Abstract
A novel process for effective separation of phospholipids, triacylglycerol and cholesterol from fresh egg yolk has been developed and validated in this study. Ethanol was the only organic solvent used in the whole procedure. Following initial separation of protein and total lipids by ethanol, most of solidified triacylglycerol was removed from total lipids by low temperature treatment of ethanol extracts within 10 h. Then, β-cyclodextrin (β-CD) was used to remove cholesterol from the remaining ethanol extracts and recycling of β-CD was also studied to obtain cholesterol and reusable β-CD powder. The highest cholesterol removal rate of nearly 99 % was obtained at β-CD: cholesterol molar ratio of 5:1, water addition of 15 g/g β-CD and reacting temperature of 50 °C. Ethanol in residual ethanol extracts was removed for obtaining phospholipids by rotary evaporation. The phospholipids produced in this procedure without cholesterol could be safety used as emulsifiers in food or cosmetic industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eggs are considered to be one of the most nutritional foods, which contain phospholipids, essential fatty acids, proteins, vitamins and minerals. Earlier researches mainly focused on total lipid extraction using multi-solvent system. Based on the principle of different solubilities of neutral lipids and polar lipids in various organic solvents, such as acetone, diethyl ether, hexane, chloroform and ethanol, phospholipids and neutral lipids containing cholesterol were fractionated (Schneider 1989). The extraction of total lipids or phospholipids from egg yolk was desirable because of the unique properties and valuable applications of these products. However, most of organic solvents used in the extraction process were toxic and the residual of them was a potential risk for the products. Moreover, the high cholesterol content of products had been a major potential detriment for human health. The increased intake of cholesterol and saturated fat could lead to the increase of plasma cholesterol in animals and human (Carleton et al. 1991; Gurr 1992; Pyorala 1987; Sieber 1993), meanwhile the high cholesterol levels in blood are a major risk factor for coronary heart disease occurrence (Chait et al. 1993). Therefore, many attempts had been made to remove cholesterol from egg yolk. Supercritical fluid extraction was used to reduce cholesterol from egg yolk in 1990s (Froning et al. 1990), but the methodology had not been developed to commercial scale for its high costs of the equipments. The removal of cholesterol by adsorption with β-cyclodextrin (β-CD) was an alternative approach. β-CD had the advantages of non-toxicity (Rao et al. 2000), non-hygroscopic and chemical stability (Nagatomo 1985). It was widely used for removing cholesterol from lard (Yen and Tsui 1995), cream (Ahn and Kwak 1999) and egg yolk or whole egg (Ravichandran and Divakar 1998; Smith et al. 1995). But commercial β-CD was expensive and its wastage during the process also led to environmental problems. Therefore, β-CD recycling during the cholesterol removing procedure was needed to be investigated.
The objective of this research was to develop a novel method for the fractionation of egg-yolk phospholipids, triacylglycerol, and cholesterol by combining ethanol extraction, low temperature crystallization and β-CD entrapment together. Non-toxic ethanol was attempted to be used as the sole organic solvent during the whole procedure. The removing of cholesterol from phospholipids products was investigated, and the recycling of β-CD was also studied in this paper.
Materials and methods
Egg yolk preparation and chemicals
Fresh eggs were collected from one flock of hens (39–46 weeks old), which received the same standard diet. Eggshells were carefully broken to separate the egg yolk from the egg white, and the combined egg yolk was kept at 4 °C before use. The moisture content of initial egg yolk was determined by using a conventional oven-drying method at 100 °C for 4 h. Standard samples of cholesterol and fatty acid methyl esters were purchased from Sigma-Aldrich Chemical Co., Ltd (Shanghai, P. R. China). Powdered β-CD (purity 99 %) was obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, P. R. China). All other chemicals and solvents used were of analytical grade.
Phospholipid extraction from fresh egg yolk
Egg yolk was placed in a homothermal reactor with a magnetic stirrer, 95 % ethanol (10 ml/g) was added and the mixture was stirred at 65 °C for 1 h. Then the ethanol-extracted fraction was filtered and collected for further processing. The extraction process was repeated twice and the ethanol-extracted fractions were combined together. The solid yolk residues were essentially egg-yolk proteins free from lipids.
Low temperature crystallization
The ethanol-extracted fraction containing more than 95 % of total egg yolk lipids was subjected to low temperature crystallization at 4 °C for different time (2 h, 4 h, 6 h, 8 h, 10 h). The solidified triacylglycerol was removed by refrigerated centrifuge (2,800 g, 10 min, 4 °C) and subjected to various analyses. The supernatant liquid was collected for next step.
Cholesterol removal
The supernatant obtained in section 2.3 was placed in a homothermal reactor with a magnetic stirrer, and β-CD aqueous solution with different concentrations was added dropwisly. The water addition for β-CD aqueous solution preparation was set to be 9 to 27 g/g β-CD. The molar ratio of β-CD: cholesterol was set to be 3:1 to 6:1 (w/w). The reaction was carried out for 30 min with different reacting temperatures (25 to 60 °C). After the reaction, the mixture was centrifuged at 2,800 g for 10 min. The precipitate and supernatant of centrifugation were collected respectively for cholesterol determination.
Recycling investigation of β-CD
The cholesterol-β-CD inclusion complex (precipitate in section 2.4) was added to absolute ethanol (1:10 w/v) and then refluxed at 95 °C for 2 h. Then, the mixture was cooled to 25 °C and centrifuged at 2,800 g for 10 min. The precipitation containing mainly β-CD was dried at 60 °C in dry oven for 6 h and reused for recycling investigation. Cholesterol was released into ethanol and it can be collected.
Phospholipids fraction
The residual ethanol extracts after cholesterol removal treatment (supernatant in section 2.4) were transferred to a previously weighed round-bottomed flask and the ethanol was removed by rotary evaporation. The dried phospholipids were subjected to phosphorus assay.
Analytical methods
Gravimetric determination
Evaporation and drying of samples were done on a 60 °C dry oven.
Total lipid determination
Total lipids were determined according to the method reported by Lee et al. (1996).
Phospholipids, cholesterol and triacylglycerol determination
Phospholipids were determined by phosphorus assay (Bartlett 1959) using a factor 25 for converting weight of phosphorus to weight of phospholipids. Cholesterol was determined colorimetrically (Nielsen 2000). Triacylglycerol was determined indirectly by subtracting phospholipids and cholesterol from total lipids.
The percentage of cholesterol reduction was calculated as follows:
where m0 is amount of cholesterol in β-CD-treated ethanol extracts, m1 is amount of cholesterol in untreated ethanol extracts. Cholesterol determination was averaged with each batch of treatments.
Fatty acid composition analysis by gas chromatograph (GC)
Fatty acid methyl esters were prepared by incubation with alkalify methanol. Briefly, triacylglycerol of egg yolk oil isolated by low temperature crystallization was mixed with methanol containing 28 g KOH/L (5.0 mL). Then the mixture was incubated at 7 °C for 20 min. The mixture was cooled and neutralized with a methanol solution (1.0 mL) containing 12.5 g BF3/L. Then 5.0 mL of hexane was added to the mixture. Fatty acid methyl esters were isolated by collection of the hexane layer after vortex mixing. Anhydrous sodium sulfate was applied for dehydration and the supernatant liquor was collected by centrifugation at 1,200 g for 10 min. Samples were transferred to GC auto sampler vials.
Fatty acids in triacylglycerol of egg yolk oil were determined as their methyl esters by a Shimadzu (Kyoto, Japan) GC-2010 gas chromatograph equipped with a Shimadzu AOC-20i auto injector (1.0 μl injections) using a BPX-70 capillary column (120 m × 250 μm × 0.25 μm, SGE) with flame ionization detection (FID). Nitrogen was supplied as the carrier gas. The initial oven temperature was 150 °C and was kept there for 1 min, then further increased to 180 °C by 10 °C/min, and finally held at 180 °C for 5 min. The temperatures of the FID and the injection port were 250 °C and 230 °C, respectively. The split ratio was 30:1 and the pressures of nitrogen, hydrogen and air were 25 kPa, 60 kPa and 50 kPa, respectively. Methyl esters of the following fatty acids were used as standards to identify the peaks on the chromatogram: 16:0, 16:1 n-7, 18:0, 18:1 n-9, 18:2 n-6, 18:3 n-3, 22:6 n-3.
Statistical analysis
All experiments on stability were carried out in triplicate. The SAS software (Version 8.0, 2000, SAS Institute, Cary, NC, USA) was used for statistical analysis of means and standard deviations. One-way variance was carried out using Duncan’s Multiple Range Test to detect significant difference between mean values. Statistical differences at P < 0.05 were considered to be significant.
Results and discussion
Total lipids extraction by 95 % ethanol
According to the quantification methods, the extraction efficiency of total lipids in ethanol-extracted fraction relative to the initial material reached 95.40 %, which consisted of 33.02 ± 1.83 % phospholipids, 62.30 ± 2.07 % triacylglycerol and 4.57 ± 0.26 % cholesterol compared to 31.58 ± 1.21 %, 64.14 ± 1.29 % and 4.24 ± 0.53 % in total egg yolk lipids. The lipids extraction efficiency was similar to the results reported by Sim (Sim 1995). The high extractability can be explained by the fact that most lipids of egg yolk were existed in the form of lipoproteins. The covalent bonds connecting with lipids and proteins in lipoproteins would crack by the energy of molecule thermal motion when extracted by polar ethanol. The conformation of lipoprotein was fractured to release triacylglycerol. Through triacylglycerol did not dissolve in 95 % ethanol, it could disperse in warm ethanol to form a non-transparent emulsion and was extracted from egg yolk system.
Low temperature crystallization
Most of triacylglycerol was solidified in low temperature (4 °C), therefore, the ethanol-extracted fraction from egg yolk system was exposed to 4 °C to isolate triacylglycerol in this study. As shown in Table 1, the precipitated of triacylglycerol in ethanol-extracted fraction were affected significantly by the exposure time at low temperature. The yield of precipitated triacylglycerol increased rapidly as the exposure time up to 8 h. Then the precipitation of triacylglycerol was slow down during the next 2 h. Approximately 75.80 % of triacylglycerol based on its amount in ethanol-extracted fraction was precipitated after cooled at 4 °C for 10 h. Meanwhile, only 1.17 % and 1.14 % of phospholipids and cholesterol based on their own weights in ethanol-extracted fraction were precipitated, respectively. The results were consistent with the results reported by Nielsen which cool the ethanol-extracted fraction to −20 °C (Nielsen and Shukla 2004). It could improve that low temperature crystallization was an efficient method to isolate triacylglycerol from the ethanol-extracted fraction of egg yolk system.
The fatty acids compositions of triacylglycerol isolated from the ethanol-extracted fraction of egg yolk system were analyzed by gas chromatography. As shown in Table 2, the unsaturated fatty acids content in triacylglycerol isolated from egg yolk achieved 64.28 %, most of which were linoleic acid (C18:2 n-6) and oleic acid (C18:1 n-9), accouting for 30.37 ± 1.47 % and 16.40 ± 0.89 %, respectively. Moreover, there were a certain amount of essential long-chain polyunsaturated fatty acids, such as linolenic acid (C18:3 n-3) (6.42 ± 0.6 %) and DHA (C22:6 n-3) (9.49 ± 0.66 %), in triacylglycerol isolated from egg yolk system. The existence of unsaturated fatty acids provided a new nutritional value of triacylglycerol of egg yolk system. Cholesterol could not be separated effectively from triacylglycerol of egg yolk in traditional processing technology. Therefore, the application of triacylglycerol isolated from egg yolk system was limited for the enriched cholesterol. However, the ratio of cholesterol in triacylglycerol isolated from egg yolk system was rather small in this research. The application potential of triacylglycerol separated according to this procedure should be investigated in the future.
Cholesterol removal
The β-CD was a kind of natural macrocyclic oligosaccharides formed by seven glucopyranose units linked by 1,4-α-glucosidic bonds. The form of β-CD molecule resembles truncated cones with the secondary hydroxyl groups located at the wider edge of the ring and the primary hydroxyl groups on the narrower edge. Hydrogen atoms are directed to the inner part of the ring resulting in a hydrophobic cavity along with a hydrophilic character outside the ring (Araújo et al. 2007). The β-CD was capable of constituting true molecular encapsulations with many highly hydrophobic molecules by taking up a whole molecule, or some part of it, into their hydrophobic cavities simultaneously expelling the few high-energy water molecules from inside (Vianna et al. 1998; Karathanos et al. 2007). No covalent bonds were formed or broken during the guest-CD complexes formation, and under physiological conditions the complexes were readily dissociated and the free guest molecules were in a rapid equilibrium with the guest molecules bound within the CD cavities. The inclusion of cholesterol by β-CD has been confirmed by previous studies and it was reported that one cholesterol molecule can be embodied by three β-CD molecules (Claudy et al. 1991). The β-CD powder was hardly dissolved in the ethanol solution, thus it was needed to be dissolved in water firstly. Therefore, the removal rate of cholesterol in ethanol extracts was mainly influenced by three factors: molar ratio of β-CD: cholesterol, the amount of water addition and reacting temperature. All the three factors were investigated in this research.
Effect of β–CD: cholesterol molar ratio
The results of effect of β-CD: cholesterol molar ratio on cholesterol removal rate was shown in Table 3. Cholesterol removal rate was markedly related to β-CD: cholesterol molar ratio. It was believed that a cholesterol molecule can be embodied by three β-CD molecules according to Claudy et al. (1991), thus β-CD: cholesterol molar ratio of 3:1 was expected to be the best molar ratio in the cholesterol removal procedure. However, cholesterol removal rate was less than 60 % at this molar ratio. In order to obtain higher removal rate, the molar ratio of β-CD: cholesterol was increased. The removal rate of cholesterol was increased from 57.18 to 94.22 % as the molar ratios of β-CD: cholesterol was raised from 3:1 to 5:1. (where the amount of water addition was 15 g/g β-CD and reacted at 50 °C). But cholesterol removal rate was decreased to less than 70 % when molar ratio was increased to 6:1. It was assumed that an excess of β-CD could compete with each other to bind to cholesterol molecules, thereby resulting in reduced cholesterol adsorption. Similar tendency was achieved in cholesterol removal researches in milk and butter oil, respectively (Micich 1990; Ji et al. 1997). Therefore, the present study indicated that β-CD: cholesterol molar ratio of 5:1 may be a sufficient amount to remove cholesterol effectively in low temperature treated fraction.
Effect of water addition
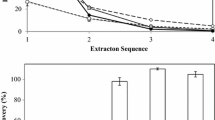
Cholesterol removal rate in ethanol solution by β-CD was markedly affected by the amount of water added to the solution according to the results shown in Fig. 1. The highest cholesterol removal rate (96.12 %) was found at water addition of 15 g/g β-CD in the sample treated with molar ratio of β-CD: cholesterol of 5:1 at 50 °C. No obvious differences were found when the amount of water addition was adjusted to 13, 17, 19 and 21 g/g β-CD. It could be explained that β-CD and cholesterol could contact effectively with each other to form β-CD-cholesterol complexes which were easily to separate at these amounts of water addition. When the amount of water addition was decreased to 9 g/g β-CD, the solubility of β-CD in ethanol solution was decreased significantly for the high ethanol concentration. The amount of β-CD dissolved in ethanol solution may be insufficient to inclusive the cholesterol in ethanol extracts, therefore the cholesterol removal rate was diminished. However, the cholesterol removal rate was significantly decreased when the amount of water addition was up to 25 g/g β-CD. This may due to the fact that higher water addition with a certain limit would increase the solubility of β-CD in ethanol solution (Kwan et al. 1991). Although β-CD added to the ethanol extracts could combine the cholesterol molecular efficiently, the inclusion complex of β-CD-cholesterol could not be removed from the ethanol extracts completely as the large solubility of β-CD with higher water addition. Therefore, the cholesterol removal rate was significantly decreased.
Effect of reacting temperature
As shown in Table 4, the cholesterol removal rates were in the range of 94.22 to 98.60 % when the reacting temperatures were chosen between 25 and 60 °C. The maximum cholesterol removal rate was 98.60 % at 50 °C. The effect rule of reacting temperature on cholesterol removal rate was not explicit according to the results in Table 4. However, it was different with some standpoint reported before. Oakenfull and Sidhu reported that removal of cholesterol from milk with β-CD was markedly influenced by temperature (Oakenfull and Sihdu 1991). Higher rate of cholesterol removal was found at lower temperatures in their study (i.e., 77, 63 and 62 % cholesterol were removed when treated with 1.0 % β-CD during 10 min of mixing at 4, 8 and 40 °C, respectively). On the other hand, Yen and Tsui reported that cholesterol removal rate from lard with β-CD stirred at 50 °C was greater than that stirred at 27 or 40 °C (Yen and Tsui 1995). The different results may be attributed to the difference of solution system of the study. The interaction mechanisms between cholesterol and β-CD still required further investigation.
Recycling investigation of β–CD
The β–CD was separated from cholesterol-β–CD complex and applied to ethanol extracts 10 times repeatedly to investigate its recycling property. As shown in Fig. 2, when β-CD was reused for up to seven times, relatively high cholesterol removal rates were found in the range of 88.63–93.37 %. The cholesterol removal rates were similar to the results using fresh β–CD each time. Thereafter, the cholesterol removal rate began to decline after reused eight times (87.21-79.80 %). The reason of the decline of cholesterol removal rate when β–CD was reused more than eight times was not clear. It was assumed that the structure of β–CD may be changed during the regeneration process, therefore the inclusive ability of β–CD to cholesterol molecular was declined. On the other hand, cholesterol molecular may not be released completely from the β–CD-cholesterol inclusion complex during the β–CD regeneration process. A few of cholesterol molecular has already been included in recycled β–CD before the β–CD was added to the ethanol extract. Thus the actual inclusive ability of β–CD to cholesterol molecular in ethanol extract was declined. The present study suggested that using recycled β-CD within seven times for cholesterol removal in the ethanol extracts might give almost the identical results to using fresh β-CD each time. However, Kwak et al. reported that recycled powdered β-CD showed 75.07 % of cholesterol removal in cream, whereas mixtures of recycled and fresh powdered β-CD (6:4) increased cholesterol removal rate to 95.59 %, suggesting that recycled powdered β-CD may not be as effective as fresh unused β-CD (Kwak et al. 2001). The different results between two studies could be attributed to the separation system used in remove reaction. Cholesterol was removed in organic phase in this study, while Kwak et al. carried out cholesterol removal in the oil phase. Another reason accounted for the differences may be the way to regenerate the used β-CD.
Phospholipids fraction
The residual ethanol extracts after cholesterol removal treatment were treated by rotary evaporation. The dried phospholipids were produced when the ethanol were removed. The purity of phospholipids produced in this procedure was 51.47 % which could be used as emulsifiers in food or cosmetic industry.
Conclusion
A novel process for effective separation of phospholipids, triacylglycerol and cholesterol from fresh egg yolk has been developed and validated in this study. The process combined ethanol extraction, low temperature crystallization and a β-CD-based process together to isolate the main three fractions. Following initial separation of protein and total lipids by ethanol, most of solidified triacylglycerol was removed from total lipids by low temperature treatment of ethanol extracts within 10 h. The optimum conditions for cholesterol removal were at β-CD: cholesterol molar ratio of 5:1, water addition of 15 g/g β-CD and reacting temperature of 50 °C. The cholesterol removal rate nearly reached 99 % and recycling β-CD can be effectively conducted. The purity of phospholipids produced in this procedure was 51.47 % which could be used as emulsifiers in food or cosmetic industry.
References
Ahn J, Kwak HS (1999) Optimizing cholesterol removal in cream using β-cyclodextrin and response surface methodology. J Food Sci 64:629–632
Araújo MVG, Vieira EKB, Lázaro GS, Conegero LS, Ferreira OP, Almeida LE, Barreto LS, Costax NB Jr, Gimenez IF (2007) Inclusion complexes of pyrimethamine in 2-hydroxypropyl-β-cyclodextrin: Characterization, phase solubility and molecular modeling. Bioorg Med Chem 15:5752–5759
Bartlett GR (1959) Phosphorus assay in column chromatography. J Biol Chem 234:466–468
Carleton RA, Finberg L, Flora J et al (1991) Report of the expert panel on population strategies for blood cholesterol reduction. Circulation 83:2154–2232
Chait A, Brunzell JD, Denke MA et al (1993) Rationale of the diet-heart statement of the American-Heart-Association report of the nutrition committee. Circulation 65:3008–3029
Claudy P, Letoffe JM, Germain P et al (1991) Physicochemical characterization of cholesterol-beta-cyclodexterin inclusion complexes. J Therm Anal 37:2497–2506
Froning RL, Wehling RL, Cuppett SL et al (1990) Extraction of cholesterol and other lipids from dried egg yolk using supercritical carbon dioxide. J Food Sci 55:95–98
Gurr MI (1992) Dietary lipids and coronary heart disease: old evidence, new perspective. Prog Lipid Res 31:195–243
Ji JR, Yoo IJ, Park WM et al (1997) Removal of cholesterol from liquid egg yolk by beta-cyclodextrin treatment. Korean J Anim Sci 39:599–604
Karathanos VT, Mourtzinos I, Yannakopoulou K, Andrikopoulos NK (2007) Study of the solubility, antioxidant activity and structure of inclusion complex of vanillin with β-cyclodextrin. Food Chem 101:652–658
Kwak HS, Suh HM, Ahn J et al (2001) Optimization of β-cyclodextrin recycling process for cholesterol removal in cream. Asian Aust J Anim Sci 14:548–552
Kwan LE, Helbig LN, Nakai S (1991) Fractionation of water soluble and insoluble components from egg yolk with minimum use of organic solvents. J Food Sci 56(6):1537–1541
Lee CM, Trevino B, Chaiyawat M (1996) A simple and rapid solvent extraction method for determining total lipids in fish tissue. J AOAC Int 79:487–492
Micich TJ (1990) Behaviors of polymer-supported digitonin with cholesterol in the absence and presence of butter oil. J Agr Food Chem 38:1839–1843
Nagatomo S (1985) Cyclodextrin-expanding the development of their formations and application. Chem Econ Eng Rev 17:28–34
Nielsen H (2000) Application of chemical methods to the determination of egg yolk contamination in commercial productions of egg white compared to enzymatic determination. LWT-Food Sci Tech 33:151–154
Nielsen H, Shukla VKS (2004) In situ solid phase extraction of lipids from spray-dried egg yolk by ethanol with subsequent removal of triacylglycerols by cold temperature crystallization. LWT - Food Sci Tech 37:613–618
Oakenfull DG, Sihdu GS (1991) Cholesterol reduction. International Patent, 91/11114
Pyorala K (1987) Dietary cholesterol in relation to plasma cholesterol and coronary heart disease. Am J Clin Nutr 45:1176–1184
Rao P, Sujatha D, Raj KR et al (2000) Safety aspects of residual β-cyclodextrin in egg treated for cholesterol removal. Eur Food Res Tech 211:393–395
Ravichandran R, Divakar S (1998) Inclusion of ring a lot of cholesterol inside the β-cyclodextrin cavity: Evidence from oxidation reactions and structural studies. J Incl Phenom Mol Recognit Chem 30:253–270
Schneider M (1989) Fractionation and purification of lecithin. In: Szuhaj BF (ed) Lecithins: sources, manufacture & uses. The American Oil Chemists’ Society, Champaign, pp 109–130
Sieber R (1993) Cholesterol removal from animal food-Can It be justified? LWT-Food Sci Tech 26:375–387
Sim JS (1995) Extraction of fresh liquid egg yolk. Canadian Patent, 1335054
Smith DM, Awad AC, Bennink MR et al (1995) Cholesterol reduction in liquid egg yolk using β-cyclodextrin. J Food Sci 60:691–694
Vianna RFL, Bentley MVLB, Ribeiro G, Carvalho FS, Neto AF, de Oliveira DCR, Collett JH (1998) Formation of cyclodextrin inclusion complexes with corticosteroids: their characterization and stability. Int J Pharm 167:205–213
Yen GC, Tsui LT (1995) Cholesterol removal from a lard-water mixture with β-cyclodextrin. J Food Sci 60:561–564
Acknowledgments
This research was supported by the Fundamental Research Funds for the Central Universities (JUSRP11222).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Su, Y., Tian, Y., Yan, R. et al. Study on a novel process for the separation of phospholipids, triacylglycerol and cholesterol from egg yolk. J Food Sci Technol 52, 4586–4592 (2015). https://doi.org/10.1007/s13197-014-1513-5
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-014-1513-5