Abstract
Carotenoids are among the main groups of bioactive compounds in fruits and are responsible for the color of fruits and processed fruit products. Liquid chromatography is the most common analytical technique employed to evaluate these compounds; however, using classical high-performance liquid chromatography-HPLC columns leads to extended running time and high consumption of organic solvents. This study aimed to develop and validate an ultra-fast method for HPLC–DAD determination of free carotenoids in Brazilian fruit juices using a new rapid-resolution column RP-C18 (50 × 4.6 mm, 1.8 µm). The method was effective in analyzing carotenoid pigments in a 10-min run, presenting linearity (R2 ≥ 0.9994), precision (CV ≤ 4.31%), recovery (90.5–92.6%), selectivity, detection limit (< 0.09 mg·L−1) and quantification limit (< 0.29 mg·L−1) acceptable for analytical purposes. The primary carotenoids in the evaluated fruit juices were β-carotene, 9-cis-β-carotene and lycopene. Pitanga, mango, and watermelon contain violaxanthin, 9-cis-β-carotene, lutein, β-carotene, and lycopene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carotenoid pigments are an important group of bioactive compounds in fruits and vegetables. In addition to the health benefits given by their consumption, carotenoids are responsible for the yellow, orange, and red colors in fruits and processed fruit products (Saini et al. 2015; Stinco et al. 2019). Due to their contribution to color and their role in preventing cardiovascular diseases and cancers (Arballo et al. 2021), quantifying the concentration of carotenoids in fruit juices is essential for the quality control of these beverages (Sakaew et al. 2018).
The most common free carotenoid found in fruits is β-carotene and its isomers, such as 9-cis-β-carotene, which has pro-vitamin A activity and contributes to the yellow and orange colors in fruits and vegetables, including mangoes, oranges, and carrots (Saini et al. 2015; Safdarian et al. 2021). Lycopene is responsible for the red color in fruits such as tomatoes, guava, and watermelon (Arballo et al. 2021). In leafy green vegetables, xanthophylls lutein and violaxanthin are the predominant carotenoids (Saini et al. 2015). Biocompounds such as β-carotene, lutein, and lycopene have been extensively studied for their nutraceutical properties in the food and pharmaceutical industries (Li et al. 2020; Saini et al. 2022).
High-efficiency liquid chromatography is one of the most common analytical techniques used to quantify carotenoid molecules in fruits and juices for its ability to separate/detect these compounds (Saini et al. 2015). Fluorometry, Near-Infrared Spectroscopy, Nuclear Magnetic Resonance, Thin Layer Chromatography-Densitometry, and UV–Vis Spectroscopy have also been used to determine specific and total forms of carotenoids (Mercadante et al. 2016; Safdarian et al. 2021). The primary columns used to separate these compounds are silica C18 and C30, in reversed phase (RP). Detection is typically made by a diode array detector (DAD) and/or a mass spectrometer (MS) (Andrés et al. 2014; Stinco et al. 2014, 2019; Daood et al. 2014; Mapelli-Brahm et al. 2017; Benmeziane et al. 2018; Crupi et al. 2020).
The use of HPLC to identify and quantify carotenoid concentrations using classical columns involves long run times and high consumption of organic solvents throughout the gradient (Stinco et al. 2014). Instead, by using ultra-performance liquid chromatography (UPLC), both run time and the use of solvents can be reduced because of more rapid resolution columns operated at higher pressures (Delpino-Rius et al. 2014). A well-known alternative to save time and reagents in HPLC analysis is the employment of rapid resolution columns (RR) (sub-2 µm particle size or poroshell), which can achieve UPLC efficiency at HPLC pressure ranges (Ali et al. 2012). Rapid-resolution columns have already been used to determine carotenoids by HPLC (Stinco et al. 2014; Benmeziane et al. 2018).
The determination of carotenoids in complex matrices or with low concentrations of analyte is a task that requires adequate steps of preparation, extraction, and pre-concentration before measurement. Furthermore, many carotenoids in fruit juices are present in values lower than the detection limits by UV–Vis spectroscopy, not allowing their direct quantification (Safdarian et al. 2021; Saini et al. 2022) or requiring complex separation methods (Mercadante et al. 2016). Therefore, methodologies that combine the extraction protocol and the rapid determination of carotenoids are needed to improve analytical routines, decrease run times, and reduce the consumption of organic solvents.
Brazil is a country with an immense variety of fruits and is also one of the leading producers of fruit and fruit products worldwide. In Brazil's northeast region, the lower-middle San Francisco Valley is a sub-region recognized for its large production of fruits in irrigated cultivation systems, responsible for more than 80% of the Brazilian exports of mango and grape. In this sub-region, there is an exceptionally high production of grape juices (Lima et al. 2014; Dutra et al. 2018, 2021) and mango, acerola, guava, cashew, pineapple, and passion fruit juices (Batista et al. 2018). In addition, there is also a vast production of other fruits mostly consumed in natura, including watermelon, melon, coconut, papaya, banana, and some species of citrus fruits (Coelho et al. 2021).
Therefore, this study sought to develop and validate an ultra-rapid HPLC–DAD method to analyze five free carotenoid pigments in fruit juices using a rapid resolution column RP-C18 (RRLC) and increase the detection limits of carotenoids by optimizing the extraction protocol. The validated method was applied to characterize whole fruit juices made from Brazilian grape cultivars and other fruits rich in carotenoids, including mango, guava, pineapple, and watermelon. All the fruits were cultivated in the lower-middle San Francisco Valley in Brazil.
Material and Methods
Fruits and the Manufacturing of Whole Fruit Juices
Brazilian grapes (Hybrid Vitis vinifera x Vitis labrusca) from Isabel Precoce, BRS Carmem, BRS Cora, BRS Magna e BRS Violeta cultivars were obtained from the farm Timbaúba (Petrolina, Brazil). In addition, Mango (cv. Palmer), guava (cv. Paluma), pineapple (cv. Pérola), watermelon (cv. Crimsom Sweet), Pitanga (cv. Tropicana), and cashew were collected from local farmers in the city of Petrolina (Pernambuco, Brazil) (9º09' S, 40º22' W). All fruits were collected, processed, and analyzed in December 2021. This region's assigned Köppen climate classification is "Bswh" or hot semi-arid. The minimum, medium, and maximum temperatures are 20.6, 26.4, and 31.7 ºC, respectively, with an average annual rainfall of 571 mm (Batista et al. 2018).
Whole fruit juices were prepared as follows: fruits were weighed to obtain 10 kg of each, and either crushed or cut into 2 × 2 cm cubes and then heated to 50 °C. Pectinase Endozym® Pectrofruit PR (AEB Bioquímica, Paraná, Brazil) was added to all fruits at a concentration of 4 ml·100 kg−1 and were continuously macerated at 50 °C for 60 min to breakdown any pectin. Macerated fruits were then pressed, and the juice was drained. Drained juice was pasteurized at 85ºC for 3 min, transferred to 300 mL colorless glass bottles, and then cooled (< 40 °C) by immersing in cold water. Juices from three independent bottles were sampled for each analysis (n = 3).
All juices were characterized for pH, temperature, °Brix, and titratable acidity, according to the methods published by the Association of Official Analytical Chemists (AOAC 2012). In addition, the color was measured by the CIE Lab system using a Konica Minolta CR-400A (Japan) colorimeter. All characteristics from these analyses are presented in Supplementary Table S1.
External Standards and Reagents
The HPLC external standards of violaxanthin (CAS 126–29-4), lutein (CAS 127–40-2), lycopene (CAS 502–65-8), β-carotene (CAS 7235–40-7), and 9-cis-β-carotene (CAS 13312–52-2) were purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLC-grade acetone, hexane, and ethanol were purchased from J.T. Baker (Phillipsburg, NJ, USA). Ultrapure water was obtained using a purification system (Marte Científica®, São Paulo, Brazil).
Carotenoids Extraction, Instrumentation, and Analytical Conditions
Carotenoids were extracted from all fruit juices using a liquid–liquid extraction method, as described by Stinco et al. (2014), with some modifications. A 2.5 ml extraction solvent (hexane: acetone, 1:1 v/v) was added to 10 ml of each fruit juice sample. Each mixture was vortexed and sonicated for two minutes. The extraction procedure was repeated twice with 2 ml hexane, and the supernatants were collected, mixed, and centrifuged at 3500 rpm for 3 min (24 ± 1 ºC). The colored layer was evaporated in a vacuum on a rotary evaporator (≤ 30 ºC), and the pigments were resuspended in 1 ml hexane: ethanol (1:1 v/v). The resuspended extract was filtered through a 0.45 µm nylon filter (Chromafil® Xtra, Macherey–Nagel—Germany) into an amber vial and immediately injected.
All analyses of β-carotene, 9-cis-β-carotene, lycopene, violaxanthin, and lutein were carried out in a liquid chromatography Agilent 1260 Infinity LC System (Santa Clara –EUA), equipped with a quaternary pump (model G1311C, 400 bar maximum pressure), vacuum degasser, thermostatic column compartment (model G1316A), automatic sampler (model G1329B) and diode array detector-DAD (model G1315D). All data acquisition and processing were performed with the OpenLab CSD ChemStation Edition software suite (Agilent Technologies, Santa Clara – EUA).
Chromatographic conditions were adapted from Daood et al. (2014), with gradient and flow rate changes to decrease the run time. A wavelength of 450 nm was used to detect all compounds. We used an RRHT Eclipse Plus RP-C18 (50 × 4.6 mm, 1.8 μm) as the rapid resolution column, alongside an RP-C18 (12.6 × 4.6 mm, 5 μm) pre-column, both manufactured by Zorbax (USA). The column temperature was maintained at 27 °C, the injection volume was 20μL, and the flow rate was set at 1.0 mL·min−1. The gradient used in the separation was 0–5 min: 80–88% B; 5–9 min: 95% B (isocratic); 9–10 min: 80% B (isocratic), where solvent A was ultrapure water, and solvent B was acetone.
Method Validation Model
To validate this altered method, assays with external standards, spiked samples, and blank samples were used to evaluate linearity, precision, recovery, specificity/selectivity, and limits of detection and quantification. All validation parameters followed the recommendations of the guide for validation and analytical quality control published by the Ministry of Agriculture of Brazil (Brasil 2011) and the European guide for the validation of analytical methods (Eurachem 2014).
Linearity
The linearity was obtained through the calibration with external standards by using five concentration points (n = 5) from the dilution of the stock solution. The calibration curve was obtained using the least squares method by correlating the peak areas with known concentrations of the external standards.
Specificity/Selectivity
The specificity evaluation was performed according to the methodology described by Padilha et al. (2017). The OpenLAB CDS 3D UV software package (Agilent Technologies, USA) was used for data processing. Samples of fruit juices were injected to assess the specificity of the method.
The analyzed carotenoid peaks were subjected to a threshold test to assess the spectral purity, allowing for an acceptable purity factor of ≥ 990 (match factor). Purity factor values greater than 990 represent the similarity between the spectra and are evidence for pure peaks, without the possible coelution with other interfering compounds in the detection.
Recovery and Precision
Precision was evaluated using the coefficient of variation (CV) obtained from six independent injections (n = 6) at three concentration ranges, using the grape juice samples spiked with external carotenoid standards. The recovery value was calculated by comparing the content obtained for the spiked sample with the content of the non-spiked sample.
Limit of Detection (LOD) and Limit of Quantification (LOQ)
LOD and LOQ values were obtained according to the method proposed by Hubaux and Vos (1970). First, three grape juice samples were spiked with external carotenoid standards in triplicate (n = 9) and diluted to reach values close to the estimated LOD. Following this, we applied the abovementioned extraction protocol "Carotenoids Extraction, Instrumentation, and Analytical Conditions". Next, all samples were analyzed, and an analytical curve was constructed by plotting the values obtained from the analysis (Axis X) vs. the sample theoretical values (Axis Y). The slope of the curve, intercept, and correlation coefficients were calculated. The residual standard deviation (RSD) was also calculated, and the LOD and LOQ were 3 and 10 times greater than the RSD value, respectively, added with the intercept of the curve.
Statistical Analysis
Statistical analysis was performed using SPSS version 20.0 for Windows (SPSS, Chicago, USA). All fruits' carotenoid contents were subject to analysis of variance (ANOVA), and means were compared using the Tukey test at 5% probability. In addition, a Principal Components Analysis (PCA) Biplot between the quantified carotenoids and the analyzed fruits was also performed using XLSTAT 2022.2.1 (Adinsoft, New York, USA).
Results and Discussion
Development and Validation of the Method
Before validation, different elution and solvent flow gradients were evaluated using ultrapure water and acetone and the separation conditions described by Daood et al. (2014) to decrease HPLC run times. These authors used a Nucleodur ISIS RP-C18 column (50 × 4.6 mm, 1.8 μm) with cross-linked end-capping. They separated 12 carotenoids within a 26-min run to analyze and quantify lycopene and β-carotene from tomato samples.
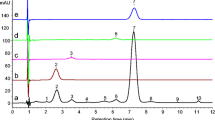
In this study, we optimized the method published by Daood et al. (2014) by altering the type of column used to a Zorbax RRHT Eclipse Plus RP-C18 (50 × 4.6 mm, 1.8 μm), which has a similar resolution capacity but does not use end-capping. In our optimized method, violaxanthin (Retention time-RT: 1.00 min), lutein (RT: 1.47 min), lycopene (RT: 7.39 min), β-Carotene (RT: 7.95 min) and 9-cis-β-Carotene (RT: 8.32 min) were separated, with a total run time of 10 min as is observed in the chromatogram obtained from the external standards (Fig. 1). Peak separation resolution was > 1.5 for all compounds.
Methods for identifying carotenoids in fruits and juices using classic silica columns RP-C18 (250 × 4.6 mm, 5 μm) and RP-C30 (250 × 3 mm, 5 μm), and elution solvents such as methanol, acetonitrile, ethyl acetate, and tert-butyl methyl ether, have presented run times ranging from 30 to 71 min (Crupi et al. 2010; Andrés et al. 2014; Bunea et al. 2012) . On the other hand, other methods using rapid resolution RP-C18 Poroshell columns (50 × 4.6 mm, 2.7 μm), with methanol and ethyl acetate as elution solvents, had significantly reduced run times to 12 min (Stinco et al. 2014; Benmeziane et al. 2018). Furthermore, carotenoid identification in fruits and juices using UPLC-DAD equipped with columns with sub-2 μm particles, RP-C18 (100 × 2.1 mm, 1.7 μm) (Delpino-Rius et al. 2014), and Zorbax RP-C18 (50 × 2.1 mm, 1.8 μm) (Bohoyo-Gil et al. 2012), can achieve run times of just 17, and 10 min, respectively.
Based on these abovementioned results, we opted to use a rapid resolution column RRLC (Zorbax RRHT Eclipse Plus RP-C18 50 × 4.6 mm, 1.8 μm) to identify carotenoids in this study, as this is considered to be an ultra-fast method, which allows for separation and analysis of violaxanthin, lutein, lycopene, β-carotene and 9-cis-β-carotene using an HPLC–DAD, with a total run time of 10 min, thus achieving the resolution power of a UPLC.
Linearity and Specificity
All parameters used in validating our method in the present study are presented in Table 1. Linearity was evaluated by the R2 (coefficient of determination) values obtained from the calibration curves with the external standards of carotenoids. According to the guide for validation and analytical quality control published by the Ministry of Agriculture of Brazil (Brasil 2011), R2 values must be > 0.99. In the present method, the R2 values ranged from 0.9994 to 0.9998 for the four quantified carotenoids, showing adequate linearity of the calibration curve (Supplementary Fig. S1).
According to Eurachem (2014), selectivity/specificity is the method's ability to determine specific analytes in complex mixtures or matrices without interference from other components with similar behaviors. In this study, the specificity of the method was evaluated by the threshold test, as shown in Fig. 2, for the peak of β-carotene in the cashew juice sample, where pure peaks are expected to have a purity factor ≥ 990 (Padilha et al. 2017). The values of spectral purity factor for the carotenoids quantified in the whole juices from grape, mango, watermelon, pitanga, guava, and pineapple ranged between 995 and 1000 (Table 1), evidencing that the separation conditions in the present method were adequate to identify and quantify without interferents. In addition to the spectral purity test of the peaks, the identification and confirmation of the carotenoids were indicated by their respective retention times and by comparing the spectra obtained in the sample peaks and the spectra obtained in the external standards (Supplementary Fig. S2).
Precision and Recovery
Precision estimates the dispersion of results among independent assays repeated for the same sample, similar samples, or standards under defined conditions. It is usually expressed by statistical parameters, such as the standard deviation and coefficient of variation (CV), calculated from the results obtained (Brasil 2011; Eurachem 2014). Brazilian legislation (Brasil 2011) recommends a maximum CV of 30% at the concentration ranges of the analyzed sample (1–10 mg·L−1). The precision in the present method ranged between 1.75 and 4.31% for β-carotene and violaxanthin, respectively, demonstrating that this parameter is adequate.
Recovery is an essential parameter for validating the method because it aims to correct the analysis result concerning systematic errors resulting from the effects of extraction, spiking, and losses in the analytical stages, until the instrumental reading. Brazilian legislation establishes that recovery values must be between 80 and 110% in the analyte ranges used in the study (Brasil 2011). In the present method, with the application of liquid–liquid extraction, rota evaporation, and resuspension of the carotenoids of the juices, the recovery values obtained ranged from 90.5 to 92.6% for lutein and β-carotene, respectively (Table 1). They were therefore considered adequate for the proposed objective.
Stinco et al. (2014) showed a recovery rate of 81.9% (β-carotene) and 92.1–107.6% (Trans-β-apo-8'-carotenal) when simultaneously determining carotenoids, chlorophylls, and tocopherols in fruits by RRLC, using liquid–liquid extractions with and without saponification. Bohoyo-Gil et al. (2012) analyzed carotenoids in food matrices by UPLC-DAD using solid–liquid extraction – they tested nectarines and honey, using acetone as the solvent. The obtained extracts were subjected to liquid–liquid extraction with hexane, and they showed recoveries of 100% (lutein), 91% (lycopene), and 97% (β-carotene). These results from previous studies agree with those obtained in the present method and demonstrate that the extraction technique applied was satisfactory.
Limit of Detection (LOD) and Limit of Quantification (LOQ)
The extraction process is one of the steps for analyzing carotenoids in fruit juices which influences the limits of detection and recovery of the applied method. Several extraction methods for carotenoids have been employed in HPLC analyses; liquid–liquid extractions with solvent mixtures such as acetone: hexane (1:1 v/v), hexane: ethanol (1:1 v/v) and hexane: ethyl ether (1:1 v/v) are most used (Andrés et al. 2014; Mapelli-Brahm et al. 2017; Stinco et al. 2019; Crupi et al. 2020). In the present work, the proportion between the juice sample (10 mL) and extraction solvent (2.5 mL hexane: acetone and 2 mL hexane twice) was modified from that described by Stinco et al. (2014) to obtain a 10 × increase of carotenoids content in resuspended extracts, and to improve the detection capacity in samples with small contents, such as the grape juice.
Table 1 shows the values for the limits of detection (LOD) and quantification (LOQ) of the modified carotenoid analysis method we propose in this study. LOD values were: 0.004 mg·L−1 (violaxanthin), 0.005 mg·L−1 (lutein), 0.08 mg·L−1 (lycopene), 0.09 mg·L−1 (β-carotene) and 0.08 mg·L−1 (9-cis-β-carotene). LOQ values were: 0.013 mg·L−1 (violaxanthin), 0.017 mg·L−1 (lutein), 0.22 mg·L−1 (lycopene), 0.29 mg·L−1 (β-carotene) and 0.23 mg·L−1 (9-cis-β-carotene). To compare the LOD of our method, we identified previous studies which evaluated both LOD and LOQ, which included the application of the extraction technique in the validation protocol, as some studies presented only the limits of detection of the instrument. For example, methods for carotenoids quantification by UPLC-DAD or HPLC–DAD in fruits and juices reported LOD ranging from 0.029 to 0.079 mg·L−1 (lutein), 0.031 mg·L−1 (violaxanthin), 0.115 to 0.118 mg·L−1 (lycopene) and 0.062 to 0.9 mg·L−1 (β-carotene). For LOQ, the values ranged from 0.10 to 0.32 mg·L−1 (lutein), 0.10 mg·L−1 (violaxanthin), 0.383 to 0.393 mg·L−1 (lycopene), and 0.206 to 1.2 mg·L−1 (β-carotene) (Delpino-Rius et al. 2014; Bohoyo-Gil et al. 2012; Andrés et al. 2014).
In our study, the LOD of our modified method was lower than those reported in other literature (Delpino-Rius et al. 2014; Bohoyo-Gil et al. 2012; Andrés et al. 2014). This result suggests that our method has high sensitivity and can be effectively applied in analyzing samples with low contents of carotenoids.
Carotenoid isomers have differing absorption coefficients; thus, it is crucial to separate them for proper quantification by LC-DAD (Daood et al. 2014). The separation between β-carotene and its 9—cis-β-carotene (synonym for (9Z)-β-carotene) isomer in the present study demonstrates an adequate separation capacity for the proposed purpose.
Carotenoids Quantification in Fruit Juices Using the Proposed Method
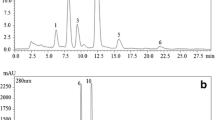
All values obtained from the carotenoids analysis of juice samples are presented in Table 2. Figure 3 shows typical chromatograms of fruit juices analyzed with our modified method, using RRLC equipped with a Diode Array Detector (DAD).
Of all the fruit juices tested, carotenoid concentrations (in decreasing order) were highest in: pitanga > mango > watermelon > guava > pineapple > cashew > grape, with a predominance of β-carotene, 9-cis-β-carotene, and lycopene in all fruits. The juice from pitanga cv. 'Tropicana' had the highest concentration of all carotenoids evaluated, with values of 64 μg·L−1 for violaxanthin, 994 μg·L−1 for lutein, 93.6 mg·L−1 for lycopene, 156.4 mg·L−1 β-carotene, 32.1–156.4 mg·L−1 9-cis-β-carotene and 283.2 mg·L−1 for total carotenoids. In juice from mango cv. Palmer, the main carotenoid present was β-carotene (156.3 mg·L−1), 9-cis-β-carotene (25.4 mg·L−1), followed by lycopene (17.7 mg·L−1), and small contents of violaxanthin (60 μg·L−1) and lutein (23 μg·L−1). The main compounds present in watermelon juice were lycopene (65.1 mg·L−1), 9-cis-β-carotene (81.1 mg·L−1), and β-carotene (45.1 mg·L−1), alongside small amounts of violaxanthin and lutein (24 and 28 μg·L−1, respectively). In the pineapple juice, β-carotene (10.1 mg·L−1) had the highest concentration, with small amounts of violaxanthin and lutein. Cashew juice contained low concentrations of all carotenoids, with β-carotene (0.88 mg·L−1) as the main compound.
The carotenoid values in the monovarietal juices from Brazilian grapes were similar amongst cultivars, with β-carotene (0.09–0.12 mg·L−1) being the main compound identified. In addition, traces of lutein were also obtained (values ≤ 5 μg·L−1).
In all analyzed fruit juices, β-carotene and its isomer 9-cis-β-carotene were the primary carotenoids, followed by lycopene. This result was true even when considering non-yellow and orange fruits such as pitanga, watermelon, guava, and grape juices.
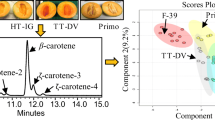
Figure 4 shows the PCA, which separated fruit juices according to the carotenoids analyzed. Component 1 had the highest proportion of variance explained (76.24%) and separated mango, pitanga, and watermelon juices in the positive part, associated with higher levels of β-carotene, 9-cis-β-carotene, lycopene, violaxanthin, and lutein, compared to other fruits. Component 2 (13.40% variance explained) separated mango juice into the negative part, associated with higher levels of β-carotene, 9-cis-β-carotene, and violaxanthin. Pitanga was associated with higher levels of lutein and lycopene than other fruits. The grapes, cashew, pineapple, and guava juices generally presented the lowest carotenoid contents (PC1 < 0).
According to Maldonado-Celis et al. (2019), β-carotene its isomer represents 60% of the carotenoids of mature mango, whereas, in its green stage, carotenoids are predominately comprised of xanthophylls such as lutein and violaxanthin. The carotenoid content varies significantly between cultivars and different maturation stages. According to Sricharoen et al. (2016), the highest β-carotene contents in fruit and vegetable juices were found in carrots (60.6 mg·L−1), tomatoes (49–54 mg·L−1), oranges (28 mg·L−1), and mangoes (12.5 mg·L−1). Other fruits, such as guava (1.74 mg·L−1) and pineapple (1.93 mg·L−1), were found to have lower levels. The authors highlighted that the difference in cultivars was the primary reason for differences in carotenoid content in fruits.
Concerning diet, the most significant nutraceutical aspects of β-carotene intake are its antioxidant and pro-vitamin A activities (Saini et al. 2015).
According to Porcu and Rodriguez-Amaya (2008), pitanga is one of the fruits with the highest carotenoid content, especially lycopene, which in fresh fruits can range from 14 to 71 mg·kg−1. In the present study, in addition to lycopene (93.6 mg·L−1), the whole pitanga juice was found to have high levels of β-carotene (156.4 mg·L−1). Therefore, Pitanga juice has the highest nutraceutical potential among the fruits we evaluated.
In a study conducted by Barba et al. (2006), a high concentration of 73 mg·kg −1 (lycopene) and 11 mg·kg −1 (β-carotene) were identified in watermelon cv. Dulce Maravilla. We found similar values of 65.1 mg·L−1 in watermelon juice cv. Crimson Sweet. The high lycopene contents in pitanga and watermelon juices make these important fruit sources of this carotenoid. According to Arballo et al. (2021), lycopene is a xanthophyll (not pro-vitamin A) strongly correlated with the reduction of several chronic diseases, including certain types of cancer and cardiovascular diseases.
Steingass et al. (2020) also reported high concentrations of β-carotene in pineapples, which corroborates with the results obtained in our study.
De Sanctis et al. (2012) reported that ~ 80% of the total carotenoids present in the grape cv. Grechetto is comprised of β-carotene and lutein. According to Oliveira et al. (2006), the main carotenoids quantified in eight varieties of grapes V. vinifera grown in the Douro winery region in Portugal were β-carotene (167–1044 μg·kg −1), lutein (210-993 μg·kg −1) and violaxanthin (3–98 μg·kg −1). In Italian grapes cv. Negro Amaro and Primitivo, analyzed during maturation, β-carotene (170–550 μg·kg−1), lutein (18–86 μg·kg−1), and violaxanthin (31–126 μg·kg−1) were also the main carotenoids identified/quantified by HPLC–DAD-MS (Crupi et al. 2020). These studies all corroborate that the carotenoids of β-carotene, lutein and violaxanthin are the main carotenoids in grapes. The carotenoids identified in Brazilian hybrid grape juices in the present study were β-carotene and lutein, which also agree with the abovementioned studies, albeit at lower values than those previously reported.
There are several cashew cultivars in Brazil, which range in color from yellow to red. According to Assunção and Mercadante (2003), the main carotenoid in cashews is β-carotene at concentrations ranging from 0.16 to 0.68 mg·kg−1. We obtained similar values in cashew juice in this study (0.88 mg·kg−1).
By characterizing the carotenoid concentrations of whole fruit juices originating from the lower-middle São Francisco Valley in Brazil, we have shown that there are high concentrations of β-carotene, 9—cis-β-carotene and lycopene in yellow, orange, and red fruits by a modified method of HPLC. We believe that pitanga, mango, and watermelon are the fruits with the highest nutraceutical potential and the highest concentration of carotenoids.
Conclusions
We have developed an ultra-fast method for extraction, identifying, and quantifying carotenoids from fruit juices using a rapid resolution column RP-C18 (50 × 4.6 mm, 1.8 μm) (RRLC) and an HPLC–DAD. The run time was reduced to approximately 10 min and achieved a separation resolution similar to that of UPLC methods. Our validation parameters demonstrate that the method has linearity, precision, recovery, selectivity, and detection limits suitable for carotenoid analysis in fruits and derivatives. Characterization of whole fruit juices originating from the lower-middle São Francisco Valley in Brazil has shown high concentrations of both β-carotene, 9-cis-β-carotene, and lycopene in yellow, orange, and red fruits. Pitanga, mango, and watermelon had the highest concentrations of violaxanthin, lutein, β-carotene, 9-cis-β-carotene, and lycopene, demonstrating potential to be explored as functional foods with nutraceutical properties in future studies.
Data Availability
The datasets generated during the current study are available from the corresponding author upon reasonable request.
References
Ali I, AL-Othman ZA, Nagae N, Gaitonde VD, Dutta KK (2012) Recent trends in ultra-fast HPLC: New generation superficially porous silica columns. J Sep Sci 35(23):3235–3249. https://doi.org/10.1002/jssc.201200454
Andrés V, Villanueva MJ, Tenorio MD (2014) Simultaneous determination of tocopherols, retinol, ester derivatives and β-carotene in milk-and soy-juice based beverages by HPLC with diode-array detection. LWT-Food Sci Technol 58(2):557–562. https://doi.org/10.1016/j.lwt.2014.03.025
AOAC (2012) Official method of analysis: Association of Analytical Chemists vol 1, 19th edn. AOAC International, Association of official analytical chemists international, ed Latimer, Horwitz W, Latimer GW (eds). Washington DC
Arballo J, Amengual J, Erdman JW (2021) Lycopene: A critical review of digestion, absorption, metabolism, and excretion. Antioxidants 10(3):342. https://doi.org/10.3390/antiox10030342
Assunção RB, Mercadante AZ (2003) Carotenoids and ascorbic acid from cashew apple (Anacardium occidentale L): variety and geographic effects. Food Chem 81(4):495–502. https://doi.org/10.1016/S0308-8146(02)00477-6
Batista PF, Lima MACD, Alves RE, Façanha RV (2018) Bioactive compounds and antioxidant activity in tropical fruits grown in the lower-middle São Francisco Valley. Rev Ciênc Agron 49:616–623. https://doi.org/10.5935/1806-6690.20180070
Barba AO, Hurtado MC, Mata MS, Ruiz VF, De Tejada MLS (2006) Application of a UV–vis detection-HPLC method for a rapid determination of lycopene and β-carotene in vegetables. Food Chem 95(2):328–336. https://doi.org/10.1016/j.foodchem.2005.02.028
Benmeziane A, Boulekbache-Makhlouf L, Mapelli-Brahm P, Khodja NK, Remini H, Madani K, Meléndez-Martínez AJ (2018) Extraction of carotenoids from cantaloupe waste and determination of its mineral composition. Food Res Int 111:391–398. https://doi.org/10.1016/j.foodres.2018.05.044
Brasil (2011) Ministério da Agricultura, Pecuária e Abastecimento. Guia de Validação e Controle de Qualidade Analítica: Fármacos em Produtos para Alimentação Animal e Medicamentos Veterinários. Secretaria de Defesa Agropecuária, Brasília, 2011. Retrieved from: http://www.agricultura.gov.br/assuntos/laboratorios/arquivos-publicacoes-laboratorio/guia-de-validacao-controle-de-qualidade-analitica.pdf/view. Accessed December 10, 2021
Bohoyo-Gil D, Dominguez-Valhondo D, Garcia-Parra JJ, González-Gómez D (2012) UHPLC as a suitable methodology for the analysis of carotenoids in food matrix. Eur Food Res Technol 235(6):1055–1061. https://doi.org/10.1007/s00217-012-1838-0
Bunea CI, Pop N, Babeş AC, Matea C, Dulf FV, Bunea A (2012) Carotenoids, total polyphenols and antioxidant activity of grapes (Vitis vinifera) cultivated in organic and conventional systems. Chem Cent J 6(1):1–9. https://doi.org/10.1186/1752-153X-6-66
Coelho EM, da Silva Haas IC, de Azevedo LC, Bastos DC, Fedrigo IMT, dos Santos Lima M, Amboni RDDMC (2021) Multivariate chemometric analysis for the evaluation of 22 Citrus fruits growing in Brazil’s semi-arid region. J Food Composi Anal 101:103964. https://doi.org/10.1016/j.jfca.2021.103964
Crupi P, Santamaria M, Vallejo F, Tomás-Barberán FA, Masi G, Caputo AR, ... Tarricone L (2020) How pre-harvest inactivated yeast treatment may influence the norisoprenoid aroma potential in wine grapes. Appl Sci 10(10):3369. https://doi.org/10.3390/app10103369
Crupi P, Milella RA, Antonacci D (2010) Simultaneous HPLC-DAD-MS (ESI+) determination of structural and geometrical isomers of carotenoids in mature grapes. J Mass Spectrom 45(9):971–980. https://doi.org/10.1002/jms.1794
Daood HG, Bencze G, Palotas G, Pek Z, Sidikov A, Helyes L (2014) HPLC analysis of carotenoids from tomatoes using cross-linked C18 column and MS detection. J Chromatogr Sci 52(9):985–991. https://doi.org/10.1093/chromsci/bmt139
Delpino-Rius A, Eras J, Marsol-Vall A, Vilaró F, Balcells M, Canela-Garayoa R (2014) Ultra-performance liquid chromatography analysis to study the changes in the carotenoid profile of commercial monovarietal fruit juices. J Chromatogr A 1331:90–99. https://doi.org/10.1016/j.chroma.2014.01.044
De Sanctis F, Silvestrini MG, Luneia R, Botondi R, Bellincontro A, Mencarelli F (2012) Postharvest dehydration of wine white grapes to increase genistein, daidzein and the main carotenoids. Food Chem 135(3):1619–1625. https://doi.org/10.1016/j.foodchem.2012.05.092
Dutra MDCP, Viana AC, Pereira GE, Nassur RDCMR, dos Santos Lima M (2021) Whole, concentrated and reconstituted grape juice: Impact of processes on phenolic composition, “foxy” aromas, organic acids, sugars and antioxidant capacity. Food Chem 343:128399. https://doi.org/10.1016/j.foodchem.2020.128399
Dutra MDCP, de Souza JF, Viana AC, de Oliveira D, Pereira GE, dos Santos Lima M (2018) Rapid determination of the aromatic compounds methyl-anthranilate, 2′-aminoacetophenone and furaneol by GC-MS: Method validation and characterization of grape derivatives. Food Res Int 107:613–618. https://doi.org/10.1016/j.foodres.2018.03.020
Eurachem Guide: The Fitness for Purpose of Analytical Methods – A Laboratory Guide to Method Validation and Related (2014) 2nd ed. (2014) ISBN 978–91–87461–59–0. Retrieved from: <https://www.eurachem.org/images/stories/Guides/pdf/MV_guide_2nd_ed_EN.pdf>. Accessed October 11, 2020. https://doi.org/10.1016/j.lwt.2010.01.011
Hubaux A, Vos G (1970) Decision and detection limits for calibration curves. Anal Chem 42(8):849–855. https://doi.org/10.1021/ac60290a013
Li LH, Lee JCY, Leung HH, Lam WC, Fu Z, Lo ACY (2020) Lutein supplementation for eye diseases. Nutrients 12(6):1721. https://doi.org/10.3390/nu12061721
Lima MdS, Silani ISV, Toaldo IM, Correa LC, Biasoto ACT, Pereira GE, Ninow JL, Bordignon-Luiz MT (2014) Phenolic compounds, organic acids and antioxidant activity of grape juices produced from new Brazilian varieties planted in the Northeast Region of Brazil. Food Chem 161:94–103. https://doi.org/10.1016/j.foodchem.2014.03.109
Maldonado-Celis ME, Yahia EM, Bedoya R, Landázuri P, Loango N, Aguillón J, ... Guerrero Ospina JC (2019) Chemical composition of mango (Mangifera indica L.) fruit: Nutritional and phytochemical compounds. Front Plant Sci 1073. https://doi.org/10.3389/fpls.2019.01073
Mapelli-Brahm P, Corte-Real J, Meléndez-Martínez AJ, Bohn T (2017) Bioaccessibility of phytoene and phytofluene is superior to other carotenoids from selected fruit and vegetable juices. Food Chem 229:304–311. https://doi.org/10.1016/j.foodchem.2017.02.074
Mercadante AZ, Rodriguez DB, Petry FB, Mariutti LRB (2016) Carotenoid esters in foods - A review and practical directions on analysis and occurrence. Food Res Int 99:830–850. https://doi.org/10.1016/j.foodres.2016.12.018
Oliveira C, Barbosa A, Ferreira AS, Guerra J, Guedes DE, Pinho P (2006) Carotenoid profile in grapes related to aromatic compounds in wines from Douro region. J Food Sci 71(1):S1–S7. https://doi.org/10.1111/j.1365-2621.2006.tb12398.x
Padilha CV da S, Mipeelis GA, Souza MEOS, Pereira GE, Oliveira D, Bordignon-Luiz MT, dos Santos Lima M (2017) Rapid determination of flavonoids and phenolic acids in grape juices and wines by RP-HPLC/DAD: Method validation and characterization of commercial products of the new Brazilian varieties of grape. Food Chem 228:106–115. https://doi.org/10.1016/j.foodchem.2017.01.137
Porcu OM, Rodriguez-Amaya DB (2008) Variation in the carotenoid composition of the lycopene-rich Brazilian fruit Eugenia uniflora L. Plant Foods Hum Nutr 63(4):195–199. https://doi.org/10.1007/s11130-008-0085-9
Sakaew C, Sricharoen P, Limchoowong N, Chanthai S (2018) Determination of β-carotene and total carotenoids in fruit juices using surfactant surface decorated graphene oxide based ultrasound-assisted dispersive solid-phase microextraction. Anal Methods 10(28):3540–3551. https://doi.org/10.1039/C8AY00670A
Safdarian M, Hashemi P, Ghiasvand A (2021) A fast and simple method for determination of β-carotene in commercial fruit juice by cloud point extraction-cold column trapping combined with UV–Vis spectrophotometry. Food Chem 343:128481. https://doi.org/10.1016/j.foodchem.2020.128481
Saini RK, Prasad P, Lokesh V, Shang X, Shin J, Keum YS, Lee JH (2022) Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements. Antioxidants 11(4):795. https://doi.org/10.3390/antiox11040795
Saini RK, Nile SH, Park SW (2015) Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res Int 76:735–750. https://doi.org/10.1016/j.foodres.2015.07.047
Sricharoen P, Limchoowong N, Techawongstien S, Chanthai S (2016) A novel extraction method for β-carotene and other carotenoids in fruit juices using air-assisted, low-density solvent-based liquid–liquid microextraction and solidified floating organic droplets. Food Chem 203:386–393. https://doi.org/10.1016/j.foodchem.2016.02.093
Steingass CB, Vollmer K, Lux PE, Dell C, Carle R, Schweiggert RM (2020) HPLC-DAD-APCI-MSn analysis of the genuine carotenoid pattern of pineapple (Ananas comosus [L.] Merr.) infructescence. Food Res Int 127:108709. https://doi.org/10.1016/j.foodres.2019.108709
Stinco CM, Szczepańska J, Marszałek K, Pinto CA, Inácio RS, Mapelli-Brahm P, ... Meléndez-Martínez AJ (2019) Effect of high-pressure processing on carotenoids profile, colour, microbial and enzymatic stability of cloudy carrot juice. Food Chem 299:125112. https://doi.org/10.1016/j.foodchem.2019.125112
Stinco CM, Benítez-González AM, Hernanz D, Vicario IM, Meléndez-Martínez AJ (2014) Development and validation of a rapid resolution liquid chromatography method for the screening of dietary plant isoprenoids: Carotenoids, tocopherols and chlorophylls. J Chromatogr A 1370:162–170. https://doi.org/10.1016/j.chroma.2014.10.044
Acknowledgements
The authors thank Timbaúba Agrícola S.A. for making their grapes available for this study.
Author information
Authors and Affiliations
Contributions
Conceptualization & Investigation, R.G.B.S.; Visualization & validation, T.C.P.; Formal analysis, T.A.A; Formal analysis, E.T.S.N; Resources, S.B.O.V.; Resources, M.E.A.O.S.; Visualization & Resources, A.J.B.A.C.; Visualization & Methodology, M.M.; Conceptualization, methodology & project administration, M.d.S.L.
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals performed by any authors.
Informed Consent
Informed consent is not applicable.
Conflicts of Interest
Renata Gomes de Barros‑Santos declares that she has no conflict of interest. Tatiana Colombo Pimentel declares that she has no conflict of interest. Tainara Araujo Amorim declares that she has no conflict of interest. Elis Tatiane da Silva Nogueira declares that she has no conflict of interest. Silvana Belém de Oliveira Vilar declares that she has no conflict of interest. Marcelo Eduardo Alves Olinda de Souza declares that he has no conflict of interest. Ana Júlia de Brito Araújo Carvalho declares that she has no conflict of interest. Marciane Magnani declares that she has no conflict of interest. Marcos dos Santos Lima declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Barros-Santos, R.G., Pimentel, T.C., Amorim, T.A. et al. Ultra-fast Determination of Free Carotenoids in Fruit Juices by Rapid Resolution Liquid Chromatography (RRLC): Method Validation and Characterization of Brazilian Whole Fruit Juices. Food Anal. Methods 16, 808–818 (2023). https://doi.org/10.1007/s12161-023-02458-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-023-02458-9