Abstract
The acidified ethanol extract of pomegranate (Punica granatum L.) peel bioactive compounds was freeze-dried and encapsulated using a combination of maltodextrin (MDX 5, 10, and 15%) with calcium alginate (0.1%) with a weight ratio (w/w) of 1:5 (extract/wall material). The effects of various parameters on pomegranate peel extraction (POPx) and POPx-encapsulated powder were analyzed on bioactive components, physicochemical, morphological properties, and storage stability. The results revealed that process yield was positively influenced by increasing MDX concentration, due to the increase on mixture content. Encapsulated powder with 15% MDX had the highest total phenolic compounds (TPC, 73.1 mg GA/kg), the total anthocyanin content (TAC, 40.2 mg c3g/kg dmp and antioxidant capacity by FRAP, 405 (Fe2+, μmol/l)), the DPPH assay (RSA, 65.1%), the lowest IC50, 0.56 ± 0.02 mg/ml, and the lowest glass transition temperature indicating that the highest antioxidant capacity among other wall materials. In respect to morphology, the particles of encapsulated powders with high concentration of MDX were larger and smoother. Stability and half-life of encapsulated powders were measured from 42 days of storage study at 4 °C and 25 °C, and 52 and 75% relative humidity. Storage tests revealed first-order degradation kinetics for anthocyanin. The TAC of the PoPx-microencapsulated powders with wall material of 15% and control decreased by 18% and 33%, respectively, after 42 days storage at 4 °C, while at the storage temperature of 25 °C, the decreases were in the order of 24% and 38%, respectively, over the same period of time. The highest anthocyanin content was observed in the freeze-dried MDX 15%-encapsulated powder at 4 °C storage temperature and 75% relative humidity with a half-life (t1/2) of 115 days, and the reaction rate constant (k) of 0.64 × 10−2 min−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Color compounds used in the food industry are mostly obtained from synthetic and chemical sources and are being replaced by natural dyes because of their negative health effects (Jafari and Mahdavi-Khazaei 2016). Anthocyanins, as one of the most valuable groups of water-soluble pigments, have gained better attention in the scientific community, mostly due to their wide range of possible applications and their antimicrobial and anticancer properties (Silva et al. 2017).
Pomegranate juice processing industry produces up to 40% of their waste in the form of peel, which is suggested and assessed as a complementary nutrient in animal feeding (Shabtay et al. 2008). The peel portion of pomegranate contains roughly 30% of all the anthocyanins in the fruit which is characterized by the presence of high molecular weight phenolics, ellagitannins, proanthocyanidins, and complex polysaccharides flavonoids (Prakash et al. 2013). The introduction of anthocyanins into dietary and medicinal formulations, however, has proved to be difficult and a technological obstacle, considering their low stability during processing and storage (Santos and Meireles 2011). As a solution, microencapsulation has been suggested for the industrial stabilization of polyphenol and anthocyanin compounds (Desai et al. 2005).
Microencapsulation is a technique involving the encapsulation of a bioactive compound by a biopolymer, and thereby shielding it from oxygen, water, light, or other environmental elements in order to increase its stability, and also to transform liquid solutions to powders for easier management (Gharsallaoui et al. 2007). Many methods of encapsulation exist in the food industry; however, freeze drying has been used as a simple technique to encapsulate water-soluble essences and natural aromas or drugs, and it is one of the most convenient processes for drying thermo-sensitive substances that are unstable in aqueous solutions (Mahdavi et al. 2014).
In various studies involved with the encapsulation of anthocyanins, diverse materials and compounds have been used, such as maltodextrins (MDX) with different dextrose equivalencies (DE) (Ersus and Yurdagel 2007; Fang and Bhandari 2012; Khazaei et al. 2014), MDX and inulin (Bakowska-Barczak and Kolodziejczyk 2011), and MDX and ascorbic acid (Ahmed et al. 2010). MDX with DE varying from 16.5 to 19.5 have been utilized as the encapsulating agents, commonly as wall material because of their high water solubility, low viscosity, low sugar content, and their colorless solutions (Nayak and Rastogi 2010). Moreover, alginates, extracted from brown seaweed, have been used to form stable gels because they contain free carboxylic groups and react with divalent cations, mainly calcium (Deladino et al. 2007).
Some efforts have been made on encapsulating of pomegranate extract and its individual components (Kanatt et al. 2010; Robert et al. 2010). However, to the best of our knowledge, the potential of acidified ethanol extraction of pomegranate peel and its encapsulation in an MDX and calcium alginate matrix has not been explored.
The objective of the present study was to investigate the effects of combinations of various levels of MDX on the anthocyanin compounds, physicochemical properties, morphological changes, and glass transition temperature of freeze-dried encapsulated pomegranate peel and also monitor the stability of the total anthocyanin of the freeze-dried products during 45 days of storage under different combinations of temperature and humidity.
Materials and Methods
Plant Materials
Pomegranate peels (Punica granatum L. variety Sishe Kape-Ferdos) (POP) were provided as a byproduct of the fruit juice industry by Anarin company, Ferdos, Iran. The peels and seeds of POP were manually separated. POP samples were packed in low-density polyethylene film with a thickness of 140 μm, and directly frozen at − 18 °C until further analyses.
Chemicals and Reagents
Chemicals and supplies used in this research were MDX with a high DE (DE = 16.5–19.5), alginate calcium salt, 2, 4, 6-tris(2-pyridyl)-s-triazine (TPTZ), Folin–Ciocalteu (FC) reagent, gallic acid 2, 2-diphenyl-1-picrylhydrazyl (DPPH); all chemicals and solvents used in this research were obtained from Sigma–Aldrich (St. Louis, MO) and Merck (Darmstadt, Germany).
Determination of Anthocyanin Content
The ethanolic extract of anthocyanins was prepared as follows: frozen POP was ground with an Armfield ball grinder without thawing. Four volumes of 96% ethanol 1:1.5 N HCl (85:15 volume/volume) blend were added to the peels in order to extract anthocyanins. The samples then were subjected to ultrasonic waves (Hielscher, Germany, UP400S, 24 kHz) for 10 min with 20% intensity. After 24 h of being stirred with a magnetic stirrer (KMC 130SH; Vision Scientific Co., Ltd., Daegu, Korea) at ambient temperature (25 ± 1 °C), samples were filtered through a Whatman grade 1 filter paper. Extraction solvent was evaporated at 45 °C under vacuum by the rotary evaporator (Laborota 4000 Efficient, Germany) to reach 8.0 ± 0.3% of soluble solid (Ersus and Yurdagel 2007).
Preparation of Microencapsulated Powders
Wall materials used for microencapsulation were combinations of MDX (MDX 5, 10, and 15%) and calcium alginate (0.1%). They were dissolved in distilled water at ambient temperature (25 ± 1 °C) and kept in a refrigerator for 24 h for completion of hydration. Following, the extract of anthocyanins from POP was mixed in a weight ratio (weight/weight) of 1.0/5.0 (extract/wall material) and centrifuged in a Mikro 220R centrifuge (Hettich Instruments, Tuttlingen, Germany) at 120 rpm, for 30 min at room temperature (∼ 22 °C) (Wang and Xu 2007).
Anthocyanin Content
The solutions were dried in a freeze dryer (Operon, Korea) for 48 h (− 55 °C, 0.15 mmHg pressure). Dried materials were ground, using a pestle and mortar, and passed through a 0.71-mm mesh, then stored in brown glass bottles with screwed caps in a freezer (− 18 °C). In order to prepare the blank sample, the concentrated extract (8.0 ± 0.3% soluble solids) without wall materials was freeze-dried in similar conditions.
Physicochemical Analysis
Measurement of pH and Moisture Content
The pH of samples was measured using a pH meter (Model 744, Metrohm, Switzerland) after thoroughly homogenizing 2 g of sample with 25 ml of distilled water. pH was measured in triplicate samples. Moisture content (%), wb (wet basis) of POP, and pomegranate peel extract (POPx)-microencapsulated powders were measured using an infrared moisture balance (MX-50, Japan) heating at 105 ± 1 °C until a constant weight (Kaushik and Roos 2007).
Bulk Density
Bulk density of POP and microencapsulated powders was measured by the Tonon et al. (2010) method. In brief, 20 g of frozen milled POP was weighed and poured into a 20 ml graduated cylinder up to the mark. The bulk density was then calculated by dividing the sample mass (g) by the sample volume (20 ml).
Color Measurement
For color measurement, fresh POP and microencapsulated powders were scanned by a photo scanner (HP, Scanjet G3010, resolution of 300 dpi) and the images saved as BMP format. To study the effect of processing parameters on color components of samples, the RGB color space images were converted to L*, a*, and b* spaces by ImageJ software version 1.4.3.67 (National Institutes of Health, USA), and the indices of hue angle (Ho = tan−1 (b/a)) and chroma (c = (a2 + b2)1/2) were calculated and the mean of three replicates was reported (Karimi et al. 2012).
Determination of the Total Anthocyanin Content
The amount of anthocyanin was evaluated by a pH differential method (Giusti and Wrolstad 2001). 0.2 g of each sample (POPx and microencapsulated powders) was dissolved in 10 ml of distilled water in a volume flask far from the light. The absorbance of the reaction solution was measured by using a UV-1601 spectrophotometer (Shimadzu, Kyoto, Japan) at 510 nm and 700 nm. The amount of anthocyanin was calculated as milligram of cyanidin-3-glucoside per gram of dry matter of powder by using A = (Aλ510 − Aλ700)pH = 1 − (Aλ510 − Aλ700)pH = 4 and extinction coefficient of 26,900 L/cm/mg with a molecular mass of 449.2 g/mol.
Determination of the Total Phenolic Content
The Folin–Ciocalteu method was used to measure the total phenolic content (TPC) of POPx and microencapsulated powders (Singleton et al. 1999). The TPC was presented as gallic acid equivalents in milligram per kilogram. For the test, 100 μl of the sample solutions (100 mg in 10 ml of methanol), 6 ml of double-distilled water, and 500 μl of Folin–Ciocalteu reagent were added. Subsequently, after waiting between 8.8 min at room temperature, 1.5 ml of sodium carbonate (20% w/v) was added to the solution. The extracts were mixed and allowed to stand for 30 min at room temperature before measuring the absorbance at 765 nm. A mixture of water and reagents was used as a blank. The calibration curve formula for gallic acid equivalents (concentration range of 0.04–0.40 mg/ml) in methanol was achieved.
where x is the absorbance in 765 nm, Y is the phenolic compounds (mg/ml), and P is the phenolic compounds (mg/g).
Determination of Antioxidant Capacity
Antioxidant capacity was determined by DPPH free radical-scavenging capacity (Brand-Williams et al. 1995) and FRAP (ferric reducing antioxidant power) by Benzie and Strain (1996).
DPPH Free Radical-Scavenging Assay
The DPPH free radical-scavenging activity of the extracts (pre-diluted to 90 mg/L concentration) was measured by DPPH assay as described by Liu et al. (2009) with a slight modification. Aliquots of each extract (100 μl) were added to 3 ml of ethanolic DPPH solutions (0.1 mM). Discolorations were measured at 517 nm using a UV-1601 spectrophotometer (Shimadzu, Kyoto, Japan) after remaining for 30 min in the dark. The %DPPH which was scavenged (%DPPH) was calculated according to (Eq. 3).
where Acont was defined as the absorbance of the control whereas Asample was defined as the absorbance of the extracts.
Graphs of scavenging efficacy percentage against extract concentration (50–200 μl) in the solution were drawn. The extract concentration providing 50% inhibition was calculated from the graphs and reported as IC50 value (Hamedi et al. 2018). All determinations were made in triplicate. The standard antioxidant of α-tocopherol was evaluated to compare the results of DPPH assay with a positive control.
FRAP
FRAP was measured using 2, 4, 6-tripyridyl-s-triazine (TPTZ) according to Benzie and Strain (1996). The results were expressed in micromole Fe2+ per mass at 595 nm, against the control solution. The stock solutions comprised 300 mM acetate buffer (pH 3.6), 10 mM TPTZ solution in 40 mM HCl, and 20 mM FeCl3·6H2O solution. The working solution was made freshly by mixing 25 ml of acetate buffer, 2.5 ml of TPTZ solution, and 2.5 ml of FeCl3·6H2O solution. The mixed solution was incubated at 37 °C for 30 min and was referred to as FRAP solution. Sample (150 μl) was mixed with 3 ml of FRAP solution and kept for 30 min in the dark. Readings of the colored product (ferrous-tripyridyltriazine complex) were then taken at 593 nm. The 1 mmol/l FeSO4 was used as the standard solution. For construction of the calibration curve, five concentrations of FeSO4 7H2O (1000, 750, 500, 250, and 125 μmol/l) were used and the absorbencies were measured as sample solution. The data (FRAP value) was expressed as millimole ferric ions reduced to ferrous form per liter.
Determination of Encapsulation Yield and Efficiency
The encapsulation yield (Y) of samples after freeze drying was calculated according to the following formula (Eq. 4), based on dry matter measurements (Fang and Bhandari 2012):
The encapsulation efficiency (EE) was determined based on the methodology described by Kaderides et al. (2015), with some modifications. The Folin–Ciocalteu method (Singleton et al. 1999) was used to determine the TPC of the freeze-dried microcapsules. For the determination of 1 g of powder was washed with 10 ml of distilled water onto the filter paper. The EE was calculated by subtracting the amount of surface phenolic content (SPC) in the TPC divided by the TPC, as shown in Eq. 5:
Determination of Glass Transition Temperature
Samples of freeze-dried pigment powders were equilibrated at 75% RH (NaCl-saturated solution) for 1 week. Glass transition temperature (Tg) was determined using a differential scanning calorimeter (DSC1 Mettler Toledo, Switzerland). The temperature ranged from − 40 to 200 °C with a heating rate of 10 °C/min (Cai and Corke 2000). Five milligrams of sample powder was weighed directly into a DSC sample pan and then sealed. An empty pan was also used as a reference. Then, a temperature scan was done to get the Tg values.
Determination of Particle Morphology and Size Distribution
Particle Size
A laser diffraction particle size analyzer (SALD-2101, Shimadzu, Japan) was used to measure particle size in terms of diameter. The microencapsulated powders were dispersed in hexane using ultrasonic waves (24 kHz and 20% intensity) for 2 min and then particle size was directly determined (Parrarud and Pranee 2010).
Scanning Electron Microscopy
Particle structures of the encapsulated powders were evaluated via scanning electron microscope (LEO-1450, Germany). Using a two-sided adhesive tape, powders were attached to SEM stubs, and then left in desiccators containing phosphorous pentoxide for 48 h. Samples were coated with 200°A gold under vacuum condition before the examination. SEM was operated at an increasing voltage of 10 kV (Ahmed et al. 2010).
Storage Stability Evaluation of Encapsulated POP Anthocyanin
Encapsulated powders were kept at controlled humidity and temperature, in the absence of light for 42 days. Samples of 3 g of each powder were transferred to a closed low-density polyethylene bags (5 cm × 5 cm). The samples were put in sealed desiccators containing saturated NaCl and MgNO3 solutions in order to obtain humidity values of 75 and 52%, then placed at 4 and 25 °C temperatures (Gradinarua et al. 2003). For determination of anthocyanin, duplicate bags were removed every 7 days. POPx without encapsulation was used as a control.
Kinetics of Degradation of Anthocyanins
Degradation kinetics of anthocyanins during storage under isothermal heating are obtained by first determining the rate constants at a given temperature against the time. The key parameters of thermal degradation kinetics, i.e., half-life (t1/2), are calculated using (Eqs. 6 and 7).
where, C0 is the initial anthocyanin contents and Ct is the anthocyanin contents after time t (min) while k is the first-order kinetic constant (Wang and Xu 2007).
Statistical Analysis
All measurements and experiments were performed in triplicate and all data was subjected to analysis of variance (ANOVA). Regression and ANOVA analyses were performed using the MSTATC and Excel software. Notable differences between means were determined via Duncan’s multiple range tests. P values of less than 0.05 were considered statistically significant.
Results and Discussion
Characterization of POP and POPx
The data on moisture content, bulk density, and color measurement of POP are presented in Table 1. The mean moisture content and bulk density were 7.84% and 0.607 kg/m3 respectively. Pan et al. (2011) reported that the moisture content of POP varied between 3.79 and 6.02% and bulk density ranged from 0.13 to 0.74 g/cm3. Color measurements a* and b* were 30.3 and 22.1, respectively. These values remained on the slightly positive side indicating a shade of redness and yellowness, and the L* value was 46.1 at the lower half of the brightness scale indicating the sample to be rather in the dark. Results also showed C and H° values were 37.5 and 36.1, respectively. Pan et al. (2011) found similar results for Indian red pomegranate (ruby) variety.
Certain physicochemical features of POPx are presented in Table 2.
The pH varied among the pomegranate peel varieties and type of fraction. The pH and acidity of POPx were 4.63 and 0.80, respectively. Opara et al. (2009) reported pH value of Indian red pomegranate “baghva” was 4.4. Anthocyanins, which belong to the flavonoid family, are the major pigments responsible for pomegranate fruit color. Environmental conditions, variety, growing region, cultivation techniques, and ripening stage of the fruit at harvest affect POP color and health beneficial compounds (Mirdehghan and Rahemi 2007). The total anthocyanin content (TAC) and TPC were 45.2 mgc3/kg dmp and 78.2 mg GA/kg, respectively. The TPC of pomegranate vary considerably from one cultivar to another and peels of the fruits were responsible for the majority of the TPC of pomegranate. Cultivars with a dark red color peel and aril displayed a higher phenolic content (Gözlekçi et al. 2011).
Radical scavenging assay (RSA) and FRAP values were 78.9% and 468.37 μmol/l, respectively. Tabaraki et al. (2012) applied ultrasound-assisted extraction (UAE) for the extraction of polyphenol and antioxidants from pomegranate (P. granatum L.) peel using ethanol–water mixture. They reported the TPC varied from 5506 to 8923 mg gallic acid equivalent/100 g of dry weight. FRAP and DPPH values varied from 24.3 to 63.4 mmol Fe2+/100 g dry weight and 60.1–83.5% inhibition, respectively. The antioxidant properties of POPx were higher than α-tocopherol (Table 2).
Physicochemical Properties of Microencapsulated POPx
Table 3 gives a summary on the result obtained for the physicochemical properties of POPx with different wall material concentrations. Increasing wall material concentration from 5 to 15% significantly improved the yield (P < 0.05); however, no statistically significant differences were observed between the amount of wall concentration of 10 and 15% (P > 0.05). Clearly, increased moisture led to an increase in the mass, thus increasing the production yield of the microcapsules. The difference in moisture content can be attributed to the difference in the number of water-bonded groups in wall material molecules.
The amount of phenolic trapped into the microcapsules is represented as the efficiency of the process. Results showed that MDX 15% had the highest efficiency. One of the reasons for the higher production efficiency of the POPx with more wall materials is probably their higher moisture content. Jalil and Nixon (1990) reported that type and concentration of polymers as wall material affected EE. There were notable differences between samples in terms of bulk density (P < 0.05). This property ranged between 228 and 312 g/cm3. The highest bulk density corresponded to the sample with MDX 15% (311.9 g/cm3). The size of the crashed particles may influence the bulk density of the produced powders (Tonon et al. 2010). Results clearly indicated statistically significant differences among three MDX concentrations on particle size (P < 0.05). The average particle size (μm) of the microcapsules prepared with different concentrations of wall materials varied from 39.8 to 47.5 μm. Microcapsules containing MDX with a concentration of 15% (as wall materials) produced larger particles than microcapsule containing MDX at a concentration of 5%.
The Tg of freeze-dried powders is a very important indicator to assess if a droplet/particle is likely to stick to the freeze dryer wall. The Tg of the freeze-dried POPx powders was around 29.2–39.5 °C (Table 3), and significant differences were obtained among MDX 5% with MDX 10% and MDX 15% (P < 0.05). The sample with MDX 15% had higher moisture content (5.4 g/100 g) presented the lowest Tg (29.2 °C), as shown in Table 3. Microparticles with lower moisture content limit the water action as a plasticizer and also promote the increase of the Tg (Da Silva Carvalho et al. 2016).
Several studies claimed that that the particle with a higher moisture content is more cohesive due to lower Tg. Selim et al. (2000) reported that with the increase of moisture content, the Tg of the microcapsules decreased. On the other hand, Tg of the encapsulated powder increased with increase in MDX concentration. This might be due to the increase in molecular weight of the component of the powders (Fang and Bhandari 2012). For the different wall material concentrations evaluated in this study, a straight influence of the moisture content on the Tg could be observed.
Changes in color of microencapsulated powder with different concentrations of MDX are compared in Table 3. Differences in concentration of MDX had significant effects (P < 0.05) on L* values. With increasing MDX from 5 to 15%, L* and b* values of powders were increased from 31.3 to 34.2 and from 13.3 to 17.7, respectively, whereas a* value decreased from 19.9 to 6.81. The changes of color values were attributed to formation of polymeric anthocyanin. The color values for all encapsulated powders showed similar trend to those reported by Ahmed et al. (2010) for spray-dried purple sweet potato flour. The amount of chroma in the microcapsules prepared with the 15% MDX wall mixtures was lower than that of it the other microcapsules, confirming their color purity. H values of MDX 15% samples were found to be higher according to other samples and difference was found statistically important (P < 0.05). Lower a* and higher H indicate dark and purple shades of red color. The changes of color values were attributed to formation of polymeric anthocyanin (Ersus and Yurdagel 2007).
Phytochemical Properties of Microencapsulated POPx
The phytochemical properties of microencapsulated POPx are summarized in Table 3. The amounts of the TPC present in the microcapsule increased by increasing the concentration of wall materials from 5 to 15%, ranging from 59.7 to 73.1 (mg GA/kg), respectively. The increased TPC might be due to interference of MDX with the phenolic compounds during analysis (Ahmed et al. 2010). Experimental data showed that encapsulation with MDX greatly improves the stability of anthocyanin to high degree and maintenance of the antiradical properties. Besides, the amount of the TAC in 15% MDX wall material was 1.5-fold higher than in 5% MDX as measured in the present study. In general, the higher amount of MDX reached higher values for anthocyanin than the lower amount of MDX, showing the ability of MDX to bind of anthocyanin. The results were in line with Ersus and Yurdagel (2007) who studied the microencapsulation of anthocyanin pigments of black carrot and found that the greatest pigment retention was when used MDX with 20–23 DE.
Antioxidant Capacity of Microencapsulated POPx
It is well known that the amounts of phenolic contents may affect the antioxidant activity of food. As antioxidant activity has been done through several mechanisms, more than one test is carried out while evaluating the antioxidant activity of natural extracts. The results revealed that the antioxidant capacity was correlated with phenol content. Ersus and Yurdagel (2007) reported that antioxidant activity has high correlation with anthocyanin content and total phenolic composition of food materials. The antioxidant capacity of the POPx-microencapsulated powders was evaluated by the hydrogen-donor ability to 2, 2-diphenyl-1-picrylhydrazyl radical (DPPH).
Microparticles containing anthocyanins were able to sequester the DPPH stable radical and to reduce ferric ion, as displayed in Table 4. In this study, the scavenging concentration of each sample at 50% (IC50) was used to compare their antioxidant capacity. Lower IC50 values mean that the required amount of a sample needed to reduce the initial DPPH concentration by 50% becomes lower, indicating a higher antioxidant capacity of the sample (Brand-Williams et al. 1995). The IC50 values of the POPx microencapsulated with 15% MDX was 0.56 mg/ml, indicating that the highest antioxidant capacity among other wall materials. The MDX 15% wall material presented higher antioxidant capacity by the FRAP method and higher antioxidant capacity by DPPH assay. Antioxidant capacity for DPPH and FRAP assays may have been influenced by bioactive compounds (anthocyanins and polyphenols) present in the POPx and of reaction mechanisms involved in each of these analyses.
Particle Size and Morphology of Microencapsulated POPx
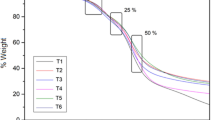
One of the most important characteristics of powder or particulate system is the distribution of particles. Figure 1 showed the particle size distribution of the microcapsules produced with different MDX concentrations. Most showed a unimodal distribution, indicating good powder homogeneity, with one peak representing a predominant size. The powders exhibited a very large size range, with diameters ranging from 3.6 to 188 μm. According to Table 3, the values of the mean diameter at MDX 15% were 47.53 μm which was higher than other concentrations. This is in agreement with the results published by (Tonon et al. 2008).
Figure 2a–c presents SEM photographs of microcapsules for the MDX concentration (5, 10, and 15%)-encapsulated powders containing anthocyanins. The comparison of electron images showed that the microsphere had a flake and irregular structure.
SEM examination showed an amorphous glass-like formation, which is considered that protects the entrapped molecules from exposure to heat and oxygen. Similar images, characteristic of freeze-dried encapsulated in MDX pigments, are reported by Khazaei et al. (2014).
Figure 2a, b, showed that MDX with 5 and 10% concentrations had crack, wrinkles, and breakages on the surface, probably reflected the effect of mechanical stress caused by the molding of particles and drying conditions on the wall materials. Freeze drying of POPx solution with MDX 15% led to the production of large, smooth particles, flake and irregular structure, and with no cracks and holes (Fig. 2c), which may be related to the feed viscosity (Tonon et al. 2008)
Storage Stability of Encapsulated POP Anthocyanin
The stability of anthocyanin in freeze-dried encapsulated POP powders and control was evaluated under various storage temperatures and relative humidity (Fig. 3). The anthocyanins of the POPx powder followed first-order kinetics, showing linear degradation with respect to time (Flores et al. 2014). Several authors also found a first-order reaction for the degradation of anthocyanins in sour cherry concentrates (Cemeroglu et al. 1994), purple and red-flesh potato extracts (Reyes and Cisneros-Zevallos 2007), and black currant nectar (Iversen 1999) during storage. Generally, the TAC of the POPx-microencapsulated powder decreased as the period of storage was increased, while higher storage temperatures were associated with lower retention rate. For example, the TAC of the PoPx-microencapsulated powders with wall material of 15% and control (POPx) decreased by 18% and 33%, respectively, after 42 days storage at 4 °C, while at a storage temperature of 25 °C, the decreases were in the order of 24% and 38%, respectively, over the same period of time. At a storage temperature of 25 °C and relative humidity of 75%, the declines in the TAC and control were even greater, between 39 and 52%, respectively. At a temperature of 25 °C and relative humidity of 52% of the TAC and control were between 44 and 50%, respectively. Fang and Bhandari (2011) present similar results on storage stability of bayberry polyphenols.
Knowledge of degradation mechanisms is important to maximize the nutritional and sensory quality of products. Thus, the degradation kinetics of the anthocyanins was monitored throughout the storage period. The half-life time (t1/2) and reaction rate constant (k) parameters are shown in Table 5. The half-life time (t1/2) and the reaction rate constant (k) parameters at the 4 °C temperature and 75 relative humidity for POPx encapsulated in MDX 15% were calculated as 115 and 0.64 × 10−2 min−1, respectively. The rate of anthocyanin degradation as a function of time in different microcapsules during 42 days of storage at 4 and 25 °C and relative humidity of 25 and 75% is shown in Fig. 3. Generally, it is accepted that the concentration of monomeric anthocyanins decreases with an increase in storage temperature. Besides decomposition, anthocyanins may also polymerize upon prolonged storage (Hager et al. 2008). With our samples, there was a slight increase in anthocyanin degradation in the first week and after that it decreased slightly. Comparing different wall material concentrations, MDX 15% anthocyanin degradation rate at 4 °C and 75% relative humidity was lower than other MDX concentrations and storage conditions.
Conclusions
Microencapsulation by freeze drying could be recommended as a suitable method for stabilizing anthocyanins of POPx. Among the wall concentrations tested in this study, the MDX 15% with 0.1% calcium alginate provided the formation of powders of higher moisture content, with a lower Tg. These formulations resulted in the highest yield and efficiency, particles with higher anthocyanin retention, high Tg, resulting in microparticles with better physical stability. Upon storage, anthocyanin degradation followed the first-order kinetics. Besides, MDX 15% presented the greatest half-life period (t1/2) with average values of 115 days. The total anthocyanin degradation decreased slightly in MDX 15% powders in 4 °C and 75% relative humidity while the antioxidant capacity increased conditions. The increase on MDX concentration also led to the production of larger particles, which is related to the increase on feed viscosity. Based on these results, the freeze-dried product may be further studied in developing a food ingredient that promotes health.
Abbreviations
- Y:
-
Yield
- E:
-
Efficiency
- TPC:
-
Total phenolic compounds
- RSA:
-
Radical scavenging activity
- FRAP:
-
Ferric reducing antioxidant power
- TAC:
-
Total anthocyanin content
- IC50 :
-
The concentration of extract required to scavenge 50% of 2, 2-diphenyl-1-picrylhydrazyl free radical
- POP:
-
Pomegranate peel
- POPx:
-
Pomegranate peel extract
- Tg:
-
Glass transition temperature
- MDX:
-
Maltodextrin
References
Ahmed, M., Akter, M. S., Lee, J. C., & Eun, G. B. (2010). Encapsulation by spray drying of bioactive components, physicochemical and morphological properties from purple sweet potato. LWT-Food Science and Technology, 43(9), 1307–1312.
Bakowska-Barczak, A. M., & Kolodziejczyk, P. P. (2011). Black currant polyphenols: their storage stability and microencapsulation. Industrial Crops and Products, 34(2), 1301–1309.
Benzie, I. F. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Analytical Biochemistry, 239(1), 70–76.
Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT-Food Science and Technology, 28(1), 25–30.
Cai, Y. Z., & Corke, H. (2000). Production and properties of spray-dried Amaranthus Betacyanin pigments. Journal of Food Science, 65(7), 1248–1252.
Cemeroglu, B., Velioglu, S., & Isik, S. (1994). Degradation kinetics of anthocyanins in sour cherry juice and concentrate. Journal of Food Science, 59(6), 1216–1218.
Da Silva Carvalho, A. G., da Costa Machado, M. T., da Silva, V. M., Sartoratto, A., Rodrigues, R. A. F., & Hubinger, M. D. (2016). Physical properties and morphology of spray dried microparticles containing anthocyanins of jussara (Euterpe edulis Martius) extract. Powder Technology, 294, 421–428.
Deladino, L., Pablo, S. A., Alba, S. N., & Miriam, N. M. (2007). Encapsulation of natural antioxidants extracted from Ilex paraguariensis. Carbohydrate Polymers, 71(1), 126–134.
Desai, K., Goud, H., & Hyun, J. P. (2005). Recent developments in microencapsulation of food ingredients. Drying Technology, 23(7), 1361–1394.
Ersus, S., & Yurdagel, U. (2007). Microencapsulation of anthocyanin pigments of black carrot (Daucus carota L.) by spray drier. Journal of Food Engineering, 80(3), 805–812.
Fang, Z., & Bhandari, B. (2011). Effect of spray drying and storage on the stability of bayberry polyphenols. Food Chemistry, 129(3), 1139–1147.
Fang, Z., & Bhandari, B. (2012). Comparing the efficiency of protein and maltodextrin on spray drying of bayberry juice. Food Research International, 48(2), 478–483.
Flores, F. P., Singh, R. K., & Kong, F. (2014). Physical and storage properties of spray-dried blueberry pomace extract with whey protein isolate as wall material. Journal of Food Engineering, 137, 1–6.
Gharsallaoui, A., Roudaut, G., Chambin, O., Voilley, A., & Saurel, R. (2007). Applications of spray-drying in microencapsulation of food ingredients: an overview. Food Research International, 40(9), 1107–1121.
Giusti, M. M., & Wrolstad, R. E. (2001). In R. E. Wrolstad (Ed.), Current protocols in food analytical chemistry (pp. 1–13). New York: John Wiley & Sons.
Gözlekçi, S., Saraçoğlu, O., Ebru Onursal, E., & Mustafa Özgen, M. (2011). Total phenolic distribution of juice, peel, and seed extracts of four pomegranate cultivars. Pharmacognosy Magazine, 7(26), 161–164.
Gradinarua, G., Biliaderisb, C. G., Kallithrakac, S., Kefalasa, P., & Garcia-Viguerad, C. (2003). Thermal stability of Hibiscus sabdariffa L. anthocyanins in solution and in solid state: effects of copigmentation and glass transition. Food Chemistry, 83(3), 423–436.
Hager, T. J., Howard, L. R., & Prior, R. L. (2008). Processing and storage effects on monomeric anthocyanins, percent polymeric color, and antioxidant capacity of processed blackberry products. Journal of Agricultural and Food Chemistry, 56(3), 689–695.
Hamedi, F., Mohebbi, M., Shahidi, F., & Azarpazhooh, E. (2018). Ultrasound-assisted osmotic treatment of model food impregnated with pomegranate peel phenolic compounds: mass transfer, texture, and phenolic evaluations. Food and Bioprocess Technology, 11(5), 1060–1074.
Iversen, C. K. (1999). Black currant nectar: effect of processing and storage on anthocyanin and ascorbic acid content. Journal of Food Science, 64(1), 37–41.
Jafari, S. M., & Mahdavi-Khazaei, K. (2016). Microencapsulation of saffron petal anthocyanins with cress seed gum compared with Arabic gum through freeze drying. Carbohydrate Polymers, 140, 20–25.
Jalil, R., & Nixon, J. R. (1990). Microencapsulation using poly(DL-lactic acid) II: effect of polymer molecular weight on the microcapsule properties. Journal of Microencapsulation, 7(2), 245–254.
Kaderides, K., Goulaa, M. A., & Adamopoulosb, K. G. (2015). A process for turning pomegranate peels into a valuable food ingredient using ultrasound-assisted extraction and encapsulation Author links open overlay panel. Innovative Food Science & Emerging Technologies, 31, 204–215.
Kanatt, S. R., Chander, R., & Sharma, A. (2010). Antioxidant and antimicrobial activity of pomegranate peel extract improves the shelf life of chicken products. International Journal of Food Science and Technology, 45(2), 216–222.
Karimi, M., Fathi, M., Sheykholeslam, Z., Sahraiyan, B., & Naghipoor, F. (2012). Effect of different processing parameters on quality factors and image texture features of bread. Journal of Bioprocessing and Biotechniques, 2(5), 1–7.
Kaushik, V., & Roos, Y. H. (2007). Limonene encapsulation in freeze-drying of gum Arabic-sucrose-gelatin systems. LWT, Food Science and Technology, 40(8), 1381–1391.
Khazaei, K. M., Jafari, S., Ghorbani, M., & Kakhki, A. H. (2014). Application of maltodextrin and gum Arabic in microencapsulation of saffron petal’s anthocyanins and evaluating their storage stability and color. Carbohydrate Polymers, 105, 57–62.
Liu, L., Sun, Y., Laura, T., Liang, X., Ye, H., & Zeng, X. (2009). Determination of polyphenolic content and antioxidant activity of kudingcha made from Ilex Kudingcha C.J. Tseng. Food Chemistry, 112(1), 35–41.
Mahdavi, S. A., Jafari, S. M., Ghorbani, M., & Assadpoor, E. (2014). Spray-drying microencapsulation of anthocyanins by natural biopolymers: a review. Drying Technology, 32(5), 509–518.
Mirdehghan, S. H., & Rahemi, H. (2007). Seasonal changes of mineral nutrients and phenolics in pomegranate (Punica granatum L) fruit. HortScience, 111, 120–127.
Nayak, C. A., & Rastogi, K. N. (2010). Effect of selected additives on microencapsulation of anthocyanin by spray drying. Drying Technology, 28(12), 1396–1404.
Opara, L. U., Al-Ani, M. R., & Al-Shuaibi, Y. S. (2009). Physico-chemical properties, vitamin C content, and antimicrobial properties of pomegranate fruit (Punica granatum L.). Food and Bioprocess Technology, 2(3), 315–321.
Pan, Z., Qu, W., Ma, H., Atungulu, G. G., & McHugh, T. H. (2011). Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrasonics Sonochemistry, 18(5), 1249–1257.
Parrarud, S., & Pranee, A. (2010). Microencapsulation of Zn-chlorophyll pigment from pandan leaf by spray drying and its characteristic. International Food Research Journal, 17, 1031–1042.
Prakash, A., Mathur, K., Vishwakarma, A., Vuppu, S., & Mishra, B. (2013). Comparative assay of antioxidant and antibacterial properties of Indian culinary seasonal fruit peel extracts obtained from Vellore, Tamilnadu. International Journal of Pharmaceutical Sciences Review and Research, 19(1), 131–135.
Reyes, L. F., & Cisneros-Zevallos, L. (2007). Degradation kinetics and colour of anthocyanins in aqueous extracts of purple-and red-flesh potatoes (Solanum tuberosum L.). Food Chemistry, 100(3), 885–894.
Robert, P., Gorena, T., Romero, N., Sepulveda, E., Chavez, J., & Saenz, C. (2010). Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. International Journal of Food Science & Technology, 45(7), 1386–1394.
Santos, D. T., & Meireles, M. A. A. (2011). Optimization of bioactive compounds extraction from jabuticaba (Myrciaria cauliflora) skins assisted by high pressure CO2. Innovative Food Science and Emerging Technologies, 12(3), 398–406.
Selim, K., Tsimidou, M., & Biliaderis, C. G. (2000). Kinetic studies of degradation of saffron carotenoids encapsulated amorphous polymer matrices. Food Chemistry, 71(2), 199–206.
Shabtay, A., Eitam, H., Tadmor, Y., Orlov, A., Meir, A., Weinberg, P., Weinberg, Z. G., Chen, Y., Brosh, A., Izhaki, I., & Kerem, Z. (2008). Nutritive and antioxidative potential of fresh and stored pomegranate industrial byproduct as a novel beef cattle feed. Agricultural and Food Chemistry, 56(21), 10063–10070.
Silva, S., Costa, E. M., Calhau, C., Morais, R. M., & Pintado, M. E. (2017). Anthocyanin extraction from plant tissues: a review. Critical Reviews in Food Science and Nutrition, 57(14), 3072–3083.
Singleton, V. L., Orthofer, R., & Lamuela-Raventos, R. M. (1999). Analysis of total polyphenols and other oxidation substrates and antioxidants by means of folin–ciocalteu reagent. Methods in Enzymology, 299, 152–178.
Tabaraki, R., Heidarizadi, E., & Benvidi, A. (2012). Optimization of ultrasonic assisted extraction of pomegranate (Punica granatum L.) peel antioxidants by response surface methodology. Separation and Purification Technology, 98, 16–23.
Tonon, R. V., Brabet, C., & Hubinger, M. D. (2008). Influence of process conditions on the physicochemical properties of açai (Euterpe oleraceae Mart.) powder produced by spray drying. Journal of Food Engineering, 88(3), 411–418.
Tonon, R. V., Brabet, C., & Hubinger, M. D. (2010). Anthocyanin stability and antioxidant activity of spray-dried acai (Euterpe oleracea Mart.) juice produced with different carrier agents. Food Research International, 43(3), 907–914.
Wang, W. D., & Xu, S. Y. (2007). Degradation kinetics of anthocyanins in blackberry juice and concentrate. Journal of Food Engineering, 82(3), 271–275.
Funding
This work is financially supported by the Iran National Scientific Foundation (INSF) and Agricultural Engineering Research Institute (AERI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Azarpazhooh, E., Sharayei, P., Zomorodi, S. et al. Physicochemical and Phytochemical Characterization and Storage Stability of Freeze-dried Encapsulated Pomegranate Peel Anthocyanin and In Vitro Evaluation of Its Antioxidant Activity. Food Bioprocess Technol 12, 199–210 (2019). https://doi.org/10.1007/s11947-018-2195-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-018-2195-1