Abstract
This study compared the stability of extracts of H. sabdariffa calyces microencapsulated with different concentrations of mesquite gum during storage. Dry Roselle calyces were mixed with 50:50 (v/v) ethanol:water solution to obtain 18°Bx concentrate. This Roselle extract concentrate was mixed with purified mesquite gum (100:1–100:5 v/w). The Roselle extract concentrate-gum (RECG) was spray dried at inlet and outlet temperatures of 180 ± 2 and 104 ± 2.3 °C, respectively, at an air flow rate of 38 m3/h. Encapsulated Roselle powders (ERP) were analyzed for moisture content, total monomeric anthocyanins (differential pH), phenolic compounds (Folin and Ciocalteu method), antioxidant capacity (ABTS), and color parameters (\( L^{*} ,a^{*} \), and \( b^{*} \)) after 5 weeks and 1 year of storage. Sorption properties (isotherms) and micrographs of powders were also obtained. The average yield of RECG powders was 15.27 ± 0.81 g/100 mL. During storage, ERP showed average values of phenolic compounds, antioxidant capacity, and anthocyanins of 3.43 ± 0.25 g gallic acid equivalents/100 g, 9.34 ± 1.4 g Trolox equivalents/100 g, and 318.7 ± 20.6 mg cyanidin-O-glycoside/100 g, respectively. Color parameters remained constant along the storage time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Roselle (Hibiscus sabdariffa L.) is a plant native to Africa that blooms annually. It is cultivated in other tropical regions around the world such as India, Philippines, Malaysia, and Mexico (Cid-Ortega and Guerrero-Beltrán 2015). Roselle calyces (or “flowers”) are a good source of natural red–purple pigments having antioxidant characteristics. The Roselle calyces are commonly used to prepare aromatic infusions to be consumed as refreshments or as hot beverages due to their tasty flavor, nutraceuticals content and alternative medicinal benefits (Da-Costa-Rocha et al. 2014). It is also used to prepare sauces, jellies, marmalades or for extracting natural pigments (Cid-Ortega and Guerrero-Beltrán 2014; Cid-Ortega and Guerrero-Beltrán 2015).
The main pigments of Roselle calyces are anthocyanins, a water soluble compounds which could be found in red, purple or blue colors, depending on the pH of the system (Giusti and Wrolstad 2001; Cid-Ortega and Guerrero-Beltrán 2016). They are frequently found in fruits, vegetables, and cereals as aglycones; a molecule possessing two benzene rings and a heterocyclic ring with an oxygen attached to it. The central nucleolus, called flavylium, constitutes the anthocyanidin which is attached to one molecule of sugar (glucose, galactose, arabinose, xylose or glucuronic acid) (Manach et al. 2004). More than 20 anthocyanidins (i.e. pelargonidin, delphinidin, cyanidin, petunidin, peonidin, malvidin, among others) are known in nature; however, more than 300 anthocyanins can be found when anthocyanidins have attached different molecules of sugar in the structure (Durst and Wrolstad 2001; García-Alonso et al. 2009).
It is well known that anthocyanins contribute to the human health due to their antioxidant, anti-carcinogenic, anti-inflammatory, and anti-angiogenic properties (Rossi et al. 2003).
Anthocyanins are relatively instable and they could undergo degradation during processing, storage, and changes in pH, temperature, light, metals, oxygen, organic acids, sugar, or copigmentation (Rein 2005). Therefore, the extracting method of anthocyanins is of paramount importance to take them out from the biological system; much care should be taken for minimizing their degradation or changes in their chemical structures. The polar characteristics of anthocyanins may permit their extraction using solvents such as alcohols, ketones, dimethyl sulfoxide or water. For food purposes, anthocyanins are commonly extracted using ethanol or water to avoid the risk of toxicity to people (Rodriguez-Saona and Wrolstad 2001) when using toxic solvents such as methanol.
Microencapsulation is considered a special technique to protect “labile” chemical compounds against damaging environments such as temperature, humidity, radiation, and microorganisms, among others. It is an accurate approach for storing, separating, and packaging these “labile” materials in a microscopy scale physical system. The spray drying method is frequently used for encapsulating heat sensitive food materials. The inlet and outlet temperatures, flow rate of the fluid, product type, residence time, and the row material conditions are some of the most important parameters to be taken into account to obtain a homogeneous powder by spray draying (García et al. 2004).
Biopolymers such as carbohydrates (starch, maltodextrins, cycledextrins, carboximethylcellulose and derivatives), gums (Arabic, sodium alginate, among others), and proteins (gelatin, soy proteins, caseinates, whey, zein) are some examples of the wide variety of compounds used as carriers in spry drying of heat sensitive chemical compounds such as flavors, lipids, oleoresins, bioactive compounds, among others (Gharsallaoui et al. 2007). Mesquite gum is an exudation of the mesquite tree (Proposis spp.), which is found in northern Mexico in arid and semiarid environments. Mesquite gum is an exudate polysaccharide material made up of an arabinogalactan protein (2–4.8%) with similar chemical and functional properties as those of Arabic gum. Its branched structure may form a water soluble compact molecular conformation. It possesses emulsifier properties and it does not increase its viscosity considerably, even at high concentrations (Orozco-Villafuerte et al. 2003); therefore, it could be an alternative to common biopolymers used as carriers to protect labile materials to be encapsulated by spray drying.
The objective of this research was to characterize H. sabdariffa calyces extracts and their microencapsulated powders obtained with mesquite gum to evaluate their stability at different storage conditions.
Materials and methods
Materials
Dehydrated “Creole” Roselle calyces (long red variety) were acquired from Chiautla, Puebla, Mexico. Mesquite gum was obtained as the exudation of the Prosopis laevigata tree grown in San Luis Potosí, Mexico.
Roselle extract concentrate (REC)
Six hundred grams of ground Roselle calyces were placed in a beaker containing 6 L of a 50:50 water:ethanol (v/v) blend. This blend was protected from light, stirred for 2 h and centrifuged for 10 min at 4500 rpm at room temperature. Supernatant was filtered at vacuum conditions through Whatman paper No. 2. The Roselle extract (RE) was then concentrated using a Büchi evaporator (Rotavapor RE 111, Büchi 461, Switzerland) at 35 °C until obtaining 1050 mL. Roselle extract and Roselle extract concentrate (REC) were analyzed for color, total soluble solids, monomeric anthocyanins, phenolic compounds and antioxidant capacity.
Mesquite gum purification
Mesquite gum was purified according to the Beristain et al. (2002) methodology.
Spray drying
100 mL of REC was mixed, separately, with 1, 2, 3, 4, or 5 g of purified mesquite gum to obtain Roselle extract concentrate-gum (RECG) blends. Blends were homogenized and then spray dried using a Büchi Mini spray dryer model B-290 (Büchi, Switzerland) at a flow rate of 10 ± 0.04 mL/min using an air flow rate of 38 m3/min. The inlet and outlet temperatures were 180 ± 2 °C and 104 ± 2.3 °C, respectively. The obtained encapsulated Roselle powders (ERP) were immediately analyzed (yield, moisture content, water activity, color, phenolic compounds, antioxidant capacity, moisture sorption properties and micrographs) or stored in jars away from light in “dry environmental” conditions (2 ± 1 °C and 23 ± 2 °C) to be analyzed (color, antioxidants) along 5 weeks and 1 year of storage.
Physicochemical analysis
Moisture content was evaluated according to the 934.06 AOAC (2000) method. Water activity (a w ) was analyzed using an AQUA-LAB hygrometer (Decagon Devices Inc., Pullman, WA, USA). Total soluble solids (TSS) were measured using a portable Atago refractometer (Tokio, Japan).
Color characteristics
Lightness (\( L^{*} \)), green–red (\( a^{*} \)), and blue–yellow (\( b^{*} \)) color parameters were measured in the Hunter scale, using a Colorgard® System/05 (Gardner, Germany) colorimeter. Color of samples was measured according to the type of Roselle product in the reflectance mode. For REC, 20 mL of extract was placed in a rectangular (10 × 53.5 × 54.7 mm) quartz cell, while for ERP, 0.7 g was placed in a cylindrical quartz cell (45 mm in diameter and 17 mm in depth) and color parameters measured. Total color change (∆E), hue (H), and chroma (C) were calculated as follow:
where \( L_{0}^{*} \), \( a_{0}^{*} \), \( b_{0}^{*} \) are the color parameters at the starting of the storage time and \( L^{*} \), \( a^{*} \), and \( b^{*} \) are the color parameters at each storage time (Marcus 1998).
Total monomeric anthocyanins
Total monomeric anthocyanins (TMA) were evaluated according to the Giusti and Wrolstad (2001) method with modifications. RE: 7 mL of buffer solutions pH 1 and 4.5 were placed, separately, in test tubes. Two hundred microliters of Roselle extract were added in each tube and then thoroughly mixed. The absorbance (A) was measured at 520 and 700 nm at the two pHs using a Cary 100 UV–visible spectrophotometer (Varian Inc., Palo Alto, CA, USA). ERP: 5 mL of solution (ethanol) was prepared in a volumetric flask with 167 mg of ERP; afterward, 800 μL of this Roselle solution was added to 7 mL of buffer solutions pH 1 or 4.5, into separate test tubes, thoroughly homogenized, and absorbance measured as explained above. REC: 100 μL of Roselle concentrate was used following the same procedure. Distilled water was used as blank for measuring absorbances. The total monomeric anthocyanins were calculated as follow:
where MW is the molecular weight (449.2 g/mol of cyanidin-3-O-glycoside), ε is the molar absorptivity coefficient (26,900 L/mol cm), F is the dilution factor (total volume/volume of extract), l is the light pathway along the quartz cell (1 cm). Then, results were calculated as mg equivalent of cyanidin-3-O-glycoside (C-3-O-G)/100 mL of Roselle extract or per 100 g of Roselle powder.
Phenolic compounds
Phenolic compounds (PC) were evaluated using the phenol Folin and Ciocalteu reagent (Cid-Ortega and Guerrero-Beltrán 2014). Fifty microliters of RE or 5 μL of REC were placed, separately, in 50 mL volumetric flasks; then, 2.5 mL of the phenol Folin and Ciocalteu solution added and thoroughly mixed. Mixtures were left at room temperature in a dark environment for 3 min. Afterward, 5 mL of a 20% sodium carbonate solution were added and mixed. Finally, a volume of 50 mL was made up with distilled water and homogenized. This blend was left for 30 min and the absorbance measured at 675 nm using a Cary 100 UV–visible spectrophotometer (Varian Inc., Palo Alto, CA, USA). For ERP, 5 mL of solution (ethanol) was prepared in a volumetric flask with 167 mg of Roselle powder. Afterward, 150 μL of this Roselle solution was reacted in similar way as explained above. Phenolic compounds were quantify as gallic acid (GA), using the following standard curve:
where x is the concentration of phenolic compounds (mg equivalent of gallic acid/100 mL of extract or per 100 g of powder).
Antioxidant capacity
The antioxidant capacity (AC) was analyzed according to the Re et al. (1999) method modified by Kuskoski et al. (2004). It is based on the formation of the ABTS∙+ radical.
ABTS ∙+ radical solution it was prepared combined 3.3 mg of potassium persulfate and 19.4 mg of the ABTS (2,2′azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid) diammonium salt (~98%)) reagent with five milliliters of distilled water. The solution was kept away from light and left for 16 h at room temperature. The ABTS∙+ radical solution was mixed up with absolute ethanol (radical-ethanol solution) in a glass beaker to obtain an absorbance of 0.70 ± 0.02 at 754 nm.
Roselle extract-ethanol solution for RE and REC, 50 and 5 μL, respectively, were diluted to 50 mL with absolute ethanol for measuring the antioxidant capacity. For ERP, 167 mg of powder was placed in a 5 mL spherical flask, absolute ethanol added, mixed, made up to volume, and completely homogenized. This solution was then diluted in a ratio of 1:30 with absolute ethanol to analyze the antioxidant capacity.
Antioxidant capacity 980 μL of the radical-ethanol solution was placed in a 1 cm quartz cell, and the absorbance was measured (A i ) using a UV–Vis spectrophotometer (Varian Inc., Palo Alto, CA, USA); then, 20 μL of Roselle extract-ethanol solution was added and left to react for 7 min and the final absorbance (A f ) measured. To obtain the percentage of inhibition the next equation was used:
The antioxidant capacity was calculated using a Trolox standard curve.
where X is the concentration of Trolox (μM).
Sorption isotherms and modeling
In order to evaluate the sorption isotherms, ERP was completely dried in a desiccator containing P2O5, until constant weight. The gravimetric static method was used for measuring the sorption properties of powders (Iglesias and Chirife 1982). About 100 mg of powder was placed in weighing bottles; bottles were placed in chambers, at three temperatures (15, 25, and 35 °C), containing oversaturated salt solutions of lithium chloride (0.111), potassium acetate (0.252), magnesium chloride (0.325), potassium carbonate (0.434), magnesium nitrate (0.524), sodium bromide (0.559), strontium chloride (0.691), sodium chloride (0.753), and potassium chloride (0.836) to create specific water activity (a w ) environments (López-Malo et al. 1994). The moisture equilibrium data were modeled, for calculating the maximum stability sorption properties of powders, using the BET (Brunauer, Emmett and Teller 1938) and the GAB (Bizot 1983) models.
BET model:
where C is the constants of BET, W is the moisture content (g water/g dry solid), and W 0 is the monolayer moisture content in dry basis (g H2O/g dry solid).
GAB model:
where C and K are the constants of the GAB model, X is the moisture content (g water/g dry solid), and X 0 is the monolayer moisture content in dry weight (g H2O/g dry solid).
Electron microscopy
The technique of backscattered electrons with an acceleration voltage of 20 kV was used for taking micrographs of powders using a Scanning Electron Microscope JEOL model JSM-6610 LV (Akyshama, Japan) (Davidov-Pardo et al. 2008).
Statistical analysis
ANOVA and Tukey test (α = 0.05) were performed to make a decision about differences within means of variables during the storage times and, or gums concentrations.
Results and discussion
REC and RE physicochemical characteristics
\( L^{*} \), \( a^{*} \), \( b^{*} \), H, and \( C^{*} \) were 1.27 ± 0.0, 3.45 ± 0.0, 0.89 ± 0.0, 14.47 ± 0.0, 3.56 ± 0.0, respectively, and for the bioactive compounds TMS, PC, and AC were 81.04 ± 12.1 mg of cyanidin-3-O-glycoside/100 mL, 1.86 ± 0.03 g of GAE/100 mL, and 0.75 ± 0.03 g of Trolox/100 mL of Roselle calyces, respectively. The lower values of color parameters of the REC indicated a deep dark-purple color which corroborated with the hue and chroma values. Values of color were different compared to results obtained by other researchers; this may be due to the amount of Roselle used for extraction and the concentration process (Salazar-González et al. 2012). On the other hand, RE showed a high concentration of TMA, PC and AC: 141.8 ± 21.2 mg of cyanidin-3-O-glycoside/100, 3.08 ± 0.06 g of GAE/100, and 1.31 ± 0.05 g of Trolox/100 g of Roselle calyxes, respectively. It is well known that several factors can affect the extraction process of bioactive compounds from vegetable materials, some of the most important are the ratio sample:solvent, type of solvent, time of extraction, and method. Cid-Ortega and Guerrero-Beltrán (2014) reported similar values to those obtained in this study. As observed, the concentration process significantly increased the bioactive compounds and antioxidant capacity by 5.7 times in REC.
Encapsulated Roselle powders (ERP) yield and physicochemical characteristics
Table 1 shows the RECG and ERP characteristics. It was observed that, increasing the gum concentration increased the TSS in RECG from 18.4% (control) to 22.5% in blends with 5% of mesquite gum; the increase of gum affected the yield and moisture content of the ERP. The water content of RECG blends had an important effect on the spray drying process. High TSS in the spray dryer solution to be spray dried reduced the amount of water by evaporation; a reduction in the moisture content of powders was achieved increasing the yield (Quek et al. 2007). High amount of gum in the solution might generate powders with low nutritional quality. No significant differences (p > 0.05) were observed for moisture content within concentrations of gum for the five powders; a global moisture content average of 2.29 ± 0.45% was obtained for all ERP; therefore, an adequate final moisture content in powders was obtained. Ananta et al. (2005) pointed out that a final moisture content of 4% is indicative of an adequate dry product. The average yield of RP was 73.66 ± 1.49%, calculated according to the TSS content in the RECG blends. Table 2 shows the color parameters of ERP. In general, ERP had average value of 40.29 ± 0.71, 31.94 ± 0.29, 9.08 ± 0.11, 15.83 ± 0.13, and 33.19 ± 0.30 for \( L^{*} \), \( a^{*} \), \( b^{*} \), H, and C respectively. Purple color was observed when color was measured in solution in a transmittance mode. These values indicated that the ERP colors were in the red-yellow segment (0°–90°) of the color space (Marcus 1998). An increase in the content of gum in the formulation significantly (p < 0.05) decreased the \( a^{*} \) and \( b^{*} \) color parameters; although these changes were not visually detected, they can be verified by the hue and chroma values. Table 2 shows \( L^{*} \), \( a^{*} \), and \( b^{*} \) color parameters after 5 weeks of storage at room temperature. Values of \( L^{*} \), \( a^{*} \), \( b^{*} \) were similar to those measured just after spray drying of solutions. ∆E values were small for reflectance (∆E = 1.08–2.02) and transmittance (∆E = 0.26–2.02); therefore, few changes occurred in color properties after 5 weeks of storage.
TMA, PC, and AC in just dried ERP
Table 3 shows the TMA, PC, and AC of dried ERP. It is observed that TMA, PC and AC decreased as the amount of mesquite gum was increased in the REC, being significant (p < 0.05) in TMA and AC. The increase of gum reduced the amount of REC; consequently, the antioxidant capacity. High concentration of mesquite gum in the ERP may have affected the microstructure of the powder, creating a crystalline surface that decreased the solubility in solvents, affecting the extraction process (Cano-Chauca et al. 2005). Similar results were reported by Mishra et al. (2014); they reported that increasing the maltodextrin concentration, the phenolic compounds content was reduced. One of the main objectives of the spray drying process was the protection of bioactive compounds (Champagne and Fustier 2007), such as anthocyanin; these compounds may have low stability to different intrinsic and extrinsic factors (Jiménez-Aguilar et al. 2011). TMA, PC, and AC showed an increase in 112–142, 6.6–24.6, and 528–734% compared to the values obtained from RE. This can be attributed to the spray drying process that generates pores in powder that enhance the contact between solvent and bioactive compounds, facilitating the extraction process (Drosou et al. 2016).
Electron microscopy
Micrographs of ERP for powders with 1% of mesquite gum magnified at 500×, 1000×, 5000×, 10,000× are shown in Fig 1. It is observed that the particle size of powders ranged from approximately 1 to 10 μm. High amount of spray dried capsules were collapsed; therefore, some wrinkles or depressions on the surface are observed. These depressions are formed by the collapse of particles during drying and cooling (Idham et al. 2012). Laohasongkrama et al. (2011) pointed out that the collapse of microcapsules is related to the temperature of the drying process. Idham et al. (2012) evaluated the effect of different carrier materials in encapsulated Roselle anthocyanins; they reported that the carrying materials affected microcapsules forming irregular surfaces (dents) and holes, although in general capsules maintained their spherical forms.
Moisture content and water activity of encapsulated Roselle powder
One of the main factors for maintaining good quality of encapsulated powders is the effect of temperature. The moisture content (4.3 ± 0.61) and water activity (0.34 ± 0.01) were low in ERP stored under refrigeration conditions, while at room temperature, the values for moisture content and water activity were 6.4 ± 0.45% and 0.47 ± 0.02, respectively. In both storage conditions, the concentration of mesquite gum did not affect significantly (p > 0.05) the moisture content and water activity.
Color of encapsulated Roselle powder stored for 1 year
Table 4 shows the effect of temperature on color characteristics (p > 0.05) of encapsulated Roselle powders (ERP) after 1 year of storage. At both temperatures, the mesquite gum concentration barely affected (p < 0.05) the color characteristics of powders (reflectance or transmittance). Hue and chroma values were higher for all ERP stored at refrigeration condition. In ERP stored at room temperature, the C and H values of powders slightly changed compared to the values at the beginning of the storage (Table 2). The main effect of the storage temperature was observed for \( L^{*} \) parameter which was significantly (p < 0.05) higher in ERP stored at room temperature (64.3 ± 1.6) than stored at refrigeration temperature (43.6 ± 2.4) probably due to fading of anthocyanins due to the light effect (Rein 2005). These results can be verified with the total change in color (∆E); this was higher for ERP stored at room temperature (24.1 ± 1.1) than at low temperature. During the storage of powders, collapse, caking, agglomeration, browning and oxidation might occur, this detrimental effect might be accelerated if the storage temperature is higher than the transition temperature (Tg) (Bhandari and Howes 1999); however, in this study, Tg was not measured.
TMA, PC, and AC in ERP stored for 1 year
Table 3 show the TMA, PC, and AC of ERP after 1 year of storage at different conditions. It is observed that for TMP, PC, and AC in powders stored at refrigeration conditions the values were significantly higher compared to those of ERP stored at room temperature; however, ERP stored at both temperatures showed a decrease in TMA, PC, and AC compared to the initial values (JD). Low temperature slightly affected TMA (9.4 ± 2.2%) and PC (14.4 ± 3.6%) of ERP. High reduction (54.9 ± 4.2%) of the antioxidant capacity was observed, probably because the reduction of other compounds in Roselle calyces such as vitamins and phytosterols (Sáyago-Ayerdi et al. 2007). The effect of temperature on bioactive compounds has been reported by several researchers; Jiménez-Aguilar et al. (2011) evaluated the effect of temperature on spray dried blueberry powder using mesquite gum; they reported that TMA, PC, and AC were reduced by 10, 7, and 15% after 1 month of storage at 4 °C. Ersus and Yurdagel (2007), reported that encapsulated anthocyanins from black carrot stored at 24 °C showed a reduction of 33% compared to 11% observed in the powder stored at 4 °C.
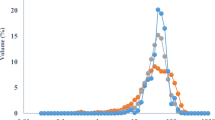
Isotherms
Figure 2 shows the sorption properties of ERP obtained at three temperatures and modeled using the GAB and BET equations. A type II sorption isotherm was obtained according to the BET classification (Brunauer et al. 1938). The BET model is useful for obtaining the water activity (0.165, 0.214, and 0.088 at 15, 25, and 35 °C, respectively) and monolayer moisture (10.6, 4.6, and 3.5 g H2O/100 g d.s. at 15, 25, and 35 °C, respectively) properties of maximum stability for food systems, in this case the ERP; the model is adequate for water activities ranging from 0 to 0.45. On the other hand, the GAB model (Bizot 1983) was used for modeling the experimental data (R2 = 0.913, 0.818, and 0.873 at 15, 25, and 35 °C, respectively); it works in a range of water activity of 0 to 0.97. The monolayers moisture content obtained with this model at the three temperatures (10.3, 7.1, 3.5 g H2O/100 g d.s. at 15, 25, and 35 °C, respectively) were similar to those obtained with the BET model. Powders should be stored at low water activity for avoiding caking, crystallization and consequently degradation of the antioxidants and color properties.
Conclusion
The physicochemical and antioxidant properties such as anthocyanins, phenolic compounds, antioxidant capacity and color of Roselle powders encapsulated with mesquite gum were obtained. These characteristics were kept almost constant along the storage at refrigeration conditions; however, the power stored at room temperature also has adequate antioxidant characteristics after 1 year. Roselle powders, beside of being used as an additive due to its flavoring and color properties could also be used as a source of antioxidants. The use of mesquite gum is an alternative for encapsulation of extracts of Roselle. Powders solubilize easily in water or in aqueous ethanol.
References
Ananta E, Volkert M, Knorr D (2005) Cellular injuries and storage stability of spray-dried Lactobacillus rhamnosus GG. Int Dairy J 15:399–409
AOAC (2000) Official methods of analysis of AOAC International, 17th edn. Gaithersburg, MD, USA
Beristain C, Azuara E, Vernon E (2002) Effect of water activity on the stability to oxidation of spray-dried encapsulated orange peel oil using mesquite gum (Prosopis juliflora) as wall material. J Food Sci 67:206–211
Bhandari BR, Howes T (1999) Implication of glass transition for the drying and stability of dried foods. J Food Eng 40:71–79
Bizot H (1983) Using the GAB model to construct sorption isotherms. In: Joeitt R, Escher F, Hallstrom B, Meffert HFT, Spiess WEL, Vos G (eds) Physical properties of foods. Applied Science Publishers, London, pp 43–54
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
Cano-Chauca M, Stringheta PC, Ramos AM, Cal-Vidal J (2005) Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innov Food Sci Emerg Technol 6:420–428
Champagne CP, Fustier P (2007) Microencapsulation for the improved delivery of bioactive compounds into foods. Curr Opin Biotechnol 18:184–190
Cid-Ortega S, Guerrero-Beltrán JA (2014) Roselle calyces particle size effect on the physicochemical and phytochemicals characteristics. J Food Res 3(5):83–88
Cid-Ortega S, Guerrero-Beltrán JA (2015) Roselle calyces (Hibiscus sabdariffa), an alternative to the food and beverages industries: a review. J Food Sci Technol 52(11):6859–6869
Cid-Ortega S, Guerrero-Beltrán JA (2016) Antioxidant and physicochemical properties of Hibiscus sabdariffa extracts from two particle sizes. J Food Res 5(2):98–109
Da-Costa-Rocha I, Bonnlaender B, Sievers H, Pischel I, Heinrich M (2014) Hibiscus sabdariffa L.—a phytochemical and pharmacological review. Food Chem 165:424–443
Davidov-Pardo G, Roccia P, Salgado D, León A, Pedroza-Islas R (2008) Utilization of different wall materials to microencapsulate fish oil. Evaluation of its behavior in bread products. Am J Food Technol 3:384–393
Drosou CG, Krokida MK, Biliaderi CG (2017) Encapsulation of bioactive compounds through electrospinning/electrospraying and spray drying: a comparative assessment of food related applications. Drying Technol 35:139–162
Durst R, Wrolstad R (2001) Separation and characterization of anthocyanins by HPLC. In: Wrolstad RE, Acree TE, Decker EA, Penner MH, Reid DS, Schwar SJ, Shoemaker CF, Smith DM, Sporns P (eds) Current protocols in food analytical chemistry. Wiley, New York
Ersus S, Yurdagel U (2007) Microencapsulation of anthocyanin pigments of black carrot (Daucus carota L.) by spray dryer. J Food Eng 80:805–812
García C, González MB, Ochoa LA, Medran R (2004) Microencapsulación de jugo de cebada verde mediante secado por aspersión. Ciencia y Tecnología Alimentaria (Mexico) 4(4):262–266
García-Alonso J, Minihane AM, Rimbach G, Rivas-Gonzalo JC, de Pascual-Teresa S (2009) Red wine anthocyanins are rapidly absorbed in humans and affect monocyte chemoattractant protein 1 levels and antioxidant capacity of plasma. J Nutr Biochem 20:521–529
Gharsallaoui A, Roudaut G, Chambin O, Voilley A, Saurel R (2007) Applications of spray-drying in microencapsulation of food ingredients: an overview. Food Res Int 40:1107–1121
Giusti M, Wrolstad R (2001) Characterization and measurement of anthocyanins by UV-Visible Spectroscopy. In: Wrolstad RE, Acree TE, Decker EA, Penner MH, Reid DS, Schwar SJ, Shoemaker CF, Smith DM, Sporns P (eds) Current protocols in food analytical chemistry. Wiley, New York
Idham Z, Muhamad II, Setapar SH, Sarmidi MR (2012) Effect of thermal processes on Roselle anthocyanins encapsulated in different polymer matrices. J Food Process Preserv 36:176–184
Iglesias HA, Chirife J (1982) Handbook of food isotherms: water sorption parameters for food and food components. Academic Press, New York, pp 1–10
Jiménez-Aguilar DM, Ortega-Regules AE, Lozada-Ramírez JD, Pérez-Pérez MCI, Vernon-Carter EJ, Welti-Chanes J (2011) Color and chemical stability of spray-dried blueberry extract using mesquite gum as wall material. J Food Comp Anal 24:889–894
Kuskoski M, Asuer A, García-Parrilla M, Troncoso A, Fett R (2004) Actividad antioxidante de pigmentos antociánicos. Ciencia y Tecnología Alimentaria (Campinas, Brasil) 24(4):691–693
Laohasongkrama K, Mahamaktudsanee T, Chaiwanichsiri S (2011) Microencapsulation of Macadamia oil by spray drying. Proc Food Sci 1:1660–1665
López-Malo A, Palou E, Argaíz A (1994) Measurement of water activity of saturated salt solutions at various temperatures. In: Argaíz A, López-Malo A, Palou E, Corte P (eds) Proceedings of the poster session, ISOPOW practicum II. Depto. Ingeniería Química y Alimentos. Universidad de las Américas-Puebla. Cholula, Puebla, Mexico, pp 113–116
Manach C, Scalbert A, Morand C, Rémés C, Jiménez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747
Marcus RT (1998) The measurement of color. In: Nassau K (ed) Color for science, art and technology. Elsevier, Amsterdam, pp 31–39
Mishra P, Mishra S, Lata Mahanta C (2014) Effect of maltodextrin concentration and inlet temperature during spray drying on physicochemical and antioxidant properties of amla (Emblica officinalis) juice powder. Food Bioprod Process 92:252–258
Orozco-Villafuerte J, Cruz-Sosa F, Ponce-Alquicira E, Vernon-Carter EJ (2003) Mesquite gum: fractionation and characterization of the gum exuded from Prosopis laevigata obtained from plant tissue culture and from wild trees. Carbohydr Polym 54:327–333
Quek S, Chok N, Swedlund P (2007) The physicochemical properties of spray-dried watermelon powders. Chem Eng Process 46:386–392
Re R, Pellegrini N, Proteggente A, Annala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Rein M (2005) Copigmentation reactions and color stability of berry anthocyanins. M.Sc. thesis. University of Helsinki, Finland
Rodriguez-Saona L, Wrolstad R (2001) Extraction, isolation and purification of anthocyanins. In: Wrolstad RE, Acree TE, Decker EA, Penner MH, Reid DS, Schwar SJ, Shoemaker CF, Smith DM, Sporns P (eds) Current protocols in food analytical chemistry. Wiley, New York
Rossi A, Serraino I, Dugo P, Di Paola R, Mondello L, Genovese T, Morabito D, Dugo G, Sautebin L, Caputi AP, Cuzzocrea S (2003) Protective effects of anthocyanins from blackberry in a rat model of acute lung inflammation. Free Radic Res 37:891–900
Salazar-González C, Vergara-Balderas F, Ortega-Regules A, Guerrero-Beltrán JA (2012) Antioxidant properties and color of Hibiscus sabdariffa extracts. Ciencia e Investigación Agraria 39:79–90
Sáyago-Ayerdi SG, Arranz S, Serrano J, Goñi I (2007) Dietary fiber content and associated antioxidant compounds in Roselle flower (Hibiscus sabdariffa L.) beverage. Journal of Agric Food Chem 55:7886–7890
Acknowledgements
This research was part of the Project Number 2006/62275-Z supported by the National Council of Science and Technology (CONACyT) in Mexico. Author Claudia Salazar-González would like to thank to CONACyT for the economic support provided for the completion of her bachelor studies. Mesquite gum was donated by Dr. J. Vernon-Carter from the Universidad Autónoma Metropolitana, Iztapalapa, Mexico City.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ochoa-Velasco, C.E., Salazar-González, C., Cid-Ortega, S. et al. Antioxidant characteristics of extracts of Hibiscus sabdariffa calyces encapsulated with mesquite gum. J Food Sci Technol 54, 1747–1756 (2017). https://doi.org/10.1007/s13197-017-2564-1
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-017-2564-1