Abstract
Arthropods, particularly insects, form successful long-term symbioses with endosymbiotic bacteria. The associations between insects and endosymbionts are remarkably stable; many stretch back several hundred million years in evolutionary time. With the exception, perhaps, of the filarial nematodes no other group of metazoans shows such a proclivility for their intracellular symbionts. The identification and classification of bacterial symbionts and hosts has grown rapidly over the last two decades and these relationships form a continuum from classical mutualism to parasitism. Complete genomes have been sequenced for many of these bacteria and some of their hosts. Now more intractable questions regarding endosymbiosis are being addressed. Investigations on the role of the host immune system in the maintenance of symbiosis, the nature of bacteriophages and transposable elements found in the genomes of many bacterial symbionts, and the molecular mechanisms involved in establishing reproductive phenotypes such as parthenogenesis, male killing, cytoplasmic incompatibility and feminization have been recently reported. This review will focus on the impact of the secondary endosymbionts Wolbachia, Cardinium, and Spiroplasma on host fitness and immunity and will revisit the question of whether these bacteria are friend or foe from an insect’s point of view.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Insects are the most speciose animal group known. They inhabit many of the earth’s environments in overwhelming numbers and their success in part is attributed to a genetic repertoire that permits them to rapidly adapt to changing environments. These may occur as the result of natural processes such as changing climates, or as a result of human interventions such as the development of a wide range of man-made molecules that initially cause substantial morbidity and mortality in numerous insect species. The relatively recent discovery of a wide array of endosymbiotic microbes in insects has revealed an expanded genetic repertoire that may account for additional mechanisms contributing to their success. The ability to utilize nutrient deficient food sources, counter parasite infection, and rapidly develop new species may result from this interaction.
Insects make good hosts for intracellular prokaryotic organisms. Arthropods are more frequently exploited by endosymbiotic bacteria than all other animal phyla and both deleterious and beneficial associations have been widely reported. For this reason insects provide a profitable field for the discovery of new symbiotic relationships and they have proven to be excellent model systems for investigating host-symbiont interactions. Bacterial symbionts are important promoters of insect diversity and speciation (Bordenstein 2003; Hurst et al. 2003b; Moran et al. 2005). They alter a variety of host cellular functions, including signal transduction (Ikeya et al. 2009), cell cycle progression (Tram et al. 2003), vesicular trafficking (Azzouna et al. 2004) and programmed cell death (Bentley et al. 2007). These responses have been reported in model systems involving intracellular pathogens, leading to a clearer understanding of the relationship between bacterium and host. However, many fastidious obligate endosymbionts cannot be maintained in culture and, therefore, the mechanisms by which they manipulate their host cells are difficult to unravel. With the availability of complete and partial genome sequences for a growing number of bacterial endosymbionts (Akman et al. 2002; Foster et al. 2005; Gil et al. 2003; Gomez-Valero et al. 2007; McLeod et al. 2004; Shigenobu et al. 2000; van Ham et al. 2003; Wu et al. 2004) postgenomic approaches are being used to characterize obligate host-endosymbiont interactions (Brennan et al. 2008; Delmotte et al. 2006; Moran 2007; Xi et al. 2008).
In this paper we review recent advances involving insects and the secondary endosymbionts, Wolbachia, Cardinium, and Spiroplasma. These symbiotic bacteria are obligate residents of insect cells and are maternally inherited, but are not nutritionally essential for their hosts, nor are they restricted to specialized host cells called bacteriocytes. Although these bacterial endosymbionts most certainly do not exhaust the list of secondary symbionts identified in the current literature (Hypsa and Novakova 2009), they were chosen because they represent three separate prokaryotic lineages, α-Proteobacteria, Bacteriodetes and Mollicutes, respectively. In this review, common characteristics of their lifestyles will be emphasized to elucidate cellular mechanisms supporting stable symbioses. Systems that can be exploited to control insect hosts and enhance bacterial survival will be discussed.
2 Secondary endosymbionts of three bacterial lineages
2.1 Wolbachia pipientis
Wolbachia appear as small rods or spheres ranging in size from 0.2 to 1.5 μm (Louis and Nigro 1989; O’Neill et al. 1997a; Popov et al. 1998; Trpis et al. 1981; Wright et al. 1978). They occur in all tissue types examined including larval salivary glands, imaginal discs and fat body (Clark et al. 2005), but are more prevalent in ovaries and testes of infected hosts (Clark and Karr 2002). Wolbachia are maternally inherited cytoplasmic particles which utilize host microtubules to localize to the anterior of the developing oocyte during oogenesis (Ferree et al. 2005b).
Wolbachia are α-Proteobacteria classified within the order Rickettsiaceae, family Anaplasmataceae, and closely related to the intracellular pathogens Ehrlichia, Anaplasma and Neorickettsia (Dumler et al. 2001; Rudakov et al. 2003). The genus Wolbachia has been divided into eight (Lo et al. 2007) and more recently, nine (Ros et al. 2009) major clades based on molecular phylogenies. According to Lo et al. (2002) clades A and B include the majority of insect Wolbachia; clades C and D Wolbachia infect filarial nematodes only (Casiraghi et al. 2001; Lo et al. 2002); clade E Wolbachia are found in the springtail Fulsomia candida (Colembola) (Bandi et al. 1999; Lo et al. 2002; Vandekerckhove et al. 1999), F Wolbachia are known from two termite (Isoptera) species (Bandi et al. 1999); clade G Wolbachia infect spiders, although its status as a clade has been disputed (Baldo and Werren 2007); clade H Wolbachia infect some arthropods and nematodes; K Wolbachia infect spider mites species of the genus Bryobia (Ros et al. 2009).
The frequency of Wolbachia in insect species measured by PCR screens seems to be about 20% (Kikuchi and Fukatsu 2003; Nirgianaki et al. 2003; Werren and Windsor 2000; Werren et al. 1995), although this number may be an underestimate (Hilgenboecker et al. 2008; Weinert et al. 2007). Consistent with this, the frequency of Wolbachia infection in Drosophila melanogaster wild-type lines maintained by the Bloomington Drosophila Stock Center is 23.3% (Clark et al. 2005). Recently, 23% of 39 species of bedbugs (Hemiptera:Cimicidae) from live and museum specimens were found to be infected with Wolbachia (Sakamoto et al. 2006). In the Acari the situation is similar; a recent study of spider mites in Japan (Gotoh et al. 2003) found that 17% of species were infected with Wolbachia alone. However, infected populations frequently carry more than one type of symbiont (Breeuwer and Jacobs 1996; Perotti and Braig 2004). In a few cases, higher frequencies have also been reported. Wolbachia was detected in all 25 populations of 19 species of sucking (Anopleura) and chewing (Mallophaga) lice (Kyei-Poku et al. 2005). In another study, when testing was limited to insect pest species or insect species used as biocontrol agents, infection was found in 46% of the 48 species tested (Floate et al. 2006).
The genomes of Wolbachia are 1.27 Mb in wMel from D. melanogaster (Wu et al. 2004), 1.48 Mb in wPip from Culex pipiens (Klasson et al. 2008) and 1.45 Mb in wRi from D. simulans (Klasson et al. 2009b). All sequenced Wolbachia genomes include a high number of mobile elements and simple repetitive DNA sequences (Brownlie and O’Neill 2006), show general plasticity and frequent rearrangement, and loss of synteny between strains (Klasson et al. 2008). A rather surprising recent discovery is the presence of Wolbachia sequences in some host genomes. Horizontal gene transfer (HGT) from Wolbachia to host DNA was first reported in the Adzuki bean beetle, Callosobruchus chinensis (Fukatsu et al. 2003; Kondo et al. 2002) and then in Drosophila ananassae, D. sechellia, D. simulans, the parsitoids Nasonia vitripennis, N. longicornis, N. giraulti, and mosquitoes Culex pipiens (Hotopp et al. 2007), Aedes aegypti and Culex quinquefasciatus (Klasson et al. 2009a). HGT has also been found in nematodes Brugia malayi, Dirofilaria immitis (Hotopp et al. 2007) and Onchocerca volvulus (Fenn et al. 2006). Transfer has also possibly occurred in the opposite direction, from A. aegypti to Wolbachia (Woolfit et al. 2009).
The ability of Wolbachia to manipulate the reproduction and/or sex ratio of their host species contributes to the prevalence of Wolbachia infections within all major insect orders and marks them as reproductive parasites. Wolbachia infections in insects and mites can lead to various phenotypes depending on the strain of Wolbachia and the genotype of the host. They induce cytoplasmic incompatibility (CI), male-killing (Dyer and Jaenike 2005; Hurst et al. 1999b; Ikeda 1970) feminization and parthenogenesis (Stouthamer et al. 1999) in a wide range of host species. In at least one insect, Asobara tabida (Hymenoptera, Braconidae), Wolbachia have become obligate mutualists. A. tabida, a parasitoid of Drosophila, is dependent on Wolbachia to support oogenesis (Dedeine et al. 2005; Dedeine et al. 2001). This insect is host to three separate Wolbachia strains, only one of which is required for oogenesis. The other two strains cause cytoplasmic incompatibility (Dedeine et al. 2004).
2.2 Cardinium hertigii
Cardinium (Flexibacteriaceae, Class Sphingobacteria, Phylum Bacteroidetes) were first seen in cell cultures established from the tick Ixodes scapularis (Kurtti et al. 1996). These unusual bacteria were observed in electron micrographs and in later studies their presence was confirmed by PCR and phylogenetic analysis. Cardinium are transovarially transmitted gram negative pleiomorphic rods 1-2 microns long and approximately 0.5 microns wide. They have a distinctive parallel array of hollow filaments resembling microtubules that extend from the inner membrane into the cytoplasm (Bigliardi et al. 2006; Nakamura et al. 2009). The function of these structures is unknown.
Cardinium were originally named Encarsia (EB, Zchori-Fein et al. 2001), and then, Cytophaga-like organisms (CLO, Hunter et al. 2003), Cytophaga-Flavobacterium-Bacteroides (CFB) (Weeks and Breeuwer 2003) and, finally, Cardinium hertigii (Zchori-Fein and Perlman 2004; Zchori-Fein et al. 2004). Like Wolbachia, the true incidence of infection with Cardinium remains to be determined. Cardinium initially appeared to be limited to Hymenoptera (Hunter et al. 2003; Matalon et al. 2007; Weeks et al. 2003; Zchori-Fein et al. 2001; Zchori-Fein et al. 2004), Hemiptera (Bigliardi et al. 2006; Weeks et al. 2003; Zchori-Fein and Perlman 2004), Acari (Enigl and Schausberger 2007; Gotoh et al. 2007; Groot and Breeuwer 2006; Hoy and Jeyaprakash 2008; Weeks et al. 2001; Weeks et al. 2003), and Areneae (Duron et al. 2008). However, Nakamura et al (2009) found 27 of 57 species (47.4%) of planthoppers, 9 of 22 species (40.9%) of spider mites, and 4 of 25 species (16%) of the biting midges Culicoides (Diptera: Ceratopogonidae) infected with Cardinium. One study found Cardinium less prevalent in mites than Wolbachia (Zchori-Fein and Perlman 2004), however, two recent reports found the opposite. Forty percent of tested mite populations representing 58% of mite species were infected in a study by Enigl and Schausberger (2007) and Gotoh et al (2007) found Cardinium in all five species of spider mites that were tested. Both predatory (Weeks et al. 2003; Zchori-Fein and Perlman 2004) and herbaceous mite species (Chigira and Miura 2005; Groot and Breeuwer 2006; Weeks et al. 2003) can be infected. In many cases, Wolbachia and Cardinium co-infect a single host species (Duron et al. 2008; Nakamura et al. 2009; Ros and Breeuwer 2009; Weeks et al. 2003; Zchori-Fein and Perlman 2004). The distribution of Cardinium may be broader than expected since a closely related bacterium has been found in a plant parasitic nematode and named “Candidatus Paeniccardinium endonii” (Noel and Atibalentja 2006).
The Cardinium genome has not been sequenced and since they have only recently come to our attention, little is currently known about their metabolism, phylogenetic diversity and distribution.
Cardinium, like Wolbachia, are reproductive parasites. They cause CI in the spider mite, Estetramychus suginamensis, (Gotoh et al. 2007), the red poultry mite, Dermanyssus gallinae (De Luna et al. 2009) the parasitoid wasp, Encarsia pergandiella (Hunter et al. 2003) and the sexual spider mite, Bryobia sarothamni (Ros and Breeuwer 2009); parthenogenesis in scale insects (Provencher et al. 2005) and Encarsia hispida; and feminization in Brevipalpus phoenicis (Groot and Breeuwer 2006; Weeks et al. 2001). Although male-killing Cardinium have not been reported, unnamed bacteria belonging to the Phylum Bacteriodetes, Class Flavobacteria cause male-killing in ladybird beetles, Coleomegilla maculata and Adonia variegate (Hurst et al. 1999a; Hurst et al. 1996). An increase in fecundity due to Cardinium has also been reported (Weeks and Stouthamer 2004). The reproductive phenotypes caused by infection with Cardinium are surprisingly similar to those induced by Wolbachia. It will be interesting to see if conserved mechanisms produce these similar outcomes in both Wolbachia and Cardinium infection.
2.3 Spiroplasma spp.
Spiroplasmas are helical, motile Gram-positive bacteria lacking a cell wall and are widely associated with plants and insects. They can be transmitted to plant hosts by phloem-feeding insects such as leafhoppers and psyllids (Hemiptera) (reviewed in Ammar and Hogenhout 2006; Regassa and Gasparich 2006). Spiroplasmas of insects are extracellular or intracellular and range from mutualists to pathogens. Some spiroplasmas are restricted to the insect gut and are non-pathogenic. Other symbiotic forms cross the gut epithelial barrier and are predominantly found in the hemolymph but are also seen in ovaries, fat bodies, hypodermis and salivary glands. Intracellular spiroplasmas are oval or flask-shaped rather than helical, with the tip of the flask-like structure used for orientation, adhesion to midgut cells, and invasion of basal lamina and plant sieve tubes (Ammar and Hogenhout 2005). Some insect-infecting spiroplasmas are entomopathogens. For example, Spiroplasma melliferum and S. apis are pathogens of the honey bee. These bacteria move into the hemolymph, where they multiply and kill the host.
The spiroplasmas belong to the Phylum Firmicutes and Class Mollicutes. Three well-studied species, S. citri, S. kunkelii, and S. phoeniceum are plant pathogens. Other spiroplasmas have been isolated from predatory and herbivorous insects (Anbutsu and Fukatsu 2003; De Luna et al. 2009; Duron et al. 2008; Fukatsu et al. 2001; Hurst et al. 1999c; Jiggins et al. 2000a; Mateos et al. 2006; Weinert et al. 2007) including Drosophila spp. (Anbutsu and Fukatsu 2006; Haselkorn et al. 2009; Watts et al. 2009), ticks (Brinton and Burgdorfer 1976; Tully et al. 1995), parasitic dermanyssoid mites (De Luna et al. 2009; Reeves et al. 2006), the predatory mite Neoseiulus californicus, and a herbivorous spider mite, Tetranychus urticae (Enigl and Schausberger 2007). Spiroplasmas are currently divided into four paraphyletic clades based on 16S rDNA phylogeny; the Ixodetis clade, the Citri-Chrysopicola-Mirum clade, the Apis sensu latu clade, and the Mycoides-Entomoplasmataceae clade (Gasparich et al. 2004). Spiroplasmas within each clade show diverse host range and broad geographical range.
Spiroplasma genomes are small, ranging in size from 760 to 2,220 kb, have a G+C content of 24 to 31% (Gasparich et al. 2004) and contain several large plasmids (Bai et al. 2004; Davis et al. 2005). Although they have a reduced genome and lack genes for basic metabolic pathways, some have been cultured outside their host in complex medium (Ammar and Hogenhout 2006).
S. poulsonii, discovered in the hemolymph of Drosophila willistoni in 1961 (Poulson and Sakaguchi 1961), are parasites that kill male progeny. As a result, they were given the name sex ratio organism, SRO. Male-killing spiroplasmas are also found in several other Drosophila species including D. hydei and D. nebulosa, in ladybird beetles including Adalia bipunctata (Hurst et al. 1999c) and Anisosticta novemdecimpunctata (Tinsley and Majerus 2006), and in butterflies including Danaus chrssippus (Jiggins et al. 2000a). Other spriroplasmas, including those infecting the pseudococcid, Antonina crawii, (Fukatsu and Nikoh 2000) the pea aphid, Acyrthosiphon pisum, (Fukatsu et al. 2001) and D. hydei (Kageyama et al. 2006) are not male killers. Spiroplasmas injected into A. pisum decrease several fitness parameters including growth, longevity and number of offspring (Fukatsu et al. 2001), however, they are not known to induce CI, parthenogenesis or feminization in their insect hosts. Although spiroplasmas are found in ticks and mosquitos these insects do not transmit them to humans or other mammals.
3 Surviving in intracellular niches
3.1 Refuge within host membranes
Intracellular bacteria characteristically reside and replicate within vacuoles of host origin (Bao et al. 1996; Finlay and Falkow 1997; O’Neill et al. 1997b; Popov et al. 1998; Wolf and Glatzel 1996). In electron micrographs of these replicative vacuoles, endosymbionts are often seen as pleiomorphic in size and shape, and single vacuoles sometimes contain numerous bacteria. Little is known about the origin of replicative vacuoles of insect endosymbionts. Host cholesterol stores of human macrophages are used to form the vacuolar membrane supporting replication and maintenance of the intracellular bacterium Coxiella burnetii (Howe and Heinzen 2006). In contrast, the lipid composition of the vacuolar membranes surrounding intracellular bacteria in insect hosts has not been studied. In electron micrographs, Wolbachia are surrounded by a triple-layered structure, the outer one derived from host membranes (Bao et al. 1996; O’Neill et al. 1997a) and associated with the endoplasmic reticulum (Wright and Barr 1980). Ultrastructural evidence suggests that these vacuoles are replicative structures which support Wolbachia cell division. In addition to its normal intracellular location, however, Wolbachia also occur in high numbers in the hemolymph of infected D. simulans and it is not known whether host membrane surrounds the bacteria in this acellular compartment. Mosquito cells (Aa23 cells) in vitro can internalize Wolbachia from the medium by phagocytosis through coated vesicles (Popov et al. 1998).
Unlike Wolbachia, Cardinium and Spiroplasma exist in the cytoplasm of host cells in ovaries, salivary gland and fat bodies without an encompassing host vacuolar membrane, (Kitajima et al. 2007). Spiroplasmas are phagocytosed into insect fat cells (Ammar and Hogenhout 2006) but their fate in the phagosomal compartment is unknown. Host vacuolar membrane is therefore not a requirement for stable symbiosis and the fact that Cardinium and Spiroplasma can exist without a host membrane is evidence that alternate evolutionary pathways can balance the needs of endosymbiotic bacteria and their host cells.
3.2 Transfer of bacterial factors between host and symbiont
Intracellular bacteria exchange molecules with their host cells through Type Three Secretion Systems (TTSS) or Type IV Secretion Systems (T4SS). T4SS of gram negative bacteria have evolved from ancestral conjugation transfer systems (Christie 2001) and are key factors in determining the intracellular fate of several well known tick-borne human pathogens, including Brucella abortus, Legionella pneumophila, C. burnetti and R. prowazekii. These bacteria use T4SS to inject virulence factors into the host cytoplasm, such as those that modulate lysosomal maturation (reviewed in Sexton and Vogel 2002). Wolbachia genomes possess homologs of the T4SS system of Agrobacterium tumifaciens (virB operon), L. pneumophila (lvhB operon) and R. conorii (Masui et al. 2000; Wu et al. 2004) which have been shown to be highly conserved among 37 Wolbachia strains (Pichon et al. 2009). It is not yet known what proteins are secreted by Wolbachia into host cells. Proteomics data indicate that Wolbachia encoded Cu-Zn-superoxide dismutase and bacterioferritin are present in the cytoplasm of Wolbachia infected Aa23 cells (Brennan et al. 2008), and an N6-adenine methyltransferase (Braig, unpublished) is present in cytoplasm of infected early embryos. These proteins are possible candidates for export from Wolbachia through the T4SS. A family of highly divergent proteins containing a variable number of ankyrin repeats are found in all Wolbachia genomes so far sequenced (Iturbe-Ormaetxe et al. 2005). Since ankyrin repeats are unusual in prokaryotic proteins but common in eukaryotic ones, they have been implicated in the CI phenotype or other host-symbiont functions involving protein-protein interactions (Iturbe-Ormaetxe and O'Neill 2007; Sinkins et al. 2005) but experimental evidence that they are secreted from Wolbachia cells is not yet available. However, in mammalian cells, C. burnetti secretes a heterogeneous group of ankyrin containing proteins through a T4SS. These proteins localize to several different host organelles and mediate a variety of host cell functions (Voth et al. 2009).
3.3 Vertical and horizontal transmission
The most important mode of transmission of insect endobacteria is vertical and transovarial. The transmission efficiency for mutualistic symbionts such as Wolbachia in nematodes and in the paraitoid wasp A. tabida is extremely high (Funk et al. 2000) and some insects have evolved elaborate mechanisms to transfer bacteria to their oocytes or embryos (Braendle et al. 2003; Mira and Moran 2002). Secondary endosymbionts are transmitted less efficiently than mutualistic ones but nevertheless have evolved mechanisms for the strict allocation of bacteria to oocytes and embryos. In Drosophila, a cytological examination of Wolbachia infected egg chambers showed that this endosymbiont engages with host microtubules and dynein to anchor themselves to the anterior half of the developing oocyte (Ferree et al. 2005a; Serbus and Sullivan 2007). Systematic studies also show a higher level of horizontal transmission (Ahrens and Shoemaker 2005; Baldo et al. 2002; Thao and Baumann 2004; Vavre et al. 1999) for secondary endosymbionts compared to the strictly mutualistic primary endosymbionts. A thorough study of eight Wolbachia infected spider species in the genus Agelenopsis provided evidence of three separate Wolbachia invasions, each one followed by extensive horizontal transfer (Baldo et al. 2008). As a result of horizontal transfer, a lack of complete concordance between host and bacterial evolution is seen (Baldo et al. 2006; Batista et al. 2009; Raychoudhury et al. 2008; Werren et al. 1995). Some species of insects, such as the fire ant Solenopsis invicta have been infected with different Wolbachia strains several times in their evolutionary history and have also lost infections in certain lineages, making it difficult to estimate the rate of horizontal transmission (Ahrens and Shoemaker 2005).
Spiroplasmas, like Wolbachia, are predominantly maternally transmitted, and show a correspondingly high degree of incongruence between the phylogenies of host and symbiont, indicating that horizontal transmission is common. S. poulsonii is transmitted horizontally between neotropical species of Drosophila by mites (Jaenike et al. 2007) and S. apis is found on the surfaces of flowers growing in the vicinity of affected beehives, suggesting that they are deposited there by contaminated insects and horizontally transferred to new hosts.
3.4 Manipulation of host reproduction
3.4.1 Cytoplasmic incompatibility
CI is a post-zygotic reproductive lethality which occurs when infected males and uninfected females are mated (Dobson 2003; Yen and Barr 1971, 1973). CI was initially described by Laven as a potential mechanism for control of the mosquito Culex pipiens (Laven 1951). It is the most common reproductive modification induced by Wolbachia and can also be induced by Cardinium infection. Sperm from infected males is rescued in eggs from females infected with the same strain of Wolbachia. Bidirectional incompatibility occurs when males and females carrying different strains of Wolbachia are mated. The developmental defect of CI is caused by a Wolbachia-induced modification of the sperm nucleus. In spite of much interest and some recent advances, investigators have yet to identify the molecular or genetic basis for the modification and rescue factors (Bourtzis et al. 1998; Harris and Braig 2003). Studies have shown that the level of expression of the CI phenotype can be modified by host nuclear genes in mosquitoes (Hoffmann 2005; Sinkins et al. 2005) and flies (Hurst et al. 2000) and is dependent on bacterial density and colonization of developing sperm cysts during spermatogenesis (Veneti et al. 2003).
3.4.2 Male-killing
Maternally inherited male-killing bacteria distort the sex ratio of the host population. Infected males die before reaching maturity, resulting in a female biased sex ratio (Dyson and Hurst 2004), altered mate competition (Jiggins et al. 2000b) and increased survival of female siblings (Hurst and Majerus 1993). Male killers have evolved in at least five different bacterial taxa: Wolbachia, (Hurst et al. 1999b), Spiroplasma (Williamson et al. 1999), γ-proteobacteria (Werren et al. 1986), Rickettsia (Lawson et al. 2000; Perlman et al. 2006; Werren et al. 1994) and Flavobacteria (Hurst et al. 1999a; Hurst et al. 1997). Roberts described a male-killing Rickettsia, R. tsutsugamushi in infected populations of Leptotrombidium (Acari: Trombiculidae) (Roberts et al. 1977). The Flavobacterium of Coleomegilla maculate (Coleoptera: Coccinellidae) is a male killer (Hurst et al. 1997); however, there has been no case of male killing induced by Cardinium reported to date (reviewed in Hunter and Zchori-Fein 2006). Wolbachia is a male killer in some hosts, most notably the ladybird beetle Adalia bipunctata (Coleoptera: Coccinellidae) (Hurst et al. 1999b), Acraea butterflies (Jiggins et al. 2001) and several Drosophila species, including D. bifasciata (Ikeda 1970), D. innubila (Dyer and Jaenike 2005) D. nebulosa (Bentley et al. 2007), and D. borealis (Sheeley and McAllister 2009). Phylogenetic evidence suggests that male-killing Wolbachia may have evolved multiple times (Jiggins et al. 2001) and changes in Wolbachia phenotype from CI to male-killing can occur frequently through recombination events, mutation or changes in hosts (Jiggens et al. 2002).
Like CI, male-killing in Drosophila requires an appropriate bacterial density in maternal tissues (Dyer and Jaenike 2005) but mechanisms for the recognition of males by male killers and virulence factors causing male death have not yet been described. Male-killing may result from diverse interactions between bacteria and hosts and this strategy has arisen in various distantly related bacterial lineages (Hurst et al. 2003b). Investigations of the genetic basis for male-killing are possible in D. melanogaster where male-killing spiroplasmas target the sex determination pathway (Veneti et al. 2005). Sex determination in dipterans occurs in response to the expression of the Sex-lethal gene, Sxl, in females and the absence of expression in males (Penalva and Sanchez 2003). Drosophila tra mutants bearing two X chromosomes express Sxl but develop into somatic males. These males are not killed by S. poulsonii (Sakaguchi and Poulson 1963). The cascade of sex determining factors in male flies leads to the formation of the dosage compensation complex (DCC), a heteromultimeric complex of the male-specific lethal MSL-2 protein with constitutively expressed MSL-1; MSL-3; maleless, MLE; and males absent on the first, MOF proteins. In D. melanogaster infected with S. poulsonii, loss-of-function mutations in the genes encoding these proteins can rescue the male-killing phenotype (Veneti et al. 2005) indicating that the formation of a functional DCC is a pre-requisite for virulence. Death of males occurs in two steps during embryogenesis (Counce and Poulson 1962). Compared to female embryos, early development in male embryos becomes arrested around stage 6 and males die prior to segmentation at stage 12. Their death is associated with widespread apoptosis induced by an unknown mechanism (Bentley et al. 2007). A stable male-killing Wolbachia in D. borealis might be used to unravel the exact mechanism of male-killing if it can successfully be transinfected into D. melanogaster (Sheeley and McAllister 2009).
Genes which lower transmission of the bacteria or increase the size of egg clutches are likely to be favored (Hurst and Jiggins 2000) if there is selection on hosts of male-killing bacteria to develop resistance. Genetic resistance to male killers has arisen in the butterfly Hypolimnas bolina infected with wBol1, a male-killing Wolbachia (Hornett et al. 2006), and D. willistoni infected with a spiroplasma. In neither case is the mechanism of resistance known. In contrast, Dyer and Jaenike (2005) were unable to find any evidence of suppression of male-killing in eight geographically isolated populations of D. innubila infected with the same Wolbachia strain. In a recent study suppression of the male killing phenotype uncovered CI in Wolbachia-infected H. bolina (Hornett et al. 2008).
4 Bacteriophages
Bacteriophages are present in Wolbachia and spiroplasmas. The presence of bacteriophage in Wolbachia was first described in Culex pipiens by Wright et al (1978) and later in D. melanogaster, (Gavotte et al. 2004), Nasonia vitripennis (Bordenstein et al. 2006), Ephestia kuehniella, (Fujii et al. 2004) E. cautella, Corcyra cepharonica and Teliogryllus taiwanemma (Kamoda et al. 2000; Masui et al. 2001). Wolbachia carry a variable number of temperate bacteriophage, prophage-like elements, and transposons in their genomes (Wu et al. 2004). Of these, at least the WO-B temperate phage appears to be capable of replication and lytic infection. The density of the resulting virions has been shown to correlate inversely with the level of CI in Wolbachia-infected Nasonia vitropennis. In testes of infected males of the parasitoid wasp, N. vitripennis, lytic phage rupture Wolbachia cells and release virions into the extracellular space (Bordenstein et al. 2006). WO-B viruses are present in the 0.22 micron filtrates of Wolbachia-infected mosquitos and D. simulans early embryonic cytoplasm (Sanogo and Dobson 2006). Bacteriophages have also been found in S. poulsonii and S. citri (Cohen et al. 1987; Jansson et al. 1982). These are dsDNA viruses ranging in size from 17 to 30 kb.
The mobile genetic elements of intracellular symbiotic bacteria are vehicles for genetic exchange and lateral gene transfer. The WO-B phage undergoes lateral exchange between Wolbachia strains colonizing the same host (Bordenstein and Wernegreen 2004) which has led Bordenstein and Reznikoff (2005) to propose an “intracellular arena hypothesis” whereby genetic information is traded through the exchange of mobile genetic elements between communities of bacteria living in the same intracellular environment. Evidence in support of this concept will come from hosts carrying multiple endosymbionts, such as two or more Wolbachia strains or two or more symbionts from separate bacterial lineages, such as Wolbachia and Spiroplasma or Wolbachia, Cardinium and Rickettsia.
5 Avoiding death from host immune systems
Although insects lack the adaptive immunity that is present in vertebrates, their innate immune defense is remarkably efficient and provides a rapid response to invasion by fungi, bacteria and parasitic organisms. Insect cellular immune responses include phagocytosis and encapsulation and inducible humoral responses involving the secretion of antimicrobial peptides into the hemolymph. Drosophila has been used as a model to study the immune response to infection by mammalian pathogens such as L. monocytogenes (Mansfield et al. 2003), S. typhimurium (Brandt et al. 2004) and Vibrio cholera (Park et al. 2005). Whole flies as well as cells in tissue culture (Ayres and Schneider 2006) respond to infection, leading to the conclusion that innate immune pathways are highly conserved (Hoffmann and Reichhart 2002). However, bacterial symbionts appear to be silent with respect to detection by host innate immune systems. How do they manage to circumvent immune responses?
5.1 Cellular immune mechanisms
Insect cellular immune defense operates against infection by microbes (Lanot et al. 2001) and invasion by parasitoid wasps (Sorrentino et al. 2002). During embryogenesis hematopoiesis initially occurs in the anterior mesoderm of the head and, following migration, hemocytes differentiate into phagocytic cells which subsequently colonize the entire embryo (Tepass et al. 1994). These cells are responsible for phagocytosing apoptotic cells produced by normal developmental pathways (Abrams et al. 1993). By the end of embryogenesis, hematopoetic tissue, composed of four to six paired lobes and containing prohemocyte stem cells, develop along the posterior portion of the dorsal vessel (Rugendorff et al. 1994). This tissue differentiates circulating hemocytes in larval and later stages, but disappears during metamorphosis and is not found in adult flies (Lanot et al. 2001). Prohemocytes give rise to cells of the hemolymph; phagocytic plasmatocytes, and granulocytes responsible for the melanization cascade. Plasmatocytes peak in number during metamorphosis and are involved in encapsulation of foreign particles too large to phagocytose, such as eggs oviposited by parasitoids (Lanot et al. 2001).
Insects, like other arthropods, respond to pathogens in the hemolymph by extracellular cascades that culminate in coagulation, melanization (Theopold et al. 2004) or phagocytosis (Roth and Kurtz 2009) of the invading particle. Spiroplasmas enter the insect hemolymph by crossing the gut epithelial barrier; Wolbachia occur in hemolymph and fat body cells, and yet these bacteria are not targeted by the cellular immune response. The reason for this is unknown; they may not be detected by host immune elicitors or they may simply be tolerated by the insect because the fitness costs of mounting an immune response is greater than the cost of maintaining the resident symbiotic population (reviewed in Schmidt 2009). In agreement with this, in D. melanogaster, a previously tolerated Spiroplasma infection became reduced when the immune response was elevated by septic shock or constitutively expressed by mutation (Hurst et al. 2003a). Recently it has been reported that Wolbachia infection in D. simulans reduces the rate of encapsulation of eggs of the parasitoid wasp Leptopilina heterotoma compared to uninfected hosts (Fytrou et al. 2006). Reduced fecundity and smaller body size in infected flies was also reported in this study but it was not determined if Wolbachia actively interferes with the host immune system in order to protect itself, or whether the cost of carrying Wolbachia infections results in a reduced expenditure on immune defense, growth and oocyte production.
5.2 Antimicrobial peptides (AMP)
Antimicrobial activity of the insect hemolymph upon septic injury has been studied for many years. The first description of the AMP cecropins and attacins was by Steiner et al. (1981). Later other AMPs were identified and it is now recognized that AMPs make an important contribution to host defense not only in insects, but also in higher animals and plants (Ganz and Lehrer 1999). In insects, antimicrobial peptides are primarily secreted from the fat body in response to systemic infections; local microbial invasions also induce secretion of AMP from epithelial tissues of the digestive and genital tracts and the Malphigian tubules (Dow and Davies 2006). Molecular and genetic studies have uncovered the mechanisms that regulate the expression of genes encoding immune peptides (Brennan and Anderson 2004; Hetru et al. 2003; Kimbrell and Beutler 2001). Two separate signaling pathways occur in Drosophila (Hoffmann and Reichhart 2002): the Toll pathway responds primarily to invasion by fungi and gram positive bacteria (Lemaitre et al. 1996; Lemaitre et al. 1997; Ligoxygakis et al. 2002); and the IMD (immune deficiency) pathway, responds primarily to attack from gram negative bacteria (Govind and Nehm 2004; Lemaitre et al. 1995). Seventeen AMPs and as many as 43 additional immune-induced molecules have been recognized following immune challenge in third instar larvae of Drosophila (Verleyen et al. 2006). AMPs target different classes of pathogenic microorganisms; for example, diptericins, cecropins, drosocins and attacins are expressed in response to IMD signaling, while the defensins, metchnikowins and drosomycins are transcribed as a result of Toll signaling (Brennan and Anderson 2004).
Components of the bacterial cell wall have been shown to be essential elements for eliciting the immune response in insects (Brennan and Anderson 2004; Hetru et al. 2003; Hoffmann and Reichhart 2002; Kaneko and Silverman 2005), as they are in vertebrates. Invertebrate hemocytes recognize and bind to conserved pathogen-associated molecular patterns (PAMPs) produced by lipopolysaccharides (LPS) or peptidoglycans (PGN) of the cell walls of invading bacteria (Kang et al. 1998; Yoshida et al. 1996). The receptors for PAMPs are the peptidoglycan recognition proteins (PGRPs) (Dziarski 2004); Drosophila have thirteen genes encoding PGRPs, including six long forms (PGRP-LA, -LB, -LC, -LD, -LE, and –LF) and seven short forms (PGRP-SA, -SB1, -SB2, -SC1A, -SC1B, -SC2 and –SD)(Werner et al. 2000).
Gram negative bacteria possess a tripartite outer wall comprising a thin peptidoglycan layer located in the periplasmic space between the outer membrane and an inner cytoplasmic membrane. Virtually all gram negative intracellular bacteria studied to date have lost genes necessary for the synthesis of cell wall lipopolysaccharide (LPS), including Wolbachia (Foster et al. 2005). However Wolbachia (wMel) possess a gene encoding one of the peptidoglycan-associated lipoprotein (Pal) (Parsons et al. 2006) family of proteins, OmpA-MotB. OmpA in the gram negative cell wall forms a linkage between the peptidoglycan layer and the outer membrane protein. In spite of this, existing components of the Wolbachia cell wall are insufficient to trigger an immune response in their hosts via the IMD pathway since Wolbachia infection does not induce diptericin or cecropin expression in D. simulans or A. albopictus (Bourtzis et al. 2000). Similarly, infection of D. melanogaster by S. poulsonii fails to induce the expression of genes encoding immune peptides (Charlat et al. 2003) even though these bacteria are widespread in hemolymph.
Symbiotic bacteria may protect themselves from host immunity in one of two ways: preventing the activation of immune signaling by hiding within a host vacuolar membrane or inhibiting immune signalling by some unknown mechanism. Some pathogenic bacteria of mammalian macrophages avoid activating anti-microbial signaling cascades; for example, A. phagocytophilum and E. chaffeensis are able to infect mammalian granulocytes and monocytes/macrophages in vivo without eliciting anti-microbial signaling. The strategies that enable them to do this have been reviewed by Rikihisa (2006). Similarly, cultured cells from D. melanogaster infected with L. monocytogenes do not show up-regulation of genes involved in immune signaling when studied using RNA interference screens (Ayres and Schneider 2006).
The effectors of the Toll pathway (Dorsal-related Immunity Factor, DIF) and the IMD pathway (Relish) are inactivated by the binding of ankyrin repeat sequences. In the case of the Toll pathway, the protein Cactus inactivates DIF and Relish is auto-inhibited in the IMD pathway. Wolbachia genomes so far sequenced encode multiple ankyrin repeat proteins; four in the case of wBm, twenty-three in the genome of wMel (Wu et al. 2004), thirty five in wRi (Klasson et al. 2009b) and sixty in wPip (Klasson et al. 2008). It has been suggested that ankyrin repeat-containing proteins play an important role in host-endosymbiont interactions (Iturbe-Ormaetxe et al. 2005), perhaps as components of the host-derived outer vacuolar membrane surrounding the endosymbiont, or otherwise modulating the host response. In wRi, one ankyrin repeat protein (WD0550) bears a significant sequence homology to the ankyrin repeat domain of Relish. This protein is a candidate for an endosymbiont-derived inhibitory factor which could modulate the host response. Experimental evidence in support of a function for ankyrin repeat proteins of Wolbachia is lacking, although the presence of at least one ankyrin repeat protein in Wolbachia harboured by Culex pipiens (wPip) shows strain and sex-specific expression in this host (Sinkins et al. 2005).
5.3 Reactive oxygen and antioxidant enzymes
Reactive oxygen species (ROS) arise during mitochondrial respiration and contribute to oxidative stress experienced by aerobic organisms. Oxidative damage due to cumulative levels of ROS contributes to ageing and disease in animals (reviewed in Dalle-Donne et al. 2006; Junqueira et al. 2004). The byproducts of respiration are partially reduced forms of O2 and include superoxide ions (O2 .-), hydrogen peroxide (H2O2) and hydroxyl radicals (OH.). ROS causes unregulated oxidation of cellular components leading to damaged membrane lipids, nucleic acids and proteins, and, ultimately, to the destruction of the cell (reviewed in Imlay 2003). In addition, ROS have been identified as important components of cell signaling pathways (reviewed in Hoidal 2001; McCord 2000; Rhee et al. 2003) and other physiological responses including early immune response to invasion by pathogens (reviewed in Kohchi et al. 2009)
In vertebrates, following phagocytosis of bacteria, superoxide is produced by the reduction of molecular oxygen by the NADPH oxidase complex, a multi-component enzyme system that assembles at the phagosomal membrane in a reaction called an oxidative burst (reviewed in Roos et al. 2003). From superoxide a number of additional ROS are formed, either directly or indirectly, which are also bactericidal (reviewed in Babior et al. 1973; Hampton et al. 1998; Kobayashi et al. 2005). ROS have been detected in the larval hemolymph of several lepidopteran species (Arakawa 1994, 1995a, b; Slepneva et al. 1999) and during encapsulation of parasitoids in immune-reactive D. melanogaster (Nappi and Vass 1998; Nappi et al. 1995). Recent work in insects has provided support for immune responsive reactions resembling the oxidative burst seen in vertebrates (Bergin et al. 2005; Ha et al. 2005; Whitten and Ratcliffe 1999).
Enzymatic and non-enzymatic systems including superoxide dismutases, catalases, peroxidases, glutathione, thioredoxin and other vitamins and metabolites (Sies 1993; Winyard et al. 2005) have evolved in response to ROS. Numerous repair pathways have also evolved in order to prevent permanent cellular damage. DNA damage in the form of lesions are often restored via base excision repair (BER), nucleotide excision repair (NER), mismatch repair (MMR), and translesion synthesis (TLS); double-strand breaks are repaired by homologous recombination (HR), and non-homologous end joining (NHEJ) (reviewed by Cline and Hanawalt 2003; Slupphaug et al. 2003). While oxidized proteins are often completely degraded by proteases and replaced by newly synthesized versions, oxidized sulfur-containing amino acids are eligible for repair (Friguet 2006). Oxidized membrane lipids may be repaired by reacylation following excision from the membrane by hydrolysis (van Kuijk et al. 1987), or within the membrane itself (Thomas et al. 1990).
Elevated levels of ROS production and an increase in both bacterial and host antioxidant proteins are a response to the presence of symbionts in A. albopictus cells naturally infected with Wolbachia (Brennan et al. 2008). Whether the excess ROS within this system is the result of the insect innate immune response to Wolbachia, or generated by additive aerobic respiration of the Wolbachia themselves, is currently under investigation. Regardless of the cause, the generation of antioxidants in response to oxidative stress appears to be an adaptation to bacterial life within eukaryotic cells and may be a key factor permitting symbiotic relationships to develop and persist over a long evolutionary timeframe.
Rickettsia rickettsii are obligate intracellular vertebrate pathogens vectored by arthropods, and closely related to Wolbachia. In vertebrate cells, R. rickettsii induce superoxide formation and the generation of superoxide dismutase, an antioxidant which converts superoxide into hydrogen peroxide (Santucci et al. 1992). At the same time, expression of antioxidants that neutralize intracellular peroxides is inhibited, leading to lipid peroxidation of membranes (Devamanoharan et al. 1994; Eremeeva and Silverman 1998). Tissue-specific antioxidant enzyme activities in mice infected with R. conorii have been reported (Rydkina et al. 2004). Such evidence lends support to the premise that intracellular bacteria other than Wolbachia interact with a host antioxidant system in a manner that is beneficial to their survival.
While generation of ROS and expression of antioxidants in Spiroplasma has not been well studied, other Mollicutes, particularly vertebrate pathogens belonging to the genera Mycoplasma have been more thoroughly researched. Hydrogen peroxide is an important virulence factor in several species of Mycoplasma (Cole et al. 1968; Hames et al. 2009; Somerson et al. 1965); and superoxide released by M. pneumoniae inhibits the activity of host-generated catalase, which functions to degrade H2O2, resulting in intracellular accumulation of H2O2 and increased cell injury (Almagor et al. 1984). ROS associated with Ureaplasma urealyticum, a close relative of Mycoplasma, is linked to lipid peroxidation of sperm cell membranes and human infertility (Potts et al. 2000) and ROS generated by M. pneumonia within human lung carcinoma cells results in DNA damage (Sun et al. 2008). Recent work on antioxidants has shown that M. pneumoniae and M. penetrans have an antioxidant function which permits their survival under conditions of oxidative stress within HeLa cells (Tarshis et al. 2004; Yavlovich et al. 2006). A peroxiredoxin believed to function primarily in the neutralization of hydrogen peroxide has recently been identified in M. hyponeumoniae (Machado et al. 2009). The Mollicutes are capable of complex interactions with their host through ROS and antioxidant pathways and research pertaining to insect hosts carrying symbiotic Spiroplasma is needed.
5.4 Antiviral defense
Insect antiviral defense mechanisms have not been well characterized. Drosophila is host to a number of pathogenic ssRNA viruses including members of the family Rhabdoviridae and Discoviridae and ds RNA viruses from the families Reoviridae, Birnaviridae, and Errantiviridae (Huszar and Imler 2008). Drosophila protects itself from attack by RNA viruses through two pathways, one involving RNAi (Galiana-Arnoux et al. 2006; Kemp and Imler 2009) and the other the JAK/STAT-dependent immune pathway (Dostert et al. 2005). Naturally occurring pathogenic DNA viruses have not been reported in Drosophila although they do occur in other insects, including other dipterans (Friesen and Miller 2001). Baculoviruses are the best studied insect viral pathogens due to their widespread use in insect pest management. The baculovirus genome is a double-stranded, covalently closed circular DNA of approximately 100–130 kb. They gain entry to the insect through the midgut after ingestion of viral particles on contaminated plants. Insect gut juices and sloughing of midgut epithelia are important viral resistance mechanisms. In addition, insect DNA viruses have evolved methods to bypass the defense mechanisms of insect cells by interfering with apoptosis. Lepidopteran baculoviruses possess p35 and iap-like genes and monitor their survival by suppressing apoptosis of host cells (reviewed in Clarke and Clem 2003). On the other hand, the RNA virus, Flock House Virus (FHV) induces apoptosis in Drosophila DL-1 cells by depleting endogenous levels of Drosophila DIAP1, and the ascovirus SfAV which attacks the fat body of larval lepidopterans encodes a caspase which induces apoptosis of Sf21 cells (Bideshi et al. 2005). The resulting apoptotic bodies form large vesicles which become the site of viral assembly.
Investigation into the immune response to lytic viruses of endosymbionts is in its infancy. It may be that Drosophila lacks an immune mechanism for responding to the bacteriophage of Wolbachia or, possibly, Wolbachia has an unrecognized means of circumventing antiviral as well as antibacterial defenses of host insects. Some of the most exciting recent work on endosymbionts has been the determination that Wolbachia protects D. melanogaster from attack by RNA viruses (Teixeira et al. 2008). However the mechanism by which they do so is unknown. Another question remains: are insect AMPs expressed in response to Wolbachia peptidoglycan fragments exposed as a result of bacteriophage lytic activity? This seems unlikely since D. simulans infected with Wolbachia produce virions (Gavotte et al. 2004) but do not show elevated expression of AMPs (Bourtzis et al. 2000).
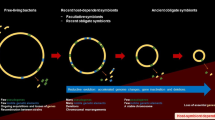
6 Concluding remarks: Tipping the balance toward insect death
It is likely that bacterial endosymbionts play an important role in the evolution of insects enabling them to succeed in restricted niches such as dependence on blood or phloem for nutrition, and to establish disease-resistant phenotypes. Teasing apart the apparent genetic crosstalk that allows for these developments will add to our understanding of evolution, evolution that works simultaneously on eukaryotic, prokaryotic and viral genomes in a single organism. This knowledge will permit the development of novel strategies to target and manipulate insect species that currently cause widespread destruction of food production and spread devastating animal and plant diseases. Despite an amazing array of novel molecules, insects rather routinely develop "resistance" to them, often very rapidly, that is, within a few generations. Short generation times provide a developmental vehicle for this capacity but it is the underlying genetics of these animals that is the engine for this change. For example gene amplification and duplication allow insects to detoxify novel insecticide molecules, even on first exposure. The response to resistance has been to develop molecules with new targets and new modes of action, with the hope that these would have more lasting effects. More recently, microbes have become the source of some of these molecules. For example, numerous isolates of Bacillus thuringiensis have provided a diverse array of insect-killing toxin molecules, and the added advantage of technology has led to the development of transgenic plants. Toxins are produced in the plants as insects consume them and this strategy has proved effective for protection of many diverse crops.
A similar strategy is not currently available for insects that feed on animals, including humans. Human-insect interactions result in the spread of diseases such as malaria, dengue and Chagas’ disease to name a few. The role of endosymbiont genes in these diseases needs to be explored. Wolbachia are obligate mutualists in filarial nematodes, some of which are human pathogens and they are required for host survival and successful oogenesis. Wolbachia play an important role in the pathogenesis of filarial disease and they offer a convenient target for treatment of these diseases. It remains to be seen if bacterial endosymbionts can be engineered to provide effective control strategies for insects, through genetic transformation or targeted manipulation of pest insect population structure.
References
Abrams JM, White K, Fessler LI, Steller H (1993) Programmed cell death during Drosophila embryogenesis. Development 117:29–43
Ahrens MJ, Shoemaker DD (2005) Evolutionary history of Wolbachia infections in the fire ant Solenopsis invicta. BMC Evol Biol 5:1–11
Akman L, Yamashita A, Hidemi W, Oshima K, Shiba T, Hattori M, Aksoy S (2002) Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat Genet 32:402–408
Almagor M, Kahane I, Yatziv S (1984) Role of superoxide anion in host cell injury induced by Mycoplasma pneumoniae infection. J Clin Investig 73:842–847
Ammar E-D, Hogenhout SA (2005) Use of immunofluorescence confocal laser scanning microscopy to study distribution of the bacterium corn stunt spiroplasma in vector leafhoppers (Hemiptera: Cicadellidae) and in host plants. Ann Entomol Soc Am 98:820–826
Ammar E-D, Hogenhout SA (2006) Mollicutes associated with arthropods and plants. In: Bourtzis K, Miller TA (eds) Insect symbiosis. CRC, Boca Raton, pp 97–118
Anbutsu H, Fukatsu T (2003) Population dynamics of male-killing and non-male-killing spiroplasmas in Drosophila melanogaster. Appl Environ Microbiol 69:1428–1434
Anbutsu H, Fukatsu T (2006) Tissue-specific infection dynamics of male-killing and nonmale-killing spiroplasmas in Drosophila melanogaster. FEMS Microl Ecol 57:40–46
Arakawa T (1994) Superoxide generation in vitro in lepidopteran larval haemolymph. J Insect Physiol 40:165–171
Arakawa T (1995a) Possible involvement of an enzymatic system for superoxide generation in lepidopteran larval haemolymph. Arch Insect Biochem Physiol 29:281–291
Arakawa T (1995b) Superoxide generative reaction in insect haemolymph and its mimic model system with surfactants in vitro. Insect Biochem Mol Biol 25:247–253
Ayres JS, Schneider DS (2006) Genomic dissection of microbial pathogenesis in cultured Drosophila cells. Trends Microbiol 14:101–104
Azzouna A, Greve P, Martin G (2004) Sexual differentiation traits in functional males with female genital apertures (fga) in the woodlice Armadillidium vulgare Latr. (Isopoda, Crustacea). Gen Comp Endocrinol 138:42–49
Babior BM, Kipnes RS, Curnutte JT (1973) The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Investig 52:741–744
Bai X, Zhang J, Holford IR, Hogenhout SA (2004) Comparative genomics identifies genes shared by distantly related insect-transmitted plant pathogenic mollicutes. FEMS Microbiol Lett 235:249–258
Baldo L, Werren JH (2007) Revisiting Wolbachia supergroup typing based on WSP: spurious lineages and discordanec with MLST. Curr Microbiol 55:81–87
Baldo L, Bartos JD, Werren JH, Bazzocchi C, Casiraghi M, Panelli S (2002) Different rates of nucleotide substitutions in Wolbachia endosymbionts of arthropods and nematodes: arms race or host shifts? Parassitologia 44:179–187
Baldo L, Dunning Hotopp JC, Jolley KA, Bordenstein SR, Biber SA, Choudhury RR, Hayashi C, Maiden MCJ, Tettelin H, Werren JH (2006) Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl Environ Microbiol 72:7098–7110
Baldo L, Ayoub NA, Hayashi CY, Russell JA, Stahlhut JK, Werren JH (2008) Insight into the routes of Wolbachia invasion: high levels of horizontal transfer in the spider genus Agelenopsis revealed by Wolbachia strain and mitochondrial DNA diversity. Mol Ecol 17:557–569
Bandi C, Slatko B, O’Neill SL (1999) Wolbachia genomes and the many faces of symbiosis. Parasitol Today 15:428–429
Bao SN, Kitajima EW, Callaini G, Dallai R (1996) Virus-like particles and rickettsia-like organisms in male germ and cyst cells of Bemisia tabaci (Homoptera, Aleyrodidaae. J Invertebr Pathol 67:309–311
Batista PD, Keddie BA, Dosdall LM, Harris HL (2009) Phylogenetic placement and evidence for horizontal transfer of Wolbachia in Plutella xylostella (Lepidoptera: Plutellidae) and its parasitoid Diadegma insulare (Hymenoptera: Ichneumonidae). Canadian Entomologist, in press
Bentley J, Veneti Z, Heraty J, Hurst GD (2007) The pathology of embryo death caused by the male-killing Spiroplasma bacterium in Drosophila nebulosa. BMC Biology 5, doi:10.1186/1741-7007-1185-1189.
Bergin D, Reeves EP, Renwick J, Wientjies FB, Kavanagh K (2005) Superoxide production in Galleria mellonella hemocytes: identification of proteins homologous to the NADPH oxidase complex of human neutrophils. Infect Immun 73:4161–4170
Bideshi DK, Tan Y, Bigot Y, Federici BA (2005) A viral caspase contributes to modified apoptosis for virus transmission. Genes Dev 19:1416–1421
Bigliardi E, Sacchi L, Genchi M, Alma A, Pajoro M, Daffonchio D, Marzorati M, Avanzati AM (2006) Ultrastructure of a novel Cardinium sp. symbiont in Scaphoideus titanus (Hemiptera: Cicadellidae). Tissue Cell 38:257–261
Bordenstein S (2003) Symbiosis and the origin of species. In: Miller KBaTA (ed) Insect symbiosis. CRC, Boca Raton, pp 283–304
Bordenstein S, Wernegreen JJ (2004) Bacteriophage flux in endosymbionts (Wolbachia): infection frequency, lateral transfer, and recombination rates. Mol Biol Evol 21:1981–1991
Bordenstein S, Reznikoff WS (2005) Mobile DNA in obligate intracellular bacteria. Nature Reviews Microbiology 3
Bordenstein SR, Marshall ML, Fry AJ, Kim U, Wernegreen JJ (2006) The tripartite associations between bacteriophage, Wolbachia and arthropods. PLoS Pathog 2:e43
Bourtzis K, Dobson SL, Braig HR, O’Neill SL (1998) Rescuing Wolbachia have been overlooked. Nature 391:852–853
Bourtzis K, Pettigrew MM, O’Neill SL (2000) Wolbachia neither induces nor suppresses transcripts encoding antimicrobial peptides. Insect Mol Biol 9:635–639
Braendle C, Miura T, Bickel R, Shingleton AW, Kambhampati S, Stern DL (2003) Developmental origin and evolution of bacteriocytes in the aphid-Buchnera symbiosis. PLoS Biol 1:70–76
Brandt SM, Dionne MS, Khush RS, Pham LN, Vigdal TJ, Schneider DS (2004) Secreted bacterial effectors and host-produced Eiger/TNF drive death in a Salmonella-infected fruit fly. PLoS Biol 2:e418
Breeuwer JAJ, Jacobs G (1996) Wolbachia: intracellular manipulators of mite reproduction. Exp Appl Acarol 20:421–434
Brennan CA, Anderson KV (2004) Drosophila: the genetics of innate immune recognition and response. Annu Rev Immunol 22:457–483
Brennan LJ, Keddie BA, Braig HR, Harris HL (2008). The endosymbiont Wolbachia pipientis induces the expression of host antioxidant proteins in an Aedes albopictus cell line. PLoS One 3, doi: 10.1371/journal.pone.0002083.
Brinton LP, Burgdorfer W (1976) Cellular and subcellular organization of the 277F agent, a spiroplasma from the rabbit tick Haemaphysalis leporispalustris. Int J Syst Bacteriol 26:554–560
Brownlie JC, O’Neill SL (2006) Wolbachia genomics: accelerating our understanding of a pervasive symbiosis. In: Bourtzis K, Miller TA (eds) Insect symbiosis. CRC, Baco Raton, pp 175–186
Casiraghi M, Favia G, Cancrini G, Bartoloni A, Bandi C (2001) Molecular identification of Wolbachia from the filarial nematode Marsonella ozzardi. Parasitol Res 87:417–420
Charlat S, Hurst GDD, Mercot H (2003) Evolutionary consequences of Wolbachia infections. Trends Genet 19:217–223
Chigira A, Miura K (2005) Detection of ‘Candidatus Cardinium’ bacteria from the haploid host Brevipalpus californicus (Acari: Tenuipalpidae) and effect on the host. Exp Appl Acarol 37:107–116
Christie PJ (2001) Type IV secretion: intercellular transfer of macromolecules by systems ancestrally related to conjugation machines. Mol Microbiol 40:294–305
Clark ME, Karr TL (2002) Distribution of Wolbachia within Drosophila reproductive tissue: implications for the expression of cytoplasmic incompatibility. Integr Comp Biol 42:332–339
Clark ME, Anderson CL, Cande J, Karr TL (2005) Widespread prevalence of Wolbachia in laboratory stocks and the implications for Drosophila research. Genetics 170:1667–1675
Clarke TE, Clem RJ (2003) In vivo induction of apoptosis correlating with reduced infectivity during baculovirus infection. J Virol 77:2227–2232
Cline SD, Hanawalt PC (2003) Who’s on first in the cellular response to DNA damage? Nat Rev Mol Cell Biol 4:361–372
Cohen AJ, Williamson DL, Oishi K (1987) SpV3 viruses of Drosophila spiroplasmas. Isr J Med Sci 23:429–433
Cole BC, Ward JR, Martin CH (1968) Hemolysin and peroxide activity of Mycoplasma species. J Bacteriol 95:2022–2030
Counce SJ, Poulson DF (1962) Developmental effects of the sex ratio agent in embryos of Drosophila willistoni. Journal of Experimental Zoology 151
Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Aldo M (2006) Biomarkers of oxidative damage in human disease. Clin Chem 52:601–623
Davis RE, Dally EL, Jomantiene R, Zhao Y, Roe B, Lin S, Shao J (2005) Cryptic plasmid pSKU146 from the wall-less plant pathogen Spiroplasma kunkelii encodes an adhesin and components of a type IV translocation-related conjugation system. Plasmid 53:179–190
De Luna CJ, Moro CV, Guy JH, Zenner L, Sparagano OAE (2009) Endosymbiotic bacteria living inside the poultry red mite (Dermanyssus gallinae). Exp Appl Acarol 48:105–113
Dedeine F, Vavre F, Fleury F, Loppin B, Hochberg ME, Bouletreau M (2001) Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc Natl Acad Sci USA 98:6247–6252
Dedeine F, Vavre F, Shoemaker DD, Bouletreau M (2004) Intra-individual coexistence of a Wolbachia strain required for host oogenesis with two strains inducing cytoplasmic incompatibility in the wasp Asobara tabida. Evolution 58:2167–2174
Dedeine F, Bouletreau M, Vavre F (2005) Wolbachia requirement for oogenesis: occurrence within the genus Asobara (Hymenoptera, Braconidae) and evidence for intraspecific variation in A. tabida. Heredity 95:394–400
Delmotte, F., Rispe, C., Schaber, J., Silva, F.J., and Moya, A. (2006). Tempo and mode of early gene loss in endosymbiotic bacteria from insects. BMC Evolutionary Biology 6, doi:10.1186/1471-2148-1186-1156.
Devamanoharan PS, Santucci LA, Hong JE, Tian X, Silverman DJ (1994) Infection of human endothelial cells by Rickettsia rickettsii causes a significant reduction in the levels of key enzymes involved in protection against oxidative injury. Infect Immun 62:2619–2621
Dobson SL (2003) Wolbachia pipientis: impotent by association. In: Bourtzis K, Miller TA (eds) Insect symbiosis. CRC, Boca Raton
Dostert C, Jouanguy E, Irving P, Troxler L, Galiana-Arnoux D, Hetru C, Hoffmann JA, Imler J-L (2005) The Jak-STAT signaling pathway is required but not sufficient for the antivial response of drosophila. Nat Immunol 6:946–953
Dow JAT, Davies SA (2006) The malpighian tubule: rapid insights from post-genomic biology. J Insect Physiol 52:365–378
Dumler JS, Barbet AF, Bekker CPJ, Dasch GA, Palmer GH, Ray SC, Rikihisa Y, Rurangirwa FR (2001) Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and HGE agent as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol 51:2145–2165
Duron O, Bouchon D, Boutin S, Bellamy L, Zhou L, Engelstadter J, Hurst GD (2008) The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol 6:27. doi: 10.1186/1741-7007-6-27
Dyer KA, Jaenike J (2005) Evolutionary dynamics of a spatially structured host-parasite association: Drosophila innubia and male-killing Wolbachia. Evolution 59:1518–1528
Dyson EA, Hurst GDD (2004) Persistance of an extreme sex-ratio bias in a natural population. Proc Natl Acad Sci USA 101:6520–6523
Dziarski R (2004) Peptidoglycan recognition proteins (PGRPs). Mol Immunol 40:877–886
Enigl M, Schausberger P (2007) Incidence of the endosymbionts Wolbachia, Cardinium and Spiroplasma in phytoseiid mites and associated prey. Exp Appl Acarol 42:75–85
Eremeeva ME, Silverman DJ (1998) Rickettsia rickettsii infection of the EA.hy 926 endothelial cell line: morphological reponse to infection and evidence for oxidative injury. Microbiology 144:2037–2048
Fenn K, Conlon C, Jones M, Quail MA, Holroyd NE, Parkhill J, Blaxter M (2006) Phylogenetic relationships of the Wolbachia of nematodes and arthropods. PLoS Pathogens 2, doi: 10.1371/journal.ppat.0020094.
Ferree PM, Frydman HM, Li JM, Cai J, Weischaus E, Sullivan W (2005a) Wolbachia utilizes host microtubules and dynein for anterior localization in the Drosophila embryo. PLoS Pathog 1:e14
Ferree PM, Frydman HM, Li JM, Cai J, Weischaus E, Sullivan W (2005b) Wolbachia utilizes host microtubules and dynein for anterior localization in the Drosophila oocyte. PLoS Pathog 1:e14
Finlay BB, Falkow S (1997) Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev 61:136–169
Floate KD, Kyei-Poku GK, Coghlin PC (2006) Overview and relevance of Wolbachia bacteria in biocontrol research. Biocontrol Sci Technol 16:767–788
Foster J, Ganatra M, Kamal I, Ware J, Makarova K, Ivanova N, Bhattacharyya A, Kapatral V, Kumar S, Posfai J et al (2005) The Wolbachia genome of Brugia malayi: endosymbiont evolution within a human pathogenic nematode. PLoS Biol 3:e121
Friesen PD, Miller LK (2001) Insect viruses. In: Knipe D, Howley P (eds) Fields Virology. Philadelphia, Lippincott, pp 599–628
Friguet B (2006) Oxidized protein degradation and repair in ageing and oxidative stress. FEBS Lett 580:2910–2916
Fujii Y, Kubo T, Ishikawa H, Sasaki T (2004) Isolation and characterization of the bacteriophage WO from Wolbachia, an arthropod endosymbiont. Biochem Biophys Res Commun 317:1183–1188
Fukatsu T, Nikoh N (2000) Endosymbiotic microbiota of the bamboo pseudococcid Antonina crawii (Insecta, Homoptera). Appl Environ Microbiol 66:643–650
Fukatsu T, Tsuchida T, Nikoh N, Koga R (2001) Spiroplasma symbiont of the pea aphid, Acyrthosiphon pisum (Insecta: Homoptera). Appl Environ Microbiol 67:1284–1291
Fukatsu T, Kondo N, Nobuyuki I, Nikoh N (2003) Discovery of symbiont-host horizontal genome transfer: a beetle carrying two bacterial and one chromosomal Wolbachia endosymbionts. In: Bourtzis K, Miller TA (eds) Insect symbiosis. CRC, Boca Raton
Funk DJ, Helbling L, Wernegreen JJ, Moran N (2000) Intraspecific phylogenetic congruence among multiple symbiont genomes. Proc Roy Soc B 267:2517–2521
Fytrou A, Schofield PG, Kraaijeveld AR, Hubbard SF (2006) Wolbachia infection suppresses both host defence and parasitoid counter-defence. Proc Roy Soc B 273:791–796
Galiana-Arnoux D, Dostert C, Schneemann A, Hoffmann JA, Imler J-L (2006) Essential function in vivo for Dicer-2 in host defense against RNA viruses in drosophila. Nat Immunol 7:590–597
Ganz T, Lehrer RI (1999) Antibiotic peptides from higher eukaryotes: biology and applications. Mol Med Today 5:292–297
Gasparich GE, Whitcomb RF, Dodge D, French FE, Glass J, Williamson DL (2004) The genus Spiroplasma and its non-helical descendants: phylogenetic classification, correlation with phenotype and roots of the Mycoplasma mycoides clade. Int J Syst Evol Microbiol 54:893–918
Gavotte L, Vavre F, Henri H, Ravallec M, Stouthamer R, Bouletreau M (2004) Diversity, distribution and specificity of WO phage infection in Wolbachia of four insect species. Insect Mol Biol 13:147–153
Gil R, Silva FJ, Zientz E, Delmotte F, Gonzalez-Candelas F, Latorre A, Rausell C, Kamerbeek J, Gadau J, Holldobler B et al (2003) The genome sequence of Blochmannia floridanus: comparative analysis of reduced genomes. Proc Natl Acad Sci US 100:9388–9393
Gomez-Valero L, Silva FJ, Simon JC, Latorre A (2007) Genome reduction of the aphid endosymbiont Buchnera aphidicola in a recent evolutionaery time scale. Gene 389:87–95
Gotoh T, Noda H, Homg X-Y (2003) Wolbachia distribution and cytoplasmic incompatibility based on a survey of 42 spider mite species (Acari: Tetranychidae) in Japan. Heredity 91:208–216
Gotoh T, Noda H, Ito S (2007) Cardinium symbionts cause cytoplasmic incompatibility in spider mites. Heredity 98:13–20
Govind S, Nehm RH (2004) Innate immunity in fruit flies: a textbook example of genomic recycling. PLoS Biol 2:E276
Groot TV, Breeuwer JA (2006) Cardinium symbionts induce haploid thelytoky in most clones of three closely related Brevipalpus species. Exp Appl Acarol 39:257–271
Ha E-M, Oh C-T, Bae Y-S, Lee W-J (2005) A direct role for dual oxidase in Drosophila gut immunity. Science 310:847–850
Hames C, Halbedel S, Hoppert M, Frey J, Stulke J (2009) Glycerol metabolism is important for cytotoxicity of Mycoplasma pneumoniae. J Bacteriol 191:747–753
Hampton MB, Kettle AJ, Winterbourn CC (1998) Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92:3007–3017
Harris HL, Braig HR (2003) Sperm chromatin remodelling and Wolbachia-induced cytoplasmic incompatibility in Drosophila. Biochem Cell Biol 81:229–240
Haselkorn TS, Markow TA, Moran NA (2009) Multiple introductions of the Spiroplasma bacterial endosymbiont into Drosophila. Mol Ecol 18:1294–1305
Hetru C, Troxler L, Hoffmann JA (2003) Drosophila melanogaster antimicrobial defense. J Infect Dis 187(Suppl 2):S327–334
Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH (2008) How many species are infected with Wolbachia?-A statistical analysis of current data. FEMS Microbiol Lett 201:215–220
Hoffmann AA (2005) Incompatible mosquitoes. Nature 436:189
Hoffmann JA, Reichhart J-M (2002) Drosophila innate immunity: an evolutionary perspective. Nat Immunol 3:121–126
Hoidal JR (2001) Reactive oxygen species and cell signaling. Am J Respir Cell Mol Biol 25:661–663
Hornett EA, Charlat S, Duplouy AMR, Davies N, Roderick GK, Wedell N, Hurst GDD (2006) Evolution of male-killer suppression in a natural population. PLoS Biol 4:e283
Hornett EA, Duplouy AM, Davies N, Roderick GK, Wedell N, Hurst GDD, Charlat S (2008) You can’t keep a good parasite down: evolution of a male-killer suppressor uncovers cytoplasmic incompatibility. Evolution 62:1258–1263
Hotopp JCD, Clark ME, Oliveira DCSG, Foster JM, Fischer P, Torres MCM, Giebel JD, Kumar N, Ishmael N, Wang S et al (2007) Wisepread lateral gene transfer from intracellular bacteria to multicellular eukaryotes. Science 317:1753–1756
Howe D, Heinzen RA (2006) Coxiella burnetti inhabits a cholesterol-rich vacuole and influences cellular cholesterol metabolism. Cell Microbiol 8:496–507
Hoy M, Jeyaprakash A (2008) Symbionts, including pathogens, of the predatory mite Metaseiulus occidentalis: current and future analysis methods. Exp Appl Acarol 46:329–347
Hunter MS, Zchori-Fein E (2006) Inherited Bacteroidetes symbionts in arthropods. In: Bourtzis K, Miller TA (eds) Insect Symbiosis. CRC, Boca Raton, pp 39–56
Hunter MS, Perlman SJ, Kelly SE (2003) A bacterial symbiont in the Bacteriodetes induces cytoplasmic incompatibility in the parasitoid wasp Encarsia pergandiella. Proc R Soc Lond B Biol Sci 270:2185–2190
Hurst GDD, Jiggins FM (2000) Male-killing bacteria in insects: mechanisms, incidence and implications. Emerg Infect Dis 6:329–336
Hurst GDD, Majerus MEN (1993) Why do maternally inherited microorganisms kill males? Heredity 71:81–95
Hurst GDD, Hammarton TC, Obrycki JJ, Majerus TM, Walker LE, Bertrand D (1996) Male-killing bacteria in a fifth ladybird beetle, Coleomegille maculata (Coleoptera: Coccinellidae). Heredity 77:177–185
Hurst GDD, Hammarton TC, Bandi C, Majerus TMO, Bertrand D, Majerus MEN (1997) The diversity of inherited parasites of insects: the male killing agent of the ladybird beetle Coleomegilla maculata is a member of the Flavobacteria. Genet Res 70:1–6
Hurst GDD, Bandi C, Sacchi L, Cochrane AG, Bertrand D, Karaca T, Majerus ME (1999a) Adonia variegata (Coleoptera:Coccinellidae) bears maternally inherited Flavobacteria that kill males only. Parasitology 118:125–134
Hurst GDD, Jiggins FM, von der Schulenburg JHG (1999b) Male-killing Wolbachia in two species of insect. Proc R Soc Lond B 266:735–740
Hurst GDD, Schulenburg JHGVD, Majerus TMO, Bertrand D, Zacharov I, Baungaard J, Volkl W, Stouthamer R, Majerus MEN (1999c) Invasion of one insect species, Adalia bipunctata, by two different male-killing bacteria. Insect Mol Biol 8:133–139
Hurst GDD, Johnson AP, von der Schulenbur JHG, Fuyama Y (2000) Male-killing Wolbachia in Drosophila: a temperature-sensitive trait with a threshold bacterial density. Genetics 156:699–709
Hurst GDD, Anbutsu H, Kutsukake M, Fukatsu T (2003a) Hidden from the host: Spiroplasma bacteria infecting Drosophila do not cause an immune response, but are suppressed by ectopic immune activation. Insect Mol Biol 12:93–97
Hurst GDD, Jiggens FM, Majerus MEN (2003b) Inherited microorganisms that selectively kill male hosts: the hidden players of insect evolution. In: Bourtzis K, Milller TA (eds) Insect symbiosis. CRC, Boca Raton, pp 177–197
Huszar T, Imler J-L (2008) Drosophila viruses and the study of antiviral host-defense. In: Maramorosch K, Shatkin AJ, Murphy F (eds) Advances in virus research. Academic, San Diego, pp 227–265
Hypsa V, Novakova E (2009) Insect symbionts and molecular phylogenies. In: Bourtzis K, Miller TA (eds) Insect symbiosis. CRC, Boca Raton
Ikeda H (1970) The cytoplasmically inherited ‘sex-ratio’ condition in natural and experimental populations of Drosophila bifasciata. Genetics 65:311–333
Ikeya T, Broughton S, Alic N, Grandison R, Partridge L (2009) The endosymbiont Wolbachia increases insulin/IGF-like signalling in Drosophila. Proceedings of the Royal Society B
Imlay JA (2003) Pathways of oxidative damage. Annu Rev Microbiol 57:395–418
Iturbe-Ormaetxe I, O’Neill SL (2007) Wolbachia-host interactions: connecting phenotype to genotype. Curr Opin Microbiol 10:221–224
Iturbe-Ormaetxe I, Burke GR, Riegler M, O’Neill SL (2005) Distribution, expression, and motif variability of ankyrin domain genes in Wolbachia pipientis. J Bacteriol 187:5136–5145
Jaenike J, Polak M, Fiskin A, Helou M, Minhas M (2007) Interspecific transmission of endosymbiotic Spiroplasma by mites. Biol Lett 3:23–25
Jansson E, Blackman A, Hakkarainen K, Miettinen A (1982) Viruses of mycoplasmasand spiroplasmas. Med Biol 60:125–131
Jiggens FM, Bentley JK, Majerus MEN, Hurst GDD (2002) Recent changes in phenotype and patterns of host specialization in Wolbachia bacteria. Mol Ecol 11:1275–1283
Jiggins FM, Hurst GDD, Jiggens CD, Schulenburg JHGVD, Majerus MEN (2000a) The butterfly Danaus chrysippus is infected by a male-killing Spiroplasma bacterium. Parasitology 120:439–446
Jiggins FM, Hurst GDD, Majerus MEN (2000b) Sex-ratio disturbing Wolbachia causes sex-role reversal in its butterfly host. Proc Roy Soc B 267:69–73
Jiggins FM, Hurst GDD, Schulenburg JHGVD, Majerus MEN (2001) Two male-killing Wolbachia strains coexist within a population of the butterfly Acraea encedon. Heredity 86:161–166
Junqueira VBC, Barros SBM, Chan SS, Rodrigues L, Giavarotti L, Abud RL, Deucher GP (2004) Ageing and oxidative stress. Mol Aspects Med 25:5–16
Kageyama D, Anbutsu H, Watada M, Hosokawa T, Shimada M, Fukatsu T (2006) Prevalence of a non-male-killing spiroplasma in natural populations of Drosophila hydei. Appl Environ Microbiol 72:6667–6673
Kamoda S, Masui S, Ishikawa H, Sasaki T (2000) Wolbachia infection and cytoplasmic incompatibility in the cricket Tetragryllus taiwanemma. J Exp Biol 203:2503–2509
Kaneko T, Silverman N (2005) Bacterial recognition and signalling by the Drosophila IMD pathway. Cell Microbiol 7:461–469
Kang D, Liu G, Lundstrom A, Gelius E, Steiner H (1998) A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proc Natl Acad Sci USA 95:10078–10082
Kemp C, Imler J-L (2009) Antiviral immunity in drosophila. Curr Opin Immunol 21:3–9
Kikuchi Y, Fukatsu T (2003) Diversity of Wolbachia endosymbionts in heteropteran bugs. Appl Environ Microbiol 69:6082–6090
Kimbrell DA, Beutler B (2001) The evolution and genetics of innate immunity. Nat Rev Genet 2:256–267
Kitajima EW, Groot T, Novelli VM, Freitas-Astua J, Alberti G, Moraes GJ (2007) In situ observation of the Cardinium symbionts of Brevipalpus (Acari: Tenuipalpidae) by electron microscopy. Exp Appl Acarol 42:263–271
Klasson L, Walker T, Sebaihia M, Sanders MJ, Quail MA, Lord A, Sanders S, Earl J, O'Neill SL, Thomson N et al (2008) Genome evolution of Wolbachia strain wPip from the Culex pipiens group. Mol Biol Evol 25:1877–1887
Klasson L, Kambris Z, Cook PE, Walker T, Sinkins SP (2009a) Horizontal gene transfer between Wolbachia and the mosquito Aedes aegypti. BMC Genomics 10:33
Klasson L, Westberg J, Sapountzis P, Naslund K, Lutnaes Y, Darby AC, Veneti Z, Chen L, Braig HR, Garrett R et al (2009b) The mosaic genome structure of the Wolbachia wRi strain infecting Drosophila simulans. Proc Natl Acad Sci USA 106:5725–5730
Kobayashi SD, Voyich JM, Burlak C, DeLeo FR (2005) Neutrophils in the innate immune response. Archivum immunologii et therapiae experimentalis 53:505–517
Kohchi C, Inagawa H, Nishizawa T, Soma G (2009) ROS and innate immunity. Anticancer Res 29:817–821
Kondo N, Nikoh N, Ijichi N, Shimada M, Fukatsu T (2002) Genome fragment of Wolbachia endosymbiont transferred to X chromosome of host insect. Proc Natl Acad Sci USA 99:14280–14285
Kurtti TJ, Munderloh UG, Andreadis TG, Magnarelli LA, Mather TA (1996) Tick cell culture isolation of an intracellular prokaryote from the tick Ixodes scapularis. J Invertebr Pathol 67:318–321
Kyei-Poku GK, Colwell DD, Coghlin P, Benkel B, Floate K (2005) On the ubiquity and phylogeny of Wolbachia in lice. Mol Ecol 14:285–294
Lanot R, Zachary D, Holder F, Meister M (2001) Postembryonic hematopoiesis in Drosophila. Dev Biol 230:243–257
Laven H (1951) Crossing experiments with Culex strains. Evolution 5:370–375
Lawson ET, Mousseau TA, Klaper R, Hunter MD, Werren JH (2000) Rickettsia associated with male-killing in a buprestid beetle. Heredity 86:497–505
Lemaitre B, Kromer-Metzger E, Michaut L, Nicolas E, Meister M, Georgel P, Reichhart JM, Hoffmann AA (1995) A recessive mutation, immune deficiency (imd) defines two distinct control pathways in the Drosophila host defense. Proc Natl Acad Sci USA 92:9465–9469
Lemaitre B, Nicolas E, Michaut L, Reichhart J-M, Hoffmann JA (1996) The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973–983
Lemaitre B, Reichhart JM, Hoffmann JA (1997) Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc Natl Acad Sci USA 94:14614–14619
Ligoxygakis P, Pelte N, Hoffmann JA, Reichhart JM (2002) Activation of Drosophila Toll during fungal infection by a blood serine protease. Science 297:114–116
Lo N, Casiraghi M, Salati E, Bazzochi C, Bandi C (2002) How many Wolbachia supergroups exist? Mol Biol Evol 19:341–346
Lo N, Paraskevopoulos C, Bourtzis K, O'Neill SL, Werren JH, Bordenstein S, Bandi C (2007) Taxonimic status of the intracellular bacterium Wolbachia pipientis. Int J Syst Evol Microbiol 57:654–657
Louis C, Nigro L (1989) Ultrastructural evidence of Wolbachia rickettsiales in Drosophila simulans and their relationships with unidirectional cross incompatibility. J Invertebr Pathol 54:39–44
Machado CX, Pinto PM, Zaha A, Ferreira HB (2009) A peroxiredoxin from Mycoplasma hyopneumoniae with a possible role in H2O2 detoxification. Microbiology 155, DOI 10.1099/mic.1090.030643-030640.
Mansfield BE, Dionne MS, Schneider DS, Freitag NE (2003) Exploration of host-pathogen interactions using Listeria monocytogenes and Drosophila melanogaster. Cell Microbiol 5:901–911
Masui S, Sasaki T, Ishikawa H (2000) Genes for the type IV secretion system in an intracellular symbiont, Wolbachia, a causative agent of various sexual alterations in arthropods. J Bacteriol 182:6529–6531
Masui S, Kuroiwa H, Sasaki T, Inui M, Kuroiwa T, Ishikawa H (2001) Bacteriophage WO and virus-like particles in Wolbachia, an endosymbiont of arthropods. Biochem Biophys Res Commun 283:1099–1104
Matalon Y, Katzir N, Gottlieb Y, Portnoy V, Zchori-Fein E (2007) Cardinium in Plagiomerus diaspidis (Hymenoptera: Encyrtidae). J Invertebr Pathol 96:106–108
Mateos M, Castrezna SJ, Nankivell BJ, Estes AM, Markow TA, Moran NA (2006) Heritable endosymbionts of Drosophila. Genetics 174:363–376
McCord JM (2000) The evolution of free radicals and oxidative stress. Am J Med 108:652–659
McLeod MP, Qin X, Karpathy SE, Gioia J, Highlander SK, Fox GE, McNeill TZ, Jiang H, Muzny D, Jacob LS et al (2004) Complete genome sequence of Rickettsia typhi and comparison with sequences of other Rickettsiae. J Bacteriol 186:5842–5855
Mira A, Moran N (2002) Estimating population size and transmission bottlenecks in maternally transmitted endosymbiotic bacteria. Microb Ecol 44:137–143
Moran N (2007) Symbiosis as an adaptive process and source of phenotypic complexity. Proc Natl Acad Sci USA 104:8627–8633
Moran N, Tran P, Gerardo NM (2005) Symbiosis and insect diversification: an ancient symbiont of sap-feeding insects from the bacterial phylum Bacteroidetes. Appl Environ Microbiol 71:8802–8810
Nakamura Y, Kawai S, Yukuhiro F, Ito S, Gotoh T, Kisimoto R, Yanase T, Matsumoto Y, Kageyama D, Noda H (2009) Prevalence of Cardinium in planthoppers and spider mites and taxonomic revision of "Candidatus Cardinium hertigii" based on detection of a new Cardinium group from biting midges. Applied and Environmental Microbiology, doi:10.1128/AEM.01583-01509.
Nappi AJ, Vass E (1998) Hydrogen peroxide production in immune-reactive Drosophila melanogaster. J Parasitol 84:1150–1157
Nappi AJ, Vass E, Frey F, Carton Y (1995) Superoxide anion generation in Drosophila during melanotic encapsulation of parasites. Eur J Cell Biol 68:450–456
Nirgianaki A, Banks GK, Frohlich DR, Veneti Z, Braig HR, Miller TA, Bedford ID, Markham PG, Savakis C, Bourtzis K (2003) Wolbachia infections of the whitefly Bemisia tabaci. Curr Microbiol 47:93–101
Noel GR, Atibalentja A (2006) “Candidatus Paenicardinium endonii”, an endosymbiont of the plant-parasitic nematode Heterodera glycines (Nemata: Tylenchida), affiliated to the phylum Bacteroidetes. Int J Syst Evol Microbiol 56:1697–1702
O’Neill SL, Pettigrew MM, Sinkins SP, Braig HR, Andreadis TG, Tesh RB (1997a) In vitro cultivation of Wolbachia pipientis in an Aedes albopictus cell line. Insect Mol Biol 6:33–39
O’Neill SL, Werren JH, Hoffmann AA (1997b) Influential passengers: inherited microorganisms and arthropod reproduction. Oxford University, Oxford
Park S-Y, Heo Y-J, Kim K-S, Cho Y-H (2005) Drosophila melanogaster is susceptible to Vibrio cholerae infection. Mol Cells 20:409–415
Parsons LM, Lin F, Orban J (2006) Peptidoglycan recognition by Pal, an outer membrane lipoprotein. Biochemistry (Mosc) 45:2122–2128
Penalva LO, Sanchez L (2003) RNA binding protein Sex-Lethal (Sxl) and control of Drosophila sex determination and dosage compensation. Microbiol Mol Biol Rev 67:343–359
Perlman SJ, Hunter MS, Zchori-Fein E (2006) The emerging diversity of Rickettsia. Proc Roy Soc B 273:2097–2106
Perotti MA, Braig HR (2004) Endosymbionts of Acari. Phytophaga XIV:457–474
Pichon S, Bouchon D, Cordaux R, Chen L, Garrett RA, Greve P (2009) Conservation of the type IV secretion system throughout Wolbachia evolution. Biochem Biophys Res Commun 385:557–562
Popov VL, Han VC, Chen SM, Dumler JS, Feng HM, Andreadis TG, Tesh RB, Walker DH (1998) Ultrastructural differentiation of the genogroups in the genus Ehrlichia. J Med Microbiol 47:235–251
Potts JM, Sharma R, Pasqualotto F, Nelson D, Hall G, Agarwal A (2000) Association of Ureaplasma urealyticum with abnormal reactive oxygen species levels and absence of leukocytospermia. J Urol 163:1775–1778
Poulson DF, Sakaguchi B (1961) Nature of “sex ratio” agent in Drosophila. Science 133:1489–1490
Provencher L, Morse GE, Weeks AR, Normark BB (2005) Parthenogenesis in the Aspidiotus nerii complex (Hemiptera: Diaspididae): a single origin of a worldwide polyphagous lineage asssociated with Cardinium bacteria. Ann Entomol Soc Am 98:629–635
Raychoudhury R, Baldo L, Oliveira DCSG, Werren JH (2008) Modes of acquisition of Wolbachia: horizontal transfer, hybrid introgression, and codivergence in the Nasonia species complex. Evolution 63:165–183
Reeves WK, Dowling APG, Dasch GA (2006) Rickettsial agents from parasitic Dermanyssoidea (Acari: Mesostigmata). Exp Appl Acarol 38:181–188
Regassa LB, Gasparich GE (2006) Spiroplasmas: evolutionary relationships and biodiversity. Front Biosci 11:2983–3002
Rhee SG, Chang T-S, Bae YS, Lee S-R, Kang SW (2003) Cellular regulation by hydrogen peroxide. J Am Soc Nephrol 14:S211–S215
Rikihisa Y (2006) Ehrlichia subversion of host innate responses. Curr Opin Microbiol 9:95–101
Roberts LW, Rapmund G, Gadigan FG (1977) Sex ratios in Rickettsia Tsutsugamushi-infected and noninfected colonies of Leptotrombidium (Acari: Trombiculidae). J Med Entomol 14:89–92
Roos D, van Bruggen R, Meischl C (2003) Oxidative killing of microbes by neutrophils. Microb Infect 5:1307–1315
Ros VID, Breeuwer JAJ (2009) The effects of, and interactions between, Cardinium and Wolbachia in the doubly infected spider mite Bryobia sarothamni. Heredity 102:413–420
Ros VID, Fleming VM, Feil EJ, Breeuwer JAJ (2009) How diverse is the genus Wolbachia? Multiple-gene sequencing reveals a putatively new Wolbachia supergroup recovered from spider mites (Acari: Tetranychidae). Appl Environ Microbiol 75:1036–1043
Roth O, Kurtz J (2009) Phagocytosis mediates specificity in the immune defence of an invertebrate, the woodlouse Porcellio scaber (Crustacea: Isopoda). Dev Comp Immunol 33:1151–1155
Rudakov NV, Shypnov SN, Samoilenko IE, Tankibaev MA (2003) Ecology and epidemiology of spotted fever group Rickettsiae and new data from their study in Russia and Kazakhstan. Ann NY Acad Sci 990:12–24
Rugendorff A, Younossi-Hartenstein A, Hartenstein V (1994) Embryonic origin and differentiation of the Drosophila heart. Roux’s Archives of Developmental Biology 203:266–280
Rydkina E, Sahni SK, Santucci LA, Turpin LC, Baggs RB, Silverman DJ (2004) Selective modulation of antioxidant enzyme activities in host tissues during Rickettsia conorii infection. Microb Pathog 36:293–301
Sakaguchi B, Poulson DF (1963) Interspecific transfer of the “sex-ratio” condition from Drosophila willistoni to D. melanogaster. Genetics 48:841–861
Sakamoto JM, Feinstein J, Rasgon JL (2006) Wolbachia infections in the Cimicidae: museum specimens as an untapped resource for endosymbiont surveys. Appl Environ Microbiol 72:3161–3167
Sanogo YO, Dobson SL (2006) WO bacteriophage transcription in Wolbachia-infected Culex pipiens. Insect Biochem Mol Biol 36:80–85
Santucci LA, Gutierrez PL, Silverman DJ (1992) Rickettsia rickettsii induces superoxide radical and superoxide dismutase in human endothelial cells. Infect Immun 60:5113–5118
Schmidt O (2009) Self-nonself recognition in symbiotic interactions. In: Bourtzis K, Miller TA (eds) Insect symbiosis. CRC, Boca Raton, pp 33–56
Serbus LR, Sullivan W (2007) A cellular basis for Wolbachia recruitment to the host germline. PLoS Pathog 3:e190
Sexton JA, Vogel JP (2002) Type IVB secretion by intracellular pathogens. Traffic 3:178–185
Sheeley SL, McAllister BF (2009) Mobile male-killer: similar Wolbachia strains kill males of divergent Drosophila hosts. Heredity 102:286–292
Shigenobu S, Watanabe H, Hattori M, Sakaki Y, Ishikawa Y (2000) Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature 407:81–86
Sies H (1993) Strategies of antioxidant defense. Eur J Biochem 215:213–219
Sinkins SP, Walker T, Lynd AR, Steven AR, Makepeace BL, Godfray HCJ, Parkhill J (2005) Wolbachia variability and host effects on crossing type in Culex mosquitoes. Nature 436:257–260
Slepneva IA, Glupov VV, Sergeeva SV, Khramtsov VV (1999) EPR detection of reactive oxygen species in hemolymph of Galleria mellonella and Dendrolimus superans sibiricus (lepodoptera) larvae. Biochem Biophys Res Commun 264:212–215
Slupphaug G, Kavli B, Krokan HE (2003) The interacting pathways for prevention and repair of oxidative DNA damage. Mutat Res 531:231–251
Somerson NL, Walls BE, Chanock RM (1965) Hemolysin of Mycoplasma pneumonia: tentative identification as a peroxide. Science 150:226–228
Sorrentino RP, Carton Y, Govind S (2002) Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev Biol 243:65–80
Steiner J, Hultmark D, Engstrom A, Bennich H, Boman HG (1981) Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 292:246–248
Stouthamer R, Breeuwer J, Hurst G (1999) Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu Rev Microbiol 53:71–102
Sun G, Xu X, Wang Y, Shen X, Chen Z, Yang J (2008) Mycoplasma pneumoniae infection induces reactive oxygen species and DNA damage in A549 human lung carcinoma cells. Infect Immun 76:4405–4413
Tarshis M, Yavlovich A, Katzenell A, Ginsburg I, Rottem S (2004) Intracellular location and survival of Mycoplasma penetrans within HeLa cells. Curr Microbiol 49:136–140
Teixeira L, Ferreira A, Ashburner M (2008) The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biology 6
Tepass U, Fessler LI, Aziz A, Hartenstein V (1994) Embryonic origin of hemocytes and their relationship to cell death in Drosophila. Development 120:1829–1837
Thao ML, Baumann L (2004) Evidence for multiple acquisition of Arsenophonus by Whitefly species (Sternorrhyncha: Aleyrodidae). Curr Microbiol 48:140–144
Theopold U, Schmidt O, Soderhall K, Dushay MS (2004) Coagulation in arthropods:defence, wound closure and healing. Trends Immunol 25:289–294
Thomas JP, Maiorino M, Ursini F, Girotti AW (1990) Protective action of phospholipid hydroperoxide glutathione peroxidase against membrane-damaging lipid peroxidation. J Biol Chem 265:454–461
Tinsley MC, Majerus MEN (2006) A new male-killing parasitism: Spiroplasma bacteria infect the ladybird beetle Anisosticta novemdecimpunctata (Coleoptera: Coccinellidae). Parasitology 132:757–765
Tram U, Ferree PM, Sullivan W (2003) Identification of Wolbachia-host interacting factors through cytological analysis. Microbes Infect 5:999–1011
Trpis M, Perrone JB, Reissig M, Parker KL (1981) Control of cytoplasmic incompatibilty in the Aedes scutellaris complex. J Hered 72:313–317
Tully JG, Rose DL, Yunker CE, Carle P, Bove JM, Williamson DL, Whitcomb RF (1995) Spiroplasma ixodetis sp. nov., a new species from Ixodes pacificus ticks collected in oregon. Int J Syst Bacteriol 45:23–28
van Ham RCHJ, Kamerbeek J, Palacios C, Rausell C, Abascal F, Bastolla U, Fernandez JM, Jimenez L, Postigo M, Silva FJ et al (2003) Reductive genome evolution in Buchnera aphidicola. Proc Natl Acad Sci USA 100:581–586
van Kuijk FJGM, Sevanian A, Handelman GJ, Dratz EA (1987) A new role for phospholipase A2: protection of membranes from lipid peroxidation damage. Trends Biochem Sci 12:31–34
Vandekerckhove TTM, Watteyne S, Willems A, Swings JG, Mertens J, Gillis M (1999) Phylogenetic analysis of the 16S rDNA of the cytoplasmic bacterium Wolbachia from the novel host Folsomia candida (Hexapoda, Collembola) and its implications for wolbachial taxonomy. FEMS Microbiol Lett 180:279–286
Vavre F, Fleury F, Lepetit D, Fouillet P, Bouletreau M (1999) Phylogenetic evidence for horizontal transmission of Wolbachia in host-parasitoid associations. Mol Biol Evol 16:1711–1723
Veneti Z, Clark ME, Zabalou S, Karr TL, Savakis C, Bourtzis K (2003) Cytoplasmic incompatibility and sperm cyst infection in different Drosophila-Wolbachia associations. Genetics 164:545–552
Veneti Z, Bentley JK, Koana T, Braig HR, Hurst GDD (2005) A functional dosage compensation complex required for male-killing in Drosophila. Science 307:1461–1463
Verleyen P, Baggerman G, D’Hertog W, Vierstraete E, Husson SJ, Schoofs L (2006) Identification of new immune induced molecules in the haemolymph of Drosophila melanogaster by 2D-nanoLC MS/MS. J Insect Physiol 52:379–388
Voth DE, Howe D, Beare PA, Vogel JP, Unsworth N, Samuel JE, Heinzen RA (2009) The Coxiella burnetii ankyrin repeat domain-containing protein family is heterogeneous, with C-Terminal truncations that influence Dot/Icm mediated secretion. J Bacteriol 191:4232–4242
Watts T, Haselkorn TS, Moran N, Markow TA (2009) Variable incidence of Spiroplasma infections in natural populations of Drosophila species. PLoS ONE 4:e5703
Weeks AR, Breeuwer JAJ (2003) A new bacterium from the Cytophaga-Flavobacterium-Bacteroides phylum that causes sex ratio distortion. In: Bourtzis K, Miller TA (eds) Insect symbiosis. CRC, Boca Raton, pp 165–176
Weeks AR, Stouthamer R (2004) Increased fecundity associated with infection by a Cytophaga-like intracellular bacterium in the predatory mite, Metaseiulus occidentalis. Proc Roy Soc B 271:S193–S195
Weeks AR, Marec F, Breeuwer JAJ (2001) A mite species that consists entirely of haploid females. Science 292:2479–2482
Weeks AR, Velten R, Stouthamer R (2003) Incidence of a new sex-ratio-distorting endosymbiotic bacterium among arthropods. Proc R Soc Lond B 270:1857–1865
Weinert LA, Tinsley MC, Temperley M, Jiggens FM (2007) Are we underestimating the diversity and incidence of insect bacterial symbionts? A case study in ladybird beetles. Biol Lett 3:678–681
Werner T, Liu G, Kang D, Ekengren S, Steiner H, Hultmark D (2000) A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proc Natl Acad Sci USA 97:13772–13777
Werren JH, Windsor DM (2000) Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proc Roy Soc B 267:1277–1285
Werren JH, Skinner SW, Huger AM (1986) Male-killing bacteria in a parasitic wasp. Science 231:990
Werren JH, Hurst DD, Zhang W, Breeuwer JAJ, Stouthamer R, Majerus ME (1994) Rickettsial relative associated with male killing in the ladybird beetle (Adalia bipunctata). J Bacteriol 176:388–394
Werren JH, Zhang W, Guo LR (1995) Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc Roy Soc B 261:53–63
Whitten MMA, Ratcliffe NA (1999) In vitro superoxide activity in the haemolymph of the west indian leaf cockroach, Blaberus discoidalis. J Insect Physiol 45:667–675
Williamson DL, Sakaguchi B, Hackett KJ, Whitcomb RF, Tully JG, Carle P, Bove JM, Adams JR, Konai M, Henegar RB (1999) Spiroplasma poulsonii sp nov., a new species associated with male-lethality in Drosophila willistoni, a neotropical species of fruit fly. Int J Syst Bacteriol 49:611–618
Winyard PG, Moody CJ, Jacob C (2005) Oxidative activation of antioxidant defence. Trends Biochem Sci 30
Wolf KW, Glatzel S (1996) Intracytoplasmic bacteria in male germ cells of Philudoria potatoria L. (Lasiocampidae, Lepidoptera, Insecta). J Invertebr Pathol 67:279–288
Woolfit M, Iturbe-Ormaetxe I, McGraw EA, O’Neill SL (2009) An ancient horizontal gene transfer between mosquito and the endosymbiotic bacterium Wolbachia pipientis. Mol Biol Evol 26:367–374
Wright JD, Barr AR (1980) The ultrastructure and symbiotic relationships of Wolbachia of mosquitoes of the Aedes scutellaris group. J Ultrastruct Res 72:52–64
Wright JD, Sjostrand FS, Portaro JK, Barr RA (1978) The ultrastructure of the rickettsia-like microorganism Wolbachia pipientis and associated virus-like bodies in the mosquito Culex pipiens. J Ultrastruct Res 63:79–85
Wu M, Sun LV, Vamathevan J, Riegler M, Deboy R, Brownlie JC, McGraw EA, Martin W, Esser C, Ahmadinejad N et al (2004) Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol 2:0327–0341
Xi Z, Gavotte L, Xie Y, Dobson SL (2008) Genome-wide analysis of the interaction between the endosymbiotic bacterium Wolbachia and its Drosophila host. BMC Genomics 9, doi:10.1186/1471-2164-1189-1181.
Yavlovich A, Kohen R, Ginsburg I, Rottem S (2006) The reducing antioxidant capacity of Mycoplasma fermentans. FEMS Microbiol Lett 259:195–200
Yen JH, Barr AR (1971) New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens. Nature 232:657–658
Yen JH, Barr AR (1973) The etiological agent of cytoplasmic incompatibility in Culex pipiens. J Invertebr Pathol 22:242–250
Yoshida H, Kinoshita K, Ashida M (1996) Purification of a peptidoglycan recognition protein from hemolymph of the silkworm, Bombyx mori. J Biol Chem 271:13854–13860
Zchori-Fein E, Perlman SJ (2004) Distribution of the bacterial symbiont Cardinium in arthropods. Mol Ecol 13:2009–2016
Zchori-Fein E, Gottlieb Y, Kelly SE, Brown JK, Wilson JM, Karr TL, Hunter MS (2001) A newly discovered bacterium associated with parthenogenesis and a change in host selection behaviour in parasitoid wasps. Proc Natl Acad Sci USA 98:1255–1260
Zchori-Fein E, Perlman SJ, Kelly SE, Katzir N, Hunter MS (2004) Characterization of a ‘Bacteroidetes’ symbiont in Encarsia wasps (Hymenoptera: Aphelinidae): proposal of Candidatus Cardinium hertigii. Int J Syst Evol Microbiol 54:961–968
Acknowledgments
The authors thank Jennifer Biliske and Philip Batista for helpful comments and suggestions in the preparation of this manuscript.
Funding
NSERC Discovery Grant to H.H.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harris, H.L., Brennan, L.J., Keddie, B.A. et al. Bacterial symbionts in insects: balancing life and death. Symbiosis 51, 37–53 (2010). https://doi.org/10.1007/s13199-010-0065-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-010-0065-3