Abstract
Egg yolk lipids are important components in egg yolk. Compared with proteins, minerals, vitamins and pigments in egg yolk, there is a lack of systematic research on extraction methods and characteristics of yolk lipid. Therefore, in this study, the extraction of egg yolk lipids by supercritical CO2 fluid extraction (SFE), subcritical propane extraction (SPE) and ethanol solvent extraction (SE) was studied, and the differences in composition and physicochemical properties of the three yolk lipids were analyzed. The egg yolk lipids extracted by SFE had the advantages of high unsaturated fatty acid content, low saponification value, acid value, peroxide value, and high content of neutral lipids such as triglyceride and cholesterol, but the polar phospholipids were completely retained in the yolk powder. The efficiency of lipid extraction of SPE was the highest among all the three methods, but the extraction capacity to the polar phospholipid was in the middle of the three methods. The efficiency of lipid extraction of SE was low, but the extraction capacity to the polar phospholipid was the highest. Suitable extraction methods could be selected based on the specific application requirements of egg yolk lipids and low-fat egg yolk powders.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Egg yolk lipids are the kinds of liquid mixture separated from egg yolk and consist mainly of triglycerides, cholesterol and phospholipids. Egg yolk lipids have anti-inflammatory effects and the function of promoting wound healing (Yenilmez et al. 2015). The egg yolk lipids contain a high content of unsaturated fatty acids, which can effectively improve the antioxidant capacity and anticancer ability, improve the formation and regulation of lipid membrane dependence (Xiao et al. 2020). Egg yolk phospholipids have high physiological activity and play a key role in the development of the brain and neural networks by improving cognitive ability and participating in signal transduction (Huang and Ahn 2019). Cholesterol is a controversial lipid component. Too much cholesterol intake may increase the risk of cardiovascular diseases, such as hypercholesterolemia and atherosclerosis (Chambers et al. 2019). Nevertheless, cholesterol is still an integral substance for the human body with many beneficial physiological activities, such as the formation of cell membranes, the synthesis of hormones and the synthesis of vitamin D3 (Chambers et al. 2019).
At present, the main extraction methods for egg yolk lipids are distillation at high temperature, solvent extraction (SE), supercritical fluid extraction (SFE), subcritical propane extraction (SPE) and enzymolysis extraction (Ohba et al. 1995). The SE method is a traditional extraction method of egg yolk lipids, which is commonly used in industrialization scale by using a single solvent (ethanol, ether) or mixed solvents (Paraskevopoulou et al. 1997). However, there are some safety problems with the solvent extraction method, such as solvent residue and active component degradation (Rui and Wei 2011). In recent years, new lipid extraction methods have been gradually applied to lipids extraction. SPE has been successfully used to separate lipids from kiwifruit seeds, palm and other materials, which has the advantages of short extraction time, less solvent residue, less thermal degradation of bioactive compounds and wide selection of solvents (Zhang et al. 2022). However, propane is flammable and explosive, which requires certain safety measures. Moreover, the extracted egg yolk lipids not only have low phospholipids content, but also contain a protein portion, which requires further processing. SFE technology overcomes many shortcomings of SE and SPE, and has been widely applied in the food industry. SFE has the characteristics of no residue, no pollution, low temperature, low energy consumption, retention of active substances, simple process and easy recovery of the solvent (Rui and Wei 2011). However, the extraction pressure of SFE is high, the requirements for equipment and the cost are high (Sun et al. 2018). Moreover, the extraction ability of SFE for polar substances is relatively low compared to SPE and SE. Each extraction method has its own advantages and disadvantages, and the characteristics of egg yolk lipids extracted by different methods are different. It may affect the subsequent application of egg yolk lipids and residue egg yolk powders. The systematic research on this aspect is insufficient by now.
In order to conduct a comparative study on the influence of different extraction methods on the characteristics of egg yolk lipids, methods of SFE, SPE and SE were used to extract egg yolk lipids from egg yolk powder (EYP), respectively. The differences in composition and physicochemical properties of the three kinds of egg yolk lipids were analyzed. The results of this study can provide guidance for the selection of suitable extraction methods of egg yolk lipids for different applications.
Materials and methods
Materials
Spray-dried egg yolk powder was provided by Jiangsu Kangde Egg Co., LTD. (Nantong, China). Cholesterol Standard was purchased from Beijing Balingwei Technology Co., LTD. (Beijing, China). N-heptane was purchased from Sinopharm Group Chemical Reagent Co., LTD. (Shanghai, China). All other chemicals used in this study were analytical grade and purchased from Sinopharmate Chemical Reagents Co., LTD. (Shanghai, China).
Sample preparation
Supercritical CO2 fluid extraction
100 g EYP sample was added into the extraction kettle of supercritical equipment (LH-2000, Huada pharmaceutical equipment Technology Co., LTD, Nantong, China), and then extracted according to the parameters of extraction temperature (35–50 °C), CO2 flow rate (16–28 L/h), and extraction pressure (25–45 MPa). The extracted egg yolk lipids were collected every 30 min, and collected five times in total. The egg yolk lipids extracted by SFE were recorded as EYL-SFE. After extraction, low-fat egg yolk powder (EYP-SFE) extracted by SFE was also collected.
Subcritical propane extraction
100 g EYP sample was added into the extraction tank of the subcritical extraction equipment (CBE-5L, Henan subcritical biological technology co., LTD, Anyang, China). The extraction conditions were referred to the method of Su et al. (2020), static extraction was performed for 30 min at 40 °C and the solid–liquid ratio was 1:9, and the extraction was repeated for 4 times. Residual low-fat egg yolk powder and egg yolk lipids extracted by SPE were collected. The low-fat egg yolk powder was air-dried in a hood to obtain egg yolk powder extracted by SPE (EYP-SPE). The egg yolk lipids were centrifuged at 11,180 ×g for 5 min to obtain clarified egg yolk lipids extracted by SPE (EYL-SPE).
Solvent extraction
The sample and the absolute ethyl alcohol (10 mL/g egg yolk powder) were added to a conical bottle, stirred at 65 °C for 4 h, and then drained and filtered, and the egg yolk powder (EYP-SE) extracted by ethanol was air-dried in a fume hood. The organic phase obtained by two extraction filtration was removed in a 50 °C vacuum rotary evaporator to obtain egg yolk lipids extracted by ethanol (EYL-SE).
The analysis of egg yolk components
Protein content
The crude protein content of egg yolk powders was determined by Kjeldahl nitrogen determination method (Lynch and Barbano 1999).
Lipid content
The lipid content of egg yolk powder was determined by acid hydrolysis method according to Perez-Palacios et al. (2008). 1 g sample was mixed with 8 mL deionized water in a 50 mL test tube, and then 10 mL hydrochloric acid was added to the mixture. The test tube was heated in a water bath of 70–80 °C until the sample was digested completely. Then 10 mL ethanol was added. After cooling, the mixture was transferred into a 100 mL stopper cylinder and 30 mL anhydrous ether was added. After phase separation, the upper organic phase was transferred into a round-bottomed flask and dried in a vacuum. Then the round-bottom flask was placed in the oven at 100 ± 5 °C at atmospheric pressure and dried to constant weight.
The lipid content of the sample was calculated according to the formula:
where, X was the lipid content, %; m1 was the mass of receiving bottle and lipid after constant weight, g; m0 was the mass of receiving bottle after constant weight, g; m was the mass of the sample, g.
Cholesterol content
The content of cholesterol was determined by colorimetry method according to Hong-xia et al. (2009) with a slight modification. 0.1 g sample was mixed with 4 mL anhydrous ethanol and 0.5 mL 50% NaOH solution and saponified in a 65 °C water bath for 2 h. 3 mL 5% NaCl solution and 10 mL petroleum ether were added to the mixture. After the stratification, the upper layer of 1 mL petroleum ether was taken in a 10 mL test tube, dried with nitrogen in a water bath at 65 °C, and mixed with 4 mL ice acetic acid and 2 mL iron vitriol color developing solution. After standing for 15 min, the absorbance was measured at 550 nm using an ultraviolet spectrophotometer (UH5300, Hitachi Production Co., LTD, Tokyo, Japan). The standard curve was prepared with 0.42.0 mL, 0.04 mg/mL cholesterol standard solution.
Phospholipids content
The content of phospholipids was determined by the content of phosphorus in total lipids. The weight of phosphorus is converted into the weight of phospholipids with a coefficient of 26.13 (Bartlett 1959). 0.3 g sample, 10 mL nitric acid, 1 mL perchloric acid and 2 mL sulfuric acid were added into the digestion tube on an adjustable electric furnace. When the digestive solution cooled, 20 mL water was added. After it was cooled, the mixture was transferred to a 100 mL volumetric bottle, washed the digestion tube with deionized water 3 times and combined the solution in the volumetric bottle. After standing for 0.5 h, the absorbance was measured at 660 nm by ultraviolet spectrophotometer (UH5300, Hitachi Production Co., LTD, Tokyo, Japan) with the blank control as reference. The standard curve was prepared with 0–5.0 mL of 10.0 mg/L phosphorus standard using liquid.
Fatty acid composition
Fatty acids (FAs) composition was determined by gas chromatography (GC) (GC-2030AF, Shimadzu Enterprise Management Co., LTD, Tokyo, Japan) according to Su et al. 2020). The strong polar stationary phase of dicyanopropyl siloxane was selected for the capillary column. The column length was 100 m, the inner diameter was 0.25 mm and the film thickness was 0.2 μm. The GC column temperature program was set to an initial temperature of 100 °C for 13 min, then a linear rise to 180 °C at 10 °C/min, to 200 °C at 1 °C/min and to 230 °C at 4 °C/min. The final temperature was 230 °C for 10 min. The temperature of the injector was 270 °C, and the temperature of the detector was 280 °C. Nitrogen with purity of 99.99% was used as carrier gas, and the shunt ratio was 100:1. The split injection volume was 1 μL.
Analysis of lipid properties
Acid value
The Acid value (AV) was determined according to the method of Sun et al. (2018). Firstly, about 4 g of the sample was added in a 250 mL conical bottle, then 80 mL of the mixture of ether-isopropyl alcohol (1:1, v/v) and 3–4 drops of phenolphthalein indicator were added. Then the sample solution was titrated manually with 0.1 mol/L NaOH solution, and the volumes of standard titration solution consumed in this titration were recorded.
The AV was calculated according to the formula:
where, XAV was acid value (AV), mg/g; V1 was the volume of the standard solution consumed by the sample determination, mL; V0 was the volume of standard solution consumed for blank determination, mL; c was the molar concentration of the standard titrant, mol/L; 56.1 was the molar mass of potassium hydroxide, g/mol; m was the sample mass, g.
Peroxide value
The Peroxide value (POV) was determined according to the method of Ming et al. (2017) with a minor modification. 5 g sample and 30 mL trichloromethane-glacial acetic acid (1:1, v/v) mixture were added into 250 mL iodine bottle. 1.0 mL saturated potassium iodide solution was accurately added, plugged the bottle tightly, shook gently for 0.5 min, and placed in the dark for 3 min. Then 100 mL water was added, and immediately titrated the precipitated iodine with 0.002 mol/L sodium thiosulfate standard solution. When the solution turned yellow, 1 mL of starch indicator was added. Then the solution was titrated until the blue color disappeared.
The POV was calculated according to the formula:
where, X was peroxide value (POV), mmol/kg; V1 was standard solution volume of sodium thiosulfate consumed by the sample, mL; V0 was standard solution volume of sodium thiosulfate consumed in blank test, mL; c was the concentration of s\odium thiosulfate standard solution, mol/L; m was the sample mass, g; 1000 was the conversion factor.
Refractive coefficient
The Abbe refractometer (WAY-2WAJ, Shenguang Instrument Co., LTD, Shanghai, China) was used to determine Refractive coefficient (RI) according to the method of Ming et al. (2017). Special attention should be paid to the constant temperature during the measurement process. The hot water in the water bath was circulated through the refractometer to keep the refractometer prism at the constant temperature required for measurement. The refractive index, accurate to 0.0001, was recorded.
Saponification value
The saponification value was determined according to the method of Sun et al. (2018). 2 g sample and 25.0 mL potassium hydroxide-ethanol solution were added in 250 mL conical bottle, then 2 zeolites were added. The solution was kept heating and boiling slightly, and then condensed and refluxed for 60 min. Then 0.5–1 mL phenolphthalein indicator was added into the hot solution and titrated with 0.5 mol/L hydrochloric acid standard solution until the pink of the indicator just disappeared. At the same time, 25.0 mL of potassium hydroxide-ethanol solution was used for blank test without adding samples.
The saponification value was calculated according to the formula:
where, Is was saponification value (in KOH), mg/g; V1 was the volume of the standard solution consumed by the sample determination, mL; V0 was the volume of standard solution consumed for blank determination, mL; c was the actual concentration of hydrochloric acid standard solution, mol/L; m was the sample mass, g.
Statistical analysis
All experiments were performed in triplicate unless otherwise stated, and the obtained resulting values are presented as the mean ± standard deviation (SD). Significant differences between groups were determined using Duncan's test and one-way analysis of variance (ANOVA) at a level of p < 0.05 (SPSS 20 software, SPSS Inc., Chicago, USA).
Results and discussion
Comparison analysis of egg yolk lipids
Component analysis
Table 1 showed the difference in the contents of cholesterol and phospholipid in three types of egg yolk lipids. EYL-SFE had the highest cholesterol content, which was 59.89 mg/g, however, the phospholipids were not detected in EYL-SFE. Because the phospholipids were polar lipids, containing polar carboxyl groups, the polarity of phospholipids was too strong (Bennett et al. 1967). The phospholipids could not be dissolved in the inert gas CO2. Therefore, nearly all the polar phospholipids were retained in the residue egg yolk powder. The phospholipid content in EYL-SPE was low. Because the non-polar solvent propane has a poor extraction ability for phospholipids. During the extraction process of SPE, the mass transfer coefficient of each stage was low, so the final lipid extraction rate was also low (Yuntong et al. 2021). EYL-SE had the highest phospholipid content because ethanol was a polar solvent and could extract phospholipids well. The extraction methods could be selected according to the different purposes. If the purpose is to remove the cholesterol in egg yolk powder and keep phospholipid in egg yolk powder as much as possible, the SFE method would be the best choice. After the extraction process of SFE, the low-fat high phospholipids egg yolk powder could be obtained, which has great potential to be used in the field of nutritional and functional foods. On the contrary, if the purpose is to obtain egg yolk lipid with high phospholipid and low cholesterol, the SE method would be the best choice. The SPE method is somewhere in between, and by adjusting the subcritical parameters, it is possible to adjust the distribution of cholesterol and phospholipids in egg yolk lipids and low-fat egg yolk powder.
Fatty acid
As shown in Table 2, there were about 20 kinds of fatty acids contained in egg yolk lipids. Among them, oleic acid, palmitic acid, linoleic acid and stearic acid were the fatty acids with the highest content. Table 2 showed that the FA contents of the three kinds of egg yolk lipids were obviously different, which was mainly caused by the extraction method. The research of Song et al. (2016) showed that different extraction methods (Soxhlet extraction, enzyme assisted organic solvent and SFE) had significant effects on the relative content of fatty acids in the obtained sturgeon eggs. The PUMA content of EYL-SPE was significantly lower than EYL-SFE and EYL-SE, which may be due to the lower PUFA extraction ability of SPE and the used solvent propane. Because different solvents and different proportions of the solvents would affect the PUFA content (Tor-Chern and Yi-Hsu 2001). When the extraction temperature increased, the MUFA content of the extracted oil would also increase (Wei et al. 2018). The MUFA content of EYL-SE was significantly lower than that of EYL-SFE and EYL-SPE, because the extraction temperature of SE was higher than SFE and SPE. Therefore, SFE and SPE may be the better choices to get egg yolk lipids with high MUFA content, while SFE and SE may be the better choices to get egg yolk lipids with high content of PUFA.
Physical properties of egg yolk lipids obtained by different methods
The RI refers to one of the most important physical constants of organic compounds, which can be accurately and easily determined, and it is often used to identify the purity of oil. At certain wavelength and temperature, the RI depends on the properties of the oil and relates to the composition and structure of the oil. As shown in Table 3, the RI of EYL-SPE was significantly lower than that of EYL-SFE and EYL-SE. It showed that the purity of EYL-SPE was significantly lower than that of EYL-SFE and EYL-SE and the reasons for the results need to be further investigated in future studies.
Saponification value refers to the mass of potassium hydroxide required for complete saponification of 1 g oil (Konuskan et al. 2019). Under normal conditions, if the molecular weight of the combined fatty acids is smaller, or the number of free fatty acids is larger, the saponification value is higher. The AV is an indicator of the content of free fatty acids in fats and can be used as an indicator of the deterioration degree of fats (Konuskan et al. 2019). Both indicators are related to free fatty acids in lipids. Table 3 showed that the saponification value and the AV of EYL-SE were significantly higher than that of EYL-SFE and EYL-SPE, the higher temperature of SE was the main reason. The higher the temperature, the easier it was to produce free fatty acids during extraction. The studying of Dudi et al. (2021) showed that cooked oil had a higher saponification value compared to uncooked oil, which could also prove the effect of temperature on free fatty acids. Considering the extraction efficiency and the yield of egg yolk lipids, the optimal extraction temperature of EYL-SE was higher than that of EYL-SFE and EYL-SPE. Therefore, EYL-SE contained more free fatty acids, which leading to the highest saponification value and the highest AV.
The POV is an indicator of the degree of oxidation of fats and fatty acids. The quality and deterioration of the food, consisted of lipid, can be judged by detecting its POV. Table 3 showed that the POV of EYL-SE was slightly higher than that of EYL-SFE and EYL-SPE. Because of the high extraction temperature of SE, the EYL-SE was easier to oxidate during the extraction process, resulting in more oxidation products, thus obtaining the highest POV (Akinoso and Adeyanju 2012). The results of these physical properties showed that the egg yolk lipids obtained by SFE and SPE had higher quality than that obtained by SE.
Component of residue egg yolk powder prepared by different methods
The compositions of residue egg yolk powders obtained by different defatted methods were shown in Table 4. Compared with EYP, the protein content was increased, the lipid content and cholesterol content were all decreased significantly for all the three residue egg yolk powders. Among the three kinds of degreased egg yolk powder, EYP-SFE had the highest protein content. The increase of protein content was closely related to the extraction of lipid. EYP-SPE had the lowest fat content, followed by EYP-SFE and EYP-SE. This indicated that the ability to extract lipids at their normal optimal operating conditions was: SPE > SFE > SE. The phospholipid content in the residue egg yolk powder obtained by supercritical extraction was the highest among all the samples. The results were consistent with that shown in the composition of egg yolk lipids (shown in Table 2). On one hand, the SPE may be the best choice to get the residue egg yolk powder with the lowest fat content. However, the cholesterol content in EYP-SPE was higher than that in EYP-SFE and EYP-SE, which may limit the application of EYP-SPE. On the other hand, the EYP-SFE had the lowest cholesterol content and the highest phospholipid content, which showed the particularity of SFE extraction, extracting neutral lipids in egg yolk powder, such as glyceryl ester and cholesterol, and retaining polar lipid phospholipids in residue yolk powder. As the phospholipids have many beneficial physiological activities, egg yolk powder with low-fat, low-cholesterol and high-phospholipid content was more in line with people's demand for health products now and should have a great application prospect. Considering the quality of both egg yolk lipids and residue egg yolk powder, SFE is the optimal method for the extraction of egg yolk lipid. The effect of operating conditions on the effectiveness of supercritical fluid extraction needed to be further clarified in the subsequent research.
The effect of operating conditions on the effectiveness of supercritical fluid extraction
For clarifying the effect of operating conditions on the effectiveness of SFE, the impact of sample morphology, CO2 flow rate, extraction temperature, extraction pressure and other factors on SFE extraction of lipids were investigated.
The influence of sample morphology on the extraction effect
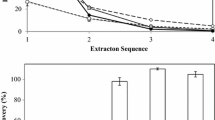
The problem of caking always existed in SFE of egg yolk powder, during the extraction process, the egg yolk powder would form a large volume of hard blocks. Therefore, the contact area of the egg yolk powder and the extraction solvent became smaller, resulting in low efficiency of lipid extraction (Sun et al. 2006). To solve this problem, the loose EYP was granulated into small yolk powder particles (EYP-G), whose length was about 45 mm and diameter was about 23 mm. Figures 1a and 2a showed that both lipid extraction rate and cholesterol extraction rate of EYP-G were significantly higher than EYP. The lipid extraction rate increased about 23% and the cholesterol extraction rate increased about 25%, when the extraction time was 2.5 h. Because EYP-G could still maintain the original small particle shape during the extraction process, and would not form large hard blocks, thus increasing the contact area with the solvent and improving the extraction efficiency.
The influence of CO2 flow rate on the extraction effect
Figures 1b and 2b showed that when the extraction time was 2.5 h and the CO2 flow rate was 28 L/h, the lipid extraction efficiency and cholesterol extraction efficiency were the highest, which were 71.86 and 75.55%, respectively. Because the greater the CO2 flow rate, the more the solvent could fully contact the sample, and increase the amount of lipids dissolved in the solvent, thus improving the extraction efficiency (Sánchez-Vicente et al. 2009). However, when the CO2 flow rate was too high, the time of CO2 staying in the extraction kettle was too short. And the material could not be fully contacted, so the extraction efficiency would decrease (Melreles et al. 2009).
The influence of extraction temperature on the extraction effect
Figures 1c and 2c showed that with the increase of the extraction temperature, lipid extraction rate and cholesterol extraction rate both increased. When the extraction time was 2.5 h and the extraction temperature was 55 °C, the extraction rates of lipid and cholesterol were the highest, which were 77.97 and 84.43%, respectively. Because the higher the temperature, the higher the movement rate and mass transfer coefficient of CO2 molecules, so the lipid extraction efficiency increased (Rebolleda et al. 2012). However, Yousefi et al. (2019) pointed out that lipid extraction efficiency decreases with the increase of temperature, because the density of CO2 molecules decreases with the increase of temperature, resulting in a decrease in extraction efficiency. Different performance, structure and parameter settings of supercritical extraction machines used all mattered. In addition, the temperature would also affect the physical and chemical indexes of the obtained yolk lipid (Sun et al. 2018). Therefore, the appropriate extraction temperature should be selected considering both the extraction rate and the properties of obtained egg yolk lipids.
The influence of extraction pressure on the extraction effect
Figures 1d and 2d showed that with the increase of extraction pressure, the extraction rate of lipid and cholesterol both increased. When the extraction time was 2.5 h and the extraction pressure was 45 MPa, the extraction rates of lipid and cholesterol were the highest, which were 80.63 and 88.45%, respectively. Because at the same temperature, increasing the pressure of the system could increase the density of the extractant, resulting at an strength increase of the extractant, a larger solubility of the solute, and an increase of extraction efficiency (Reverchon 1997). However, increasing the pressure of the system would also increase the requirements of the extraction equipment. Therefore, the appropriate extraction pressure should be selected according to the extraction system (Zhou and Wu 2003).
If the equipment conditions allowed, the higher flow rate, temperature and extraction pressure could be chosen to obtain a higher extraction effect. The egg yolk powder needed to be granulated before extraction to improve the extraction effect. However, this study only considered the effect of operating conditions on the extraction rate of lipids and cholesterol. The effect of operating conditions on the physicochemical properties of lipids should be further studied in the future.
Conclusions
The results of this study showed that the egg yolk lipids and residual egg yolk powder obtained by different methods had different properties and each extraction method has its own advantages. Compared with the subcritical fluid method and solvent method, the supercritical fluid method can obtain high-quality egg yolk lipids and low-fat, low-cholesterol and high-phospholipid yolk powder. The supercritical fluid method was a suitable extraction method to obtain both high-quality egg yolk lipid and egg yolk powder.
Data availability
The data for this article is transparent.
Code availability
Not applicable.
Abbreviations
- SE:
-
Solvent extraction
- SFE:
-
Supercritical fluid extraction
- SPE:
-
Subcritical propane extraction
- EYL-SFE:
-
Egg yolk lipid extracted by supercritical extraction
- EYP-SFE:
-
Low-fat egg yolk powder extracted by supercritical extraction
- EYL-SPE:
-
Egg yolk lipid extracted by subcritical extraction
- EYP-SPE:
-
Low-fat egg yolk powder extracted by subcritical extraction
- EYL-SE:
-
Egg yolk lipid extracted by solvent extraction
- EYP-SE:
-
Low-fat egg yolk powder extracted by solvent extraction
- EYP-G:
-
Granulated egg yolk powder
- FA:
-
Fatty acid
- RI:
-
Refractive coefficient
- AV:
-
Acid value
- POV:
-
Peroxide value
References
Akinoso R, Adeyanju JA (2012) Optimization of edible oil extraction from ofada rice bran using response surface methodology. Food Bioprocess Tech 5:1372–1378. https://doi.org/10.1007/s11947-010-0456-8
Bartlett GR (1959) Phosphorus assay in column chromatography. Jbc 234:466–468. https://doi.org/10.1016/s0021-9258(18)70226-3
Bennett PB, Papahadjopoulos D, Bangham AD (1967) The effect of raised pressure of inert gases on phospholipid membranes. Life Sci 6(25):27–33. https://doi.org/10.1016/0024-3205(67)90317-7
Chambers KF, Day PE, Aboufarrag HT, Kroon PA (2019) Polyphenol effects on cholesterol metabolism via bile acid biosynthesis, CYP7A1: a review. Nutrients. https://doi.org/10.3390/nu11112588
Dudi L, Jillellamudi NV, Chanda C, Kanuri G (2021) Assessment of quality parameters in edible vegetable oils. Int J Pharm Investig 11:296–299. https://doi.org/10.5530/ijpi.2021.3.52
Hong-xia H, Xiao-bin Z, Sheng-hua Z (2009) Cholesterol analysis in duck’s eggs by direct saponification-colorimetric and HPLC methods. Hubei Agricultural Sciences 48:455–457
Huang X, Ahn DU (2019) How can the value and use of egg yolk be increased? J Food Sci 84:205–212. https://doi.org/10.1111/1750-3841.14430
Konuskan DB, Arslan M, Oksuz A (2019) Physicochemical properties of cold pressed sunflower, peanut, rapeseed, mustard and olive oils grown in the Eastern Mediterranean region. Saudi J Biol Sci 26:340–344. https://doi.org/10.1016/j.sjbs.2018.04.005
Lynch JM, Barbano DM (1999) Kjeldahl nitrogen analysis as a reference method for protein determination in dairy products. J Aoac Int 82:1389–1398
Melreles MAA, Zahedi G, Hatami T (2009) Mathematical modeling of supercritical fluid extraction for obtaining extracts from vetiver root. J Supercrit Fluid 49:23–31. https://doi.org/10.1016/j.supflu.2008.12.009
Ming Y, Shaohua L, Mengyuan W, Man Z (2017) Physicochemical properties and fatty acid compositions of egg yolk oils from four avian specie. J Henan Univ Technol Nat Sc Edn 38:47–51
Ohba R, Ide S, Yoshida A, Nagata Z, Ueda S (1995) Effects of mixed enzyme preparation on the solubility of proteins for separating egg-yolk oil from a fresh yolk suspension. Biosci Biotech Bioch 59:949–951. https://doi.org/10.1271/bbb.59.949
Paraskevopoulou A, Kiosseoglou V, Alevisopoulos S, Kasapis S (1997) Small deformation properties of model salad dressings prepared with reduced cholesterol egg yolk. J Texture Stud 28:221–237. https://doi.org/10.1111/j.1745-4603.1997.tb00112.x
Perez-Palacios T, Ruiz J, Martin D, Muriel E, Antequera T (2008) Comparison of different methods for total lipid quantification in meat and meat products. Food Chem 110:1025–1029. https://doi.org/10.1016/j.foodchem.2008.03.026
Rebolleda S, Rubio N, Beltrán S, Sanz MT, González-Sanjosé ML (2012) Supercritical fluid extraction of corn germ oil: study of the influence of process parameters on the extraction yield and oil quality. J Supercrit Fluid 72:270–277. https://doi.org/10.1016/j.supflu.2012.10.001
Reverchon E (1997) Supercritical fluid extraction and fractionation of essential oils and related products. J Supercrit Fluid 10:1–37. https://doi.org/10.1016/s0896-8446(97)00014-4
Rui LI, Wei X (2011) Extraction methods and applications of egg yolk oil. Sci Technol Food Ind 32:444–446
Sánchez-Vicente Y, Cabañas A, Renuncio JAR, Pando C (2009) Supercritical fluid extraction of peach (<i>Prunus persica</i>) seed oil using carbon dioxide and ethanol. J Supercrit Fluid 49:167–173. https://doi.org/10.1016/j.supflu.2009.01.001
Song Y, Li Y, Qi L, Xin Q, Xia Y, Song L (2016) Extraction and fatty acid composition of lipid from sturgeon eggs. Food Sci 37:92–96
Su YJ, Ji MY, Li JH, Chang CH, Dong SJ, Deng YD, Yang YJ, Gu LP (2020) Subcritical fluid extraction treatment on egg yolk: product characterization. J Food Eng. https://doi.org/10.1016/j.jfoodeng.2019.109805
Sun R, Sivik B, Larsson K (2006) The fractional extraction of lipids and cholesterol from dried egg yolk using supercritical carbon dioxide. Lipid Fett 97:214–219. https://doi.org/10.1002/lipi.19950970605
Sun D, Cao C, Li B, Chen H, Li J, Cao P, Liu Y (2018) Antarctic krill lipid extracted by subcritical n-butane and comparison with supercritical CO2 and conventional solvent extraction. Lwt 94:1–7. https://doi.org/10.1016/j.lwt.2018.04.024
Tor-Chern C, Yi-Hsu J (2001) Polyunsaturated fatty acid concentrates from borage and linseed oil fatty acids. J Am Oil Chem Soc 78:485–488. https://doi.org/10.1007/s11746-001-0290-3
Wei X, Wei Y, Xue J, Zhang X, Shao X (2018) Effect of high-temperature heating on fatty acid composition and physicochemical properties of peony seed oil. Food Sci 39:15–20
Xiao N, Zhao Y, Yao Y, Wu N, Xu M, Du H, Tu Y (2020) Biological activities of egg yolk lipids: a review. J Agr Food Chem 68:1948–1957. https://doi.org/10.1021/acs.jafc.9b06616
Yenilmez E, Basaran E, Arslan R, Berkman MS, Guven UM, Baycu C, Yazan Y (2015) Chitosan gel formulations containing egg yolk oil and epidermal growth factor for dermal burn treatment. Pharmazie 70:67–73. https://doi.org/10.1691/ph.2015.4126
Yousefi M, Rahimi-Nasrabadi M, Pourmortazavi SM, Wysokowski M, Jesionowski T, Ehrlich H, Mirsadeghi S (2019) Supercritical fluid extraction of essential oils. Trac 118:182–193. https://doi.org/10.1016/j.trac.2019.05.038
Yuntong L, Mohammed O, Yajing Q, Jiachen S, Shuyi L, Jun S, Zhongwei C, Bin X (2021) Extraction of oat lipids and phospholipids using subcritical propane and dimethyl ether: experimental data and modeling. Eur J Lipid Sci Tech. https://doi.org/10.1002/ejlt.202000092
Zhang F, Li JH, Chang CH, Gu LP, Su YJ, Yang YJ (2022) Selective removal effect of subcritical fluid extraction on egg yolk lipids and characterization and enzymatic improvement of defatted egg yolk powder. Innov Food Sci Emerg. https://doi.org/10.1016/j.ifset.2022.103090
Zhou Q, Wu M (2003) Application of supercritical co2 extraction technology in oils and fats industry. China Oils Fats 28:17–20
Acknowledgements
The authors would like to thank for the financial support from the National Key Research and Development Program of China (2022YFD2101004) and the Collaborative innovation center of food safety and quality control in Jiangsu Province, China.
Funding
The financial support was from the National Key Research and Development Program of China (2022YFD2101004) and the Collaborative innovation center of food safety and quality control in Jiangsu Province, China.
Author information
Authors and Affiliations
Contributions
These authors above contributed equally to the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
No conflict of interest exists in the submission of this manuscript.
Ethics approval
Not applicable.
Consent to participate
We agree to participate.
Consent for publication
We agree to publish the individual’s data and images.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Su, Y., Cai, Y., Chang, C. et al. Comparative analysis on the properties of egg yolk lipids extracted by different extraction methods. J Food Sci Technol (2024). https://doi.org/10.1007/s13197-024-05981-7
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13197-024-05981-7