Abstract
The effect of ohmic heating method (OHM) on quality and quantity attributes of black grape molasses was investigated and compared with the conventional heating method (CHM). Results showed that the samples prepared by OHM had the highest antioxidant activity than CHM. Increasing of voltage gradient had a positive effect on the saving of antioxidant activity. Changes in pH for OHM were lower than CHM. Heating methods had no significant effect on phenol content. Antioxidant capacity and phenol content of treated samples were lower than the fresh sample at the same water content. The titratable acidity of treated samples using CHM was higher than the OHM. The OHM saved about 2.4–7.2-fold of processing time and 6.3-fold of energy consumption than the CHM. Heat generation and electrical conductivity depended on sample moisture content. OHM provides minimal damage to the sensory characteristics. As a final result, the OHM significantly improved the quality and saved the quantity parameters of the grape molasses processing than the CHM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Grape molasses commonly used in Middle Eastern food because of their high sugar content like glucose and fructose, minerals and organic acids (Özcan et al. 2015; Gurak et al. 2010). Conventional thermal processing including, classical and vacuum evaporation methods, is the most common method in the processing industry of grape juice concentrate (Helvacıoğlu et al. 2018; Kayışoğlu and Demirci 2006). Processing under vacuum stated to lower the boiling point and possibly prevent the decomposition of fruit juice components due to excessive and long-term application of heat. This is while Helvacıoğlu et al. (2018) showed that the quality of grape molasses (phenolic compounds, total phenol contents, and antioxidant activity) produced by the traditional method was higher than that produced by conventional vacuum evaporation technique (CVET). Also, Kayışoğlu and Demirci (2006) reported that the sedimentation during heating and color of grape molasses in CVET were two important problems because the color of molasses using CVET was not much preferred in the market by consumers. The internal resistance by conventional heating resulted in very heterogeneous treatment and the notable quality loss of product (Hosainpour et al. 2014; Özcan and Al-Juhaimi 2017). Therefore, the use of new methods to process products that can overcome or minimize these problems is essential.
The modern food industry has focused on the development of new techniques to produce a safe, high-quality product with a long shelf life. Various emerging techniques in the food industry have been studied, including pulsed electric field, cold atmospheric plasma, ultrasound, high-pressure processing and ohmic heating (Ahmad et al. 2019). Among the emerging methods of food processing, ohmic heating seems to be an interesting alternative for processing of different food materials such as fruit and fruit juice (Icier et al. 2017; Fadavi et al. 2018), meat cooking (Inmanee et al. 2019; Engchuan et al. 2014), dairy productions (Costa et al. 2018; Ferreira et al. 2019a, b), and cereals (Deleu et al. 2019; Dias-Martins et al. 2019), when compared to the conventional processing. The advantages of ohmic heating compared to conventional heating includes maintaining the color and nutritional value of food, uniform heating, short processing time, and higher yield (Costa et al. 2018; Fadavi et al. 2018). The electric current passing through food material causes rapid heat generation and thus faster heating of food in the ohmic heating system. The amount of heat generated inside the food is directly related to the voltage gradient and the electrical conductivity (Darvishi et al. 2015). In recent years, the interest in using the ohmic heating method has been increased for the concentration of fruit juice such as pomegranate juice (Icier et al. 2017), sour cherry juice (Sabancic and Icier 2017), tomato juice (Fadavi et al. 2018), and orange juice (Darvishi et al. 2019).
Although several researches have been conducted on the conventional grape molasses production (Helvacıoğlu et al. 2018; Özcan et al. 2015; Kayışoğlu and Demirci 2006), but to the best of our knowledge no investigation was found on the production of grape molasses by the ohmic heating method. Therefore, the aim of the present study was to comparatively evaluate (1) quality parameters (antioxidant activity, phenol content, pH, titratable acidity, and total soluble solids) and sensory evaluation, (2) processing time and heating rate, heat generation, energy consumption of grape molasses produced by ohmic heating and conventional methods. Also, variations of internal heat generation and electrical conductivity of grape juice during the concentration process were evaluated as a function of moisture content and sample temperature.
Materials and methods
Grape juice preparation
Amount of 100 kg of fresh black grape was purchased from the local fruit market, Kurdistan, Iran. Based on seller information, the grape samples were harvested in 5/10/2018 from a grape farm in the mountainous areas of Kurdistan. The experiments were conducted at 11/10/2018 (1 week after harvest). Samples washed using tap-water then processed to grape juice using a juice extractor. Juice samples passed through the filter.
Ohmic heating method
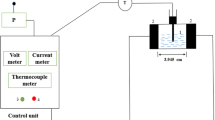
Figure 1 shows the schematic and picture of the static ohmic heating system. The ohmic heating unit consisted of a rectangular cell, two removable electrodes (316L stainless steel) with a 100 mm gap between them, a power analyzer (Dina Co, Iran) for measurement of electrical current, voltage and power consumption, three thermocouples (NTC- type) with Teflon coated, a voltage regulating transformer (1 kW, 0–330 V, 50 Hz, MST-3, Toyo, Japan), a microcontroller board, and a personal computer. Variation of the mass sample recorded by a digital balance (EK-5055, China) with ± 0.1 g accuracy which is placed under the ohmic cell, as shown in Fig. 1.
About 225 ± 1 g of fresh black grape juice with 19 ± 0.5 °C initial temperature was used in each experiment. Ohmic heating process was carried out until the juice moisture ratio reached to 0.22 ± 0.03 by using different voltage gradients 15, 20, 25 and 30 V/cm at 50 Hz frequency. Voltage, current, mass and temperature data were measured at 1 s time intervals (Δt = 1 s) during heating and passed this information to the computer with a RS 232. After each test, the electrodes were rinsed using a brush and distilled water.
Conventional heating method
About 225 ± 0.5 g of fresh grape juice was poured into a laboratory beaker (Pyrex glass) and then heated using a laboratory heater (Aryan Azma Co, Iran) with maximum power. The temperature of the juice sample measured using thermocouples (NTC- type) and recorded at 1 s time intervals (Δt = 1 s) during the heating process.
Moisture content
The instantaneous moisture content of juice sample was determined as follows (Darvishi et al. 2015):
where Mt is the moisture content at any time of heating (kg water/kg dry matter), m0 and M0 are the initial mass (kg) and moisture content (kg water/kg dry matter) of fresh juice sample, respectively, and mt is the mass of sample at any time of heating (kg). The moisture content of the juice sample at any time of the concentration process than the fresh juice presented as follows:
where Xw is the moisture content ratio (dimensionless) of juice sample at any time of the concentration process.
Electrical conductivity
The electrical conductivity was calculated as follows (Icier and Ilicali 2004):
where σ is the electrical conductivity (S/m), I is the current flowing through the sample (A); V is the applied voltage (V), A is the sample contact surface with the electrode (m2).
Due to evaporation of the sample water during the heating process, the sample contact surface with the electrode was reduced and the sample density raised continuously, thus an effective contact area and instantaneous density should be considered in Eq. (3) as follows (Fadavi et al. 2018; Darvishi et al. 2015):
where ρt and mt are the instantaneous sample density (kg/m3) and mass of sample (kg) at any time of heating, respectively. The variation of density of grape juice samples with concentration ratio and temperature calculated using the following equation (Bayindir 1993):
where T is the juice sample temperature (°C) and X is the concentration ratio of juice sample (dimensionless).
Heat generation
The volumetric heat generation in the ohmic system was determined as follows (Sabancic and Icier 2017):
where VHG is the volumetric heat generation (W/m3) and ∇V is the applied voltage gradient (V/m). Heat generation rate was determined as follows:
where HG is the heat generation (W) and ut is the volume of the sample at any time of the heating process (m3).
Energy consumption
The energy consumption during OHM and CHM was calculated, respectively, as follows (Nouroallahi et al. 2018):
where P is the power consumption of electrical heater (W), t is the processing time (s), Eoh and Econ are the energy consumption (J) by OHM and CHM, respectively.
Quality assessment
Total soluble solids (TSS) were determined by refractometer (Atago, Japan) at 20 °C and results were expressed as percentage. Titratable acidity (TA) was measured by titrating 3 mL of sample in 27 ml DW with 0.1 N NaOH up to pH 8.2. The results were expressed as percentage of tartaric acid. The pH of samples was measured using a pH meter (Metrohm 827, Switzerland).
TP was extracted from 0.5 mL of sample in ice cold 2 mL HCl-methanol-distilled water (1: 80: 19% v/v) for 12 h at 4 °C and then centrifuged at 12,000×g for 15 min at 4 °C. The TP content in extracts was determined according to the Folin–Ciocalteu procedure (Singleton et al. 1999) with some modification as described previously (Koushesh Saba and Moradi 2016). Briefly, 20 µL of the prepared extract was added to 250 µL distilled water, 750 µL of 1:10 Folin–Ciocalteu reagent: water solution and 800 µL of Na2CO3 (7.5%, w/v). The mixture was incubated at 30 °C for 60 min then; absorption was measured at 765 nm. The results were expressed as mg of gallic acid equivalents (GAE) per 100 mL of the sample using aqueous gallic acid standard.
Antioxidant activity (AA) was measured using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay as described by Patras et al. (2009). The absorbance was measured at 515 nm using a spectrophotometer (Unico UV-2100, USA). AA was expressed as the percentage inhibition of the DPPH radical and was determined using the following equation:
Sensory profiling
The sensory evaluation of samples was done by a panel of five experts on the hedonic scale according to a 1–10 point scale ranging from “very strong dislike” to “very strong like”. Color, viscosity, flavor, and aroma were evaluated by this method and the average values were used for assessing the acceptability by the consumers.
Statistical analysis
All experiments were performed at least three times. The results were expressed as average ± standard deviation, and ANOVA analysis and Duncan test were used for determining statistical significance. Microsoft Excel was used for plotting 2D view of the results.
Results and discussion
Heating rate and processing time
The trend of juice sample temperature during the heating process is shown in Fig. 2. Temperature of sample reached to 90.7 ± 0.9 °C and then the evaporation process started. The temperature of juice samples under OHM increased faster than the CHM.
Heating rate of OH treatments is 4 to 17-fold higher than the conventional treatment (Table 1). Heating rate increased with increasing voltage gradient because increased input energy increased the activity of water molecules (Icier and Ilicali 2005; Sarkis et al. 2013; Darvishi et al. 2015). Processing time for OHM is 2.4 to 7.2-fold lower than the CHM (Table 1). Furthermore, processing time reduced about threefold by increasing voltage gradient from 15 to 30 V/cm. These findings were in line with previous reports (Icier and Ilicali 2005; Sarkis et al. 2013; Darvishi et al. 2015).
Electrical conductivity
Variations of electrical conductivity as function of sample temperature and moisture content ratio are shown in Fig. 3. Applied voltage gradient was not significantly affected the electrical conductivity during warming (P > 0.05). During warming up, the electrical conductivity increased linearly from 0.2 to 0.75 S/m with increasing temperature of juice sample from 18 °C (as initial temperature) up to boiling point. At high gradient voltages of 25 and 30 V/cm, for the temperatures between 72 and 74 °C, a sudden bubbling took place and impaired the linearity of electrical conductivity. The increase in the electrical conductivity values with temperature is related to the reduced drag of ions (Fadavi et al. 2018; Icier and Ilicali 2004). Icier et al. (2008) reported that the electrical conductivity of grape juice increased 0.3-0.8 S/m with increasing temperature from 20 to 80 °C at 20–40 V/cm voltage gradient. Also, Tankesh (2018) found that the electrical conductivity of red grape juice increased linearly from 0.25 to 0.8 S/m with increasing temperature from 30 to 85 °C at 20–40 V/cm voltage gradient.
The electric conductivity has been decreased rapidly when the boiling process started due to the formation of gas bubbles which worked as electrical insulation (Darvishi et al. 2015; Fadavi et al. 2018). The rate of formation of gas bubbles in high voltage gradient was more. Therefore, the decrease of electrical conductivity was visible at the beginning of boiling. After the boiling step, the electrical conductivity decreased with decreasing moisture content ratio of juice sample (0.22 ≤ Xw < 1). The concentration of sugar and soluble solid in the juice samples increased with decreasing moisture content. The concentration dependence of the electrical conductivity of the juices has been explained by the increased drag for the movement of ions with increasing concentration (Darvishi et al. 2015). The sugar might act as an electric insulator and reduced the flow of electrical current from the juice sample. A similar trend has been observed by Castro et al. (2003), Icier (2003) and Icier and Ilicali (2004). Castro et al. (2003) reported that an increase in soluble solids and sugar content of strawberry-based products decreased electrical conductivity from 0.5 to 0.05 S/m. Similarly, Icier (2003) concluded that, as the sugar content increased, the electrical conductivities of the liquid solutions decreased while the acidity of the juices enhanced their electrical conductivities.
Heat generation and energy consumption
According to Fig. 4a, the heat generation in juice sample increased sharply during warming up due to increasing of electrical conductivity. The amount of the generated heat rapidly decreased with the starting of boiling and evaporation process. The same trend of heat generation reported by Sabancic and Icier (2017) and Darvishi et al. (2015) for ohmic concentration of sour cherry juice and tomato juice, respectively. The heat generation in high voltage gradient was larger than the low voltage gradient. The heat generation values at the beginning of boiling were 400 W, 702 W, 1058 W and 1395 W for 15 V/cm, 20 V/cm, 25 V/cm and 30 V/cm, respectively. However, the heat generation values at the end of concentration process decreased dramatically to 40.5 W for 15 V/cm, 112 W for 20 V/cm, 112.5 W for 25 V/cm and 264 W for 30 V/cm.
Variations of moisture content ratio of juice sample during the concentration process are shown in Fig. 4b. The warming up period for juice sample (Xw = 1) were 333 s for 15 V/cm, 145 s for 20 V/cm, 92 s for 25 V/cm, and 77 s for 30 V/cm. The warming up period decreased with increasing voltage gradient (P < 0.05) due to more heat generation in higher voltage gradient at the same time. Decreasing of heat generation in juice sample caused to reduction rate of evaporation and increment of processing time. The average values of energy consumption are shown in Table 1. The voltage gradient had no significant effect on the specific energy consumption using CHM (P > 0.05). The needed energy for the production of grape molasses using OHM is 6.4-fold lower than the CHM.
Quality parameters
According to Fig. 5a, there were no significant differences in TSS values among treatments (P > 0.05). The TA of samples prepared by OHM varied from 0.60 to 0.87%, while the TA of samples processed using CHM was 1.50% (Fig. 5b). The concentrated samples prepared by CHM had the highest TA. The TA at high voltage gradient levels (25 and 30 V/cm) was lower than the low voltage gradient levels (15 and 20 V/cm). The decrease in TA might be due to the higher rate of heating at higher voltage gradient, which resulted in the conversion of organic acids into sugars in large extent. The findings of Chakrabortya and Athmaselvi (2014) for guava juice and Tankesh (2018) for grape juice are in line with these results. Results also revealed that TA of concentrated samples acquired with OHM was significantly lower than CHM. It could be due to the electrochemical degradation of organic acids during OHM. Moreover, TA reduction of subjected samples to the higher voltages gradient enhanced the possibility of an electrochemical reaction. Mercali et al. (2014) proposed electrolysis of water and electrode corrosion as mechanisms of ascorbic acid degradation during the ohmic heating of acerola pulp.
Quality parameters of grapemolasses versus heating conditions. a–dMeans with different letters on them in columns and rows are significantly different at 5% level (Results are mean of replication (n = 3) ± standard deviation). *It should be noted that the moisture content of fresh juice and concentrated samples are 92.2% and 26.2% wet basis. The values of TP and AA are presented in 100 mL of material. For comparison between TP and AA of fresh and concentrated samples, values of TP and AA of concentrated samples should be divided into 3.52 because juice samples concerned about 3.52-fold
The average pH of the concentrated samples prepared with OHM varied between 2.64 and 3.42, while the pH of samples prepared with CHM was2.14 (Fig. 5c). The pH values of grape juice concentrated by reverse osmosis varied between 2.91 and 2.98 (Gurak et al. 2010) in the temperature range of 20–50 °C. Cosme et al. (2018) and Gurak et al. (2010) reported that the low value of pH indicated a high acidity due to the existence of tartaric, malic and citric acids in concentration grape juice. The pH of fresh grape juice in the current study was 3.69. The pH difference of concentrated samples (using OHM) with the fresh sample decreased when higher voltage gradient was used. Also, the pH difference of CHM treatment with the fresh sample was higher than OHM. Heating rate and processing time have been reported as effective parameters on change percentage of pH (Fadavi et al. 2018; Darvishi et al. 2019). It has been reported that heating rate increased pH because the organic acids converted into sugars or utilized for energy synthesis (Chattopadhyay et al. 1992). The heating rate increased by increasing the voltage gradient and the processing time of the product decreased that might reduce the pH changes. The same results have been reported when ohmic heating was used for tomato, orange and pomegranate juices (Fadavi et al. 2018; Darvishi et al. 2013, 2019).
The key phenolic compounds of all grape variety were gallic acid, 3,4-dihydroxybenzoic acid, catechin, 1,2-dihydroxybenzene (Özcan et al. 2017a, b). When comparing the TP and AA properties of treatment and fresh samples, it should be noted that the fresh sample had more moisture content than the treated samples and should be considered the concentration ratio. Figure 5d showed the TP of grape juice concentrate in different thermal treatments. No significant difference was observed among TP of concentrated samples using CHM and OHM (P > 0.05). The TP of OHM and CHM treatments was lower than the fresh juice at the same moisture content. As can be seen, both heating processes, OHM and CHM, significantly reduced the TP of treated samples than the fresh samples in the same moisture content (P < 0.05). Also, TP was not significantly influenced by the applied voltage gradient (P > 0.05). The presented results by Yildiz et al. (2009) suggested that conducted ohmic heating at the gradient voltage of 10–40 V/cm did not cause any difference in TP content of pomegranate juice. In contrast, the decreasing trend of TP during the concentration of pomegranate juice produced by ohmic heating has been reported by Sabanci et al. (2018). Based on these results, it seems that several factors such as the technological treatment, food matrices, and processing conditions might have an effect on the TP content of fruit and vegetable juice during thermal and electro-thermal treatment.
The antioxidant potential of samples was assessed by scavenging effects on the DPPH radicals (Fig. 5e). There was no significant difference between treated samples with CHM and OHM performed at 15 V/cm (P > 0.05), while the grape juice concentrated by OHM-30 V/cm had the greatest AA. The AA of submitted samples to OHM increased from 77.0 to 85.3% with increasing voltage gradient from 15 to 30 V/cm. Under OHM, the processing time was greater and the degradation of AA was more at lower voltage gradient that was in line with previous findings (Darvishi et al. 2019). The AA of both heating methods was lower than the fresh sample when these values compared at the same moisture content. Helvacıoğlu et al. (2018) reported that the pro-oxidant and antioxidant molecules might be formed depending on the degree of applied thermal treatment in grape molasses production. During heat treatment antioxidants in food can be degraded and new components with antioxidant activity can be formed.
Sensory profiling
The results of sensory evaluation are shown in Table 1. For the four traits studied, the samples processed in the ohmic heating method had a higher score than the conventional heating samples (P < 0.01). It was not observed significant difference among the score of color, aroma and viscosity at different gradient levels for treated samples using OHM (P > 0.05).
Conclusion
The effect of two heating methods including ohmic and conventional heating was evaluated on the quality parameters and engineering aspects of the grape molasses processing. The samples prepared by CHM had the highest TA and lowest pH. The TSS and TP values of the final product were not significantly affected by the heating method and voltage gradient. Increasing the voltage causes less reduction in the pH of the concentrated juice than the fresh juice. The AA of samples in OHM treatment was higher than the OHM (P < 0.05). Results showed that the processing time in OHM was 2.4 to 7.2-fold lower and the heating rate was 4 to 17-fold higher than the CHM. The OHM saved up to 6.3-fold in energy consumption compared with CHM. Applied voltage gradient had no significant effect on energy consumption (P > 0.05). The heat generated in the juice sample during the concentration process decreased with decreasing moisture content of juice sample and it caused to the reduction rate of evaporation and increment of processing time. The electrical conductivity increased linearly from 0.2 to 0.75 S/m with increasing temperature up to the boiling point during warming up (Xw = 1) and then decreased with decreasing moisture content ratio of juice sample during evaporation process (0.22 ≤ Xw < 1). It can be noted that the OHM provided better quality and significantly saved the engineering parameters than the CHM during the processing of grape molasses.
References
Ahmad T, Butt MZ, Aadil RM, Inam-ur-Raheem M, Bekhit A, Guimarães JT, Balthazar CF, Rocha RS, Esmerino EA, Freitas MQ, Silva MC, Sameen S, Cruz AG (2019) Impact of nonthermal processing on different milk enzymes. Int J Dairy Technol 70:1–15. https://doi.org/10.1111/1471-0307.12622
Bayindir L (1993) Density and viscosity of grape juice as a function of concentration and temperature. J Food Process Preserv 17:147–151
Castro I, Teixeira JA, Salengke S, Sastry SK, Vicente AA (2003) The influence of field strength, sugar and solid content on electrical conductivity of strawberry products. J Food Process Eng 26:17–29
Chakrabortya I, Athmaselvi KA (2014) Changes in physicochemical properties of guava juice during ohmic heating. J Ready Eat Food 1(4):152–157
Chattopadhyay N, Hore JK, Sen SK (1992) Extension of storage life of sweet orange (C. sinensis Osbeck) cv. Jaffa. Indian J Plant Physiol 35:245–255
Cosme F, Pinto T, Vilela A (2018) Phenolic compounds and antioxidant activity in grape juices: a chemical and sensory view. Beverages 4(22):2–14
Costa NR, Cappato LP, Ferreira MVS, Pires RPS, Moraes J, Esmerino EA, Silva R, Neto RPC, Tavares MIB, Freitas MQ, SilveiraJúnior RN, Rodrigues FN, Bisaggio RC, Cavalcanti RN, Raices RSL, Silva MC, Cruz AG (2018) Ohmic heating: a potential technology for sweet whey processing. Food Res Int 106:771–779
Darvishi H, Khoshtaghza MH, Najafi G (2013) Ohmic heating of pomegranate juice: electrical conductivity and pH change. J Saudi Soc Agric Sci 12:101–108
Darvishi H, Hosainpour A, Nargesi F, Fadavi A (2015) Exergy and energy analyses of liquid food in an Ohmic heating process: a case study of tomato production. Innov Food Sci Emerg Technol 31:73–82
Darvishi H, Mohammadi P, Fadavi A, Koushesh Saba M, Behroozi-Khazaei N (2019) Quality preservation of orange concentrate by using hybrid ohmic—vacuum heating. Food Chem 289:292–298
Deleu LJ, Luyts A, Wilderjans E, Haesendonck IV, Brijs K, Delcour J (2019) Ohmic versus conventional heating for studying molecular changes during poundcake baking. J Cereal Sci 89:102708
Dias-Martins AM, Cappato LP, da Costa Mattos M, Rodrigues FN, Pacheco N, Carvalho CWP (2019) Impacts of ohmic heating on decorticated and whole pearl millet grains compared to open-pan cooking. J Cereal Sci 85:120–129
Engchuan W, Jittanit W, Garnjanagoonchorn W (2014) The ohmic heating of meat ball: modeling and quality determination. Innov Food Sci Emerg Technol 23:121–130
Fadavi A, Yousefi S, Darvishi H, Mirsaeedghazi H (2018) Comparative study of ohmic vacuum, ohmic, and conventional-vacuum heating methods on the quality of tomato concentrate. Innov Food Sci Emerg Technol 47:225–230
Ferreira M, Cappato L, Silva R, Rocha R, Guimarães JT, Balthazar CF, Esmerino EA, Freitas MQ, Rodrigues FN, Granato D, Neto R, Tavares MIB, Silva PHF, Raices RSL, Silva MC, Cruz A (2019a) Ohmic heating for processing of whey-raspberry flavored beverage. Food Chem. https://doi.org/10.1016/j.foodchem.2019.125018
Ferreira M, Cappato L, Silva R, Rocha R, Neto R, Tavares MIB, Esmerino EA, Freitas MQ, Bissagio RC, Ranadhera R, Raices RSL, Silva MC, Cruz A (2019b) Processing raspberry-flavored whey drink using ohmic heating: physical, thermal and microstructural considerations. Food Res Int 123:20–26
Gurak PD, Cabral LMC, Maria HMRL, Matta VM, Freitas SP (2010) Quality evaluation of grape juice concentrated by reverse osmosis. J Food Eng 96:421–426
Helvacıoğlu S, Charehsaz M, Güzelmeriç E, Türköz AE, Yeşilada E, Aydın A (2018) Comparatively investigation of grape molasses produced by conventional and industrial techniques. Marmara Pharm J 22(1):44–51
Hosainpour A, Nargesi F, Darvishi H, Fadavi A (2014) Ohmic pre-drying of tomato paste. Food Sci Technol Int 20:193–204
Icier F (2003) The experimental investigation and mathematical modeling of ohmic heating of foods. PhD Thesis. Ege University, Food Engineering Department, Izmir, Turkey
Icier F, Ilicali C (2004) Electrical conductivity of apple and sourcherry juice concentrates during ohmic heating. J Food Process Eng 27(3):159–180
Icier F, Ilicali C (2005) The use of tylose as a food analog in ohmic heating studies. J Food Eng 69(1):67–77
Icier F, Yildiz H, Baysal T (2008) Polyphenoloxidase deactivation kinetics during ohmic heating of grape juice. J Food Eng 85 (3):410–417
Icier F, Yildiz H, Sabancic S, Cevik M, Cokgezme OF (2017) Ohmic heating assisted vacuum evaporation of pomegranate juice: electrical conductivity changes. Innov Food Sci Emerg Technol 39:241–246
Inmanee P, Kamonpatana P, Pirak T (2019) Ohmic heating effects on Listeria monocytogenes inactivation, and chemical, physical, and sensory characteristic alterations for vacuum packaged sausage during post pasteurization. LWT 108:183–189
Kayışoğlu S, Demirci M (2006) Effects of storage time and condition on mineral contents of grape pekmez produced by vacuum and classical methods. J Tekirdag Agric Fac 3(1):1–7
Koushesh Saba M, Moradi S (2016) Internal browning disorder of eight pear cultivars affected by bioactive constituents and enzyme activity. Food Chem 205:257–263
Mercali GD, Schwartz S, Marczak LDF, Tessaro IC, Sastry S (2014) Ascorbic acid degradation and color changes in acerola pulp during ohmic heating: effect of electric field frequency. J Food Eng 123:1–7
Nouroallahi SB, Azadbakht M, Darvishi H (2018) Ohmic blanching of white mushroom and its pretreatment during microwave drying. Heat Mass Transf 54:3715–3725
Özcan MM, Al-Juhaimi F (2017) Effect of microwave roasting on yield and fatty acid composition of grape seed oil. Chem Nat Compd 53(1):132–134
Özcan MM, Alpar S, Al-Juhaimi F (2015) The effect of boiling on qualitative properties of grape juice produced by the traditional method. J Food Sci Technol 52(9):5546–5556
Özcan MM, Al-Juhaimi F, Gülcü M, Uslu N, Geçgel U (2017a) Determination of bioactive compounds and mineral contents of seedless parts and seeds of grapes. S Afr J Enol Viticult 38(2):212–220
Özcan MM, Al Juhaimi F, Gulcu M, Uslu N, Gecgel Ü, Ghafoor K, Dursun N (2017b) The effect of harvest time on physico-chemical properties and bioactive compounds of pulp and seeds of grape varieties. J Food Sci Technol 54(8):2230–2240
Patras A, Tiwari BK, Brunton NP, Butler F (2009) Modelling the effect of different sterilisation treatments on antioxidant activity and colour of carrot slices during storage. Food Chem 114 (2):484–491
Sabanci S, Cevik M, Cokgezme OF, Yildiz H, Icier F (2018) Quality characteristics of pomegranate juice concentrates produced by ohmic heating assisted vacuum evaporation. J Sci Food Agric 99(5):2589–2595
Sabancic S, Icier F (2017) Applicability of ohmic heating assisted vacuum evaporation for concentration of sour cherry juice. J Food Eng 212:262–270
Sarkis JR, Jaeschke DP, Tessaro IC, Marczak LDF (2013) Effects of ohmic and conventional heating on anthocyanin degradation during the processing of blueberry pulp. LWT Food Sci Technol 51:79–85
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol 299:152–178
Tankesh K (2018) Development of an ohmic heating system for pasteurization of grape (Vitis vinifera L.) Juice. M.Sc. thesis. Acharya N.G. Ranga Agricultural University, India
Yildiz H, Buzkurt H, Icier F (2009) Ohmic and conventional heating of pomegranate juice: effects on rheology, color, and total phenolics. Food Sci Technol Int 15:503–512
Acknowledgements
Financial support for this research (Research Project No. 4/48202) was provided by University of Kurdistan, Sanandaj, Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Darvishi, H., Koushesh Saba, M., Behroozi-Khazaei, N. et al. Improving quality and quantity attributes of grape juice concentrate (molasses) using ohmic heating. J Food Sci Technol 57, 1362–1370 (2020). https://doi.org/10.1007/s13197-019-04170-1
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-04170-1