Abstract
The present work reports the compositional analysis of thirteen different packed fruit juices using high performance liquid chromatography (HPLC). Vitamin C, organic acids (citric and malic) and sugars (fructose, glucose and sucrose) were separated, analyzed and quantified using different reverse phase methods. A new rapid reverse phase HPLC method was developed for routine analysis of vitamin C in fruit juices. The precision results of the methods showed that the relative standard deviations of the repeatability and reproducibility were <0.05 and <0.1 respectively. Correlation coefficient of the calibration models developed was found to be higher than 0.99 in each case. It has been found that the content of Vitamin C was less variable amongst different varieties involved in the study. It is also observed that in comparison to fresh juices, the packed juices contain lesser amounts of vitamin C. Citric acid was found as the major organic acids present in packed juices while maximum portion of sugars was of sucrose. Comparison of the amount of vitamin C, organic acids and sugars in same fruit juice of different commercial brands is also reported.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fruit juice is considered to be one of the healthiest foods in human diet. Commercially prepared juices claim to retain their nutritional effects. Juices contain water, sugars, acids and minerals. Sugars are the most commonly found compounds in all beverages. They are present naturally and as additives to impart sweetness and texture. Sugars are also used as preservatives. Sucrose, glucose and fructose are main sugars found in fruit juices. Organic acids are another important component of fruit juices. Their presence and concentration determine tartness and flavor. Malic acid (MA) and citric acid (CA) are the major organic acids of fruit juices. The MA is predominant in apple, pear and stone fruits (Eisele and Drake 2004) while CA is most abundant in citrus and tropical fruits (Vera et al. 2006). The principal vitamin available in fruit juices is vitamin C, chemically known as ascorbic acid (AA). It is a powerful antioxidant naturally present in food and food products. It plays an important role in the prevention of infectious diseases.
Different kinds of fruits are processed into juices. Due to the loss of some nutrients in the processing of juice, cheaper juices, sugars, acids, colorants, water and other additives are often added into the pure juice. The taste and health benefits of the processed juices are influenced by variations in the main ingredients, particularly the composition of the sugars, organic acids and vitamins. Vitamin loss during processing is also of great concern for nutritionists, processors and consumers. Since vitamin C is an important indicator and used as a parameter for the determination of fruit quality as well as deterioration of juices due to storage, the quantitative determination of vitamin C becomes vital. It is a labile substance, which is easily degraded by atmospheric oxygen and enzymes. It also undergoes oxidation under the influence of light and heat. This makes it important to monitor its variation during manufacturing and storage.
Quantification of sugars can give an estimation of the amount of artificial sugar present in packed juice (Jahren et al. 2006). Organic acids including citric and malic acids can be used as “fingerprints” representing characteristics of individual fruit juice (Shui and Leong 2002; Soyer et al. 2003). This allows detection of the addition of a juice of different origin and fermentation in fruit juices. All these factors require the need for reliable techniques to detect the quality of juices.
Several methods have been reported for the determination of above-mentioned components in food items (Castellari et al. 2000; Zeppa et al. 2001). The most traditional method to detect the organic acids was developed by enzymatic procedures, where precision and time consumption were considered as the main defaults (Han and Cui 1996). The analysis of sugars, acids and vitamins by traditional methods is often complicated (Timpa and Burke 1986). In view of these disadvantages some techniques using high precision instruments came in use. In the field of food chemistry some commonly employed techniques are: fluorescence analysis (Chen et al. 2008), photospectrometric analysis (Fish et al. 2002), electroanalysis (Kapor et al. 2001), and capillary electrophoresis (Blatny et al. 1995). Gas chromatographic methods are also reported for food component analysis (Silva and Ferraz 2004; Lehotay and Hajslova 2002; Ikeda et al. 2006) However most of these methods have some limitations, viz in case of gas chromatography, in spite of good separation and sensitivity, tedious and time-consuming derivatization steps are required. Moreover, toxic derivatization agents are used in the analysis. High temperature required by some analyzing techniques also lead to sample decomposition.
High performance liquid chromatography (HPLC) is a powerful technique for the analysis of carbohydrates, organic acids, preservatives and various other components in food items (Muntean et al. 2003; Gattuso et al. 2007; Belajova and Suhaj 2004; Cunha et al. 2002; Nour et al. 2010; Bhattacherjee et al. 2010; Chen et al. 2011; Venkateswaran and Vijayalakshmi 2010). It is a faster and easier technique for the analysis of sugars and acids in food (Kafkas et al. 2006; Versari et al. 2008; Chinnici et al. 2005; Arora et al. 2011; George et al. 2010). It does not require derivatization and provides accuracy. The resolving power of HPLC is invaluable for accurately quantifying many food components. It has been used to analyze acids in beverages like tomato juice (Marconi et al. 2007) and wine (Fang et al. 2007; Sergio et al. 2007)
To the best of our knowledge, no article related to the compositional analysis of commercially available fruit juices is reported in literature. Packing of commercial juices also does not provide information about the individual sugar and acid content. Considering the prevalence of these juices and frequent use by all age groups, knowledge of their contents need attention. In the present work, the occurrence and quantity of vitamin C, organic acids and sugars in commercial fruit juices from different brands is investigated using reverse phase high performance liquid chromatography technique.
Experimental
Chemicals and reagents
All the chemicals used were of analytical grade. D-fructose, D-glucose and sucrose were procured from Qualigens fine chemicals, India. Citric and malic acid were procured from Merck Ltd., India and ascorbic acid was brought from Sisco Research Laboratories Pvt. Ltd., India. Acetonitrile, ammonium di-hydrogen phosphate buffer and potassium di-hydrogen orthophosphate buffer used were of HPLC grade procured from Qualigens fine chemicals, India. Water used for dilutions and making solutions was obtained through Milli Pore water purification system. Rankem 0.45 μm nylon membrane filters and 0.22 μm syringe filters were used for filtration of mobile phase and samples. Tropicana apple, pineapple, Appy classic apple, Frooti mango, Real guava, litchi, pineapple, mixed fruit, mango, mausami, orange, Onjus mixed fruit, apple juices were purchased from local stores.
Instrument
Analysis was carried out using Shimadzu HPLC (UFLC Prominence) equipped with a binary pump (LC-20 AD), a variable wavelength UV-VIS (SPD-20A) detector, a refractive index detector (RID-10A), a column oven (CTO-20A), a degasser and a manual injection valve fitted with 20 μl sample loop. The instrument was controlled by LC solution software.
Chromatographic conditions
Vitamin C (Ascorbic acid)
Vitamin C was analyzed using Phenomenex Luna C18 column with a mobile phase of acetonitrile and 10 mM potassium di-hydrogen ortho phosphate buffer mixed in a ratio of 40:60 (pH = 2.1) at a wavelength of 268 nm with UV detector. The flow of the mobile phase was maintained at a speed of 1 ml/min and the temperature of the column oven was set at 50°C.
Organic acids
The acids were isocratically separated using a Phenomenex Luna C18 column at a flow rate of 1 ml/min. The mobile phase used was 2% (w/v) ammonium di-hydrogen ortho phosphate (NH4H2PO4) buffer (pH 2.18). Column oven temperature was set at 32°C. UV-VIS detector at wavelength 214 nm was used for detection.
Sugars
For the analysis of sugars, binary gradient elution was used for separation with a flow rate of 1 ml/min. The mobile phase used consisted of acetonitrile and water (75:25,v/v). The separating column for sugars was Phenomenex Luna NH2. Column oven temperature was maintained at 40°C. A refractive Index detector was used for detection.
For all the components analyzed, the conditions like column equilibration and mobile phase flow rate were standardized according to the method used.
Standard solutions
Standard solutions of vitamin C, citric acid, malic acid, fructose, glucose and sucrose were prepared in volumetric flask by dissolving the required amount of reagent (weighed by Mettlar Analytical Balance) in 100 ml of water. Flanking ranges of concentration commonly found in commercial fruit juices, were chosen. The standard ranges of concentration were 0.01%–0.1% for vitamin C, 0.05%–1.6% for citric acid and malic acid, 1%–10% for fructose and 0.5%–10% for glucose and sucrose. Twelve standard solutions of each component were prepared and 20 μl of each standard solution was subjected to chromatographic run and their chromatograms were recorded.
Analysis of commercial juices
Juice samples were analyzed under the same conditions as of standards. Chromatographic peaks were identified by comparing retention times of separated components in chromatograms of juice samples against known standards and also by spiking samples with pure compounds. Data acquisition and all computation were done with Shimadzu LC solution software. Calculation of concentration (expressed in %) of vitamin C, organic acids and individual sugars of various juices was carried out by using external standard method. Quantitation values were obtained by applying their peak areas to the calibration curve.
Three different samples of same fruit juice were analyzed. Good repeatability was obtained in terms of retention times of different constituents under study. For quantification the values were averaged for the three values obtained for each juice.
Results and discussion
Method development
Different methods are available for the analysis of vitamins by reversed phase HPLC (Anyakora et al. 2008; Almagro et al. 2002). Many of these methods utilize silica bonded columns, a mobile phase consisting of a phosphate buffer with acidic pH and some percentage of acetonitrile or methanol (Maia et al. 2006). These conditions were used as the base for method development for vitamin C. As the mobile phase was highly aqueous, columns that were stable under those conditions were investigated. Phenomenex Luna 5 μ (250 × 4.6 mm) C8, C18 and NH2 column were tried. The NH2 column did not provide sufficient retention of vitamin C, while the peak with C8 column was not sharp and clear. C18 column provided acceptable retention of vitamin C at 12 min with 100% phosphate buffer as mobile phase at a flow rate 0.5 ml/min. However the peak, while symmetric, was broad. After several trials, the mobile phase consisted of a mixture of acetonitrile and phosphate buffer (40:60) with C18 column at a wavelength of 268 nm with UV detector gave excellent peak shape and retention time was also reduced significantly. The method using these conditions was then optimized: the flow rate was increased to reduce run time and column oven temperature was also increased. The pH of the mobile phase was reduced to 2.1. The final conditions for the analysis of vitamin C are presented in Table 1. The chromatographic conditions achieved satisfactory resolution, reasonable retention and symmetric peak shape for the separation and determination of vitamin C.
Precision of the method developed for vitamin C is assessed by performing replicate analysis of a standard vitamin C solution. For this purpose 0.02% solution is injected to the HPLC five times at intra and inter-day intervals. The results showing the slope, correlation coefficient, mean response factor, percent relative standard deviation of response factor of calibration made at intra and interday intervals are tabulated in Table 2.
Calibration and validation
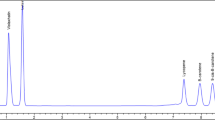
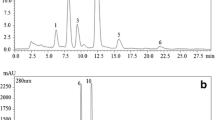
Figure 1a shows the chromatogram of standard vitamin C (ascorbic acid) under the chromatographic conditions described in previous section. Vitamin C elutes at 3.4 min. Calibration graphs for standard vitamin C is obtained by plotting peak area against concentration. A straight-line relationship between concentration and intensity is observed as evident from Fig. 1D. Some outliers (points lying outside of the curve) are detected, which are not discarded. These outliers can be attributed to the experimental error due to inaccuracy in weight measurement or standard solution preparation. Figure 1b and c depicts the chromatogram of standard organic acids: citric and malic acid respectively. Citric acid elutes at 7.5 min and malic acid elutes at 4.0 min. Figure 2a, b and c shows the chromatograms of standard sugars: fructose, glucose and sucrose respectively. Fructose eluted at 6.3 min while the retention times of glucose and sucrose were 7.8 and 9.4 min respectively. Calibration of organic acids and sugars is achieved in the same manner as of vitamin C. Calibration graphs for citric acid, malic acid, fructose, glucose and sucrose are shown in Fig. 1b and c and Fig. 2d, e and f respectively.
Table 3 shows the results of the analysis of linearity of the calibration curves and their regression equations. The correlation coefficients obtained are found to be higher than 0.99, showing a good relationship between peak area and concentrations in the studied ranges. To verify the linearity, the response factor (RF) is computed based on the linear regression of a plot of peak area vs. concentration. Validation of all the calibration curves is achieved by subjecting standard samples of vitamin C, organic acids and sugars. The results obtained after the concentration determination using calibration curve are in good agreement with the standard values.
The repeatability of all the methods used is established by injecting the standard vitamin C, organic acids and sugar samples five times in a day. The reproducibility is established by analyzing each standard sample on three different days for over 2 weeks. The standard deviation for repeatability is in the range of 0.01 to 0.04 while that of reproducibility is of 0.06 to 0.1.
Traceability and recovery
To check the traceability of the HPLC methods developed for sugars, organic acids and ascorbic acids, an analyte was quantified in the juice sample. The sample was then spiked with five increasing concentrations of the same analyte. A calibration model was then constructed with six calibration points including the unspiked juice sample. A linear calibration curve accounted for the traceability of the method used in the study. Three injections of each sample were given over a period of 3 days. Good repeatability and reproducibility in terms of retention time, peak area, peak height and peak asymmetry was found in the pure real samples and the samples spiked with analytes.
Recovery studies were conducted by calculating the concentration of an analyte in spiked samples of real juices by developed calibration model. The obtained values were then compared with the expected values. Percent recovery obtained for each spiked sample was found to be between 97–100%.
Determination of different components in commercial fruit juices
Vitamin C (Ascorbic acid)
Chromatograms of ten different varieties of juices are recorded for vitamin C determination. Figure 3a shows the chromatogram of Real orange juice showing a significant peak of vitamin C at 3.4 min. Less variation is observed in the content of vitamin C when same fruit juice from different brands is analyzed (Table 4). Although all juices are found to contain not more than 0.03% of vitamin C, Real litchi has maximum amount, i.e. 0.025% amongst the varieties under study, while Tropicana apple contains the minimum amount (0.002%). The lesser amount of vitamin C found in packed juices as compared to fresh juices (Kabasakalis et al. 2000) can be attributed to the processing and storage conditions and its oxidation.
Organic acids
Chromatograms of eleven different juice samples are recorded and efficient separation of organic acids found in packed fruit juices are achieved using the reversed-phase C18 column. Figure 3b shows the chromatogram depicting organic acids separation in Real orange juice. The peak at 4.0 min is identified as malic acid while the one at 7.5 min is ascribed to citric acid. Various concentrations of organic acids determined by external standard method in juice samples are given in Table 4. It has been observed that distinct brands of same fruit juice are composed of different concentrations of acids. The malic acid content of apple juice from Tropicana is 0.30%. Apple juice from Appy Classic contains 0.20% of malic acid, while its content in Onjus apple juice is 0.07%. The amount of citric acid in the above labels is found to be 0.004%, 0.24% and 0.50% respectively. Maximum concentration of malic acid is found in Real mausami (0.61%), while Onjus mixed fruit juice is found to contain maximum concentration of citric acid (0.61%).
Sugars
Ten different juice samples are subjected to chromatographic analysis and a good resolution is observed under the method used for sugar separation. The reverse phase method employed in this study succeeded in eluting all sugars in a single chromatographic run. All sugars are separated and detected within 10 min under the described conditions after column equilibration. The order of sugar elution and peak identity in juice samples is confirmed when peak retention times are found identical to those of pure standards of each sugar. Figure 3c depicts the HPLC chromatogram of Real orange juice with major sugars separation. The peak at 6.4 min is identified as of fructose and peak at 7.7 min is of glucose, while the peak at 9.5 min is assigned to sucrose. It is quiet evident from the chromatographic study that major sugars in all packed fruit juices are fructose, glucose and sucrose as their peaks are significantly observed.
Table 4 reports different concentrations of sugars obtained after applying external standard quantification method. It is evident from the results that different commercial brands of even same fruit juice show characteristic variation in their sugar content. Tropicana apple juice contains 5.89% of fructose, 3.03% of glucose and 0.94% of sucrose, while the apple juice from Appy classic is composed of 6.13% of fructose, 5.16% of glucose and 1.95% of sucrose. Apple juice from another brand Onjus contains 4.23% of fructose, 3.62% of glucose and 1.37% of sucrose. Amongst the commercial labels under study Appy classic apple juice is found to contain maximum amount of fructose (6.13%) and glucose (5.16%), while maximum amount of sucrose (6.87%) is found in Real orange juice.
Conclusions
Vitamin C, the major vitamin, malic and citric acid, the main organic acids and fructose, glucose and sucrose, the main sugar markers of fruit juices can be efficiently analyzed, compared and quantified by presented RP-HPLC methods. The results obtained in terms of retention times of sugars and acids agree well with earlier reported work (Karadeniz 2003; Sanchez-mata Maria et al. 2002). A novel and rapid reverse phase method is developed for the analysis of vitamin C, which significantly reduced its retention time. Difference is observed in the content of organic acids and sugars and vitamin C of same fruit juice belonging to different brands. The present procedures can be easily applied for routine analysis in the fruit juice industry for determining composition of packed fruit juices. Moreover, information on wholesomeness of juices and exogenous addition can be provided. The information provided in this study can be useful for the knowledge of individual components of commercially available packed fruit juices.
References
Almagro I, San Andres MP, Vera S (2002) Determination of water soluble vitamins in pharmaceutical preparations by reversed-phase high performance liquid chromatography with a mobile phase containing sodium dodecyl sulphate and n-propanol. Chromatographia 55:185–188
Anyakora C, Afolami I, Ehianeta T, Onwumere F (2008) HPLC analysis of nicotinamide, pyridoxine, riboflavin and thiamin in some selected food products in Nigeria. Afr J Pharm Pharmaco 2(2):29–36
Arora S, Shendurse AM, Sharma V, Wadhwa BK, Singh AK (2011) Assessment of stability of binary sweetener blend (aspartame x acesulfame-K) during storage in whey lemon beverage. J Food Sci Technol. doi:10.1007/s13197-011-0386-0
Belajova E, Suhaj M (2004) Determination of phenolic constituents in citrus juices: Method of high performance liquid chromatography. Food Chem 86:339–343
Bhattacherjee AK, Tandon DK, Dikshit A, Kumar S (2010) Effect of pasteurization temperature on quality of aonla juice during storage. J Food Sci Technol 48(3):269–273
Blatny P, Kvasnicka F, Kenndler E (1995) Determination of phytic acid in cereal grains, legumes, and feeds by capillary isotachophoresis. J Agric Food Chem 43:129–133
Castellari M, Versari A, Spinabelli U, Galassi S, Amati A (2000) An improved HPLC method for the analysis of organic acids, carbohydrates, and alcohols in grape musts and wines. J Liq Chromatogr Relat Technol 23:2047–2056
Chen H, Zhang Y, Lu X, Qu Z (2011) Comparative studies on the physicochemical and antioxidant properties of different tea extracts. J Food Sci Technol. doi:10.1007/s13197-011-0291-6
Chen Y, Chen J, Luo Z, Ma K, Chen X (2008) Synchronous fluorescence analysis of phytate in food. Michrochim Acta 164:35–40
Chinnici F, Spinabelli U, Riponi C, Amati A (2005) Optimization of the determination of organic acids and sugars in fruit juices by ion-exclusion liquid chromatography. J Food Compos Anal 18:121–130
Cunha SC, Fernandes JO, Ferriera IM (2002) HPLC/UV determination of organic acids in fruit juices and nectars. Eur Food Res Technol 214:67–71
Eisele TA, Drake SR (2004) The partial compositional characteristics of apple juice from 175 apple varieties. J Food Comp Anal 18:213–221
Fang F, Li JM, Pan QH, Huang WD (2007) Determination of red wine flavonoids by HPLC and effect of aging. Food Chem 101(1):428–433
Fish WW, Perkins-Veazie P, Collins JK (2002) A quantitative assay for lycopene that utilizes reduced volumes of organic solvents. J Food Compos Anal 15(3):309–317
Gattuso G, Barreca D, Caristi C, Gargiulli C, Leuzzi U (2007) Distribution of flavonoids and furocoumarins in juices from cultivars of citrus bergamia risso. J Agric Food Chem 55(4):9921–9927
George V, Arora S, Wadhwa BK, Singh AK (2010) Analysis of multiple sweeteners and their degradation products in lassi by HPLC and HPTLC plates. J Food Sci Technol 47(4):408–413
Han YS, Cui YL (1996) Determination of d-isocitrate in orange and its juice by enzymatic method. Acta Nutrimenta Sinica 18(1):120–125
Ikeda G, Tomizawa A, Imayoshi Y, Iwabuchi H, Hinata T, Sagara Y (2006) Flavor design of sesame-flavored dressing using gas chromatography/olfactometry and food kansei model. Food Sci Technol Res 12:261–269
Jahren AH, Saudek C, Yeung EH, Linda Kao WH, Kraft RA, Caballero B (2006) An isotopic method for quantifying sweeteners derived from corn and sugar cane. Am J Clin Nutr 84:1380–1384
Kabasakalis V, Moshatou E, Siopidou D (2000) Ascorbic acid content of commercial fruit juices and its rate of loss upon storage. Food Chem 70:325–328
Kafkas E, Kosar M, Turemis N, Baser KHC (2006) Analysis of sugars, organic acids and vitamin C contents of blackberry genotypes from Turkey. Food Chem 97(4):732–736
Kapor MA, Yamanaka H, Carneiro PA, Zanoni MVB (2001) Electroanalysis of food dyes: determination of indigo-carmine and tartrazine. Eclet Quím 26:53–68
Karadeniz F (2003) Main organic acid distribution of authentic citrus juices in Turkey. Turk J Agric For 28:267–271
Lehotay SJ, Hajslova J (2002) Application of gas chromatography in food analysis. Trends Anal Chem 21(9):686–697
Maia AM, Baby AR, Yasaka WJ, Suenaga E, Kaneko TM, Velasco VMR (2006) Validation of HPLC stability-indicating method for vitamin C in semisolid pharmaceutical/cosmetic preparations with glutathione and sodium metabisulfite, as antioxidants. Talanta 71:639–643
Marconi O, Floridi S, Montanari L (2007) Organic acids profile in tomato juice by HPLC with UV detection. J Food Qual 30:253–266
Muntean E, Bele C, Socaciu C (2003) HPLC analysis of carotenoids from fruits of Cucurbita pepo L. var. melopepo Alef. Acta Agro Hung 51:455–459
Nour V, Trandafir I, Ionica ME (2010) HPLC organic acid analysis in different citrus juices under reversed phase conditions. Not Bot Hort Agrobot Cluj 38(1):44–48
Sanchez-mata Maria DE, Camara-hurtado M, Diez-marques C (2002) Identification and quantification of soluble sugars in green beans by HPLC. Eur Food Res Technol 214:254–258
Sergio GA, Esteban GR, Isidro HG (2007) HPLC analysis of diverse grape and wine phenolics using direct injection and multidetection by DAD and fluorescence. J Food Compos Anal 20:618–629
Shui G, Leong LA (2002) Separation and determination of organic acids and phenolic compounds in fruit juices and drinks by high-performance liquid chromatography. J Chromatogr A 977:89–96
Silva FO, Ferraz V (2004) Microwave-assisted preparation of sugars and organic acids for simultaneous determination in citrus fruits by gas chromatography. Food Chem 88(4):609–612
Soyer Y, Koca N, Karadeniz F (2003) Organic acid profile of Turkish white grapes and grape juices. J Food Compos Anal 16:629–636
Timpa JD, Burke JJ (1986) Monitoring organic acids and carbohydrates in cotton leaves by high performance liquid chromatography. J Agric Food Chem 34(5):910–913
Venkateswaran V, Vijayalakshmi G (2010) Finger millet (Eleusine coracana)—an economically viable source for antihypercholesterolemic metabolites production by Monascus purpureus. J Food Sci Technol 47(4):426–431
Vera E, Sandeaux J, Persin F, Pourcelly G, Dornier M, Rouales J (2006) Deacidification of clarified tropical fruit juices by electrodialysis. Part I. Influence of operating conditions on the process performances. J Food Eng 78(4):1427–1438
Versari A, Parpinello GP, Mattioli AU, Galassi S (2008) Characterization of Italian commercial apricot juices by high-performance liquid chromatography analysis and multivariate analysis. Food Chem 108(1):334–340
Zeppa G, Conterno L, Gerbi V (2001) Determination of organic acids, sugars, diacetyl, and acetoin in cheese by high-performance liquid chromatography. J Agric Food Chem 49:2722–2726
Acknowledgement
Authors are thankful to Department of Biotechnology, India for providing the financial support (Grant No. BT/PR7221/MED/14/970/2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tyagi, G., Jangir, D.K., Singh, P. et al. Rapid determination of main constituents of packed juices by reverse phase-high performance liquid chromatography: an insight in to commercial fruit drinks. J Food Sci Technol 51, 476–484 (2014). https://doi.org/10.1007/s13197-011-0502-1
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-011-0502-1