Russ J Genet 44:6–20
Ravi AV, Musthafa KS, Jegathammbal G, Kathiresan K, Pandian SK (2007) Screening and evaluation of probiotics as a biocontrol agent against pathogenic Vibrios in marine aquaculture. Lett Appl Microbiol 45:219–223
Raza W, Yang W, Shen Q-R (2008) Paenibacillus polymyxa: antibiotics, hydrolytic enzymes and hazard assessment. J Plant Pathol 90:419–430
Renni RJ, Thomas JB (1987) 15N-determined effect of inoculation with N2-fixing bacteria on nitrogen assimilation in Western Canadian wheats. Plant Soil 100:213–223
Rezzonico F, Zala M, Keel C, Duffy B, Moënne-Loccoz Y, Défago G (2007) Is the ability of biocontrol fluorescent pseudomonads to produce the antifungal metabolite 2,4-diacetylphloroglucinol really synonymous with higher plant protection? New Phytol 173:861–872
Römling U, Sierralta WD, Eriksson K, Normark S (1998) Multicellular and aggregative behaviour of Salmonella typhimurium strains is controlled by mutations in the agfD promoter. Mol Microbiol 28:249–264
Rosado AS, Seldin L (1993) Production of a potentially novel anti-microbial substance by Bacillus polymyxa. World J Microbiol Biotechnol 9:521–528
Ryu CM, Kim J, Choi O, Kim SH, Park CS (2006) Improvement of biological control capacity of Paenibacillus polymyxa E681 by seed pelleting on sesame. Biol Control 39:282–289
Safronova IY, Botvinko IV (1998) Intercellular matrix of Bacillus subtilis 271: composition and functions. Microbiology (Moscow, Russ Fed) 67:45–50
Santaella C, Schue M, Berge O, Heulin T, Achouak W (2008) The exopolysaccharide of Rhizobium sp. YAS34 is not necessary for biofilm formation on Arabidopsis thaliana and Brassica napus roots but contributes to root colonization. Environ Microbiol 10:2150–2163
Santhiya D, Subramanian S, Natarajan KA (2002) Surface chemical studies on sphalerite and galena using extracellular polysaccharides isolated from Bacillus polymyxa. J Colloid Interface Sci 256:237–248
Seldin L, de Azevedo SF, Alviano DS, de Freire Bastos MC (1999) Inhibitory activity of Paenibacillus polymyxa SCE2 against human pathogenic microorganisms. Lett Appl Microbiol 28:423–427
Selim S, Negrel J, Goveraets C, Gianinazzi S, van Tuinen D (2005) Isolation and partial characterization of antagonistic peptides produced by Paenibacillus sp. strains B2 isolated from the sorghum mycorrhizosphere. Appl Environ Microbiol 71:6501–6507
Sharma PK, Rao KH (2003) Adhesion of Paenibacillus polymyxa on chalcopyrite and pyrite: surface thermodynamics and extended DLVO theory. Coll Surf B 29:21–38
Sheludko AV, Kulibyakina OV, Shirokov AA, Petrova LP, Matora LY, Katsy EI (2008) The effect of mutations affecting synthesis of lipopolysaccharides and calcofluor-binding polysaccharides on biofilm formation by Azospirillum brasilense. Microbiology (Moscow, Russ Fed) 77:313–317
Shishido M, Massicotte HB, Chanway CP (1996) Effect of plant growth promoting Bacillus strains on pine and spruce seedling growth and mycorrhizal infection. Ann Bot 77:433–441
Shishido M, Breuil C, Chanway CP (1999) Endophytic colonization of spruce by plant growth-promoting rhizobacteria. FEMS Microbiol Ecol 29:191–196
Shoda M, Sugano Y (2005) Recent advances in bacterial cellulose production. Biotechnol Bioprocess Eng 10:1–8
Siddiqui ZA, Baghel G, Akhtar MS (2007) Biocontrol of Meloidogyne javanica by Rhizobium and plant growth-promoting rhizobacteria on lentil. World J Microbiol Biotechnol 23:435–441
Singh HP, Singh TA (1993) The interaction of a rock phosphate, Bradyrhizobium, vesicular-arbuscular mycorrhizae and phosphate-solubilizing microbes on soybean grown in a sub-Himalayan mollisol. Mycorrhiza 4:37–43
Skvortsova NG, Umarov MM, Kostina NV (1998) The effect of nonleguminous plant inoculation with Bacillus polymyxa – Pseudomonas mixed cultures on nitrogen transformations in the rhizosphere. Microbiology (Moscow, Russ Fed) 67:201–204
Sletmoen M, Maurstad G, Sikorski P, Paulsen BS, Stokke BT (2003) Characterisation of bacterial polysaccharides: steps towards single-molecular studies. Carbohydr Res 338:2459–2475
Smirnova TA, Didenko LV, Tiganova IG, Andreyevskaya SG, Alexeyeva NV, Stepanova TV, Romanova YM (2009) Study on the structure of biofilm formed by Salmonella typhimurium bacteria on abiotic surfaces by the methods of light and transmission electron microscopy. Biotechnology 5:16–23 (in Russian)
Smirnova TA, Didenko LV, Azizbekyan RR, Romanova YM (2010) Structural and functional characteristics of bacterial biofilms. Microbiology (Moscow, Russ Fed) 79:413–423
Solano C, García B, Valle J, Berasain C, Ghigo J-M, Gamazo C, Lasa I (2002) Genetic analysis of Salmonella enteritidis biofilm formation: critical role of cellulose. Mol Microbiol 43:793–808
Stanley NR, Britton RA, Grossman AD, Lazazzera BA (2003) Identification of catabolite repression as a physiological regulator of biofilm formation by Bacillus subtilis by use of DNA microarrays. J Bacteriol 185:1951–1957
Sutherland IW (1972) Bacterial exopolysaccharides. Adv Microbiol Physiol 8:143–212
Sutherland IW (1986) Industrially useful of microbial polysaccharides. Microbiol Sci 3:5–9
Sutherland IW (1994) Structure-function relationships in microbial exopolysaccharides. Biotechnol Adv 12:393–448
Sutherland IW (2001) Exopolysaccharides in biofilm, flocs and related structures. Water Sci Technol 43:77–86
Syu MJ (2001) Biological production of 2,3-butanediol. Appl Microbiol Biotechnol 55:10–18
Tanaka M, Machida Y, Tanaka S, Okamatsu H, Yatake T (1982) Microbial process for the production of a polysaccharide and its kationic salt and their use in depressing serum and liver cholesterol level and thachogenic index. UK Patent № 2,080,818
Thoendel M, Horswill AR (2010) Biosynthesis of peptide signals in gram-positive bacteria. Adv Appl Microbiol 71:91–112
Tien TM, Gaskins MN, Hubbell DH (1979) Plant growth substances produced by Azospirillum brasilense and their effect on the growth of pearl millet (Pennisetum americanum L.). Appl Environ Microbiol 37:1016–1024
Tien TM, Diem HG, Gaskins MH, Hubbell DH (1981) Polygalacturonic acid transeliminase production by Azospirillum species. Can J Microbiol 27:426–431
Tikhonovich IA, Provorov NA (2003) Symbiotic genetics of plant-microbe interactions. Ekologicheskaya Genetika 1:36–46 (in Russian)
Timmusk S (2003) Mechanism of action of the plant growth promoting bacterium Paenibacillus polymyxa. PhD thesis, Uppsala University, Uppsala
Timmusk S, Wagner EGH (1999) The plant-growth-promoting rhizobacterium Paenibacillus polymyxa induces changes in Arabidopsis thaliana gene expression: a possible connection between biotic and abiotic stress responses. Mol Plant Microbe Interact 12:951–959
Timmusk S, Nicander B, Granhall U, Tillberg E (1999) Cytokinin production by Paenibacillus polymyxa. Soil Biol Biochem 31:1847–1852
Timmusk S, van West P, Gow NAR, Wagner EGH (2003) Antagonistic effects of Paenibacillus polymyxa towards the oomycete plant pathogens Phytophthora palmivora and Pythium aphanidermatum. In: Timmusk S (ed) Mechanism of action of the plant growth promoting bacterium Paenibacillus polymyxa. Uppsala University, Uppsala, Sweden, pp 1–28
Timmusk S, Grantcharova N, Wagner EGH (2005) Paenibacillus polymyxa invades plant roots and forms biofilms. Appl Environ Microbiol 71:7292–7300
Timmusk S, Paalme V, Lagercratz U, Nevo E (2009a) Detection and quantification of Paenibacillus polymyxa in the rhizosphere of wild barley (Hordeum spontaneum) with real-time PCR. J Appl Microbiol 107:736–745
Timmusk S, van West P, Gow NAR, Huffstutler RP (2009b) Paenibacillus polymyxa antagonizes oomycete plant pathogens Phytophthora palmivora and Pythium aphanidermatum. J Appl Microbiol 106:1473–1481
Tkachenko AA, Sevryugina TV (1989) Levan biosynthesis by Bacillus polymyxa. Mikrobiologiya 58:457–461 (in Russian)
Tran H, Ficke A, Asiimwe T, Höfte M, Raaijmakers JM (2007) Role of the cyclic lipopeptide massetolide A in biological control of Phytophthora infestans and in colonization of tomato plants by Pseudomonas fluorescens. New Phytol 175:731–742
Trüper HG (2005) The type species of the genus Paenibacillus Ash et al. 1994 is Paenibacillus polymyxa. Opinion 77 judicial commission of the international committee on systematics of prokaryotes correspondence. Int J Syst Evol Microbiol 55:513
Tupinambá G, da Silva AJR, Alviano CS, Souto-Padron T, Seldin L, Alviano DS (2008) Antimicrobial activity of Paenibacillus polymyxa SCE2 against some mycotoxin-producing fungi. J Appl Microbiol 105:1044–1053
Ui S, Masuda H, Muraki H (1983) Laboratory-scale production of 2,3-butanediol isomers (D(–), L(+), and meso) by bacterial fermentations. J Ferment Technol 61:253–259
Van Loosdrecht MCM (1988) Bacterial adhesion. PhD thesis, Agricultural University of Wageningen, the Netherlands
Vandevivere P, Kirchman DL (1993) Attachment stimulates exopolysaccharide synthesis by a bacterium. Appl Environ Microbiol 59:3280–3286
Verhoef R, Schols HA, Blanco A, Siika-aho M, Ratto M, Buchert J, Lenon G, Voragen AG (2005) Sugar composition and FTIR analysis of exopolysaccharides produced by microbial isolates from paper mill slime deposits. Biotechnol Bioeng 91:91–105
Vijayalakshmi SP, Raichur AM (2002) Bioflocculation of high-ash Indian coals using Paenibacillus polymyxa. Int J Miner Process 67:199–210
Vlamakis H, Aguilar C, Losick R, Kolter R (2008) Control of cell fate by the formation of an architecturally complex bacterial community. Genes Dev 22:945–953
Vogt C, Simon D, Alfreider A, Babel W (2004) Microbial degradation of chlorobenzene under oxygen-limited conditions leads to accumulation of 3-chlorocatechol. Environ Toxicol Chem 23:265–270
Voloshin SA, Kaprelyants AS (2004) Cell-cell interactions in bacterial populations. Biochemistry 69:1268–1275
Voloshin SA, Shleeva MO, Kaprelyants AS, Syroeshkin AV (2005) The role of intercellular contacts in the initiation of growth and in the development of a transiently nonculturable state by cultures of Rhodococcus rhodochrous grown in poor media. Microbiology (Moscow, Russ Fed) 74:420–427
Von der Weid IA, Paiva E, Nobrega A, van Elsas JD, Seldin L (2000) Diversity of Paenibacillus polymyxa strains isolated from the rhizosphere of maize planted in Cerrado soil. Res Microbiol 151:369–381
Vu B, Chen M, Crawford RJ, Ivanova EP (2009) Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 14:2535–2554
Watnick P, Kolter R (2000) Biofilm, city of microbes. J Bacteriol 182:2675–2679
Weller DM, Thomashow LS (1994) Current challenges in introducing beneficial microorganisms into the rhizosphere. In: O’Gara F, Dowling DN, Boesten B (eds) Molecular ecology of rhizosphere biopolymers microorganisms. VCH, Germany, pp 1–18
Weller DM, Raaijmakers JM, McSpadden Gardener BB, Thomashow LS (2002) Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu Rev Phytopathol 40:309–348
Yahalom E, Dovrat A, Okon Y, Czosnek H (1991) Effect of inoculation with Azospirillum brasilense strain Cd and Rhizobium on the root morphology of burr medic (Medicago polymorpha L.). Isr J Bot 40:155–164
Yang J, Kharbanda PD, Mirza M (2004) Evaluation of Paenibacillus polymyxa pkb1 for biocontrol of Pythium disease of cucumber in a hydroponic system. Acta Hortic 635:59–66
Yegorenkova IV, Konnova SA, Sachuk VN, Ignatov VV (2001) Azospirillum brasilense colonisation of wheat roots and the role of lectin–carbohydrate interactions in bacterial adsorption and root-hair deformation. Plant Soil 231:275–282
Yegorenkova IV, Tregubova KV, Matora LY, Burygin GL, Ignatov VV (2008) Composition and immunochemical characteristics of exopolysaccharides from the rhizobacterium Paenibacillus polymyxa 1465. Microbiology (Moscow, Russ Fed) 77:553–558
Yegorenkova IV, Tregubova KV, Matora LY, Burygin GL, Ignatov VV (2010) Use of ELISA with antiexopolysaccharide antibodies to evaluate wheat-root colonization by the rhizobacterium Paenibacillus polymyxa. Curr Microbiol 61:376–380
Yegorenkova IV, Tregubova KV, Matora LY, Burygin GL, Ignatov VV (2011) Biofilm formation by Paenibacillus polymyxa strains differing in the production and rheological properties of their exopolysaccharides. Curr Microbiol 62:1554–1559
Yegorenkova IV, Tregubova KV, Ignatov VV (2013) Paenibacillus polymyxa rhizobacteria and their synthesized exoglycans in interaction with wheat roots: colonization and root hair deformation. Curr Microbiol 66:481–486
Yi K, Rasmussen AW, Gudlavalleti SK, Stephens DS, Stojiljkovie I (2004) Biofilm formation by Neisseria meningitidis. Infect Immun 72:6132–6138
York GM, González JE, Walker GC (1996) Exopolysaccharides and their role in nodule invasion. In: Stacey G, Mullin B, Gresshoff PM (eds) Biology of molecular plant-microbe interactions. International Society for Molecular Plant-Microbe Interactions, St. Paul, MN, pp 325–330
Zamudio M, Bastarrachea F (1994) Adhesiveness and root hair deformation capacity of Azospirillum strains for wheat seedlings. Soil Biol Biochem 26:791–797
Zanchetta P, Lagarde N, Guezennec J (2003) A new bone-healing material: a hyaluronic acid-like bacterial exopolysaccharide. Calcif Tissue Int 72:74–79
Zengguo H, Duygu K, Liwen Z, Chunhua Y, Kari BG-C, Ahmed EY (2007) Isolation and identification of a Paenibacillus polymyxa strain that coproduces a novel lantibiotic and polymyxin. J Appl Environ Microbiol 73:168–178
Zhang XQ, Bishop PL, Kupferle MJ (1998) Measurement of polysaccharides and proteins in biofilm extracellular polymers. Water Sci Technol 37:345–348
Zherebtsov NA, Abramova IN, Shelamova SA, Popova TN (2003) Identification of catalytically active groups in inulinase from Bacillus polymyxa 722. Appl Biochem Microbiol 39:544–548
Zogaj X, Nimtz M, Rohde M, Bokranz W, Römling U (2001) The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol Microbiol 39:1452–1463
Acknowledgments
I express my sincere gratitude to my colleagues who have been involved in different stages of the P. polymyxa research described in this chapter. Thanks are due to Mr. Dmitry N. Tychinin (this institute) for translating the original manuscript into English.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Yegorenkova, I.V. (2013). Exopolysaccharides of Paenibacillus polymyxa Rhizobacteria in Plant–Bacterial Interactions. In: Maheshwari, D., Saraf, M., Aeron, A. (eds) Bacteria in Agrobiology: Crop Productivity. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-37241-4_17
Download citation
DOI: https://doi.org/10.1007/978-3-642-37241-4_17
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-37240-7
Online ISBN: 978-3-642-37241-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)