Abstract
Azospirillum brasilense is a non-photosynthetic rhizobacterium that promotes the growth of plants. In this work, we evaluated the effects of different light qualities on the growth, viability, and motility in combination to other culture conditions such as temperature or composition of the culture medium. Exponential cultures of A. brasilense Az39 were inoculated by drop-plate method on nutritionally rich (LB) or chemically defined (MMAB) media in the presence or absence of Congo Red indicator (CR) and exposed continuously to white light (WL), blue light (BL), and red light (RL), or maintained in dark conditions (control). The exposure to BL or WL inhibited growth, mostly in LB medium at 36 °C. By contrast, the exposure to RL showed a similar behavior to the control. Swimming motility was inhibited by exposure to WL and BL, while exposure to RL caused only a slight reduction. The effects of WL and BL on plant growth-promoting rhizobacteria should be considered in the future as deleterious factors that could be manipulated to improve the functionality of foliar inoculants, as well as the bacterial effects on the leaf after inoculation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Light is an essential environmental factor for the development and continuity of life. Certain organisms, known as “photosynthetic” organisms, have developed the ability to capture light and transform it into chemical energy, a transformation through which they regulate metabolic functions (Elías-Arnanz et al. 2011). However, light can also cause photophysical and photochemical damage to the structure of nucleic acids, proteins, and lipids, and/or trigger the synthesis of highly toxic metabolites (van der Horst and Hellingwerf 2004). Particularly, the wavelengths responsible for such lethal effects (Christie et al. 1999; Hitomi et al. 2000) are found in the violet (380–450 nm) and blue (440–480 nm) parts of the visible spectrum. In presence of visible light, oxygen, and certain photosensitizing molecules, compounds known as reactive oxygen species (ROS) can appear, such as singlet oxygen, which can cause photooxidative stress (Ziegelhoffer and Donohue 2009). Several physical factors, intensity and wavelength among them, determine whether light has a beneficial or deleterious effect on a specific microorganism (Purcell et al. 2007). Thus, a very wide variety of bacteria (photosynthetic or non-photosynthetic) are able to control different motility mechanisms through the perception of light, which gives them a series of physiological advantages (Häder 1987; Armitage and Hellingwerf 2005; Wilde and Mullineaux 2017). Also there are reports based on the bactericidal effect of light on non-photosynthetic bacteria and of the interaction between light receptors and mobility-associated proteins (Lubart et al. 2011; Wu et al. 2013; Amin et al. 2016). Azospirillum is one of the best studied genera among non-photosynthetic plant growth-promoting rhizobacteria, but the effect of visible light on growth has not been elucidated, and it is still difficult to determine when this is beneficial or noxious. It is able to colonize numerous species of plant and significantly improve their growth, development and productivity under field conditions. In South America, Azospirillum is used as commercial inoculants in approximately 3.0 million ha cultivated, mainly with cereal crops (i.e., maize and wheat) and legumes (i.e., soybean). Specifically, A. brasilense Az39, which is presented as a model of beneficial bacteria for plants, is the active principle for 75% of registered products (Cassán and Diaz-Zorita 2016). In the last 2 decades, different inoculation practices have been developed under field conditions with several beneficial bacteria (i.e., farmer or industrial seed treatments, in-furrow, foliar, or soil-sprayed applications, etc.). In particular, combined inoculation of soybean seeds and leaves with rhizobia and azospirilla, respectively, has improved plant performance and crop yield under controlled and agricultural conditions (Puente et al. 2017, 2018). After foliar inoculation, Azospirillum spp. remains on the leaf surface for a while before colonizing the plant tissues. In such conditions, the effects of environmental factors (i.e., light, temperature, water potential, etc.) are restrictive for bacterial survival and the whole colonization process. This way, bacterial exposure to direct light on the leaf surface could modify bacterial survival and plant growth promotion capacity. Therefore, in the formulation of foliar microbial inoculants, deleterious effect of light during the spraying should be taken into account to ensure the functionality of these products by adding different protective compounds such as light absorbers (Congo Red and stilbene derivatives) or nanoparticles (zinc oxide, titanium oxide, silica, and graphene oxide) to prevent microbe deactivation by sunlight (Preininger et al. 2018). In the case of Azospirillum, there is only one paper in the literature, by Kumar et al. (2012), which reported that exposure of A. brasilense Sp7 to red light does not modify bacterial growth or motility under controlled environmental conditions.
The aim of this work was to evaluate how different wavelengths in the visible non-UV spectrum in combination with other factors such as temperature and composition of the culture medium affected the growth, survival, and motility of A. brasilense Az39 in controlled environmental conditions.
Materials and methods
Biological material and growth conditions
A. brasilense Az39 was obtained from the Microbiology and Zoology Institute for Agriculture (IMyZA) from the National Institute for Agricultural Technologies (Instituto Nacional de Tecnología Agropecuaria, INTA) (Castelar, Buenos Aires, Argentina). A. brasilense Az39 was grown in sterile Luria–Bertani (LB medium) (Bertani 1951) in darkness until OD595 1.2 at 36 °C overnight. Then, 40 µl from this pre-inoculum were transferred into a glass tube-containing sterile LB medium, which was incubated in a dark growth chamber at 36 °C with 200 rpm shaking until exponential growth phase corresponding to OD595 = 1.0, equivalent to approximately 5 × 108 CFU/ml. Aliquots from these dilutions, containing LB as a rich growth medium or MMAB as a minimal growth medium (Vanstockem et al. 1987), were distributed on Petri dishes by the drop-plate method (Miles and Misra 1938). Briefly, from each pure culture of A. brasilense Az39, decimal dilutions were made in borosilicate tubes containing sterile solution ClNa 0.85% until reaching a dilution of 10−6. From each tube, four aliquots of 20 μl were taken and inoculated in triplicate in polystyrene Petri dishes containing the LB or MMAB culture media with UV radiation absorption at 280–290 nm (Deltalab, Spain). The inoculated plates were independently exposed to experimental conditions related to the presence or absence of light: white light (WL) [56 µW/mm2], generated by a 75 W incandescent light lamp (General Electric, USA), blue light PAR 38 (BL) [11 µW/mm2], and red light PAR 38 (RL) [13.9 µW/mm2], generated by 450 nm and 660 nm LED lamps, respectively (OSRAM, Germany), at 36 °C for 72 h. In all cases, a control treatment was prepared by maintaining the same growth media and growth conditions in darkness with aluminum foil. After the incubation period, the typical colonies were counted in those spots (drops) containing between 5 and 50 colonies. The colony-forming units per milliliter (CFU/ml) were calculated considering dilution and inoculation factors [CFU/ml = dF × iF × 50 (dF: dilution factor; iF: inoculation factor)] and converted to log10 for the figures. A Vernier, Spectro Vis-Plus spectrophotometer (USA) was used to confirm the wavelength values for each light source, and their intensity was measured using a Tenmars TM-201 lx meter (Taiwan).
Effect of light on Az39 growth at different incubation temperatures and medium culture
20 µl aliquots were sampled and sown on Petri dishes by drop-plate method with LB agar (Bertani 1951) or LB agar modified by the addition of 15 mg/l of Congo Red indicator (LB-CR), (Molina et al. 2014) and MMAB agar (Vanstockem et al. 1987) or MMAB modified by the addition of 15 mg/l Congo Red indicator (MMAB-CR). After the inoculation, plates were incubated at either 28 °C or 36 °C for 72 h.
Effect of light on Az39 growth in modified LB and MMAB medium
20 µl aliquots from each different growth medium (LB, LB-CR, MMAB, and MMAB-CR) were sown on Petri dishes by drop-plate method (Rivera et al. 2014). Petri dishes containing LB medium modified by the addition of distilled sterile water were prepared as experimental variants, so the medium was diluted at 25% (v/v) or 50% (v/v). The same was done for MMAB medium modified by the addition of LB medium at 25%, 50%, and 75%. In all cases, plates were either incubated under dark conditions (control) or exposed to WL [56 µW/mm2], BL [11 µW/mm2] and RL [13.9 µW/mm2] as previously described.
Effect of exposure time to light
Plates with LB-CR medium were used to evaluate the effect of light at different exposure times. Plates were placed in a growth chamber and covered with aluminum foil after 2, 4, 8, and 24 h of growth at 36 °C. Plates exposed from the start (time 0) to conditions of exposure to light or darkness and covered with aluminum foil were used as controls until the end of the experiment.
Effect of light intensity
From dilutions previously obtained, 20 µl aliquots were sown using the drop-plate method on Petri dishes with MMAB medium. Once inoculated, the plates were incubated under different light intensities which were regulated by adjusting the distance between the plate and the light source. These intensities were 67, 56, and 25 µW/mm2 for WL, and 51, 11, and 8 µW/mm2 for BL, with respective controls in conditions of darkness at 36 °C for 72 h.
Effect of light on Az39 motility
From a pure culture in LB medium, 1 µl was sown on Petri dishes with Minimal Swim medium (SM) (Atkinson et al. 2006). This medium was formulated with 0.3% agar (w/v). After sowing, plates were incubated at 36 °C under dark conditions (control) or exposed to WL [56 µW/mm2], BL [11 µW/mm2], and RL [13.9 µW/mm2]. Plates were incubated for 72 h, after which bacterial displacement was measured in centimeters. Petri dishes completely covered with aluminum foil were used as controls in darkness conditions. The assay was performed in triplicate.
Statistical analyses
All assays were performed in triplicate with three repetitions for each growth condition. Data obtained were tested with ANOVA and Honestly-Significant-Difference (HSD) Tukey, with a 95% confidence level. Infostat software (Universidad Nacional de Córdoba, Argentina) was used to carry out the analyses, and the graphs were designed with GraphPad Prism 5.0 software (GraphPad Software, USA).
Results
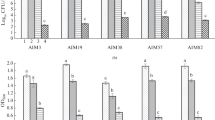
Growth and development in A. brasilense Az39 were affected by the presence of different light qualities. As can be seen in Fig. 1, both BL and WL had an inhibitory effect on growth. There were no significant differences (P < 0.05) neither in biomass production nor cell number per unit volume compared to the control in dark conditions when bacterium was exposed to RL.
Evaluation of A. brasilense Az39 growth (log10 CFU/ml) in LB-CR medium under dark conditions (Control) or exposed to WL (56 µW/mm2), RL (13.9 µW/mm2), and BL (11 µW/mm2). The plates were incubated for 72 h at 36 °C. The bars correspond to the mean ± standard deviation of a total of four biological repetitions. The absence of bars indicates growth inhibition. Same letters indicate no significant differences between treatments according to Tukey’s test (P < 0.05)
Complexity of the culture medium increases the sensitivity of Az39 to WL and BL
BL was lethal for the microorganism growing in LB and LB 50% (v/v), but WL only inhibited growth in undiluted LB medium (Fig. S1b).
Congo Red (CR) is an indicator normally used for the recognition of Azospirillum colonies. Colonies appear dark red or scarlet with typical colony characteristics (rough appearance, and hardened and dry surface). In addition, this indicator is capable of absorbing light in the blue part of the spectrum. For this reason, we considered to evaluate the possible protective effect of Congo Red on the survival of Az39 exposed to light at different wavelengths. When LB modified with the addition of Congo Red indicator was used as growth medium, exposure to either BL (Fig. S1d) or WL caused differential bacterial behaviors: in LB-CR 50% medium, the bacterium continued growing after the exposition to BL, but when exposed to WL, no growth was observed in the same medium (Fig. S1b–d). It is likely that the effect that each type of light has on the bacterium is impacted by the growth medium and the presence or absence of color indicators in the formulation. Exposure to BL only affected growth in A. brasilense Az39 when a 75% LB concentration was added to MMAB medium (Fig. S2b). However, exposure to WL, while growing in minimal medium modified by the addition of 50% and 75% LB resulted in bacterial growth inhibition (Fig. S2a). Dimorphic colonies were prevalent, with an approximate 2:1 ratio between smooth colonies (S) and rough colonies (R) when no growth inhibition existed after exposure to WL in MMAB medium enriched with LB. Exposure to BL under the same experimental conditions only brought about smooth colonies (S) (data not shown). These results suggest that the effect of WL on the bacterium depends on the growth medium richness, that is, how much more complex is the medium nutritionally, Az39 is less tolerant to exposure to WL. Given the behavior of Az39 when exposed to different wavelengths and grown in media with different compositions, it appears that high nutrient availability makes the bacterium less tolerant to WL or BL exposure. It is likely that the presence of certain types and amounts of nutrients and/or photoactivator molecules affects bacterial growth patterns, in particular at the level of cell cover, which might determine a non-specific tolerance to light.
Temperature impacts survival in WL and BL
As shown in Fig. 2, growth of A. brasilense Az39 was not affected by exposure to RL at 36 °C (Fig. 2a). Furthermore, BL (Fig. 2d) and WL (Fig. 2b) proved lethal when the bacterium was sown in LB media at 36 °C regardless of the presence or absence of Congo Red indicator. Exposure to BL or WL in minimal medium MMAB, by contrast, was not detrimental for growth at the same temperature (Fig. 2b–d). Figure 3 shows that a 28 °C temperature did not inhibit the growth of Az39 after exposure to dark conditions (Fig. 3a) and RL (Fig. 3c), and a similar observation was made at 36 °C. Exposure to WL (Fig. 3b) and BL (Fig. 3d) at 28 °C inhibited bacterial growth in both rich LB and minimal MMAB media in presence or absence of Congo Red indicator.
Evaluation of A. brasilense Az39 growth (log10 CFU/ml) in minimal medium (MMAB), minimal medium modified by addition of Congo Red (MMAB-CR), Luria–Bertani medium (LB), and Luria–Bertani medium modified by addition of Congo Red (LB-CR): a under dark conditions (Control) or exposed to b whith light (WL) 56 µW/mm2, c red light (RL) 13.9 µW/mm2, or d blue light (BL) 11 µW/mm2. The plates were incubated for 72 h at 36 °C. The bars correspond to the mean ± standard deviation of a total of four biological repetitions. The absence of bars indicates growth inhibition. Same letters indicate no significant differences according to Tukey’s test (P < 0.05) between the culture medium treatments exposed to the same light conditions
Evaluation of A. brasilense Az39 growth (log10 CFU/ml) in minimal medium (MMAB), minimal medium modified by addition of Congo Red (MMAB-CR), Luria–Bertani medium (LB), and Luria–Bertani medium modified by addition of Congo Red (LB-CR): a under dark conditions (Control) or exposed to b white light (WL) 56 µW/mm2, c red light (RL) 13.9 µW/mm2, or d blue light (BL) 11 µW/mm2. The plates were incubated for 72 h at 28 °C. The bars correspond to the mean ± standard deviation of a total of 4 biological repetitions. The absence of bars indicates growth inhibition. Same letters indicate no significant differences according to Tukey’s test (P < 0.05) between the culture medium treatments exposed to the same light conditions
Deleterious effect of WL and BL is proportional to the exposure time and light intensity
As shown in Fig. 3, WL decreased bacterial growth from 8 h and it proved to be lethal after 24 h of exposure (Fig. S3a). However, at the last exposure time, BL only caused a decrease in bacterial viability, probably due to the beginning of cell damage process (Fig. S3c). In the case of RL, there was no growth inhibition in any of the times analyzed and viability was similar to that of the control under dark conditions. These results suggest that the duration of the exposure to WL can determine the effects on growth, while the impact on viability is observed with a minimum exposure time of 24 h. For BL, partial inhibition was observed at 24 h, which makes it likely that a longer exposure time is required for inhibition to occur compared with WL. There was no growth inhibition after 24 (Fig. S3b), 48, or 72 h when bacterium was exposed to RL (data not shown).
In nature, sunlight intensity varies according to factors such as temperature and season, but in general terms, 1000 lx intensity usually reaches the ground. Therefore, we evaluated the effect of decreasing light intensities on Az39 growth and development in minimal medium. As can be seen in Fig. 4, the growth in MMAB culture medium of Az39 was inhibited after exposure to intensities of 51 µW/mm2 and 67 µW/mm2 corresponding to BL and WL, respectively. Exposure to either 11 µW/mm2 or 56 µW/mm2, corresponding to BL and WL, did not inhibit growth or development.
Evaluation of A. brasilense Az39 growth (log10 CFU/ml) in minimal medium MMAB exposed to different intensities of: a withe light (WL) [> 67, 67, 56 and 25 µW/mm2] and b blue light (BL) [> 51, 51, 11 and 8 µW/mm2]. The plates were incubated for 72 h at 36 °C. The bars correspond to the mean ± standard deviation of a total of 4 biological repetitions. The absence of bars indicates growth inhibition. Same letters indicate no significant differences according to Tukey’s test (P < 0.05) between the light intensity treatments exposed to the same light condition
Light modifies the swimming motility of Az39 independently of the wavelength
Figure 5 shows how bacterial swimming motility is affected by light exposure. There was a decrease in A. brasilense Az39 motility after exposure to each light wavelength, but mainly with WL and BL, which caused a decrease of 87 and 88% in motility (in cm), respectively, compared to the control in darkness, which showed the highest motility under the established experimental conditions. RL also modified the swimming motility, but the reduction percentage was approximately 60% compared to the control. A. brasilense Az39 viability was previously evaluated using Swim Medium. In such experiments, the bacterium had the same viability as the control when the plates were exposed to BL and WL at 36 °C. This pattern was identical to that observed with MMAB medium (see Fig. 2b–d), and the possibility of the reduction in cell number (CFU/ml) was ruled out due to a lethal effect of WL and BL in Swim Medium plates (data not shown).
Evaluation of swimming motility on agar plates inoculated with A. brasilense Az39 and incubated under dark conditions (Control) or exposed to white light (WL) 56 µW/mm2, red light (RL) 13.9 µW/mm2, or blue light (BL) 11 µW/mm2. Motility was measured in terms of bacterial displacement under exposure to the light (measured in cm). The plates were incubated for 72 h at 36 °C. The bars correspond to the mean ± standard deviation of a total of three biological repetitions. The absence of bars indicates no displacement. Same letters indicate no significant differences according to Tukey’s test (P < 0.05) between treatments under the same experimental conditions
Discussion
The effect of light on microorganisms has been studied more than the molecular bases for the effects of light on microbial life (Elías-Arnanz et al. 2011). Most research studies about the effect of light on bacteria began in the 20th century, but it is only in the last 40 years that its molecular pathways have been approached (Ulrich et al. 2005; Wuichet et al. 2010). Several cellular responses have proven to be dependent on light, such as pigment formation, DNA repair, and stress response, as well as multicellular behavior such as the formation of biofilm or sporocarps in the case of myxobacteria (Takano et al. 2005; Sinha and Hader 2002). However, these responses have not been confirmed in Azospirillum spp. Kumar and coworkers reported that exposure to red light does not modify the growth or motility of A. brasilense Sp7 under controlled environmental conditions. Moreover, they discarded any possible mechanism of tolerance induced by phytochromes in this bacterium (Kumar et al. 2012).
In our experiments, A. brasilense Az39 was affected at the growth level by exposure to WL and BL (see Fig. 1). Additionally, BL was lethal in most cases, but there was no difference between RL and the control. Therefore, we assume that the bacterium adapts well to this last type of wavelength, but not to BL or WL. Kumar et al. (2012) support that exposure to red light does not affect bacterial growth in a minimal or rich medium and does not cause significant morphological changes in A. brasilense Sp7. Similarly, A. brasilense Az39 does not show morphological changes when exposed to RL, regardless of the medium or the culture conditions. However, exposure to BL or WL affected its growth depending on the incubation temperature and the composition of the culture medium.
Kaushik et al. (2002) showed that temperatures below the optimal decreased biomass production and metabolic activity in A. brasilense CDJA and A. brasilense A40. It is known that a temperature of 28 °C decreases growth in A. brasilense, increases the formation of protective structures or substances, and decreases metabolic functions in a way that prevents the deterioration of the bacterial cell, but not its growth (Díaz-Saez et al. 2013). In our experiments, the growth and development of Az39 were completely inhibited when bacteria were exposed to WL or BL (see Fig. 3b–d) at 28 °C, which allows us to suppose that Az39 was unable to prevent cell death by light exposure at that temperature.
Furthermore, exposure to light at 36 °C showed different results. Bacterial growth was inhibited by WL or BL in the rich LB medium (see Fig. 2b–d), while growth in a minimal medium (MMAB) was not affected. In this sense, the composition of culture media affects cellular physiology, and, therefore, alters bacteria survival capacity (Kram and Finkel 2015). In fact, the enrichment of the culture medium can cause drastic changes in the physiology of the cells and, thus, generates a modification in oxidative states, which in turn leads to an increase in the levels of glycation and the frequency of mutation, and, finally, death (Mironova et al. 2001; Dimitrova et al. 2004). Metabolic stress implies significant morphological changes, including loss of motility, increased accumulation of reserve substances, and increased production of exopolysaccharides, suggesting that cell surface remodeling is a strategy against stress (Bible et al. 2015). For Caulobacter crescentus, a non-photosynthetic phototactic and pigment-producing species, it was reported that the biosynthesis of cell surface structures is activated under blue light exposure (Foreman et al. 2012). Konnova et al. (1994) reported that the composition of the outer membrane of Azospirillum spp. differs according to the growth phase and culture conditions, which offers certain advantages under unfavorable conditions. In our experiments, the bacterium might have been less tolerant to BL and WL exposure under conditions of high nutrient availability (LB medium) due to the presence of certain nutrients that affected bacterial physiology and cell packing, both of which decreased the non-specific tolerance to light, or due to presence of a higher concentration of photosensitizer molecules (see Figs. 1, 2, S1b, S1d and S2a).
A novel aspect of the effects of visible light on microorganisms has been related to their capacity to biosynthesize photosensitizer molecules that can trigger cell death (Maclean et al. 2008). Several reports indicate that many bacteria have a large number of genes that code for the synthesis of endogenous photosensitizers, which respond to the presence of visible light, but particularly to violet and blue light at 405 and 470 nm, respectively (Ashkenazi et al. 2003; Guffey and Wilborn 2006). The absorption of these wavelengths by photosensitizers generates reactive oxygen species (ROS) that damage cell structures and lead to bacterial cell death (Hessling et al. 2017). In our experiments, bacterial growth was inhibited by exposure to BL or WL but not RL, and this pattern was modified by the composition of the culture medium. Under optimal nutrient availability conditions (LB medium), the bacterium might be less tolerant to BL and WL exposure than under the limited conditions of a minimal medium (MMAB). This is probably due to the presence of a higher concentration of precursors for the biosynthesis of photosensitizers, or nutrients necessary for cell packing, with a consequent decrease in non-specific tolerance to light.
In the last decades, it has been recognized that many bacteria have a high concentration of endogenous photosensitizers (Ashkenazi et al. 2003). Porphyrins, coproporphyrin III, protoporphyrin IX, and uroporphyrin III are the main endogenous photosensitizers responsible for bacterial photoinactivation (Feuerstein et al. 2004, Maclean et al. 2008). As mentioned, the absorption of visible light by these molecules generates ROS that damage cell structures, leading to cell death. The genomic analysis in A. brasilense Az39 showed the presence of at least 14 coding sequences for different photosensitizing molecules. These molecules are synthesized from the metabolism of several amino acids, such as glycine, alanine, aspartate, and glutamate, among others. In our experiments, we used rich medium (LB), with high amino acid concentrations which could increase total porphyrins (data not shown).
A change in motility is common in photosynthetic bacteria, which are capable of detecting alterations in the quantity and quality of the light. Three general types of response to light have been described for bacteria (Häder 1987; Ragatz et al. 1995; Gest 1995): scotophobia or fear of darkness, photokinesis, alteration in motility caused by a light stimulus, and phototaxis, which involves movement towards or away from a source of light. Notably, the direction in which the light radiates is critically determining in phototaxis (Jiang et al. 1998). Bacteria of the genus Azospirillum are motile and capable of chemotaxis toward organic acids, sugars, and some aminoacids. Additionally, they are able to follow gradients of oxygen, alternative electron acceptors, and redox active compounds. Most attractant and repellent compounds described so far for this bacterial genus include those that affect intracellular metabolism, leading to the suggestion that most taxis responses correspond to energy taxis in Azospirillum spp. (Alexandre 2015). Besides this, no reports have been published until now in relation to the bacterial capacity to respond to light in terms of phototaxis or motility. In this work, we observed a decrease in motility under exposure to WL and BL (see Fig. 5a), in comparison to the displacement reached in darkness (maximum value). However, under RL, motility was lower than in the control, although at the same time greater than under conditions of WL and BL exposure (full-plate irradiation). These results are consistent with the other reports which show that light represses mobility in A. tumefaciens and, by contrast, those bacteria grown in the dark are more mobile, adhere to plants better and are more virulent. The reduction in motility under different light wavelengths is a general behavior present in other bacteria of the Rhizobiaceae family (A. tumefaciens, A. vitis, and Rh. Leguminosarum) (Oberpichler et al. 2008). Likewise, motility in Az39 was higher in darkness (with half of plate being irradiated) (see Fig. 5b). In both experimental conditions, WL was found to be highly inhibitory for the motility of this bacterium. RL and BL were inhibitory as well, although in a lower proportion.
Some reports showed that light as a stimulus or environmental factor is capable of inducing or repressing the expression of genes related to the synthesis of flagella (Ramos et al. 2004). The polar flagellum is not only involved in chemotaxis (Grishanin et al. 1991), but also in the first adsorption step when A. brasilense colonizes plants (Michiels et al. 1991). Inoculation with Azospirillum spp. usually resulted in increased internal colonization of roots, stems, and leaves, indicating high bacterial motility through the plants. Several authors (Baldani et al. 1992; James et al. 1994; Souza et al. 2014) have suggested that the presence of diazotrophic bacteria in xylem vessels may point them out as a route of bacterial migration to different organs. Internal colonization of plant leaves also increased in response to foliar inoculation, suggesting that in this case, the stomata acted as a passive doorway for bacterial migration (Fukami et al. 2016). In summary, in this study, we show that A. brasilense Az39 is lethally affected by BL and WL, and that this behavior should be considered when using A. brasilense in agricultural practices, including the foliar spray inoculation of soybean, maize, or wheat, among other crops (Puente et al. 2017, 2018; Fukami et al. 2016, 2018). Preininger et al. (2018) have recently compiled the most significant concepts around foliar microbial inoculants and they suggest that efficacy of this products significantly depends on environmental conditions at the time of spraying. Light has a great impact on the viability of microorganisms and their interaction with plant leaf surface. Thereby, formulation should contain protective compounds against the deleterious effect of light to ensure their activity. In our experimental conditions, BL exposure did not inhibit Az39 growth when the bacterium was cultured in a medium modified by the addition of an aliquot of MMAB containing Congo Red indicator (MMAB-CR), in comparison with exposure to WL in LB-CR or BL in rich medium alone (LB). These results show that the Congo Red offers to A. brasilense Az39 some protection against exposure to BL when the cell surface composition changes (i.e., production exopolysaccharide) (Budanova et al. 2018) and/or because it absorbs light at 497 nm, in the blue part of the spectrum.
This is the first evidence on the differential behavior by Az39 when exposed to different wavelengths in culture media modified by the addition of the indicator.
Taking into account the consolidation of certain practices in agriculture and the use of Azospirillum spp. for foliar spray inoculation in several crops, it is of utmost importance to understand the effect of sunlight or specific wavelengths on such microorganisms, to generate a basic functional model and optimize inoculant functionality in the future.
Change history
17 June 2020
In the original article, last name and first names of all the authors are inverted. The correct names should appears as ���Romina Molina, Gast��n L��pez, Bel��n Rodr��guez, Susana Rosas, Ver��nica Mora, Fabricio Cass��n���.
References
Alexandre G (2015) Minireview: chemotaxis control of transient cell aggregation. J Bacteriol 197(20):3230–3237
Amin RM, Bhayana B, Hamblin MR, Dai T (2016) Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: in vitro and in vivo studies. Lasers Surg Med 48(5):562–568
Armitage JP, Hellingwerf KJ (2005) Light-induced behavioral responses (‘phototaxis’) in prokaryotes. Discoveries in photosynthesis. Springer, Dordrecht, Berlin, pp 985–995
Ashkenazi H, Malik Z, Harth Y, Nitzan Y (2003) Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol Med Microbiol 35:17–24
Atkinson S, Chang CY, Sockett RE, Cámara M, Williams P (2006) Quorum sensing in Yersinia enterocolitica controls swimming and swarming motility. J Bacteriol 188(4):1451–1461
Baldani VLD, Baldani JI, Olivares F, Döbereiner J (1992) Identification and ecology of Herbaspirillum seropedicae and the closely related Pseudomonas rubrisubalbicans. Symbiosis Rehovot 12:65
Bertani G (1951) Studies on lysogenesis I.: the mode of phage liberation by lysogenic Escherichia coli. J Bacteriol 62(3):293–300
Bible AN, Khalsa-Moyers GK, Mukherjee T, Green CS, Mishra P, Purcell A, Alexandre G (2015) Metabolic adaptations of Azospirillum brasilense to oxygen stress by cell-cell clumping and flocculation. Appl Environ Microbiol 81:8346–8357
Budanova AA, Shirokov AA, Shchyogolev SY, Matora LY (2018) Analysis of Congo red-induced changes in the cell surface and macrocolony structure of the bacterium Azospirillum brasilense. Microbiology 87(1):60–65
Cassán F, Diaz-Zorita M (2016) Azospirillum sp. in current agriculture: from the laboratory to the field. Soil Biol Biochem 103:117–130
Christie JM, Salomon M, Nozue K, Wada M, Briggs WR (1999) LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): binding sites for the chromophore flavin mononucleotide. Proc Natl Acad Sci USA 96:8779–8783
Díaz-Saez Y, Díaz-de los Ríos M, Alberto-Casas M, Nuñez-Caraballo A, Martínez-Mora M (2013) Crecimiento de Azospirillum brasilense en presencia de disacáridos: sacarosa y lactosa. ICIDCA. Sobre los Derivados de la Caña de Azúcar 47(2)
Dimitrova R, Mironova R, Ivanov I (2004) Glycation of proteins in Escherichia coli: effect of nutrient broth ingredients on glycation. Biotechnol Biotechnol Equip 18:99–103
Elías-Arnanz M, Padmanabhan S, Murillo FJ (2011) Light-dependent gene regulation in nonphototrophic bacteria. Curr Opin Microbiol 14:128–135
Feuerstein O, Persman N, Weiss EI (2004) Phototoxic effect of visible light on Porphyromonas gingivalis and Fusobacterium nucleatum: an in vitro study. Photochem Photobiol 80:412–415
Foreman R, Fiebig A, Crosson S (2012) The LovK-LovR two-component system is a regulator of the general stress pathway in Caulobacter crescentus. J Bacteriol 194(12):3038–3049
Fukami J, Nogueira MA, Araujo RS, Hungria M (2016) Accessing inoculation methods of maize and wheat with Azospirillum brasilense. AMB Express 6(1):3
Fukami J, de la Osa C, Ollero FJ, Megías M, Hungria M (2018) Co-inoculation of maize with Azospirillum brasilense and Rhizobium tropici as a strategy to mitigate salinity stress. Funct Plant Biol 45(3):328–339
Gest H (1995) Phototaxis and other sensory phenomena in purple photosynthetic bacteria. FEMS Microbiol Rev 16:287–294
Grishanin RN, Chalmina II, Zhulin IB (1991) Behaviour of Azospirillum brasilense in a spatial gradient of oxygen an in a ‘redox’ gradient of an artificial electron acceptor. J Gen Microbiol 137:2781–2785
Guffey JS, Wilborn J (2006) In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomed Laser Surg 24:684–688
Häder DP (1987) Photosensory behavior in procaryotes. Microbiol Rev 51(1):1
Hessling M, Spellerberg B, Hoenes K (2017) Photoinactivation of bacteria by endogenous photosensitizers and exposure to visible light of different wavelengths—a review on existing data. FEMS Microbiol Lett 364(2):fnw270
Hitomi K, Okamoto K, Daiyasu H, Miyashita H, Iwai S, Toh H, Todo T (2000) Bacterial cryptochrome and photolyase: characterization of two photolyase-like genes of Synechocystis sp. PCC6803. Nucleic Acids Res 28(12):2353–2362
James EK, Reis VM, Olivares FL, Baldani JI, Döbereiner J (1994) Infection of sugar cane by the nitrogen-fixing bacterium Acetobacter diazotrophicus. J Exp Bot 6(1):757–766
Jiang ZY, Rushing BG, Bai Y, Gest H, Bauer CE (1998) Isolation of Rhodospirillum centenum mutants defective in phototactic colony motility by transposon mutagenesis. J Bacteriol 180:1248–1255
Kaushik R, Saxena A, Tilak KVBR (2002) Can Azospirillum strains capable of growing at a sub-optimal temperature perform better in field-grown-wheat rhizosphere. Biol Fert Soils 35(2):92–95
Konnova SA, Makarov OE, Skvortsov IM, Ignatov VV (1994) Isolation, fractionation and some properties of polysaccharides produced in a bound form by Azospirillum brasilense and their possible involvement in Azospirillum-wheat root interactions. FEMS Microbiol Lett 118(1–2):93–99
Kram KE, Finkel SE (2015) Rich medium composition affects Escherichia coli survival, glycation, and mutation frequency during long-term batch culture. Appl Environ Microbiol 81:4442–4450
Kumar S, Kateriya S, Singh VS, Tanwar M, Agarwal S, Singh H, Tripathi AK (2012) Bacteriophytochrome controls carotenoid-independent response to photodynamic stress in a non-photosynthetic rhizobacterium, Azospirillum brasilense Sp7. Sci Rep 2:1
Lubart R, Lipovski A, Nitzan Y, Friedmann H (2011) A possible mechanism for the bactericidal effect of visible light. Laser Therapy 20(1):17–22
Maclean M, MacGregor SJ, Anderson JG, Woolsey G (2008) High-intensity narrow-spectrum light inactivation and wavelength sensitivity of Staphylococcus aureus. FEMS Microbiol Lett 285:227–232
Michiels KW, Croes CL, Vanderleyden J (1991) Two different modes of attachment of Azospirillum brasilense Sp7 to wheat roots. Microbiology 137(9):2241–2246
Miles A, Misra S (1938) The estimation of the bactericidal power of blood. J Hyg 38(06):732–737
Mironova R, Niwa T, Hayashi H, Dimitrova R, Ivanov I (2001) Evidence for non-enzymatic glycosylation in Escherichia coli. Mol Microbiol 39:1061–1068
Molina R, Obando D, Torres D, Rivera D, Cassán F (2014) Utilización de un medio de cultivo para la cuantificación y la diferenciación de bacterias presentes en la misma formulación. Libro de resúmenes digital, pp 249
Oberpichler I, Rosen R, Rasouly A, Vugman M, Ron EZ, Lamparter T (2008) Light affects motility and infectivity of Agrobacterium tumefaciens. Environ Microbiol 10(8):2020–2029
Preininger C, Sauer U, Bejarano A, Berninger T (2018) Concepts and applications of foliar spray for microbial inoculants. App Microbiol Biotechnol 102(17):7265–7282
Puente ML, Gualpa JL, Lopez GA, Molina RM, Carletti SM, Cassán FD (2017) The benefits of foliar inoculation with Azospirillum brasilense in soybean are explained by an auxin signaling model. Symbiosis 76:41–49
Puente ML, Zawoznik M, de Sabando ML, Perez G, Gualpa JL, Carletti SM, Cassán FD (2018) Improvement of soybean grain nutritional quality under foliar inoculation with Azospirillum brasilense strain Az39. Symbiosis 77:41–47
Purcell EB, Siegal-Gaskins D, Rawling DC, Fiebig A, Crosson S (2007) A photosensory two-component system regulates bacterial cell attachment. Proc Natl Acad Sci USA 104(46):18241–18246
Ragatz L, Jiang ZY, Bauer CE, Gest H (1995) Macroscopic phototactic behavior of the purple photosyn thetic bacterium Rhodospirillum centenum. Arch Microbiol 163:1–6
Ramos HC, Rumbo M, Sirard JC (2004) Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol 12:509–517
Rivera D, Revale S, Molina R, Gualpa J, Puente M, Maroniche G, Spaepen S (2014) Complete genome sequence of the model rhizosphere strain Azospirillum brasilense Az39, successfully applied in agriculture. Genome announc 2(4):e00683-14
Sinha RP, Hader DP (2002) UV-induced DNA damage and repair: a review. Photochem Photobiol Sci 1:225–236
Souza R, Meyer J, Schoenfeld R, Costa PB, Passaglia LMP (2014) Characterization of plant growth-promoting bacteria associated with rice cropped in iron-stressed soils. Ann Microbiol 65:951–964
Takano H, Obitsu S, Beppu T, Ueda K (2005) Light-induced carotenogenesis in Streptomyces coelicolor A3 (2): identification of an extracytoplasmic function sigma factor that directs photodependent transcription of the carotenoid biosynthesis gene cluster. J Bacteriol 187(5):1825–1832
Ulrich LE, Koonin EV, Zhulin IB (2005) One-component systems dominate signal transduction in prokaryotes. Trends Microbiol 13:52–56
van der Horst MA, Hellingwerf KJ (2004) Photoreceptor proteins, “star actors of modern times”: a review of the functional dynamics in the structure of representative members of six different photoreceptor families. Acc Chem Res 37(1):13–20
Vanstockem M, Michiels K, Vanderleyden J, Van Gool AP (1987) Transposon mutagenesis of Azospirillum brasilense and Azospirillum lipoferum: physical analysis of Tn5 and Tn5-Mob insertion mutants. Appl Environ Microbiol 53(2):410–415
Wilde A, Mullineaux CW (2017) Light-controlled motility in prokaryotes and the problem of directional light perception. FEMS Microbiol Rev 41(6):900–922
Wu L, McGrane RS, Beattie GA (2013) Light regulation of swarming motility in Pseudomonas syringae integrates signaling pathways mediated by a bacteriophytochrome and a LOV protein. MBio 4(3):e00334-13
Wuichet K, Cantwell BJ, Zhulin IB (2010) Evolution and phyletic distribution of two-component signal transduction systems. Curr Opin Microbiol 13:219–225
Ziegelhoffer EC, Donohue TJ (2009) Bacterial responses to photo-oxidative stress. Nat Rev Microbiol 7:856–863
Funding
Funding was provided by FONCyT (Grant No. PICT-2015-1599).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no conflicts of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Romina, M., Gastón, L., Belén, R. et al. Evaluation of growth and motility in non-photosynthetic Azospirillum brasilense exposed to red, blue, and white light. Arch Microbiol 202, 1193–1201 (2020). https://doi.org/10.1007/s00203-020-01829-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-01829-8