Abstract
Background and Aims
Salt stress negatively affects alfalfa (Medicago sativa L.) production and biological nitrogen fixation. We investigated whether rhizobium symbiosis has an effect on host plant tolerance to salt stress.
Methods
We determined the survival rate, oxidative damage level, activities of antioxidant enzymes, and contents of osmotic solutes in the leaf and root of 4-month-old alfalfa with active nodules, inactive nodules or without nodules, and under short-term salt stress.
Results
Alfalfa with active nodules showed higher survival rate. Higher survival rate was associated with reduced lipid peroxidation, higher activities of superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and ascorbate peroxidase (APX) as well as higher concentrations of reduced glutathione (GSH) and soluble sugar, especially in roots under salt stress. Variance analysis indicated nodulation affected the activities of SOD, CAT, POD, and APX along with concentrations of GSH, soluble sugar, and soluble protein. Inoculation also resulted in higher basal levels of superoxide anion radical (O2 −·) without salt stress.
Conclusions
Rhizobium symbiosis had a positive effect on alfalfa salt tolerance by improving the activity of antioxidant enzymes and osmotic adjustment capacity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Symbiotic nitrogen fixation (SNF) has profound agronomic, economic, and ecological impacts because the availability of fixed nitrogen most frequently limits agricultural production throughout the world (Hoffman et al. 2014). Symbiosis of rhizobia with more than 100 agriculturally important legumes contributes at least half of the annual nitrogen fixed in soil ecosystems (Peoples and Craswell 1992). Moreover, SNF could replace fertilizer that degrades lands and pollutes groundwater (Bolanos et al. 2006), and SNF may constitute a sustainable alternative to chemical fertilization in salinity-affected areas (El-Akhal et al. 2013). Therefore, understanding and improving the tolerance mechanism of rhizobial legumes to saline environments are particularly important for both agricultural and environmental conservation.
In SNF, plants provide carbon compounds and energy sources needed by rhizobia in exchange for fixed nitrogen. In addition to nitrogen fixation, rhizobia have been shown to benefit plants in many different ways. Rhizobia produce molecules that can influence plant development, including phytohormones, lipochitooligosaccharide nod factors, lumichrome, riboflavin, and H2 generated by nitrogenase (Dakora 2003). When present in soil, nod factors can stimulate seed germination, promote plant growth, and increase grain yields, as well as increase photosynthetic rates. Very low concentrations of lumichrome and H2 released by rhizobia also promote plant growth and increase biomass (Dakora 2003). Rhizobia are known to suppress the population of soil pathogens (Berendsen et al. 2012), and the legume itself releases phenolics that can suppress pathogens and promote growth of mutualistic microbes (Siqueira et al. 1991). The inoculation of legumes with rhizobia, which resulted in sink stimulation of photosynthesis, improved the photosynthetic nutrient use efficiency and the proportion of seed yield in relation to the total plant biomass (Kaschuk et al. 2009). Root nodules have a stronger antioxidant defense capacity than the host roots: higher amounts of antioxidant molecules (ascorbate, reduced glutathione (GSH), and homoglutathione (hGSH)) and higher activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), peroxidases (POD), and ascorbate-glutathione cycle enzymes. Therefore, plant-associated rhizobia can impart some degree of tolerance by plants to abiotic stresses (Grover et al. 2011; Shrivastava and Kumar 2015). Recent studies showed that the expression of genes related to plant defense might initially be upregulated after inoculation with rhizobia (Deakin and Broughton 2009). These effects imply that symbiosis may enhance the overall health status of plants and improve their tolerance to abiotic stresses, including salinity.
The low number of studies available provides a mixed picture for how the symbiotic relation impacts plant tolerance to salinity. It has been reported that N-fixing legumes were more sensitive to salt stress than those depending on mineral nitrogen (Bouhmouch et al. 2005). Compared with N-fertilized plants, those fixing nitrogen showed a larger degree of decreased yield (Lauter et al. 1981), less nitrogen accumulation (Serraj and Drevon 1998), and greater uptake of Na+ and Cl− under NaCl stress, inducing ionic imbalance in the host plant (Yousef and Sprent 1983). SNF is a process requiring high energy input from host plants and may compete for energy that is required for powering plant salt tolerance mechanisms. As a result, maintaining high SNF might compromise plant tolerance to salinity and even survival of stress. Interestingly, Cordovilla et al. (1999) reported that pea plants (Pisum sativum L.) dependent on nitrogen fixation were more tolerant to salt stress than those N-fertilized, in contrast to the results obtained with faba bean (Vicia faba L.) where growth inhibition of shoot and root was observed in nitrogen-fixing plants. Thus, the effects of symbiosis on legume salt tolerance appear to depend heavily on species. More studies are needed to address how this symbiotic relationship impacts salt tolerance in legumes and to understand the physiological processes affected.
Salt tolerance is a complex phenomenon that involves morphological and developmental changes as well as physiological and biochemical processes. Many salt tolerant plants adapt to salinity by minimizing the direct effects of Na or Cl ions through mechanisms such as salt exclusion, salt sequestration, or salt excretion (Munns and Tester 2008). However, these mechanisms are usually not sufficient to limit the amount of salt entering the plants. Thus, many plants need to deal with the direct effects of salt toxicity. One adverse effect of salinity is the production of reactive oxygen species (ROS), such as superoxide anion (O2 −·) and hydrogen peroxide (H2O2), inducing oxidative stress (Ertani et al. 2013). ROS not only damage host plants but also negatively affect nitrogen fixation (Becana et al. 2000). The production of oxygen radicals stimulated by high salinity facilitates lipid peroxidation (Sreenivasulu et al. 1999). Prevention of ROS production in the nodule and reduction of oxidative stress could provide a protective effect on nodule structure and function in salinity stress (Redondo et al. 2012). Plants employ antioxidants (e.g., ascorbate and glutathione) and detoxifying enzymes, such as SOD, CAT, and ascorbate peroxidase (APX), to combat oxidative stress induced by salinity (Chinnusamy et al. 2005; Hernandez et al. 1995). It has been shown that a stronger antioxidant capacity is associated with salt tolerance. For example, the cotton callus from a NaCl-tolerant cultivar exhibited enhanced activities of SOD, CAT, and APX, whereas the callus tissue from a NaCl-sensitive cultivar showed a little change in the activities of these enzymes after salt stress (Gossett et al. 1994). Reduced glutathione, the major source of non-protein thiols in most plant cells, acts as an antioxidant buffer and is involved directly in scavenging ROS (Shao et al. 2008). An increase of GSH under salt stress has been shown to be important in mitigating the salt-induced oxidative stress in Brassica plants (Ruiz and Blumwald 2002). Dehydration often occurs concurrently with salinity stress, and the accumulation of solutes, like proline and soluble sugars, helps plant systems adapt to a saline environment by reducing water loss (Bohnert et al. 1995). Plants accumulate proline, soluble sugar, and stress responsive proteins that participate actively in the osmotic adjustment when plants are under salt stress (Evelin et al. 2009; Szabados and Savouré 2010).
Alfalfa (Medicago sativa L.) is one of the most important leguminous forage crops worldwide, and the SNF effect on its salt tolerance has not been studied. We hypothesize that rhizobium symbiosis may improve the alfalfa salt tolerance by affecting its physiological and biochemical processes such as enhancing the antioxidant capacity during stress response. In this study, we studied the response of well-developed alfalfa plants with active nodules (AN), inactive nodules (IN), and no nodules (NN) to salt stress by assessing the survival rate and their ability to deal with oxidative and osmotic stress induced by salt shock.
Materials and Methods
Plant materials and growth conditions
Seeds of alfalfa (M. sativa Ladak+) introduced from the USA, with a germination rate of more than 90 %, were surface-sterilized with 70 % ethanol for 30 s and 5 % sodium hypochlorite for 20 min, and thoroughly rinsed four to five times with sterile water. Seeds were then germinated in Petri dishes in a growth chamber for 5 days with a relative humidity of 70 % at 25 °C. Seedlings were transferred to conical plastic pots (H 30 cm × D 7 cm, one plant per pot) containing sterilized quartz sand without added nutrients and cultured in the greenhouse with a relative humidity of 55 ± 5 % and 70 ± 5 %, at 30 ± 5 °C and 20 ± 5 °C, during day and night, respectively.
Inoculation
When seedlings were about 10 cm in height, they were randomly and equally divided into three groups: (1) inoculated with Rhizobium meliloti strain Dormal and watered daily with 1/4 strength nitrogen-free Hoagland nutrient solution (Hoagland and Arnon 1950), which resulted in development of AN; (2) inoculated and watered with 1/4 strength Hoagland nutrient solution daily, which led to development of IN due to inhibition of rhizobia by sufficient nitrogen in the nutrient solution; and (3) not inoculated and watered with 1/4 strength Hoagland nutrient solution, which led to plants with NN. Shoots were cut on the sixtieth day and ninetieth day after inoculation to promote root and/or nodule growth. After 120 days of growth, plants were subjected to salt stress for survival tests and biochemical analyses.
Plant growth and nodule characterization
Plants were harvested before salt treatment for nodule examination as well as dry weight and nitrogen content analysis. Dry weight of shoots and roots was recorded after drying at 105 °C for 15 min and then at 70 °C to a constant weight. The nitrogen content was estimated using the Kjeldahl method. Nodule volume was calculated based on the diameter.
Survival rate tests
Beginning on day 120 after inoculation, each of the three groups of alfalfa was further divided into four salt treatments subgroups: 0 (control), 100, 200, and 300 mM NaCl dissolved in 1/4 strength Hoagland nutrient solution (for IN and NN) and 1/4 strength nitrogen-free Hoagland nutrient solution (for AN). Plants were treated twice daily for 30 days, followed by irrigation with the original nutrient solution without NaCl for another 20 days. Plants with regenerated green shoots were scored and survival rate (%) was defined as the number of live plants in proportion to total plants. Plant regrowth capacity was evaluated by measuring the fresh weight of regenerated shoots after salt stress.
Biochemical analyses
Another salt stress experiment was conducted to determine the biochemical changes in alfalfa plants immediately after salt shock. Plants were cultivated as described above and were subjected to salt shock 120 days after inoculation: 1/4 strength Hoagland nutrient solution (for IN and NN) and 1/4 strength nitrogen-free Hoagland nutrient solution (for AN) both containing 150 mM NaCl. Samples were harvested at 0 (control), 1, 2, 4, 6, and 12 h after salt shock. Leaves and roots were collected separately, frozen immediately in liquid nitrogen, and stored at −80 °C for further analysis. All experiments were repeated four times.
Lipid peroxidation
Lipid peroxidation was determined using the thiobarbituric acid (TBA) reaction as described by Puckette et al. (2007) with some modifications. Samples were homogenized in 5 mL of 5 % (w/v) trichloroacetic acid (TCA) on ice and then centrifuged at 3000×g for 15 min. To 2 mL of the supernatant aliquot, 5 mL of 5 % TCA containing 0.5 % TBA were added. The mixture was heated to 100 °C for 15 min, chilled in an ice bath, and centrifuged at 3000×g for 15 min. Absorbance of the supernatant was measured at 450, 532, and 600 nm. The concentration of malondialdehyde (MDA) was calculated as a measure of lipid peroxidation and expressed as millimole per gram dry weight (DW).
Determination of antioxidant enzyme activity
Samples were ground in a mortar on ice with 0.1-mM potassium phosphate buffer (PBS) (pH 7.8) within 1 % polyvinylpyrrolidone (PVP). Homogenate was centrifuged in a refrigerated centrifuge (10,000×g, 4 °C, 15 min), and the supernatant was used to assay the activity of all antioxidant enzymes.
Content of superoxide anion radical (O2 −·) was measured according to Elstner and Heupel (1976) with some modifications. To 1 mL of supernatant, 0.75 mL of PBS (0.65 mM, pH 7.8) and 1 mL of hydroxylamine hydrochloride (10 mM) were added. The mixture was incubated at 25 °C for 20 min, then mixed with 1 mL of sulfanilic acid (17 mM) and 1 mL of α-naphthylamine (7 mM), and incubated again at 30 °C for 30 min. The absorbance of the mixture was measured at 530 nm and sodium nitrite was used as standard solution.
Superoxide dismutase (SOD, EC, 1.15.1.1) activity was determined on the basis of its ability to inhibit the reduction of nitroblue tetrazolium (NBT) by superoxide anion generated by the riboflavin system under 4000 W (light intensity) at 25 °C. One unit of SOD was defined as the amount of enzyme required to inhibit the reduction rate of NBT by 50 % (Giannopolitis and Ries 1977). Peroxidase (POD, EC, 1.11.1.7) activity was measured using guaiacol (1-hydroxy-2-methoxybenzene, C7H8O2) as a substrate. The level of enzyme activity was expressed as the amount of guaiacol oxidized by POD per minute. Ascorbate peroxidase (APX, EC, 1.11.1.11) activity was assayed, as described by Nakano and Asada (1981), using ascorbate as a substrate. Oxidation of ascorbate was initiated by hydrogen peroxide (H2O2), and the decrease in absorbance at 290 nm was monitored. The concentration of oxidized ascorbate was calculated using an extinction coefficient of 2.8 mM−1 cm−1. One unit of APX activity was defined as the amount of enzyme required to oxidise 1 mM ascorbate per minute. Catalase (CAT, EC, 1.11.1.6) activity was measured by monitoring the consumption of H2O2 at 240 nm according to the method of Chance and Maehly (1955). One unit of CAT activity was defined as an absorbance change of 0.01 unit min−1.
Determination of reduced glutathione
Reduced glutathione content was determined by the following procedures described by Hissin and Hilf (1976) with certain modifications. Samples were ground with 3 mL of HPO3 (5 %) and then centrifuged at 14,000×g for 10 min. To 0.2 mL of supernatant, 2.6 mL of PBS (pH 7.0, 150 mM) and 0.2 mL of dithiobis-2-nitrobenzoic acid (DTNB, 0.1 mM) were added. After the mixture was incubated at 30 °C for 5 min for reaction, the absorbance of the mixture was measured at 412 nm.
Determination of compatible solutes
Proline content was determined following the method of Bates et al. (1973). Root and leaf tissues were homogenized in 4 mL of 3 % (w/v) sulfosalicylic acid and centrifuged at 10,000×g for 30 min. The supernatant (2 mL) was transferred to a test tube and mixed with 2 mL of glacial acetic acid and 2 mL ninhydrin reagent. The reaction mixture was kept in a water bath at 100 °C for 60 min to develop color. After cooling, the reaction mixture was mixed with 4 mL of toluene and vortexed for 30 s. The upper phase containing proline was measured with a spectrophotometer at 520 nm using toluene as a blank. Total soluble protein concentration was determined according to Bradford (1976) using bovine serum albumin as a standard. Five hundred milligrams of fresh materials were ground well in 10 mL of 50 mM cooled phosphate buffer (pH 7.8). The homogenate was centrifuged at 6000×g for 20 min at 4 °C. The supernatant was used to determine the total soluble proteins. The content of soluble sugar was determined following the method of Dreywood (1946). Roots or leaves were dried at 80 °C for 48 h to constant weight, incubated in 80 % ethanol at 80 °C for 30 min, and then centrifuged at 3500×g for 10 min. The mixture containing 2 mL of supernatant and 5 mL of anthrone reagent was boiled in a water bath for 10 min. The absorbance of the mixture was determined at 620 nm.
Statistical analyses
The experiment was carried out in a split plot design with a completely randomized block layout. Four salt levels (0, 100, 200, and 300 mM NaCl), three nodulation treatments (AN, IN, and NN), and two replicates comprised the survival assay. Salt levels were replaced by six sampling times (0, 1, 2, 4, 6, and 12 h after salt application) for determination of physiological characters. SPSS (version 16.0) was used for statistical analysis. Nodule volume was analyzed using independent T test. Other data were subjected to one-way analysis of variance and means were compared using S-N-K test at the level of significance (defined as α = 0.05). To explore the physiological responses of alfalfa leaf and root to nodulation under salt stress, between-factors effects were analyzed using univariate analysis of variance. Figures were created using Microsoft Excel (version 2007).
Results
Plant growth and nodule characterization
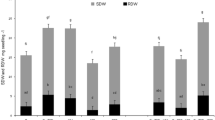
Plants of three nodulation treatments, with AN, IN, and NN, showed similar growth before salt treatment, indicated by no difference in shoot and root dry weight (Fig. 1a, b). Close examination showed NN plants lacked nodules, IN plants developed small but white nodules, and AN plants produced larger and pink nodules (Fig. 2a). Nodules of AN plants were almost four times larger than those of IN plants (Fig. 2c). Nitrogen content among these plants was similar in both shoots and roots, indicating the amount of nitrogen from SNF in AN plants is similar to that in NN and IN when obtaining nitrogen from a nutrient solution (Fig. 1c, d).
Dry weight (a and b) and nitrogen content (c and d) in shoot and root of alfalfa prior to salt treatment (AN alfalfa with active nodules, IN alfalfa with inactive nodules, NN alfalfa with no nodules. The data are means ± SE. Means are compared among the all groups. The significance is labeled with letters. The same as below except for specific notification.)
Survival and plant regrowth
Nodulation treatment affected alfalfa survival under salt stress and shoot regrowth after salt treatment (Table 1). Under 200 mM NaCl stress, AN and IN plants showed higher survival compared to NN plants which showed 50 % reduction in survival rate (Fig. 3a). AN produced greater fresh weight of shoot than NN during regrowth after 100 and 200 mM salt stress (Fig. 3b). While IN showed the lowest shoot weight under 100 mM salt treatment in regrowth, it showed better regrowth than NN plants after 200 mM treatment.
Oxidative damage
Methane dicarboxylic aldehyde (MDA) concentration, reflecting lipid peroxidation level, was influenced by nodulation under salt shock in alfalfa leaf and root (Table 2). In AN and IN leaves, MDA content was nearly 70 % higher than that of NN in control conditions and the first hour of salt shock (Fig. 4a). However, MDA content in NN leaf increased and stayed at a relatively constant but higher level from 2 to 6 h after salt shock compared to AN and IN. In particular, MDA level was 2.5 and 5.6 times higher than in AN and IN leaves, respectively, 6 h after salt shock. There was also a notable difference in MDA content among roots of AN, IN, and NN plants (Fig. 4b). MDA content in AN root was the lowest compared to NN and IN roots in control conditions and during salt shock for 1 and 2 h, while NN root maintained the highest MDA content during the same time. The highest level of root MDA content in NN appeared at the second hour after salt application while it delayed to the forth hour in AN.
Under control conditions and the first hour of salt shock, superoxide anion radical (O2 −·) content in the leaves and roots of AN and IN plants was higher than that in NN (Fig. 4c, d). However, O2 − · content of NN leaf increased with time, except for 4 h, while AN plants showed a significant reduction of O2 − · content and maintained a much lower level than NN plants. O2 − · content of NN leaf was about three times higher than AN and IN leaves 6 and 12 h after salt shock. O2 − · content in roots of AN, IN, and NN plants was similar from 4 to 12 h of salt shock.
Antioxidant defense
To explore the influence of nodulation on how plants deal with oxidative stress induced by a saline environment, we measured enzymatic activities of SOD, POD, APX, and CAT. Activities of these antioxidant enzymes were significantly affected by nodulation and salt shock, except SOD activity in the leaf was not influenced by nodulation (Fig. 4a and Table 1). The SOD activity was nearly doubled in NN and IN leaves 1 h after salt shock and returned to the original level 2 h after stress. The SOD activity in AN leaf was lower during the first 2 h but doubled 4 h after stress, reaching a similar level as NN and IN plants 12 h after stress (Fig. 4a). The SOD activity in AN root displayed an increasing trend with salt shock and activity was higher than that of IN and NN roots except for the first hour of salt shock (Fig. 4b). The POD activity in both leaves and roots of AN and IN plants was significantly higher than NN plants in control conditions (Fig. 5c, d). Salt shock had little effect on POD activity in NN plants but resulted in significant reduction in AN and IN, reaching similar levels for all three types of plants 4 h after salt shock. CAT activity in AN leaf was the lowest compared to NN and IN plants and showed a little change in response to salt shock, except at 4 h. CAT activity in NN and IN leaves showed gradual reduction with time of salt shock but maintained relatively higher levels than AN plants (Fig. 4e). In contrast to the leaf, the root of AN plants showed higher CAT activity without salt shock and maintained a relatively constant but higher level than NN and IN plants (Fig. 4f). The APX activity in leaves of AN and IN plants was higher than NN plants before salt stress (Fig. 4g). APX activity decreased with time after salt shock in all plants, although IN and AN plants maintained higher activities during the first 4 h of salt shock. The APX activity was the same in roots of AN and NN plants before salt shock and showed limited change during salt shock (Fig. 4h). The APX activity in IN root was higher than AN and NN plants and fluctuated during salt shock but reached similar levels as AN and NN plants 6 h after salt shock.
Nodulation effected GSH content during salt shock. Both root and leaf of NN plants showed an increase in GSH level, reaching a higher level than AN and IN plants 2 h after salt shock. GSH levels in NN leaf returned to original levels 4 h after salt shock. The GSH level in root and leaf of AN plants showed small increases with time after salt shock and was significantly higher than NN plants 4 and 12 h after salt shock. GSH content remained relatively constant in IN plants before and after salt shock (Fig. 6a, b).
Effect of nodulation on contents of GSH (a and b), proline (c and d), soluble sugar (e and f), and soluble protein (g and h) in alfalfa leaf and root under salt shock (Data are means ± SE of four experiments. Different letters indicate significant difference (p < 0.05) amount AN, IN, and NN plants at the same time point.)
Compatible solutes and soluble protein content
Proline content in alfalfa leaves was affected by nodulation (Table 2). Proline content in AN leaf was lower than in IN and NN plants before salt stress. Salt shock increased proline content significantly in all the plants during the first 4 hours and remained at that level in all treatments. Proline content in AN plants reached that same level as IN and NN plants at 6 h after salt shock (Fig. 5c). No difference in root proline content among AN, IN, and NN plants before salt treatment was evident. Salt shock resulted in great fluctuation of proline content. A clear difference was observed in plants 1 h after salt shock. AN showed the lowest proline content while NN plants showed the greatest proline content (Fig. 5d). Soluble sugar content was similar in leaves of AN, IN, and NN plants before salt stress. Salt shock appeared to increase the soluble sugar content in all plants, which reached the highest levels at 2, 4, and 6 h after salt shock in IN, NN, and AN leaves, respectively (Fig. 5e). AN and NN roots showed the same amount of soluble sugar before salt treatment. Salt shock had little effect on soluble sugar in AN roots, but caused a continuous decline in NN roots. Soluble sugar content fluctuated in IN plants but was maintained at levels similar to the control (Fig. 5f). No difference in soluble protein content was observed in AN, IN, and NN plant before salt treatment in either roots or leaves. Both root and leaf of AN plants maintained relatively constant levels of soluble protein after salt shock, while NN plants showed drastic fluctuation of soluble protein content. IN plants also showed fluctuating soluble protein level after salt shock but changes were less drastic compared to NN plants (Fig. 6g, h).
Discussion
In our experiments, we demonstrated that rhizobium symbiosis can improve salt tolerance in alfalfa based on survival rate at 200 mM NaCl treatment. Better salt tolerance is probably not simply due to a difference in nitrogen nutrition level since there was a little difference in nitrogen content in AN, IN, and NN plants prior to salt treatment. The notion is further supported by better shoot regrowth of AN plants at 100 and 200 mM NaCl treatments, compared to NN plants, suggesting that the interaction between rhizobia and host plants inherently improves the tolerance of alfalfa to salinity. However, our data also showed that AN plants exhibited better shoot regrowth than IN plants under salt stress. There may be two possible explanations for this observation. First, the production of some of the active molecules supporting salt tolerance requires functional symbiosis. Second, non-functional nodules may be parasitic, competing for energy required by host plants in response to salt stress, which may explain why IN plants even performed worse than NN plants at 100 mM NaCl stress. In fact, a study has shown that soybean plants will sanction Rhizobium by limiting oxygen supply to the bacterium if Rhizobium does not fix nitrogen (Kiers et al. 2003).
Our data indicated that symbiosis modified physiological and biochemical responses in alfalfa immediately after salt shock, providing a physiological basis for improved salt tolerance in symbiotic alfalfa plants during the long-term salt stress experiments. Our biochemical analysis clearly showed how symbiosis and nodulation affect the antioxidant response. The production of ROS is an inevitable consequence of aerobic respiration. ROS are capable of reacting indiscriminately to cause oxidative damage to biomolecules such as lipid peroxidation, denaturation of proteins, and mutation of DNA (Porcel et al. 2012). Common ROS include O2 −·, H2O2, and hydroxyl free radical (OH·) (Gill and Tuteja 2010). Estimates suggest that 2 to 5 % of the photosynthetically produced electrons are dissipated by this reaction under normal conditions and up to 30 % under stress (Polle 2001). Therefore, O2 − · could be an indicator of oxidative injury to plant cells and tissue. In our experiment, higher O2 − · content was found in leaves of IN and AN plants under control conditions, potentially due to higher photosynthetic activity required to meet the symbiosis needs. It has been suggested that, during the symbiotic interaction, bacteria are exposed to oxidative stress during the oxidative burst that follows the infection process (Peleg-Grossman et al. 2012; Santos et al. 2001). This may also explain why leaves, as well as roots, of AN and IN plants showed a higher O2 − · content during control conditions. However, AN and IN plants were able to control O2 − · content at lower levels during salt shock, while O2 − · content increased with time in NN leaf. AN maintained higher O2 − · content than non-nodulated alfalfa at the first hour of salt shock in the leaf and root, which suggested that O2 − · content may be affected by the rhizobium symbiosis with the host plant. The functional nodule is prone to high levels of ROS due to the high respiration rate necessary to supply energy required by the nitrogen reduction process (Harrison et al. 2005). While O2 − · is produced during the infection process, it is also produced in the later stages of nodulation. O2 − · can be observed in infected cells of young nodules, revealing the prolonged production of O2 − · during nodule development (Pauly et al. 2006). It is not clear whether a higher production of O2 − · in AN roots 1 h after salt shock is due to a hindered nodulation process since an increase in O2 − · production was not observed at that time in IN plants.
MDA, one of the decomposition products of polyunsaturated fatty acids (PUFA) of biomembranes, accumulated under salinity treatment (Chaudhuri and Chouchuri 1993). MDA content was the most important marker for salt tolerance (Ashrafi et al. 2015). High salinity could modify the plant membrane structure and might stimulate oxygen radical production which facilitated lipid peroxidation (Sreenivasulu et al. 1999). Stable and lower MDA content at the early stage of salt shock in AN and IN roots indicated that the membrane structure damage and oxidative stress are less in alfalfa root with nodules. Similarly, the lower MDA content during the period from 2 to 6 h salt shock in the AN and IN leaves suggests that inoculated alfalfa is more tolerant of salt shock compared with alfalfa without nodules. This is consistent with the study conducted by Wu et al. (2010), who found that there is notably lower MDA content in the leaves of citrus seedlings with mycorrhiza than in non-mycorrhizal seedlings.
Plant cells are capable of suppressing the buildup of harmful intracellular ROS concentrations. This is achieved by antioxidative defense systems consisting of enzymatic and nonenzymatic ROS scavengers (Mittova et al. 2015). SOD is a critical enzyme, responsible for the elimination of O2 −·, and is considered a key antioxidant in aerobic cells (Rasool et al. 2013). Combined action of SOD, POD, APX, and CAT is critical in mitigating effects of oxidative stress, since SOD catalyzed the conversion of O2 − · into H2O2, and POD, APX, and CAT scavenged H2O2 into H2O. The notable high level and continuous increase of SOD activity in AN root indicated that alfalfa with active nodules has a better capacity to enhance the SOD activity to scavenge O2 − · induced by salt shock. This result might explain why plants with nodules can survive adverse conditions when O2 − · levels increased. Although the SOD activity in AN leaves was the highest and nearly constant at later stages of salt shock, variance analysis indicated that nodules have no effect on leaf SOD activity. The POD activity has been found to be higher in salt-tolerant, compared with salt-sensitive alfalfa under salt or drought stress (Wang et al. 2009). Inoculated alfalfa displayed a high level of POD activity in both the leaf and root under normal conditions and the first 2 h of salt shock, which means POD may play a crucial role to scavenge ROS when host plants are infected by rhizobia and exposed to early stages of salt shock. Another major hydrogen peroxide detoxifying system in plant cells is the ascorbate-glutathione cycle, in which APX enzymes play a key role in catalyzing the conversion of H2O2 into H2O, using ascorbate as a specific electron donor (Caverzan et al. 2012). Even though APX activity fluctuated in the root and declined in the leaf over the period of salt shock, AN root, in general, maintained a comparatively high level of APX activity. The high level of CAT activity in AN root suggests a strong capability for detoxifying H2O2.
GSH plays a crucial role in plant defense against abiotic and biotic stresses, including salt tolerance (Pauly et al. 2006) and also appears to be essential for proper development of root nodules resulting from symbiotic interaction (Frendo et al. 2005). In legumes, there is a strong positive correlation between nitrogenase activity and nodule GSH along with hGSH contents (Groten et al. 2006). hGSH concentration regulates the biological nitrogen fixation efficiency in nodules and a deficiency of hGSH impairs nodule growth (El Msehli et al. 2011). A gradual increase of GSH content in AN leaves indicated alfalfa with functional nodules had a continuous capacity to increase defense against oxidative stress induced by salt shock, despite the fact that GSH content was not affected by inoculation.
One of the main consequences of NaCl stress is loss of intracellular water. Plants accumulate many metabolites that are also known as “compatible solutes” in the cytoplasm to reduce the salt stress-induced water loss. This mechanism of osmotic adjustment plays a vital role in the protection of plants against stress, particularly against salinity (Siddiqui et al. 2008). It was reported that inoculation with native rhizobium can improve osmotic stress tolerance in common bean (Sassi-Aydi et al. 2012). Plants accumulate proline, soluble sugar, and protein that participate actively in osmotic adjustment when they are under salt stress (Evelin et al. 2009; Szabados and Savouré 2010). However, the role of proline in osmotic tolerance in legumes remains controversial (Ashraf and Harris 2004; Khadri et al. 2006). In our study, proline content increased significantly during the first 4 h in all the plants, suggesting that proline may be important for initial dehydration stress response. However, AN plants showed consistently lower proline contents, especially in leaves, than IN and NN plants during the initial response, implying that proline may not the key contributor to the improved salt tolerance in symbiotic alfalfa plants.
Within the plant-rhizobium symbiotic relationship, the plant provides photoassimilates that supply energy and carbon compounds required by the bacteria to fix nitrogen. Photoassimilates are delivered to the nodules as sucrose. The bacteria return ammonium (NH4 +) to the host, and this is assimilated in the form of amino acids (Aranjuelo et al. 2015). In this investigation, inoculation affected root total protein and soluble sugar content. In general, AN and IN plants showed more stable content of soluble protein content in both leaf and root under salt shock compared to NN plants. While it is not clear why soluble protein content fluctuated so greatly in NN plants, it is consistent that a significant decrease in total soluble protein content 1 h after salt shock was observed in both roots and leaves of NN plants. This reduction may indicate a greater sensitivity in protein production of NN plants to salt shock compared to AN and IN plants. Some scientists reported that the increase in soluble protein as a result of salt stress was higher in salt-sensitive plants than in salt-tolerant spring wheat and maize (Ashraf and O'Leary 1999; Azevedo Neto et al. 2009). On the other hand, a higher content of soluble protein has also been reported in salt tolerant cultivars of barley and rice under salt stress conditions (Hurkman et al. 1989; Lutts et al. 1996). It was reported that soluble protein generally accumulated or remained unchanged in plants grown under saline conditions and they might provide a storage form of nitrogen which was re-utilized when stress was alleviated. Thus, the significance of change in soluble protein content in association with nodulation and salt tolerance in alfalfa requires further study.
Total soluble sugar content increased over time more in the leaves of nodulated plants than in the leaves of the non-nodulated plants after the onset of the salt shock. Higher content of soluble sugar in nodulated plants suggests a potential salt tolerance mechanism in these plants in maintaining sugar production and transport to roots compared to NN plants. A reduction of soluble sugar in roots of NN plants can be deleterious, leading to severe dehydration stress and death of the plants under salinity stress. Results we observed for soluble sugar are consistent with Feng et al. (2002), who reported that in maize, mycorrhizal plants maintained higher total soluble sugar than non-mycorrhizal plants. Furthermore, Morant-Manceau et al. (2004) reported that total soluble sugar contribution to osmotic adjustment was higher in salt-tolerant plants. Palma et al. (2013) found that concentrations of soluble sugar are higher in nodules than in alfalfa leaves and roots and that nodules are organs specially protected in order to maintain this crucial function, even under stress conditions. Thus, future work can be done to distinguish whether the change in soluble sugar content occurs in alfalfa roots or nodules under salinity stress. In summary, our research demonstrated that symbiotic nitrogen fixation has a positive effect on the response of alfalfa to salt stress. Active nodules improved the survival of host plants under salinity stress potentially through enhancing various anti-oxidative stress mechanisms and maintaining a stable production of soluble sugar and total soluble proteins.
Abbreviations
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- POD:
-
Peroxidase
- APX:
-
Ascorbate peroxidase
- GSH:
-
Reduced glutathione
- O2 −·:
-
Superoxide anion radical
- ROS:
-
Reactive oxygen species
- AN:
-
Alfalfa with active nodules
- IN:
-
Alfalfa with inactive nodules
- NN:
-
Alfalfa with no nodules
References
Aranjuelo I, Molero G, Erice G, Aldasoro J, Arrese-Igor C, Nogués S (2015) Effect of shoot removal on remobilization of carbon and nitrogen during regrowth of nitrogen-fixing alfalfa. Physiol Plant 153:91–104
Ashraf M, Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–16
Ashraf M, O'Leary JW (1999) Changes in soluble proteins in spring wheat stressed with sodium chloride. Biol Plant 42:113–117
Ashrafi E, Razmjoo J, Zahedi M, Pessarakli M (2015) Screening alfalfa for salt tolerance based on lipid peroxidation and antioxidant enzymes. Agron J 107:167–173
Azevedo Neto AD, Prisco JT, Gomes Filho E (2009) Changes in soluble amino-N, soluble proteins and free amino acids in leaves and roots of salt-stressed maize genotypes. J Plant Interact 4:137–144
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Becana M, Dalton DA, Moran JF, Iturbe‐Ormaetxe I, Matamoros MA, Rubio CM (2000) Reactive oxygen species and antioxidants in legume nodules. Physiol Plant 109:372–381
Berendsen RL, Pieterse CM, Bakker PA (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17:478–486
Bohnert HJ, Nelson DE, Jensen RG (1995) Adaptations to environmental stresses. Plant Cell 7:1099
Bolanos L, Martín M, El-Hamdaoui A, Rivilla R, Bonilla I (2006) Nitrogenase inhibition in nodules from pea plants grown under salt stress occurs at the physiological level and can be alleviated by B and Ca. Plant Soil 280:135–142
Bouhmouch I, Souad-Mouhsine B, Brhada F, Aurag J (2005) Influence of host cultivars and Rhizobium species on the growth and symbiotic performance of Phaseolus vulgaris under salt stress. J Plant Physiol 162:1103–1113
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Caverzan A, Passaia G, Rosa SB, Ribeiro CW, Lazzarotto F, Margis-Pinheiro M (2012) Plant responses to stresses: role of ascorbate peroxidase in the antioxidant protection. Genet Mol Biol 35:1011–1019
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775
Chaudhuri K, Chouchuri M (1993) Effects of short-term NaCl salinity stress on free radical mediated membrane damage in two jute species. Indian J Exp Biol 31:327–331
Chinnusamy V, Jagendorf A, Zhu J-K (2005) Understanding and improving salt tolerance in plants. Crop Sci 45:437–448
Cordovilla MDP, Ligero F, Lluch C (1999) Effects of NaCl on growth and nitrogen fixation and assimilation of inoculated and KNO3 fertilized Vicia faba L. and Pisum sativum L. plants. Plant Sci 140:127–136
Dakora FD (2003) Defining new roles for plant and rhizobial molecules in sole and mixed plant cultures involving symbiotic legumes. New Phytol 158:39–49
Deakin WJ, Broughton WJ (2009) Symbiotic use of pathogenic strategies: rhizobial protein secretion systems. Nat Rev Microbiol 7:312–320
Dreywood R (1946) Qualitative test for carbohydrate material. Ind Eng Chem, Anal Ed 18:499–499
El-Akhal M, Rincón A, Coba de la Peña T, Lucas MM, El Mourabit N, Barrijal S, Pueyo JJ (2013) Effects of salt stress and rhizobial inoculation on growth and nitrogen fixation of three peanut cultivars. Plant Biol 15:415–421
El Msehli S et al (2011) Crucial role of (homo) glutathione in nitrogen fixation in Medicago truncatula nodules. New Phytol 192:496–506
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem 70:616
Ertani A, Schiavon M, Muscolo A, Nardi S (2013) Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil 364:145–158
Evelin H, Kapoor R, Giri B (2009) Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann Bot 104:1263–1280
Feng G, Zhang F, Li X, Tian C, Tang C, Rengel Z (2002) Improved tolerance of maize plants to salt stress by arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots. Mycorrhiza 12:185–190
Frendo P, Harrison J, Norman C, Jiménez MJH, Van de Sype G, Gilabert A, Puppo A (2005) Glutathione and homoglutathione play a critical role in the nodulation process of Medicago truncatula. Mol Plant-Microbe Interact 18:254–259
Giannopolitis CN, Ries SK (1977) Superoxide dismutases I. Occurrence in higher plants. Plant Physiol 59:309–314
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gossett DR, Millhollon EP, Lucas M (1994) Antioxidant response to NaCl stress in salt-tolerant and salt-sensitive cultivars of cotton. Crop Sci 34:706–714
Groten K et al (2006) Redox regulation of peroxiredoxin and proteinases by ascorbate and thiols during pea root nodule senescence. FEBS Lett 580:1269–1276
Grover M, Ali SZ, Sandhya V, Rasul A, Venkateswarlu B (2011) Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J Microbiol Biotechnol 27:1231–1240
Harrison J, Jamet A, Muglia CI, Van de Sype G, Aguilar OM, Puppo A, Frendo P (2005) Glutathione plays a fundamental role in growth and symbiotic capacity of Sinorhizobium meliloti. J Bacteriol 187:168–174
Hernandez J, Olmos E, Corpas F, Sevilla F, Del Rio L (1995) Salt-induced oxidative stress in chloroplasts of pea plants. Plant Sci 105:151–167
Hissin PJ, Hilf R (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74:214–226
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Calif Agric Exp Stn, Circ 347
Hoffman BM, Lukoyanov D, Yang ZY, Dean DR, Seefeldt LC (2014) Mechanism of nitrogen fixation by nitrogenase: the next stage. Chem Rev 114:4041–4062
Hurkman WJ, Fornari CS, Tanaka CK (1989) A comparison of the effect of salt on polypeptides and translatable mRNAs in roots of a salt-tolerant and a salt-sensitive cultivar of barley. Plant Physiol 90:1444–1456
Kaschuk G, Kuyper TW, Leffelaar PA, Hungria M, Giller KE (2009) Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biol Biochem 41:1233–1244
Khadri M, Tejera NA, Lluch C (2006) Alleviation of salt stress in common bean (Phaseolus vulgaris) by exogenous abscisic acid supply. J Plant Growth Regul 25:110–119
Kiers ET, Rousseau RA, West SA, Denison RF (2003) Host sanctions and the legume-rhizobium mutualism. Nature 425:78–81
Lauter D, Munns D, Clarkin K (1981) Salt response of chickpea as influenced by N supply. Agron J 73:961–966
Lutts S, Kinet J, Bouharmont J (1996) Effects of salt stress on growth, mineral nutrition and proline accumulation in relation to osmotic adjustment in rice (Oryza sativa L.) cultivars differing in salinity resistance. Plant Growth Regul 19:207–218
Mittova V, Volokita M, Guy M (2015) Antioxidative systems and stress tolerance: insight from wild and cultivated tomato species. Reactive oxygen and nitrogen species signaling and communication in plants. Springer, In, pp 89–131
Morant-Manceau A, Pradier E, Tremblin G (2004) Osmotic adjustment, gas exchanges and chlorophyll fluorescence of a hexaploid triticale and its parental species under salt stress. J Plant Physiol 161:25–33
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Palma F, Tejera NA, Lluch C (2013) Nodule carbohydrate metabolism and polyols involvement in the response of Medicago sativa to salt stress. Environ Exp Bot 85:43–49
Pauly N et al (2006) Reactive oxygen and nitrogen species and glutathione: key players in the legume–Rhizobium symbiosis. J Exp Bot 57:1769–1776
Peleg-Grossman S, Melamed Book N, Levine A (2012) ROS production during symbiotic infection suppresses pathogenesis-related gene expression. Plant Signaling Behav 7:409–415
Peoples MB, Craswell ET (1992) Biological nitrogen fixation: investments, expectations and actual contributions to agriculture. Plant Soil 141:13–39
Polle A (2001) Dissecting the superoxide dismutase-ascorbate-glutathione-pathway in chloroplasts by metabolic modeling. Computer simulations as a step towards flux analysis. Plant Physiol 126:445–462
Porcel R, Aroca R, Ruiz Lozano JM (2012) Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron Sustainable Dev 32:181–200
Puckette MC, Weng H, Mahalingam R (2007) Physiological and biochemical responses to acute ozone-induced oxidative stress in Medicago truncatula. Plant Physiol Biochem 45:70–79
Rasool S, Ahmad A, Siddiqi T, Ahmad P (2013) Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol Plant 35:1039–1050
Redondo FJ, de la Pena TC, Lucas MM, Pueyo JJ (2012) Alfalfa nodules elicited by a flavodoxin-overexpressing Ensifer meliloti strain display nitrogen-fixing activity with enhanced tolerance to salinity stress. Planta 236:1687–1700
Ruiz J, Blumwald E (2002) Salinity-induced glutathione synthesis in Brassica napus. Planta 214:965–969
Santos R, Hérouart D, Sigaud S, Touati D, Puppo A (2001) Oxidative burst in Alfalfa-Sinorhizobium meliloti symbiotic interaction. Mol Plant-Microbe Interact 14:86–89
Sassi-Aydi S, Aydi S, Abdelly C (2012) Inoculation with the native Rhizobium gallicum 8a3 improves osmotic stress tolerance in common bean drought-sensitive cultivar. Acta Agric Scand, Sect B 62:179–187. doi:10.1080/09064710.2011.597425
Serraj R, Drevon JJ (1998) Effects of salinity and nitrogen source on growth and nitrogen fixation in alfalfa. J Plant Nutr 21:1805–1818
Shao HB, Chu LY, Shao MA, Jaleel CA, Mi HM (2008) Higher plant antioxidants and redox signaling under environmental stresses. C R Biol 331:433–441
Shrivastava P, Kumar R (2015) Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22:123–131
Siddiqui MH, Khan MN, Mohammad F, Khan MMA (2008) Role of nitrogen and gibberellin (GA3) in the regulation of enzyme activities and in osmoprotectant accumulation in Brassica juncea L. under salt stress. J Agron Crop Sci 194:214–224
Siqueira JO, Nair MG, Hammerschmidt R, Safir GR, Putnam AR (1991) Significance of phenolic compounds in plant‐soil‐microbial systems. Crit Rev Plant Sci 10:63–121
Sreenivasulu N, Ramanjulu S, Ramachandra-Kini K, Prakash HS, Shekar-Shetty H, Savithri HS, Sudhakar C (1999) Total peroxidase activity and peroxidase isoforms as modified by salt stress in two cultivars of fox-tail millet with differential salt tolerance. Plant Sci 141:1–9
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89
Wang WB, Kim YH, Lee HS, Kim KY, Deng XP, Kwak SS (2009) Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol Biochem 47:570–577
Wu QS, Zou YN, Liu W, Ye XF, Zai HF, Zhao LJ (2010) Alleviation of salt stress in citrus seedlings inoculated with mycorrhiza: changes in leaf antioxidant defense systems. Plant Soil Environ 56:470–475
Yousef A, Sprent J (1983) Effects of NaCl on growth, nitrogen incorporation and chemical composition of inoculated and NH4NO3 fertilized Vicia faba (L.) plants. J Exp Bot 34:941–950
Acknowledgments
This work was supported by the Project of National Natural Science Foundation of China (31372357, 31272490), the National Key Technology R&D Program in the 12th Five-Year Plan of China (2011BAD17B05), the major project for Tibetan forage industry (Z2014C02N02), and China Agriculture Research System (CARS-35). The authors thank Drs. Yajun Wu and Roger N Gates from South Dakota State University for their advice on statistical analysis and constructive comments to this manuscript, as well as the anonymous reviewers for their thoughtful critique and suggestions.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Timothy J. Flowers.
Rights and permissions
About this article
Cite this article
Wang, Y., Zhang, Z., Zhang, P. et al. Rhizobium symbiosis contribution to short-term salt stress tolerance in alfalfa (Medicago sativa L.). Plant Soil 402, 247–261 (2016). https://doi.org/10.1007/s11104-016-2792-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2792-6