Abstract
The objective of this work was to validate a method for direct determination in grape juice and wine of 1-kestose, nystose and raffinose oligosaccharides by reversed-phase high-performance liquid chromatography with refractive index detection using a new type of RP-C18 column (150 × 4.6 mm, 4 µm) with polar end-capping. The validated methodology was also used to characterize grape juice and fine wine products from Northeastern Brazil; and presented suitable linearity, precision, recovery, limits of detection and quantification. The method presented good specificity, revealing that sugars, organic acids, and ethanol (the main interferences in refraction detection) did not influence the quantification of the studied oligosaccharides. The main oligosaccharide found was 1-kestose (approximately 50% of the samples), followed by raffinose (20% of the samples). The results obtained in this are an indication that grape juices and wines have the potential to be functional beverages in relation to the presence of prebiotics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Consumption of prebiotics has gained popularity among consumers, since in addition to being recognized for their functional properties, epidemiological and experimental studies have demonstrated positive effects on intestinal health after consumption. These benefits are related to modulating the intestinal microbiota, which contribute to preventing and treating chronic diseases through attenuating inflammatory processes, improving the immune system, and regulating lipid and glucose metabolism, among other benefits (Florowska et al. 2016; Aquino et al. 2017).
The most commonly utilized prebiotics in human food include fructans, inulin, fructooligosaccharides (FOS), galactooligosaccharides (GOS), and xylooligosaccharides (XOS) (Ambalam et al. 2016). Due to the importance of prebiotics in modern human food, they are added to beverages, meat products, dairy and bakery products (Al-Sheraji et al. 2013), and many commercial infant formulas are enriched with FOS and GOS, such as kestose, nystose, and galactosyl lactose (Sabater et al. 2016).
Recent studies have characterized FOS and GOS in various fruits and vegetables, including grapes (Malinovska et al. 2014; Ghfar et al. 2015; Maeda et al. 2017). In a study by Blanch et al. (2011), FOS 1-kestose, neokestose, nystose, nystose and kestopentaose were quantified in the “Cardinal” table grape variety. The oligosaccharides were present in small quantities, especially 1-kestose, which was the main FOS found.
According to Rodríguez-Costa et al. (2018), extracts rich in phenolic compounds obtained from wine grape residues present prebiotic properties since they increase in vitro growth of the Lactobacillus and Bifidobacterium population, which are beneficial intestinal bacteria whose in vivo growth is stimulated by ingestion of these substances. However, studies that quantify the presence of prebiotic oligosaccharides such as 1-kestose, nystose and raffinose in these beverages are scarce.
Studies have shown that oligosaccharide content in wines vary according to factors such as grape variety, region of origin, maturation stage, vinification technique, types of yeast and pectinases used. In these studies, oligosaccharides were isolated and fractionated, and their profiles and concentrations were then estimated by the presence of residual compounds such as rhamnose, arabinose, galactose, mannose, glucose, xylose, fucose, galacturonic acid, and xylitol (Doco et al. 2014; Apolinar-Valiente et al. 2015; Lapuente et al. 2016; García et al. 2017).
The Vale do Submédio São Francisco (VSMSF), located in Northeastern Brazil, is a region that has been distinguished for producing grape juice with Brazilian varieties (V. labrusca and hybrids) and wines that have high antioxidant activity, associated with phenolic compounds (Lima et al. 2014; Padilha et al. 2017a; Dutra et al. 2018a). In addition to phenolic compounds, the products of this region have recently been characterized by the presence of simple sugars, organic acids, minerals and aromatic compounds (Padilha et al. 2017b; Coelho et al. 2018; Dutra et al. 2018b).
Determination methods of FOS by HPLC typically utilize amino-bonded columns in HILIC mode, where the main isocratic solvent used in separating the compounds is acetonitrile in concentrations > 70% (Doco et al. 2014; Malinovska et al. 2014; Ghfar et al. 2015; Marrubini et al. 2018). Ion exchange columns have also been used as an alternative to using amino-bonded columns and consuming organic solvents (Muir et al. 2009; Ball et al. 2013; Benkeblia 2013).
Common RP-C18 columns do not present good stability for use with water as the mobile phase as it may exhibit phase collapse, and thus show reduced retention or selectivity changes over time, leading to irreproducible results (Long et al. 2009). This makes it difficult to use in separating highly polar substances such as simple sugars and oligosaccharides. A number of reversed phase modifications directly dedicated to the operation in mobile phase rich in water are available on the market, such as alkyl reversed-phase bonded phase designed to retain hydrophilic and other compounds when using highly aqueous mobile phases, including 100% water (Long et al. 2009). RP-C18 columns containing silanol groups in a stationary phase bonded by trimethylsilyl groups (endcapped) present good stability, and solvents like water have been an alternative for applications involving determinations of organic acids in wines (Snow et al. 2015).
In a study by Barthomeuf et al. (1997), it was possible to separate and identify FOS 1-kestose, 1-nystose and 1-fructo-furanosyl nystose in a mixture of sugars containing glucose, fructose, and saccharose using a RP-C18 column (250 × 3.9 mm, 5 μm) with bonding of alkyl groups using ultrapure water as the mobile phase.
For the absence of chromophores in their molecules, oligosaccharides like FOS and GOS are normally detected by refraction (RID) (Zhu et al. 2012; Malinovska et al. 2014), pulsed amperometric detection (PAD) (Blanch et al. 2011), mass detection (MS) (Doco et al. 2014; Ghfar et al. 2015), and evaporative light scattering detection (ELSD) (Muir et al. 2009; Maeda et al. 2017).
In this context, the objective of this work was to develop and validate a method for direct determination of prebiotic oligosaccharides (1-kestose, nystose and raffinose by RP-HPLC/RID) in grape juice and wine using a new RP-C18 polar endcapping column. Additionally, the methodology was used to characterize products from Northeastern Brazil.
Materials and methods
Chemicals and external standards
External standards of 1-kestose, nystose and raffinose oligosaccharides were obtained from Sigma-Aldrich (St. Louis, MO, USA). Ultrapure water was obtained in a Marte Científica purification system (São Paulo, SP, Brazil). Acetonitrile and ethanol were obtained from Merck (Darmstadt, Germany). Rhamnose and succinic acid were obtained from Sigma-Aldrich (St. Louis, MO, USA). Glucose, fructose, maltose, saccharose, tartaric acid, citric acid, lactic acid, acetic acid and malic acid were obtained from Merck (Darmstadt, Germany).
Determination of oligosaccharides using RP-HPLC/RID
The HPLC system consisted of an Agilent 1260 coupled to a refractive index detector (RID) (G1362A model) (Agilent Technologies, Santa Clara, CA, USA). Grape juice and wine samples which had been previously diluted in ultrapure water were then filtered through a 0.45 μm PTFE membrane (Millex Millipore, Barueri, SP, Brazil), and injected at volumes of 20 μL. Separation of the compounds was done on a Synergi™ Hydro-RP C18 column with polar endcapping (150 × 4.6 mm, 4 μm) (Phenomenex, Torrance, CA, USA) at a temperature of 35 °C.
The solvent flow used for separation of the compounds was 0.7 mL min−1 with a run time of 21 min. The gradient used was 0–8 min: 100% A; 8–9 min: 80% B; 9–12 min: 80% B; 12–13 min: 100% A; 13–20 min: 100% A (flowrate of 1.5 mL min−1); where solvent A was ultrapure water and solvent B was acetonitrile. The temperature in the RID cell was kept at 35 °C. Data were processed using OpenLAB CDS ChemStation Edition software (Agilent Technologies).
Method validation protocol
For the validation study, the following parameters were evaluated in order to ensure the method quality: linearity, limit of detection (LOD), limit of quantification (LOQ), recovery, precision and selectivity/specificity. The validation protocol used was in accordance with Brazilian legislation (Brasil 2011), and also followed the guidelines contained in the European guide for validation of analytical methods (Eurachem 2014).
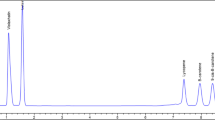
Linearity: The linearity range was established by injecting five differing concentrations (0.25, 0.5, 1.0, 2.5 and 5.0 g L−1) obtained by dilutions of a standard mixture of compounds in ultrapure water. The results were plotted for evaluating the linear relationship between the peak areas of each respective compound and their concentrations. A calibration curve for each oligosaccharide was established based on the data recorded during triplicate analysis. The chromatograms obtained by injecting external standards compounds can be seen in Fig. 1.
Obtaining the limits of detection and instrumental quantification
In order to obtain the method’s limits of detection and quantification, three external standards for the studied compounds were prepared in concentrations smaller than the minimum limit of the method’s calibration curve and injected in triplicate (n = 9). The residual standard deviation (RSD) was calculated by comparing the values obtained in the analysis of the current values. LOD and LOQ were established at 3 and 10 times the RSD, respectively, added with the intercept of the curve (Hubaux and Vos 1970).
Precision and Recovery
Tests were performed to evaluate the accuracy of the method, addition and recovery by comparing the concentrations of the analytes found in six wine/grape juice samples spiked with known amounts of each oligosaccharide. The precision was evaluated using the coefficient of variation (CV%) obtained from an analysis of the six fortified samples, in triplicate. Recovery was calculated comparing the values obtained for each “spiked” compound in relation to the initial value contained in the sample.
Selectivity/specificity
The study of method selectivity was performed by evaluating the interference of the main refractive substances present in the grape juice and wine, such as sugars, organic acids and ethanol (Coelho et al. 2018). The ability to separate the studied oligosaccharides from the main sugars contained in juices and wines was evaluated by injection of a mixed standard containing glucose, fructose, saccharose and the substances of interest: raffinose, 1-kestose and nystose, all at the concentration of 2.5 g L−1 in ultrapure water. The method selectivity against a complex matrix of interferents was evaluated by comparing the retention times of the studied FOS with the peak retention times obtained in a chromatogram of a synthetic wine containing: glucose (20 g L−1), fructose (5 g L−1), maltose (5 g L−1), tartaric acid (5 g L−1), malic acid (5 g L−1), succinic acid (5 g L−1), lactic acid (5 g L−1), acetic acid (2 g L−1) and ethanol (100 mL L−1) in ultrapure water.
Characteristics of the analyzed samples with the validated methodology
Table 1 presents descriptions of the studied samples and their basic analytical characteristics (mean ± standard deviation). Basic analysis of the quality of the samples were carried out using classic techniques described by L’Organization Internationale de la Vigne et du Vin (L’OIV) (2011).
Fifteen Brazilian products were analyzed, all originating from VSMSF, Petrolina, PE (09°21′S, 40°40′W), and Lagoa Grande, PE (8°59′S, 40°16′W). The monovarietal juices were produced with one Vitis labrusca L., “Isabel Precoce”, and three hybrid grapes varieties, “BRS Cora” (Muscat Belly A × H. 65.9.14), “BRS Violeta” (BRS Rúbea × IAC 1398-21) and “BRS Magna” (BRS Rúbea × IAC 1398-21 (Traviú), using 20 kg of grapes by the hot press method (Morris and Striegler 2005). The commercial and monovarietal grape juices were produced in May 2017. The commercial wines were from classic international varieties of grape (V. vinifera L.), “Tempranillo”, “Syrah”, “Sauvignon Blanc” and “Barbera”, all of the 2016 vintage. Three bottles from different lots were collected for the commercial juices and wines, corresponding to three replications.
Statistical analysis
Means obtained from the analysis of the grape juices and wines were submitted to analysis of variance (one-way ANOVA) and compared by applying the Tukey test (p < 0.05). Statistical analysis was performed using SPSS version 17.0 for Windows (SPSS, Chicago, IL, USA).
Results and discussion
Validation of the method
Different chromatographic conditions were tested for separating compounds and the best condition was used for the method validation and to characterize the studied samples. Acidification levels of the mobile phase (0, 4, 10 and 50 mmol of H2SO4), different solvent flows (0.4–1.0 mL min−1), different temperatures in the column furnace (35, 50 and 70 °C), and different solvents (water, 30% v/v acetonitrile and 70% v/v acetonitrile) were evaluated in the method development. The best separation conditions were using water, where acidification and heating did not exert a positive influence on the separation of the compounds. Separation resolution was lost at solvent flows above 0.7 mL min−1. Finally, we reduced the sample injection volume from 20 to 10 µL in order to accomplish separation between raffinose and kestose close to 1.5 resolution. Acetonitrile was only used in the gradient method for column reconditioning, because a decrease in the retention time of the compounds after successive injections in isocratic pumping with ultrapure water as mobile phase was observed in the preliminary tests, even with the manufacturer’s declaration that the column is stable with water.
Typical chromatograms of the studied grape and wine juice samples are shown in Fig. 2.
Linearity and limits of detection (LOD) and quantification (LOQ)
The validation parameter results for determining the prebiotics can be seen in Table 2. In the linearity evaluation, the values obtained for the “R” correlation coefficient ranged from 0.99988 to 0.99998. According to Brasil (2011), the R values for linearity in analytical methodologies should be > 0.99.
The limits of detection (LOD) ranged from 0.074 to 0.090 g L−1 for nystose and 1-kestose, respectively. The limits of quantification (LOQ) ranged from 0.179 to 0.214 g L−1 for nystose and 1-kestose, respectively. The LOD and LOQ for raffinose ware 0.082 and 0.192 g L−1, respectively.
In another methodology validated for quantification of oligosaccharides studied in fruits and vegetables by HPLC-ELSD, Muir et al. (2009) obtained an LOD of 0.05 and 0.10 g L−1 for raffinose and 1-kestose, respectively. These values are in accordance with those obtained in the present detection method by refraction.
In a study by Ghfar et al. (2015), detection limits ranging from 0.38 to 0.69 mg L−1 (LOD), and 1.29–2.38 mg L−1 (LOQ) were determined for 1-kestose and nystose by RP-HPLC/MS in dates. However, it is well known that mass detectors have much higher detection sensitivity compared to refraction detectors, such as those used in the present study.
Although the present determination method of oligosaccharides in RP-HPLC/RID presents higher LOD and LOQ values than other methods with more sensitive detectors, in the refraction it is possible to directly quantify sugars without the need for complex sample preparation to eliminate interferents such as phenolic compounds (Doco et al. 2014).
Based on the linearity, LOD and LOQ values obtained in the present study for the direct detection of the oligosaccharides by refraction, we consider the methodology suitable for the proposed purpose, being a simple method, which can handle large sample preparations.
Precision and recovery
The method’s precision was evaluated by coefficient of variation (CV%), where the obtained values varied from 8.3 to 12.2 for grape juices, and from 2.6 to 9.4 for wine (Table 2). For the concentration range used in the calibration curve for oligosaccharides in the present method (0.25–5 g L−1), Brazilian legislation establishes that CV% values should be less than 15% (Brasil 2011), which demonstrates that the presented results were adequate in relation to this parameter.
The European guide for validation of analytical methods (Eurachem 2014), although presenting guidelines on how a method should be validated and the methodology for obtaining values for the chosen parameters, does not mention acceptable ranges for the parameters of a method.
In the evaluation using the recovery method (RC%) the obtained values ranged from 90.3 and 108.8 (1-kestose and nystose, respectively) for the wine samples. For the wine samples, the RC% values ranged from 68.5 to 110.3 (nystose and 1-kestose, respectively). For the Brazilian legislation, the acceptable RC% values vary according to the concentration range of the analyte, and the RC% in the concentration range of the oligosaccharides in this study should be between 80 and 110% (Brasil 2011). The recoveries obtained in the present method were considered adequate, except for nystose in wine (68.5%) which was outside the recommended range. The low recovery of nystose in wine (68.5%) is possibly related to the different physicochemical characteristics of this beverage when compared to grape juice, and a correction factor was added in the method based on this premise to make it possible to consistently quantify this in wine samples.
In another method validation study for determining oligosaccharides in dates, Ghfar et al. (2015) obtained RC% values ranging from 89 to 90 for nystose and 1-kestose using RP-HPLC/MS. Based on the values obtained for precision and recovery, we consider the present method suitable for the proposed purpose.
Selectivity/specificity
According to Muir et al. (2009), separating and identifying carbohydrates is a complex and challenging area of research. One of the major problems is coelution of the different carbohydrate sugars.
In the present work, the ability of the method to identify and quantify 3 oligosaccharides in grape juice and wine by HPLC-RID using a new RP-C18 (150 × 4.6 mm, 4 μm) with polar endcapping column was evaluated. The method used herein presented good separation capacity for raffinose, 1-kestose, and nystose (Fig. 3a) in evaluating the interference of the main sugars in the juice/wine (glucose, fructose and saccharose). The analysis conditions led to coelution of the sugars glucose and fructose, which would be a limitation in applying this method for determining these monosaccharides.
In order to verify the interference of the main refractive compounds contained in juices and grapes and wines (sugars, organic acids and ethanol), a synthetic wine containing these substances was injected. From the retention time of the peaks obtained in the chromatogram in the present method, as shown in Fig. 3b, it is evident that sugars (glucose, fructose, maltose, and rhamnose), organic acids (citric, tartaric, malic, lactic, succinic and acetic acids) and ethanol did not interfere in the raffinose, 1-kestose and nystose determinations.
In a study by Ball et al. (2013), it is mentioned that amino-bonded type RP-C18 columns present better interaction and retention and separation power for high molecular weight sugars such as raffinose (MW = 549.5) and stachyose (MW = 666), and co-elute lower molecular weight compounds such as glucose (MW = 180), galactose (MW = 180), mannitol (MW = 182), and sorbitol (MW = 182). This was observed in our study, where the retention and separation capacity of the RP-C18 column for raffinose (MW = 549.51), 1-kestose (MW = 504.44) and nystose (MW = 666.58) was better than the sugars glucose (MW = 180), fructose (MW = 180), saccharose (MW = 342), Maltose (MW = 342.3), rhamnose (MW = 164.16); and the organic acids (Fig. 3a, b), being lower molecular weight compounds.
The selectivity/specificity of the present method for determining prebiotics in juices and wines by RP-HPLC/RID is demonstrated by the results obtained in the interference study.
Based on the separations obtained for the studied compounds, we consider the new RP-C18 column suitable for determining oligosaccharides with the advantages of minimizing organic solvent consumption, as compared to RP-C18 type amino-bonded columns used for separation of the same substances in HILIC mode (Doco et al. 2014; Malinovska et al. 2014; Ghfar et al. 2015).
Characterization of grape juice and wines from Northeast Brazil
The characterization results for the prebiotic oligosaccharides in the studied samples are presented in Table 3. In general, the main compound present in all studied samples was 1-kestose. Raffinose was only present in the BRS Violeta organic juice (0.196 g L−1), Syrah (0.098 g L−1), and Sauvignon Blanc (0.091 g L−1) Samples. Nystose was only present in Tempranillo wine (0.334 g L−1).
In relation to 1-kestose, the highest values were obtained in the organic Barbera samples (1.776 g L−1), and in conventional and organic Tempranillo (1.512 and 1.412 g L−1, respectively), however not being detected in Syrah and Sauvignon Blanc. In monovarietal grape juices, 1-kestose was only present in the BRS Violeta and BRS Cora varieties, in respective values of 0.224 and 0.399 g L−1. In the blended commercial juices, 1-kestose was also only present in the Isabel Precoce blends with BRS Violeta and BRS Cora in values of 0.445 and 0.098 g L−1, respectively.
FOS is abundantly found in natural products such as cereals, fruits and vegetables, especially honey, barley, tomato, onion, banana, rye and garlic, in values ranging from 0.15 to 0.75% (Belorkar and Gupta 2016). In table grapes of the “Cardinal” variety, Blanch et al. (2011) found nystose in values ranging from 0.93 to 3.26 mg kg−1 fresh weight (FW), and 1-kestose in values of 10.11–17 mg kg−1. We note a study conducted by Malinovska et al. (2014), wherein 32 fruits and 41 plants were characterized in relation to FOS, mostly nectarines (0.89 g 100 g−1 FW), scallions (4.10 g 100 g−1 FW), and white onions (2.22 g 100 g−1 FW).
Given the general lack of studies quantifying fructooligosaccharides (1-kestose and nystose) and galactooligosaccharide (raffinose) in grape-derived beverages, it was not possible to compare the values obtained in the present study with products of the same nature. However, the presence of 1-kestose in approximately 50% of the juice and wine samples studied, with a maximum value of 1.776 g L−1 (0.18%), is an indication that these beverages have functional potential in relation to prebiotics.
Studies evaluating isolated and fractionated oligosaccharides in wines have reported total values varying from 0.185 to 0.425 g L−1; arabinooligosaccharides, rhamno-arabino-oligosaccharides, and differing rhamnogalacturonan-arabino-oligosaccharides. Values vary according to origin, grape variety, and the amount of oligosaccharides increasing with advancement in grape maturation stage, with the use of pectinases, and with specific yeast strains for the vinification process (Doco et al. 2014; Apolinar-Valiente et al. 2015; Lapuente et al. 2016; García et al. 2017). The results may explain the higher values of 1-kestose in the wines presently studied in relation to grape juice.
Quantifying oligosaccharides in grape products, which are recognized sources of phenolic compounds, is important because the consumption of foods containing these two groups of compounds may potentiate the beneficial effects described for prebiotics due to a possible synergistic effect between these substances in the body (Aquino et al. 2017).
Conclusion
The method for determining raffinose, 1-kestose and nystose oligosaccharides in wine and juices by RP-HPLC/RID using a new RP-C18 column (150 × 4.6 mm, 4 μm) with polar endcapping proved to be suitable for use in research on these compounds. The method presented suitable linearity, precision, recovery, and limits of detection and quantification for characterizing juices and wines. The method selectivity showed that sugars, organic acids and ethanol (the main interferences in refraction detection) did not influence the determination of the studied oligosaccharides. The applicability of the validated method was verified by characterization of prebiotics in grape juices and commercial wines from Brazil, where the main compound found was 1-kestose in approximately 50% of samples, followed by raffinose in 20% of the samples. The 1-kestose amount in grape juices and wines found in this study evidence that the main bulk of oligosaccharides in wine probably come from the activity of pectinases and yeast strains used during the vinification process. The values obtained for oligosaccharides in this study are an indication that grape juices and wines from Northeast Brazil have the potential to be functional beverages in relation to the presence of prebiotics.
References
Al-Sheraji SH, Ismail A, Manap MY, Mustafa S, Yusof RM, Hassan FA (2013) Prebiotics as functional foods: a review. J. Funct. Foods 5:1542–1553
Ambalam P, Ramam M, Purama RK, Doble M (2016) Probiotics, prebiotics and colorectal cancer prevention. Best Pract Res Clin Gastroenterol 30:119–131
Apolinar-Valiente R, Romero-Cascales I, Williams P, Gómez-Plaza E, López-Roca JM, Ros-García JM, Doco T (2015) Oligosaccharides of Cabernet Sauvignon, Syrah and Monastrell red wines. Food Chem 179:311–317
Aquino JS, Batista KS, Duarte FND, Lins PP, Gomes JAS, & Silva LA (2017) Models to evaluate the prebiotic potential of foods. In: Chavarri M (ed), Functional food—improve health through adequate food. In tech, Rijeka, pp 235–256. https://www.intechopen.com/books/functional-food-improve-health-through-adequate-food/models-to-evaluate-the-prebiotic-potential-of-foods
Ball S, Mapp K, Lloyd L (2013) Sugar analysis: an aqueous alternative to the use of amino-bonded HPLC columns with acetronitrile eluents. Poster, Agilent Technologies Inc., Santa Clara, CA, USA. https://www.agilent.com/cs/library/posters/public/SI-2576%20HPLC%202009%20sugar%20analysis,%20no%20ACN.pdf Accessed 21 May 2018
Barthomeuf C, Grizard D, Teulade J-C (1997) Assay and structural determination of fructo-oligosaccharides synthesized by an enzymatic system from Penicillium rugulosum. Biotechnol Tech 11:845–848
Belorkar SA, Gupta AK (2016) Oligosaccharides: a boon from nature’s desk. AMB Express 6(82):1–11
Benkeblia N (2013) Fructooligosaccharides and fructans analysis in plants and food crops. J Chromatogr A 1313:54–61
Blanch M, Sanchez-Ballesta MT, Escribano MI, Morodio C (2011) Fructo-oligosaccharides in table grapes and response to storage. Food Chem 129:724–730
Brasil (2011) Ministério da Agricultura, Pecuária e Abastecimento. Guia de Validação e Controle de Qualidade Analítica: Fármacos em Produtos para Alimentação Animal e Medicamentos Veterinários. Secretaria de Defesa Agropecuária, Brasília, 2011. http://www.agricultura.gov.br/assuntos/laboratorios/arquivos-publicacoes-laboratorio/guia-de-validacao-controle-de-qualidade-analitica.pdf/view Accessed 20 May 2018
Coelho EM, da Silva Padilha CV, Miskinis GA, de Sá AGB, Pereira GE, de Azevêdo LC, dos Santos Lima M (2018) Simultaneous analysis of sugars and organic acids in wine and grape juices by HPLC: method validation and characterization of products from northeast Brazil. J Food Compos Anal 66:160–167
Doco T, Williams P, Meudec E, Cheynier V, Sommerer N (2014) Complex carbohydrates of red wine: characterization of the extreme diversity of neutral oligosaccharides by ESI-MS. J Agric Food Chem 63:671–682
Dutra MCP, De Souza JF, Viana AC, De Oliveira D, Pereira GE, Dos Santos Lima M (2018a) Rapid determination of the aromatic compounds methyl-anthranilate, 2′-aminoacetophenone and furaneol by GC-MS: method validation and characterization of grape derivatives. Food Res Int 107:613–618
Dutra MCP, Rodrigues LL, Viana AC, De Oliveira D, Pereira GE, Dos Santos Lima M (2018b) Integrated analyses of phenolic compounds and minerals of Brazilian organic and conventional grape juices and wines: validation of a method for determination of Cu, Fe and Mn. Food Chem 269:157–165
Eurachem (2014). Eurachem guide: the fitness for purpose of analytical methods—a laboratory guide to method validation and related topics. In: Magnusson B, Örnemark U (eds) 2nd ed. ISBN 978-91-87461-59-0. www.eurachem.org Accessed 18 Aug 2018
Florowska A, Krygier K, Florowski T, Duewska E (2016) Prebiotics as functional food ingredients preventing diet-related diseases. Food Funct 7:2147–2155
García M, Apolinar-Valiente R, Williams P, Zarzoso BE, Arroyo T, Crespo J, Doco Thierry (2017) Polysaccharides and Oligosaccharides Produced on Malvar Wines Elaborated with Torulaspora delbrueckii CLI 918 and Saccharomyces cerevisiae CLI 889 native yeasts from D.O. “Vinos de Madrid”. J Agric Food Chem 65:6656–6664
Ghfar AA, Wabaidur SM, Ahmed AYBH, Alothman ZA, Khan MR, Al-Shaalan NH (2015) Simultaneous determination of monosaccharides and oligosaccharides in dates using liquid chromatography–electrospray ionization mass spectrometry. Food Chem 176:487–492
Hubaux A, Vos G (1970) Decision and detection limits for linear calibration curves. Anal Chem 42:849–855
Lapuente LM, Valiente RA, Guadalupe Z, Ayestarán B, Magariño SP, Williams P, Doco T (2016) Influence of grape maturity on complex carbohydrate composition of red sparkling wines. J Agric Food Chem 64:5020–5030
Lima MS, Silani ISV, Toaldo IM, Correa LC, Biasoto ACT, Pereira GE, Bordignon-Luiz MT, Ninow JL (2014) Phenolic compounds, organic acids and antioxidante activity of grape juices produced from new Brazilian varieties planted in the Northeast Region of Brazil. Food Chem 161:94–103
Long WJ, Brooks AE, Biazzo W (2009) Analysis of polar compounds using 100% aqueous mobile phases with agilent ZORBAX eclipse plus phenyl-hexyl and other ZORBAX Phenyl columns. Application note. Agilent Technologies Inc., Santa Clara, CA, USA, pp 1–7. https://www.agilent.com/cs/library/applications/5990-3616EN.pdf Accessed 11 Nov 2018
Maeda T, Watanabe A, Zadrak WD, Osanai S, Honda K, Oku S, Shimura H, Suzuki T, Yamasaki A, Okabe Y, Ueno K, Onodera S (2017) Analysis of varietal differences in the fructo-oligosaccharide accumulation profile among Onion (Allium cepa L.) cultivars grown by spring-sown cultivation. Hortic J 86:501–510
Malinovska RJ, Kuzmanova S, Winkelhausen A (2014) Oligosaccharide profile in fruits and vegetables as sources of prebiotics and functional foods. Int J Food Prop 17:949–965
Marrubini G, Appelblad P, Maietta M, Papetti A (2018) Hydrophilic interaction chromatography in food matrices analysis: an updated review. Food Chem 257:53–66
Morris JR, Striegler KR (2005) Processing fruits: science and technology, 2nd edn. CRC Press, Boca Raton
Muir JG, Rose R, Rosella O, Liels K, Barret JS, Shepherd SJ, Gibson PR (2009) Measurement of short-chain carbohydrates in common australian vegetables and fruits by high-performance liquid chromatography (HPLC). J Agric Food Chem 57:554–565
OIV—Organisation Internationale de la Vigne et du Vin (2011). Recueil des methods internationals d’analyse des vins et des mouts, edition 2011. 8th Assemblée Générale, 21 June 2010, Paris
Padilha CVS, Biasoto ACT, Corrêa LC, Lima MS, Pereira GE (2017a) Phenolic compounds profile and antioxidant activity of commercial tropical red wines (Vitis vinifera L.) from São Francisco Valley, Brazil. J Food Biochem 41:1–9
Padilha CVS, Miskinis GA, De Souza MEAO, Pereira GE, Bordignon-Luiz MT, Lima MS (2017b) Rapid determination of flavonoids and phenolic acids in grape juices and wines by RP-HPLC/DAD: method validation and characterization of commercial products of the new Brazilian varieties of grape. Food Chem 228:106–115
Rodríguez-Costa S, Cobas AC, Saavedra PR, Arias JJP, Miranda JM, Cepeda A (2018) In vitro evaluation of the prebiotic effect of red and white grape polyphenolic extracts. J Physiol Biochem 74:101–110
Sabater C, Prodanov M, Olano A, Corzo N, Montilla A (2016) Quantification of prebiotics in commercial infant formulas. Food Chem 194:6–11
Snow L, Trass M, Klein M, Orlowicz S, Rivera B (2015) Fast and Robust Analysis of Organic Acids from Wine using HPLC-UV. Application note TN-1189, pp 1–8. Phenomenex, Torrance, CA, USA. https://az621941.vo.msecnd.net/documents/0d4a0bb0-f83c-4a26-b928-43649c3ed58a.pdf
Zhu ZY, Lian HY, Si CL, Liu Y, Liu N, Chen J, Ding LN, Yao Q, Zhang Y (2012) The chromatographic analysis of oligosaccharides and preparation of 1-kestose and nystose in yacon. Int J Food Sci Nutr 63:338–342
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. The authors thank the CAPES (Brazil) for a scholarship awarded to P.C. Nunes and T.H.F do Bonfim.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
dos Santos Lima, M., Nunes, P.C., de Lourdes de Araújo Silva, B. et al. Determining 1-kestose, nystose and raffinose oligosaccharides in grape juices and wines using HPLC: method validation and characterization of products from Northeast Brazil. J Food Sci Technol 56, 4575–4584 (2019). https://doi.org/10.1007/s13197-019-03936-x
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-03936-x