Abstract
To investigate salt stress and biochar application effects on nodulation and nitrogen metabolism of soybeans (Glycine max cv. M7), an experiment was conducted under the control condition. The treatments comprised three biochar rates (non, 50 and 100 g kg−1 soil) and three salinities (0, 5 and 10 dS m−1 NaCl), with four replications of treatments. Salt stress diminished the number of nodules and their weights in the soybean roots. Nitrogen content and metabolism decreased in nodules, roots and shoots, while reducing the activity of glutamate dehydrogenase (GDH), glutamine synthetase (GS), glutamine oxoglutarate aminotransferase (GOGAT) and nitrate reductase (NR). Also, salinity brought down root and shoot weight, total plant biomass, chlorophyll content, leaf area (LA) and rubisco activity in the soybean. On the other hand, application of biochar improved nodulation, nitrogen content, rubisco activity, GDH, GS, GOGAT and NR activities in different parts of the soybean and nodules under salt stress, and consequently improved chlorophyll content, LA, root and shoot weight. Both the 50 and 100 g kg−1 biochar rates showed similar effects in improving nitrogen metabolism and plant performance under salt stress. Generally, biochar increased nodulation and nitrogen metabolism of the soybean under saline conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nitrogen plays a critical role in plant growth (Singh et al. 2016). Although nitrogen appears in the atmosphere as gaseous nitrogen molecules, that form of nitrogen is not directly accessible to plants, which nevertheless require the element for their chlorophyll structure, proteins, amino acids, nucleic acids, and enzymes (Sinclair and Horie 1989). In leguminous plants such as the common bean (Phaseolus vulgaris L.), chickpeas (Cicer arietinum L.), and soybeans (Glycine max L.), rhizobia such as the bacteria Bradyrhizobium japonicum fix atmospheric nitrogen in a process driven by energy created through photosynthesis (Berman-Frank et al. 2003) and this biological process is done with nitrogenase enzyme, and other enzymes such as, glutamate dehydrogenase (GDH), glutamine synthetase (GS) and glutamine oxoglutarate aminotransferase (GOGAT) have a main roles in nitrogen assimilation. Briefly, the nitrogenase enzymatic system, reduces molecular nitrogen (N2) to ammonium (NH4 +) in root nodules during the biological process of nitrogen fixation (Chakrabarti and Mukherji 2003). The enzymes GDH, GS, and GOGAT are also critical in nitrogen assimilation and amino acid synthesis in crops. In particular, GS is central in multifunctional enzymes, and improving the activity of this enzyme can facilitate nitrogen assimilation and translocation in crops (Becker et al. 2000; Wang et al. 2003).
Environmental stresses such as salinity can harm nodulation and enzymatic activities of nitrogen metabolism in legumes (Chakrabarti and Mukherji 2003; Flowers et al. 2010), including their growth, nodulation, and crop productivity (Tu 1981; Singleton and Bohlool 1984; Faghire et al. 2013). Abd-Alla et al. (1998) observed that salinity significantly inhibited the activity of nitrogenase, as well as reduced the nodule number and dry weight per plant, in four cultivars of soybeans. That same year, Comba et al. (1998) reported that though sodium toxicity did not affect nodules and nitrogen metabolism under moderate salt stress, severe salinity adversely affected nodulation and the activity of nitrogenase, GDH, GS, and GOGAT. Earlier, James et al. (1993) found that short-term salt stress greatly reduced nitrogenase activity and oxygen permeability in soybean nodules, as they do in other legumes (Chakrabarti and Mukherji 2003; Araújo et al. 2015). In the upper organs of soybeans, salt stress reduces the leaf area, nitrate reductase (NR) activity, compromises the activation of chlorophyll degradation enzymes such as chlorophyllase, and inhibits chlorophyll synthesis. Salt toxicity also affects other pivotal enzymes in photosynthesis, including rubisco, the capacity of which suffers in terms of photosynthetic electron transport and cell energy limits (Koyro and Huchzermeyer 1999; Huchzermeyer and Heins 2000).
The carbon base of soil is vital for nitrogen fixation, especially given its influence in forming a medium suitable for physical reactions, chemical processes, and biological activities. Among new carbon additives for soil, biochar is produced via the thermal degradation of organic materials such as plant residues in environments with little or no oxygen (Antal and Gronli 2003; Fahad et al. 2016). An extremely constant form of carbon that can linger in soil for many years (Ascough et al. 2009; Steinbeiss et al. 2009; Lehmann et al. 2009), biochar is used to not only improve soil’s capacity to act as carbon sink on farms, but also to increase soil fertility (Chan and Xu 2009; Ogawa and Okimori 2010). Using biochar on farms can also increase water retention in soil, directly due to its high surface area (Lehmann et al. 2009) and indirectly via subsequent increases in organic carbon in the soil (Blanco-Canqui and Lal 2009). Biochar not only increases crop yields under regular conditions, but also raises those yields amid environmental stresses such as salinity by decreasing sodium toxicity’s harmful effects after stimulating the potassium uptake of plants (Akhtar et al. 2015). Recently, Farhangi-Abriz and Torabian (2017) have reported that biochar protected bean seedlings against sodium toxicity by alleviating oxidative stress, and earlier, Quilliam et al. (2013) found that applying biochar improved the activity of nitrogenase in clover plants, but reduced nodulation. Van Zwieten et al. (2015) also observed that biochar improved nitrogen fixation in acidic soils.
Up to date, the interaction of biochar in the nodulation, nitrogen fixation, and metabolism of legumes under salt stress has not been addressed in published research. In response, the aim of the study reported here was to investigate the influence of biochar on soybean nodulation and nitrogen metabolism under salt stress.
2 Material and methods
2.1 Preparation of biochar
Biochar was produced according to the method of Qian et al. (2013). Maple residues (Acer pseudoplatanus L.) were split and passed through a 0.5 mm mesh and heated at 580 °C for 6 h in an environment without oxygen at a rate of 7 °C min−1. The element concentration of biochar in terms of carbon, nitrogen, hydrogen, and oxygen was assayed with an elemental analyzer (Elementar, Hanau, Germany). The chief properties of biochar appear in Table 1.
2.2 Experimental conditions
A pot experiment with four replications was conducted on March 5, 2016, in a glass greenhouse with a factorial design based on the randomized complete block design. Three levels of salinity (i.e., non-saline and 5 and 10 dS m−1 of NaCl) chosen according to the range of the salinity tolerance of soybean (Grieve et al. 2012) and three biochar treatments (i.e., non-biochar and 50 and 100 g kg−1 soil) were used to test the soybean (Glycine max cv. M7). The soil was mixed with biochar and distributed into pots 10 cm in radius and 25 cm in height in amounts of 2.5 kg per pot. Table 1 presents properties of the experimental soil. Next, six soybean seeds inoculated with B. japonicum (Strain RS146) in an amount of 108 bacteria per gram were sown in each pot. The pots were kept in controlled conditions in a glass greenhouse with a day and night temperature cycle of 25 and 23 °C, respectively, as well as 55–60% relative humidity, 150 W m−2 light intensity, and a 13 h photoperiod. Plants were irrigated every day with tap water in an amount comparable to field water capacity. After the first trifoliate leaf emerged, NaCl was added to the irrigation water supplied to the treatments involving saline. During the experiment, the electric conductivity of each pot was measured with a digital conductivity meter (inoLab Model, Weilheim, Germany). Conductivity was preserved at a favorable level by adding water or concentrated NaCl to the pots.
2.3 Nodule formation and plant performance
Nodule number and weight, as well as shoot and root dry weights, of the soybean were measured 58 d after sowing (i.e., during the flowering stage). The dry weights of tissues were determined after oven drying at 70 °C for 72 h.
2.4 Nitrogen content assay
To measure the nitrogen content of nodules and plants during the flowering stage, root and leaf tissues were washed with deionized water at least twice, dried in an oven at 70 °C for 48 h, and powdered, and their nitrogen contents were measured by using a CHNS elemental analyzer (Elementar, Hanau, Germany).
2.5 Enzyme assay
All enzymatic activity was assayed in soybean nodules, roots and leaves at the flowering stage. GS was assayed by measuring the creation of y-glutamyl-hydroxamate, which reacts with ferric chloride, and the brown color in the acid medium was measured at 540 nm (100 Conc U/V Visible Spectrophotometer, Varian, CA, USA). Tissue in portions of 100 mg was homogenized in an extraction buffer containing 0.1 M of potassium phosphate buffer with a pH of 7.8, 0.4 M of sucrose, 10 mM of KCI and DTT, 1 mM of MgCl2, and 10 mM of EDTA. Enzyme activity was estimated according to the standard curve with commercial y-glutamyl-hydroxamate (100–800 μg) (Sawhney and Singh 1985). GDH activity was assayed after the oxidation of NADH at 340 nm. Fresh tissue in amounts of 200 mg was homogenized in 3 mL of extraction buffer containing 0.05 M of Tris-HCL with a pH of 7.5, 0.4 M of sucrose, and 0.01 M of β-mercaptoethanol, and the mixture was centrifuged at 20,000 g for 30 min. Prepared samples in amounts of 1 mL were added to the reaction mixture in amounts of 2 mL consisting of 1.6 mL of 0.1 M of Tris-HCI buffer with a pH of 7.5, 0.1 mL of 2-oxoglutarate (0.33 M), 0.1 mL of NH4CI 3 M, and 0.2 mL of NADH. Absorbance was read at 340 nm. Measuring GOGAT activity involved using the same extraction buffer for GDH. The reaction mixture for the enzyme included 0.7 mL of 0.1 M of Tris-HCI buffer at a pH of 7.5, 1 mL of glutamine at a pH of 7.0, 0.1 mL of 0.33 M 2-oxoglutarte, 0.2 mL of 10−3 M NADH, and 1 mL of extraction enzyme. Absorbance was read at 340 nm by following the method of Duke and Ham (1976). Lastly, NR activity was measured spectrophotometrically at 540 nm per the method of Jaworski (1971).
2.6 Physiological performance
The content of chlorophyll a, chlorophyll b, and total chlorophyll in leaves was measured by following Arnon’s (1949) method. A 200 mg of fresh leaf sample was cut and extracted with 80% acetone at −4 °C, and the extracted samples were centrifuged at 10,000 g for 10 min. Supernatant was collected and absorbance read at 645 and 663 nm using a spectrophotometer (100 Conc U/V Visible Spectrophotometer, Varian, CA, USA). Leaf area was measured by a portable area meter (model ADC-AM 300 UK). The activity of the rubisco enzyme from 100 mg of the leaf samples was assayed by following the methods of Lobo et al. (2015), which involved spectrophotometrically measuring the oxidation of NADH at 340 nm. The assayed buffer contained 100 mM of bicine, 25 mM of KHCO3, 20 mM of MgCl2, 3.5 mM of ATP, 5 mM phosphocreatine, 80 nkat of G-3-P dehydrogenase, 80 nkat of 3-phosphoglyceric phosphokinase, 80 nkat of creatine-phosphokinase, and 0.25 mM of NADH.
2.7 Analysis of variance
Data were analyzed according to the experimental design using MSTATC software, and means were compared with least significant difference test at p ≤ 05. All figures were drawn by using Microsoft Excel 2016.
3 Results
3.1 Nodule number and weight
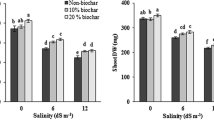
The interactive effects of salinity and biochar significantly affected nodule number and weight per plant (p ≤ 0.01). In general, salinity reduced nodule number and weight (Fig. 1). Although no tangible difference emerged among biochar treatments in terms of nodule number and weight in conditions of non-salinity, the application of biochar significantly increased the nodule number and weight of soybean in conditions of moderate and severe salinity.
3.2 Plant performance
Salinity and biochar significant affected the dry weights of the roots, shoots, total plant biomass and total nitrogen content of the soybean (p ≤ 0.01). Although increased salinity noticeably reduced their dry weights, total plant biomass and total nitrogen content. The difference in root weight in conditions of 5 and 10 dS m−1 was not significant (Fig. 2). Applying biochar particularly improved the dry weights of the roots and shoots as well as total plant biomass and total nitrogen content, although no difference emerged between biochar in amounts of 50 and 100 g kg−1.
3.3 Nitrogen metabolism in nodules
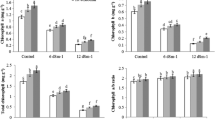
Nitrogen content in the soybean nodules decreased in conditions of 5 and 10 dS m−1 of NaCl. When biochar was added to the soil, nitrogen content in the nodules improved. Both treatments involving biochar showed a statistically similar effect on the nitrogen content of nodules (Table 2). GDH, GOGAT, and NR activity decreased under salt stress; however, GS activity in amounts of 5 dS m−1 did not decrease, yet did when salinity reached 10 dS m−1. All enzyme activity in soybean nodules significantly improved with biochar. Interestingly, biochar usage increased GS activity and nitrogen content in conditions without saline, which was not observed regarding the other enzymes in nodules (Table 2).
3.4 Nitrogen metabolism in roots
Salinity and biochar significantly influenced the nitrogen content of roots as well. The nitrogen content in roots diminished with rising salinity, yet increased with the addition of biochar to the soil. GDH, GS, GOGAT, and NR activity in the roots decreased as salinity increased, yet improved with the addition of biochar. Results presented in Table 3 show that with biochar added to the soil, GDH and NR activity and nitrogen content markedly improved under non-saline condition.
3.5 Nitrogen metabolism in leaves
The nitrogen content of soybean leaves decreased with higher salinity, yet improved with the addition of biochar. All enzyme activity in nitrogen metabolism in the leaves decreased as salt stress increased, yet improved with the addition of biochar as well. In short, adding biochar to the soil enhanced the nitrogen content and GDH, GS, GOGAT, and NR activity of leaves in conditions with and without saline (Table 4).
3.6 Physiological performance
Salt stress and biochar treatments markedly affected chlorophyll a, chlorophyll b, and total chlorophyll. The chlorophyll content of soybean leaves decreased in amounts of 5 and 10 dS m−1; however, the a/b chlorophyll ratio increased with increased salinity. On average, the application of biochar improved chlorophyll content (i.e., a, b, and total) and the a/b chlorophyll ratio in soybeans. Biochar at the rates of 50 and 100 g kg−1 showed similar results on improving chlorophyll contents of leaves (Table 5).
Lastly, the effects of salt stress and biochar on leaf area (LA) and the rubisco activity in soybeans were significant. Increased salt stress strongly reduced LA and rubisco activity (Table 5), whereas adding biochar to the soil at both experimental rates improved them.
4 Discussion
Among the most common forms of environmental stress worldwide (López-Gómez et al. 2016), salinity can wreak numerous adverse effects on nitrogen metabolism and nodulation processes in plants (Faghire et al. 2011). In particular, decreased nodule numbers and weights of soybeans under salt stress (Fig. 1) stem from the harmful effects of salt ions on rhizosphere biota and soil pH, while salt stress reduces nodulation factors in legumes. More generally, salinity reduces the growth of plants (Ghoulam et al. 2002), increases oxidative damage in plant tissues (Farhangi-Abriz and Torabian 2017) and, in soybeans, compromises root and shoot growth. The results of the study reported here clearly show that salt stress diminished nitrogen content, the nitrogen metabolism of soybean (Tables 3 and 4), nodulation processes (Fig. 1, Table 2), root and shoot growth (Fig. 2). Many factors related to soil quality, including pH, calcium level, form of nitrogen, and nitrogen availability, can affect the degree of legume nodulation (Slattery et al. 2001). All of those parameters can be improved with the addition of biochar (Jones et al. 2012). Even despite salt stress, improved nodulation in soybean with the application of biochar (Fig. 1) could derive from improved nodulation factors such as root flavonoids and special components (e.g., richadsin). Moreover, biochar improved plant performance under salt stress (Table 5) by providing more photosynthetic products, including carbohydrates for rhizobium microorganisms in root zones. Biochar can also alter soil biota under sodium toxicity. However, future research should further explore all reported benefits of biochar on soybean. Increased root and shoot growth under salt stress (Fig. 2) relates to the increased nitrogen content in plant tissues due to improved nitrogen metabolism in different parts of the soybean (Tables 2, 3, and 4). Improved plant performance in terms of increased chlorophyll content, leaf area, and the rubisco activity of leaves (Table 5) play a vital role in the successful growth of soybean under salt stress. Previous research has indicated that under salt stress, biochar alters the oxidative stress in plant tissues and thereby improves plant growth (Farhangi-Abriz and Torabian 2017).
Decreasing the nitrogen content in nodules and plant tissues under salt stress (Tables 2, 3, and 4 and Fig. 2) could relate to the diminished nitrogen uptake, and nitrogen metabolism in the different parts of the soybean (Tables 2, 3, and 4). GS and GOGAT or an alternative GDH pathway may incorporate nitrogen from both nitrate reduction and soil into plant cells. The decreased activity of those enzymes under salt stress reduced nitrogen content in the roots and leaves of soybean, and diminished enzyme activity with increased salt stress could relate to the generally harmful effects of salt stress in plants, including ion imbalances, toxicity, oxidative damage, and the down regulation of some genes (Kusano et al. 2011). Oxygen permeability in nodules is crucial for controlling nitrogenase activity. Reducing the nitrogen content of nodules under salt stress could stem from the reduced oxygen concentration in root zones and the nodule cortex (James et al. 1993; Serraj et al. 1994; Fernández-Pascual et al. 1996). Moreover, salt stress has been observed to reduce the mobilization of assimilates to the nodules (Fougere et al. 1991; Munns and James 2003) and consequently inhibit nitrogen metabolism (Table 2). From the results of the study reported here, it is clear that all biochar treatments improved nitrogen fixation and the nitrogen metabolism of soybean under salt stress. Enriching nitrogen to plant tissues by applying biochar presumably resulted from improved enzymatic activity in the nodules, roots, and leaves of the plants (Tables 2, 3, and 4). The improved nitrogen content in nodules and plants due to treatment with biochar could have resulted from the altered pH of the soil.
Nitrogen supply greatly affects leaf growth by increasing the LA of crops and, in turn, altering the influences of photosynthesis. Chlorophyll content is approximately proportional to leaf nitrogen content (Evans 1983), and studies have also suggested a positive correlation between chlorophyll content and the level of nitrogen (Yu-kui et al. 2012; Rambo et al. 2010). The results reported here clearly indicate that by reducing nitrogen metabolism and nitrogen content, salt stress also decreased chlorophyll synthesis, LA, and rubisco activity in soybeans (Tables 2, 3, and 4). The observed reduction of chlorophyll content in soybeans grown in conditions involving saline could stem from both the increased degradation and inhibited synthesis of the pigment (Garsia-Sanchez et al. 2002). Taffouo et al. (2010) reported that sodium toxicity decreased the content of photosynthetic pigments in treated plants. Improved chlorophyll content, LA, and rubisco activity in treatments with biochar (Table 5) resulted from increased nitrogen content and metabolism in soybean (Tables 2, 3, and 4). Consistent with the results reported here, Wang et al. (2014) found that biochar noticeably increased the photosynthetic rate and chlorophyll content in the leaves of Malus hupehensis Rehd seedlings. Several other studies have indicated that the general performance of plants increases with the addition of biochar (Van Zwieten et al. 2010; Lehmann et al. 2011; Thomas et al. 2013; Akhtar et al. 2015; Hammer et al. 2015).
With regard to the results, salt stress adversely affects the nodulation and nitrogen metabolism of soybean. Applying biochar can improve the nodulation, nitrogen content and nitrogen metabolism of the plants by stimulating nitrogen fixation and GDH, GS, GOGAT, and NR activity. With improved nitrogen content in soybean, general plant performance, including shoot and root dry weight, chlorophyll content, LA, and rubisco activity all increased. The results reported here show that applying biochar under salt stress could mitigate the harmful effects of salt stress by enhancing the nitrogen metabolism in soybean and increasing the plants’ general performance. The study reported here involved conducting a pioneering evaluation of the effects of biochar on the nodulation and nitrogen metabolism of soybean under salt stress. Future work should seek to confirm the effects of applying biochar on nitrogen fixation and metabolism in other legumes, as well as examine changes in nitrogen metabolism with organic matter under other environmental conditions.
References
Abd-Alla MH, Vuong TD, Harper JE (1998) Genotypic differences in dinitrogen fixation response to NaCl stress in intact and grafted soybean. Crop Sci 38:72–77
Akhtar SS, Andersen MN, Liu F (2015) Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric Water Manag 158:61–68
Antal MJ, Gronli M (2003) The art, science, and technology of charcoal production. Ind Eng Chem Res 42:1619–1640
Araújo SS, Beebe S, Crespi M, Delbreil B, González EM, Gruber V, Lejeune-Henaut I, Link W, Monteros MJ, Prats E, Rao I (2015) Abiotic stress responses in legumes: strategies used to cope with environmental challenges. Crit Rev Plant Sci 34:237–280
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant physiol 24:1–15
Ascough PL, Bird MI, Brock F, Higham TFG, Meredith W, Snape CE, Vane CH (2009) Hydropyrolysis as a new tool for radiocarbon pre-treatment and the quantification of black carbon. Quat Geochronol 4:140–147
Becker TW, Carrayol E, Hirel B (2000) Glutamine synthetase and glutamate dehydrogenase isoforms in maize leaves: localization, relative proportion and their role in ammonium assimilation or nitrogen transport. Planta 211:800–806
Berman-Frank I, Lundgren P, Falkowski P (2003) Nitrogen fixation and photosynthetic oxygen evolution in cyanobacteria. Res Microbiol 154:157–164
Blanco-Canqui H, Lal R (2009) Crop residue removal impacts on soil productivity and environmental quality. Crit Rev Plant Sci 28:139–163
Chakrabarti N, Mukherji S (2003) Effect of phytohormone pretreatment on nitrogen metabolism in Vigna radiata under salt stress. Biol Plant 46:63–66
Chan KY, Xu Z (2009) Biochar: nutrient properties and their enhancement. Biochar for environmental management: science and technology. Earthscan, London, pp 67–84
Comba ME, Benavides MP, Tomaro ML (1998) Effect of salt stress on antioxidant defense system in soybean root nodules. Funct Plant Biol 25:665–671
Duke SH, Ham GE (1976) The effect of nitrogen addition on N2-fixation and on glutamate dehydrogenase and glutamate synthase activities. In nodules and roots of soybeans inoculated with various strains of Rhizobium japonicum. Plant Cell Physiol 17:1037–1044
Evans JR (1983) Nitrogen and photosynthesis in the flag leaf of wheat (Triticum aestivum L.) Plant Physiol 72:297–302
Faghire M, Bargaz A, Farissi M, Palma F, Mandri B, Lluch C, García NAT, Herrera-Cervera JA, Oufdou K, Ghoulam C (2011) Effect of salinity on nodulation, nitrogen fixation and growth of common bean (Phaseolus vulgaris) inoculated with rhizobial strains isolated from the Haouz region of Morocco. Symbiosis 55:69–75
Faghire M, Mohamed F, Taoufiq K, Fghire R, Bargaz A, Mandri B, Oufdou K, Laury A, Drevon JJ, Ghoulam C (2013) Genotypic variation of nodules’ enzymatic activities in symbiotic nitrogen fixation among common bean (Phaseolus vulgaris L.) genotypes grown under salinity constraint. Symbiosis 3:115–122
Fahad S, Hussain S, Saud S, Hassan S, Tanveer M, Ihsan MZ, Shah AN, Ullah A, Khan F, Ullah S, Alharby H (2016) A combined application of biochar and phosphorus alleviates heat-induced adversities on physiological, agronomical and quality attributes of rice. Plant Physiol Biochem 103:191–198
Farhangi-Abriz S, Torabian S (2017) Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicol Environ Saf 137:64–70
Fernández-Pascual M, De Lorenzo C, de Felipe MR, Rajalakshmi S, Gordon AJ, Thomas BJ, Minchin FR (1996) Possible reasons for relative salt stress tolerance in nodules of white lupin cv. Multolupa J Exp Bot 47:1709–1716
Flowers TJ, Gaur PM, Gowda CL, Krishnamurthy L, Samineni S, Siddique KH, Colmer TD (2010) Salt sensitivity in chickpea. Plant Cell Environ 33:490–509
Fougere F, Le Rudulier D, Streeter JG (1991) Effects of salt stress on amino acid, organic acid, and carbohydrate composition of roots, bacteroids, and cytosol of alfalfa (Medicago sativa L.) Plant Physiol 96:1228–1236
Garsia-Sanchez F, Jufon JL, Carvaial M, Syverstem JP (2002) Gas exchange, chlorophyll and nutrient contents in relation to Na+ and cl− accumulation in ‘sunburst’ mandarin grafted on different rootstocks. Plant Sci 162:705–712
Ghoulam C, Foursy A, Fares K (2002) Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ Exp Bot 47:39–50
Grieve CM, Grattan SR, Maas EV (2012) Plant salt tolerance. Agricultural salinity assessment and management, 2nd edn. ASCE Manual Rep Eng Pract 71:405–459
Hammer EC, Forstreuter M, Rillig MC, Kohler J (2015) Biochar increases arbuscular mycorrhizal plant growth enhancement and ameliorates salinity stress. Appl Soil Ecol 96:114–121
Huchzermeyer B, Heins T (2000) Energy metabolism and salt stress. INCO-DC annual report. University of Osnabrück Publication, Osnabrück, pp 48–73
James EK, Sprent JI, Hay GT, Minchin FR (1993) The effect of irradiance on the recovery of soybean nodules from sodium chloride-induced senescence. J Exp Bot 44:997–1005
Jaworski EG (1971) Nitrate reductase assay in intact plant tissues. Biochem Biophys Res Commun 43:1274–1279
Jones DL, Rousk J, Edwards-Jones G, DeLuca TH, Murphy DV (2012) Biochar-mediated changes in soil quality and plant growth in a three-year field trial. Soil Biol Biochem 45:113–124
Koyro HW, Huchzermeyer B (1999) Influence of high NaCl-salinity on growth, water and osmotic relations of the halophyte Beta Vulgaris Ssp. Maritima. Development of a quick check. Prog Biometeorol 13:87–101
Kusano M, Fukushima A, Redestig H, Saito K (2011) Metabolomic approaches toward understanding nitrogen metabolism in plants. J Exp Bot 62:1439–1453
Lehmann J, Czimczik C, Laird D, Sohi S (2009) Stability of biochar in soil. Biochar for environmental management: science and technology. Earthscan, London, pp 183–206
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota-a review. Soil Biol Biochem 43:1812–1836
Lobo AKM, de Oliveira MM, Neto MCL, Machado EC, Ribeiro RV, Silveira JAG (2015) Exogenous sucrose supply changes sugar metabolism and reduces photosynthesis of sugarcane through the down-regulation of Rubisco abundance and activity. J Plant Physiol 179:113–121
López-Gómez M, Hidalgo-Castellanos J, Lluch C, Herrera-Cervera JA (2016) 24-Epibrassinolide ameliorates salt stress effects in the symbiosis Medicago Truncatula-Sinorhizobium meliloti and regulates the nodulation in cross-talk with polyamines. Plant Physiol Biochem 108:212–221
Munns R, James RA (2003) Screening methods for salinity tolerance: a case study with tetraploid wheat. Plant Soil 253:201–218
Ogawa M, Okimori Y (2010) Pioneering works in biochar research, Japan. Soil Res 48:489–500
Qian L, Chen B, Hu D (2013) Effective alleviation of aluminum phytotoxicity by manure-derived biochar. Environ Sci Technol 47:2737–2745
Quilliam RS, Rangecroft S, Emmett BA, Deluca TH, Jones DL (2013) Is biochar a source or sink for polycyclic aromatic hydrocarbon (PAH) compounds in agricultural soils? GCB Bioenergy 5:96–103
Rambo L, Ma BL, Xiong Y, da Silvia Ferreira PR (2010) Leaf and canopy optical characteristics as crop-N-status indicators for field nitrogen management in corn. J Plant Nutr Soil Sci 173:434–443
Sawhney V, Singh R (1985) Effect of applied nitrate on enzymes of ammonia assimilation in nodules of Cicer arietinum L. Plant Soil 86:241–248
Serraj R, Roy G, Drevon JJ (1994) Salt stress induces a decrease in the oxygen uptake of soybean nodules and in their permeability to oxygen diffusion. Physiol Plant 91:161–168
Sinclair TR, Horie T (1989) Leaf nitrogen, photosynthesis, and crop radiation use efficiency: a review. Crop Sci 29:90–98
Singh M, Singh VP, Prasad SM (2016) Responses of photosynthesis, nitrogen and proline metabolism to salinity stress in Solanum lycopersicum under different levels of nitrogen supplementation. Plant Physiol Biochem 109:72–83
Singleton PW, Bohlool BB (1984) Effect of salinity on nodule formation by soybean. Plant Physiol 74:72–76
Slattery JF, Coventry DR, Slattery WJ (2001) Rhizobial ecology as affected by the soil environment. Anim Prod Sci 41:289–298
Steinbeiss S, Gleixner G, Antonietti M (2009) Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol Biochem 41:1301–1310
Taffouo VD, Wamba OF, Yombi E, Nono GV, Akoe A (2010) Growth, yield, water status and ionic distribution response of three bambara groundnut (Vigna subterranean L. verdc.) landraces grown under saline conditions. Int J Bot 6:53–58
Thomas SC, Frye S, Gale N, Garmon M, Launchbury R, Machado N, Melamed S, Murray J, Petroff A, Winsborough C (2013) Biochar mitigates negative effects of salt additions on two herbaceous plant species. Environ Manag 129:62–68
Tu JC (1981) Effect of salinity on rhizobium-root-hair interaction, nodulation and growth of soybean. Can J Plant Sci 61:231–239
Van Zwieten L, Kimber S, Morris S, Chan KY, Downie A, Rust J, Joseph S, Cowie A (2010) Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327:235–246
Van Zwieten L, Rose T, Herridge D, Kimber S, Rust J, Cowie A, Morris S (2015) Enhanced biological N2 fixation and yield of faba bean (Vicia faba L.) in an acid soil following biochar addition: dissection of causal mechanisms. Plant Soil 395:7–20
Wang YF, Jiang D, Yu ZW, Cao WX (2003) Effects of nitrogen rates on grain yield and protein content of wheat and its physiological basis. Sci Agric Sin 36:513–520 in Chinese with English abstract
Wang Y, Pan F, Wang G, Zhang G, Wang Y, Chen X, Mao Z (2014) Effects of biochar on photosynthesis and antioxidative system of Malus hupehensis Rehd. Seedlings under replant conditions. Sci Hort 175:9–15
Yu-kui R, Yun-feng P, Zheng-rui W, Jian-bo S (2012) Stem perimeter, height and biomass of maize (Zea mays L.) grown under different N fertilization regimes in Beijing, China. Int J Plant Prod 3:85–90
Acknowledgements
We appreciate the University of Tabriz for providing greenhouse and laboratory.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farhangi-Abriz, S., Torabian, S. Biochar improved nodulation and nitrogen metabolism of soybean under salt stress. Symbiosis 74, 215–223 (2018). https://doi.org/10.1007/s13199-017-0509-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-017-0509-0