Abstract
Wetland construction has been used as a tool to mitigate wetland loss, but constructed wetlands might not provide the same functions as natural wetlands. Hundreds of long-hydroperiod wetlands have been constructed within the Daniel Boone National Forest, Kentucky, in a ridge-top ecosystem where natural wetlands dry annually (i.e., have short hydroperiods). The constructed wetlands have been colonized by several amphibian species not historically associated with this ecosystem and that could have negative impacts on native amphibian species. We compared wood frog (Lithobates sylvaticus) reproductive success at constructed and natural wetlands and benefits of wood frog presence in constructed wetlands to eastern newts (Notophthalmus viridescens). Wood frog reproductive success was zero when eggs were laid in constructed wetlands: 7–70 % of eggs were consumed and no wood frog larvae were found. Eastern newts, present at all constructed wetlands, benefited from wood frog presence, i.e., newts in constructed wetlands with wood frog eggs had higher body condition than newts in natural wetlands. Wetland construction techniques should be altered so their hydrology mimics that of natural wetlands to support historically occurring species. Understanding the influence of species interactions, as habitat loss and modification increase, will continue to be critical for amphibian conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wetlands are important habitats that are used by many taxa at various life stages (Gibbons 2003). However, wetlands are also an ecosystem type heavily impacted by humans (e.g., urban development, agriculture; Dahl 2011). Wetland construction has been used as a tool to mitigate wetland loss, but constructed wetlands might not be providing the same functions or habitat conditions as natural wetlands (Lichko and Calhoun 2003; Moreno-Mateos et al. 2012; Calhoun et al. 2014). Previous research on constructed wetlands has focused on quantifying water chemistry, plant and wildlife communities, and general wetland condition (Brown and Veneman 2001; Pechmann et al. 2001; Shulse et al. 2010; Denton and Richter 2013; Strand and Weisner 2013), but information on how constructed wetlands impact historical species interactions is limited.
Amphibian species distributions in freshwater wetlands are largely a result of two factors, hydroperiod (the length of time a wetland contains ponded surface water) and predator-prey interactions (Wellborn et al. 1996). Hydroperiod influences species use of wetlands because some species require long hydroperiods (i.e., >1 year) for successful reproduction and development (e.g., American bullfrog [Lithobates catesbeianus]; Wang and Li 2009), while others develop more quickly and are able to flourish in more ephemeral habitats (e.g., eastern spadefoot [Scaphiopus holbrookii]; Hansen 1958). Still other species are able to breed in both long-hydroperiod and short-hydroperiod wetlands (e.g., spotted salamander [Ambystoma maculatum]; Rubbo and Kiesecker 2005; Denton and Richter 2013). More predatory insect, anuran, and salamander species occur in long-hydroperiod wetlands. Therefore, such habitats may represent a hostile environment for organisms that typically inhabit short-hydroperiod wetlands (Wellborn et al. 1996) and limit a species’ ability to reproduce successfully (Wellborn et al. 1996; Azevedo-Ramos et al. 1999; Lardner 2000).
Predators have important top-down effects on amphibian community structure (Morin 1986; Walls and Williams 2001; Rowe and Garcia 2014). High predator densities can impact reproductive success of some amphibians breeding in long-hydroperiod wetlands by reducing embryonic and larval survival (Walls and Williams 2001). Thus, long-hydroperiod wetlands can act as ecological sinks or traps for species that typically breed in more ephemeral wetlands (Cortwright and Nelson 1990; Vasconcelos and Calhoun 2006). As natural habitats continue to be modified and destroyed, and replaced with longer-hydroperiod wetlands, more ecological traps are likely to be formed (Battin 2004). Understanding how these ecological traps function in natural systems and what makes them attractive for use is important for amphibian conservation and management.
The ridge-top, forested ecosystem of Daniel Boone National Forest (DBNF) in eastern Kentucky is an altered landscape where community-level changes in the amphibian species assemblage have occurred (Drayer 2011; Denton and Richter 2013). Short-hydroperiod wetlands are a key characteristic of the ridge-top ecosystem in eastern Kentucky. During the last 25 years, over 400 wetlands have been constructed on ridge tops of the DBNF for the purpose of game and wildlife management (Drayer 2011; Denton and Richter 2013). However, most of these ridge-top wetlands were constructed to serve as permanent water sources and have much longer hydroperiods than wetlands historically present on the landscape (Brown and Richter 2012).
Constructed wetlands have allowed amphibians that require long-hydroperiods, many of which are predatory, to colonize the ridge-top ecosystem in DBNF. Thus, the constructed wetlands have a different assemblage of amphibians from those historically present in natural wetlands (Drayer 2011; Denton and Richter 2013). Many of the constructed wetlands have amphibians present that require larval overwintering (e.g. American bullfrog and green frog [L. clamitans]) or have a fully aquatic adult stage (e.g. eastern newt). These species are top predators of other amphibian species (Morin 1986; Boone et al. 2004) and potential reservoirs of disease (Greenspan et al. 2012; Richter et al. 2013). In contrast, natural wetlands contain amphibians that metamorphose quickly and do not require a long hydroperiod (e.g. wood frogs). Predators in these shorter hydroperiod habitats are limited, but when present, consist primarily of predatory insects and ambystomatid salamander larvae (Drayer 2011; Denton and Richter 2013).
Presence of long-hydroperiod constructed wetlands among shorter hydroperiod natural wetlands provides potential for interactions between species of natural and constructed wetland assemblages (Brown and Richter 2012). Interactions between one predatory species from constructed wetlands, the eastern newt, and a species from natural wetlands, the wood frog, have been observed (S. Richter and A. Drayer unpubl. data). Wood frog larvae were only detected in natural wetlands by Drayer (2011) and Denton and Richter (2013). However, Richter and Drayer (unpubl. Data) conducted egg masses surveys and found wood frog eggs in constructed wetlands, and observed eastern newts consuming them. We explored the potential effects of this predator/prey interaction. Based on published and anecdotal accounts of constructed wetland amphibian assemblages in DBNF, it appeared that constructed wetlands were potentially functioning as ecological traps for wood frogs, in particular because of predation from eastern newts. Additionally, we hypothesized that presence of wood frogs in constructed wetlands could benefit newts and be reflected by an increase in body condition.
Our objectives were to determine if amphibian species of natural and constructed wetland assemblages interact, and to evaluate potential positive and negative influences of having constructed wetlands on the landscape. Specifically, the following questions were addressed: (1) Do wood frogs reproduce successfully in ridge-top constructed wetlands? (2) Do newts in constructed wetlands with wood frogs benefit from this additional food source?
Methods
Site Selection and Description
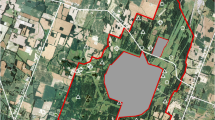
We explored species interactions in six constructed and six natural wetlands over two breeding seasons from February 2013 to May 2014 within the Cumberland District of the DBNF in eastern Kentucky (Fig. 1). Our sample size was limited by the need to maintain a balanced sample and the occurrence of only six natural ridge-top wetlands in this region of the DBNF. We selected constructed wetlands by randomly selecting from those located within 1 km of each of the six natural wetlands. We also stratified our sample of constructed wetlands such that half had a known presence of wood frog breeding and half did not (S. Richter and A. Drayer unpubl. Data). This design allowed for an evaluation of effects of wood frog occurrence on newt body condition and abundance in constructed wetlands. All wetland sites (both constructed and natural) were fishless, not connected by surface waters, located on a ridge-top, and surrounded by deciduous forest. Previous studies characterized wetland vegetation, shape, and slope typical of natural and constructed wetlands in DBNF (Drayer 2011; Denton and Richter 2013).
Wood Frog Sampling
We conducted egg mass surveys at each wetland every other week throughout the wood frog breeding season (February–March) in 2013 and 2014. At each wetland, we performed a visual inspection of the entire wetland to determine egg mass occurrence and abundance. Locations of all detected egg masses were recorded. To ensure accuracy of our egg mass counts, we repeated each count. In the event the first and second counts were different, we performed a third count and used an average of the three values as our egg mass abundance estimate. During our surveys, we recorded the number of potential amphibian predators (e.g. eastern newts, bullfrogs, green frogs) in the wetlands and any evidence of egg predation. The percent of egg predation and egg mortality due to abiotic factors (e.g. freezing and wetland drying) were estimated based upon visual inspection of egg masses. Mortality caused by freezing was estimated by counting the number of white eggs within an egg mass. Mortality caused by drying was estimated by counts of the number of egg masses in an area of the wetland not containing water. We calculated the proportion of egg mass loss due to abiotic factors (i.e., freezing, drying) after all egg mass surveys were completed in March. The percent of egg predation was determined by estimating the percentage of an egg mass with missing embryos or where only fragments of jelly remained. Once hatching began, estimation of egg-mass predation was discontinued.
After eggs hatched, we placed mesh minnow traps evenly along the outer edge of the wetland. Wetland area was calculated prior to setting traps during each sampling period and the number of traps set was adjusted based on the estimated area; six minnow traps were set for every 100-m area. We visually inspected traps for tears and placed traps deep enough that water covered the funnel opening without completely submerging the trap. We checked all traps after being in place for a 24-h period. We counted the number of wood frog larvae in each trap. In the event we could not set traps due to low water level, we determined larval wood frog presence by dipping a D-frame net across the substrate in a 180-degree arc every 2 m around the entire shoreline. We counted and identified all larvae captured in traps and dipnets to species and life stage. We released all animals when sampling was completed.
Newt Sampling
We sampled for eastern newts once per month in May, July, September, and November of 2013 and January to May of 2014, for a total of nine sampling events. We used minnow traps and dipnetting during each sampling event. Minnow trap sampling followed the same protocol used for wood frogs. Following trap removal, we dipnetted to supplement and maximize the number of newts caught at each wetland. We repeatedly jabbed a D-frame net into the substrate in 1 m arcs along the edge and shallow areas (i.e., less than 5 ft. deep) of the wetland and repeated until no newts were caught within 20 dips. Due to time constraints, we did not dipnet if more than 75 newts were captured during a single trapping event; this occurred in only two constructed wetlands and zero natural wetlands. To limit the amount of disturbance to egg masses, we did not dipnet when wood frog eggs were present (March 2014 only).
We gave each captured eastern newt an individual code using Visual Implant Elastomer (VIE; Northwest Marine Technology, Inc., Shaw Island, WA) for mark-recapture in four body locations (behind each fore-limb and in front of each hind-limb). We measured snout-vent length (SVL), tail length, and tail width to the nearest millimeter. Mass was measured using a Pesola spring scale to 0.01 g. Animals were released post-processing.
Data Analyses
Body condition is related to the health of an organism (e.g., Legagneux et al. 2013; Maceda-Veiga et al. 2014). We used the Scaled Mass Index (SMI; Peig and Green 2009) to estimate body condition because SMI is able to account for changes in the relationship between mass and length as an organism grows and body size changes (Peig and Green 2010). The SMI accounts for ontogenetic variation and sexual dimorphism in body size, and is ideal to use when comparing multiple populations (Peig and Green 2010). The SMI is also a better estimator of mass and length relationship when compared to dry weight measurements (Peig and Green 2009, 2010; Legagneux et al. 2013, Maceda-Vega et al. 2014). Snout-vent length was the morphological metric (L0) most correlated with body mass (Mi) on a log-log scale (r = 0.44, p < 0.01), and was used as the indicator of body size (Li). Bivariate plots, one for each wetland type (i.e. wood frog absent and wood frog present), were created to determine which M and L data were most correlated. The correlation between M and L was highest for the wood frog absent group (r = 0.545); thus, we ran a standardized major axis (SMA) regression using ln-transformed M and L to determine the slope of the fitted line, or bsma value. For L0, the average SVL from the whole group (i.e. newts from both wetland types) was used (Table 2). Finally, SMI was calculated for each individual from both wetland types (n = 1263).
Once SMI was calculated, we performed statistical analyses to test if the presence of wood frogs positively affected newt body condition. First, we used Pearson’s correlations to determine multi-collinearity among predictive variables of newt body condition. Correlations with an r ≥ 0.70 resulted in the elimination of one parameter per correlated pair by removing the variable that was correlated to multiple parameters or had the lowest correlation to body condition. Ranid catch per unit effort (CPUE) was highly correlated with eastern newt CPUE and was removed. Wetland type (whether wood frogs were present or absent) was highly correlated with number of wood frog clutches and was removed. Additionally, we performed log-transformations on the SMI and wetland size data to reduce heteroscedasticity. The global model to predict SMI included newt sex, newt CPUE, number of wood frog clutches, wetland size, and an interactive effect between number of wood frog clutches and newt CPUE. We performed model selection using second-order Akaike’s Information Criterion (AICc) in SPSS v. 20 (IBM Corp 2011). The estimated theta values of negative binomial distributed global models were used across all candidate models (Mazerolle 2015). We developed candidate models a priori, and each model consisted of a different combination of parameters or a reduction in the number of parameters. If more than one model had a ΔAIC <2, we followed the method for model averaging described in Burnham and Anderson (2002). We used regression coefficients (β) and 85 % confidence intervals to represent effect sizes and to determine significance of each variable based on the recommendation of Arnold (2010).
Results
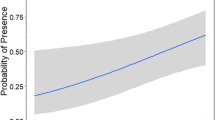
All natural wetlands dried during the summer and all constructed wetlands were permanently inundated. Wetland size varied among wetlands (Table 1). Wood frog eggs were laid at all natural wetlands and three constructed wetlands during the 2013 breeding season (February–March), and at all natural wetlands and four constructed wetlands during the 2014 breeding season (February–March; Table 1). Nine newt sampling events were completed at the constructed wetlands and seven were completed at natural wetlands throughout 2013 and 2014. A total of 14,286 amphibians of 11 species were identified from funnel traps (Table 2), although these data should not be used to infer community composition of wetlands because we were specifically targeting wood frogs and newts. See Drayer (2011) and Denton and Richter (2013) for community data. Larval ranid predators (i.e., bullfrogs, green frogs) were only captured in constructed wetlands (total captures =886). A total of 1275 eastern newts, including 162 recaptures, were captured at six constructed (n = 1263) and two natural (n = 12) wetlands. All recaptures were caught in constructed wetlands. Eighty-three percent of all captured newts were male. Most females were captured during the newt breeding season (Fig. 2).
Wood Frog Reproductive Success
The number of wood frog egg clutches deposited at each wetland varied by wetland and year (Table 1). In constructed wetlands, wood frog egg mortality was caused by predation or freezing. Newts were observed actively eating eggs at each constructed wetland, and while larval bullfrogs and green frogs were not observed actively eating eggs, they likely ate eggs based on our observations of 5–20 individuals scattering from egg masses as we approached a wetland to conduct surveys. No invertebrate predators (e.g., Belostomatidae; Odonata larvae) were observed near wood frog egg masses. Few were captured in minnow traps and only in constructed wetlands. In contrast, freezing and pond drying were the primary causes of wood frog egg mortality in natural wetlands. One natural wetland dried prior to eggs hatching in 2014 resulting in 100 % egg mortality. Post-hatching, hundreds to thousands of free-swimming wood frog larvae were captured in natural wetlands while none were captured in constructed wetlands (Table 1).
Newt Body Condition
Average L, M, and SMI values of eastern newts varied across all wetlands (Table 3). Average SMI was significantly higher in constructed wetlands with wood frogs than in constructed wetlands without wood frogs (Mann-Whitney U-test, W = 402,474.00, p < 0.001). However, there was temporal variation in SMI values. Newt SMI in constructed wetlands with wood frogs was higher after wood frog eggs were laid (March–May), but newts in constructed wetlands without wood frogs had a higher SMI during the fall and winter months (Fig. 3). Average SMI of newts within constructed wetlands with wood frogs decreased from 2013 to 2014, while average SMI of newts within constructed wetlands without wood frogs decreased slightly from 2013 to 2014 (Fig. 3). The model that best explained newt SMI included sex, wetland size, number of wood frog clutches, newt CPUE, and the interaction between wood frog clutches and newt CPUE. The second top model did not include wetland size. The ∆ i was 1.062 and the w i total was 0.99 between the top two models. All parameters were significant (p < 0.001), except for newt CPUE (Table 4).
Discussion
We studied the use of permanent constructed wetlands as breeding habitat by wood frogs. Previous research in the DBNF system did not detect the use of constructed wetlands by wood frogs (Drayer 2011; Denton and Richter 2013); however, that research was based on larval surveys. Because we included egg mass surveys, we quantified wood frog use of constructed wetlands as breeding habitat. We found strong support that these constructed wetlands act as sink habitats for wood frogs in this anthropogenically altered wetland ecosystem. Finally, our study indicated that eastern newts benefit from the presence of wood frog eggs and larvae resulting in increased newt body condition when emerging from winter conditions.
Wood frogs are early breeders and their eggs provide an easy food source for predators during late winter and early spring (e.g. Vasconcelos and Calhoun 2006). Wood frog larvae are small and remain relatively immobile on top of the egg mass immediately after hatching; thus, they are especially susceptible to predators before becoming free-swimming larvae. We were unable to quantify post-hatching death. However, if larvae survive post-hatching, they might be more active foragers because wood frogs are usually in low-predator wetlands (Julian et al. 2006). Under natural conditions, wood frog larvae have a high detectability because they occur in high abundance (Drayer 2011; Denton and Richter 2013, this study) and generally are active on the pool surface during the day. Thus, we feel confident that they were absent or in very low abundance in constructed wetlands. Wood frogs were likely depredated at both the egg and larval stages, and multi-stage predation can have important consequences on population growth and species distributions (Rubbo et al. 2006). It is also a possibility that disease was a factor in the absence of free-swimming larvae (Harp and Petranka 2006; Greenspan et al. 2012). However, no mass mortality events were observed at any of the study wetlands, as were observed in other years (S. Richter, unpubl. Data).
Repeated failure to successfully reproduce can lead to local population decline and extinction (Semlitsch 2000) unless a source habitat is able to provide individuals for recolonization (Calhoun et al. 2014). In the DBNF, populations of wood frogs at natural wetlands appear to act as a source for colonization of constructed wetlands. Egg predation was not observed and hundreds to thousands of wood frog larvae were captured in all but one natural wetland. All of the natural wetlands in this study dried during the summer effectively excluding eastern newts and other amphibians that require longer hydroperiods. In these natural wetlands, wood frogs were reproductively successful because of the absence of top predators.
Eastern newts benefited from the presence of wood frogs. Overall, average newt body condition was higher in wetlands that had wood frog eggs. Specifically, newt body condition increased in constructed wetlands with wood frogs directly after wood frogs bred, while newt body condition in wetlands without wood frogs remained lower and relatively constant or decreased during the same period. The number of wood frog clutches available likely influenced the fluctuation of newt body condition observed at most constructed wetlands with wood frogs present. An increase in prey availability can lead to a higher body condition in predators (Pope and Matthews 2002; Brown and Shine 2007; Sztatecsny et al. 2013). Our results suggest that in wetlands without wood frogs, there is more competition for potentially fewer resources, leading to lower body condition. Wood frogs are likely a nutritious prey item for newts at a time when few other resources are available. During fall and winter, newts in wetlands without wood frogs had a higher body condition than newts in wetlands with wood frogs. During this time very few newts were captured (0.3 % of total captures at wood frog present sites [n = 16] and 4 % of total captures at wood frog absent sites [n = 25]); thus, newts that remained in wetlands were likely able to consume more resources and increase in body condition. Overall, the body condition fluctuation observed was likely due to seasonal changes, as prey availability is highest during the spring and summer months and typically lowest during the fall and winter (Pope and Matthews 2002). Although, the small sample size during the winter months might account for some of the variation in body condition observed.
Wood frog eggs were typically laid in lower abundance at constructed wetlands compared to natural wetlands. The difference in abundance between wetland types provides support for the idea that constructed wetlands are a secondary choice for breeding adults. Additionally, the higher number of wood frogs present in natural wetlands might influence individuals that breed later in the season to breed in constructed wetlands to reduce competition. This hypothesis requires further testing, but the potential for competition among conspecifics has led to female amphibians ovipositing at sites containing predators where they might not otherwise breed (e.g. Crump 1991; Matsushima and Kawata 2005). Some other possible explanations for the lower abundance of egg masses in constructed wetlands include active avoidance of breeding wetlands with predators (e.g. Petranka et al. 1994; Hopey and Petranka 1994) or a preference for breeding in wetlands with short hydroperiods (Rodolf and Rödel 2005; Julian et al. 2006).
An understanding of how different amphibian species interact in human-altered habitats is key to their conservation (Boone et al. 2004; Vasconcelos and Calhoun 2006). Anthropogenic alterations within DBNF have led to an increase in predator-prey interactions that might lead to local population declines of amphibians that use ephemeral habitats. Our research demonstrates the negative impacts one species of one community assemblage can have on a species of a different assemblage. Although newts, bullfrogs, and green frogs are native to the DBNF; historically in our study area, they remained in lowland basins where permanent water was available for breeding habitat. The persistence of water in constructed wetlands has allowed newts and these other predators to colonize these wetlands, reducing the ability of wood frogs to successfully breed in the constructed sites (Drayer 2011; Denton and Richter 2013).
Anthropogenic alteration to natural habitats is an important factor related to the decline of amphibians (Vitousek 1994; McKinney 2002). Improving construction techniques to discourage amphibians that do not naturally occur within an ecosystem could reduce the possibility of local population declines (Calhoun et al. 2014). Our results shed light on the importance of creating wetlands with hydroperiods that mimic those of natural wetlands on the landscape to support historically occurring species and maintain biodiversity. Replacing natural wetlands with long-hydroperiod constructed wetlands often results in a loss of original wetland function (Dahl 2011; Moreno-Mateos et al. 2012). Our study provides important baseline data for quantifying how altered landscapes alter species interactions (Gibbons et al. 2006). Understanding community composition in constructed wetlands is an important first step, but studying species interactions and abiotic and biotic characteristics in the context of historic ecosystems more accurately measures construction success (Calhoun et al. 2014; Moreno-Mateos et al. 2015).
References
Arnold TW (2010) Uninformative parameters and model selection using Akaike’s information criterion. J Wildl Manag 74:1175–1178
Azevedo-Ramos C, Magnusson WE, Bayliss P (1999) Predation as the key factor structuring tadpole assemblages in a savanna area in Central Amazonia. Copeia 1999:22–33
Battin J (2004) When good animals love bad habitats: ecological traps and the conservation of animal populations. Conserv Biol 18:1482–1491
Boone MD, Little EE, Semlitsch RD (2004) Overwintered bullfrog tadpoles negatively affect salamanders and anurans in native amphibian communities. Copeia 2004:683–690
Brown DR, Richter SC (2012) Meeting the challenges to preserving Kentucky’s biodiversity. Sustain 25:22–33
Brown GP, Shine R (2007) Rain, prey and predators: climatically driven shifts in frog abundance modify reproductive allometry in a tropical snake. Oecologia 154:361–368
Brown SC, Veneman PLM (2001) Effectiveness of compensatory wetland mitigation in Massachusetts, USA. Wetlands 21:508–518
Burnham KP, Anderson DR (2002) Model selection and multimodal inference: a practical information-theoretic approach. Springer, New York
Calhoun AJK, Arrigoni J, Brooks RP, Hunter ML, Richter SC (2014) Creating successful vernal pools: a literature review and advice for practitioners. Wetlands 34:1027–1038
Cortwright SA, Nelson CE (1990) An examination of multiple factors affecting community structure in an aquatic amphibian community. Oecologia 83:123–131
Crump ML (1991) Choice of oviposition site and egg load assessment by a treefrog. Herpetologica 47:308–315
Dahl TE (2011) Status and trends of wetlands in the conterminous United States 2004 to 2009. U.S. Department of the Interior; Fish and Wildlife Service, Washington, DC
Denton RD, Richter SC (2013) Amphibian communities in natural and constructed ridge top wetlands with implication for wetland construction. J Wildl Manag 77:866–869
Drayer A (2011) Efficacy of constructed wetlands of various depths for natural amphibian community conservation. Eastern Kentucky University, Richmond, Kentucky, Thesis
Gibbons JW (2003) Terrestrial habitat: a vital component for herpetofauna of isolated wetlands. Wetlands 23:630–635
Gibbons JW, Winne CE, Scott DE, Willson JD, Glaudas X, Andrews KA, Todd BT, Fedewa LA, Wilkinson LA, Tsaliagos RN, Harper SJ, Greene JL, Tuberville TD, Metts B, Dorcas ME, Nestor JP, Young CA, Akre T, Reed RN, Buhlmann KA, Norman J, Croshaw DA, Hagan C, Rothermel BB (2006) Remarkable amphibian biomass and abundance in an isolated wetland: implications for wetland conservation. Conserv Biol 20:1457–1465
Greenspan SE, Calhoun AJK, Longcore JE, Levy MG (2012) Transmission of Batrachochytrium dendrobatidis to wood frogs (Lithobates sylvaticus) via a bullfrog (L. catesbeianus) vector. J Wildl Dis 48:575–582
Hansen KL (1958) Breeding pattern of the Eastern Spadefoot toad. Herpetologica 14:57–67
Harp EM, Petranka JW (2006) Ranavirus in wood frogs (Rana sylvatica): potential sources of transmission within and between ponds. J Wildl Dis 42:307–318
Hopey ME, Petranka JW (1994) Restriction of wood frogs to fish-fee habitats: how important is adult choice? Copeia 4:1023–1025
IBM Corp (2011) IBM SPSS Statistics for Windows, Version 20.0. IBM Corp. Armonk
Julian JT, Snyder CD, Young JA (2006) The use of artificial impoundments by two amphibian species in the Delaware Water Gap National recreation area. Northeast Nat 13:459–468
Lardner B (2000) Morphological and life history responses to predators in larvae of seven anurans. Oikos 88:169–180
Legagneux P, Simard A, Gauthier G, Bệty J (2013) Effects of neck collars on the body condition of migrating greater snow geese. J Field Ornithol 84:201–209
Lichko LE, Calhoun AJK (2003) An evaluation of Vernal pool creation projects in New England: project documentation from 1991 to 2000. Environ Manag 32:141–151
Maceda-Veiga A, Green AJ, De Sostoa A (2014) Scaled body-mass index shows how habitat quality influences the condition of four fish taxa in North-Eastern Spain and provides a novel indicator of ecosystem health. Freshw Biol 59:1145–1160
Matsushima N, Kawata M (2005) The choice of oviposition site and the effects of density on oviposition timing on survivorship in Rana japonica. Ecol Res 20:81–86
Mazerolle MJ (2015) Model selection and multimodal inference based on (Q)AIC(c). [Available on internet at https://cran.r-project.org/web/packages/AICcmodavg/AICcmodavg.pdf] Accessed 25 November 2015
McKinney ML (2002) Urbanization, biodiversity, and conservation. Bioscience 52:883–890
Moreno-Mateos D, Power ME, Comin FA, Yockteng R (2012) Structural and functional loss in restored wetland ecosystems. PLoS Biol 10:e1001247
Moreno-Mateos D, Meli P, Vera-Rodríguez MI, Aronson J (2015) Ecosystem response to interventions: lessons from restored and created wetland ecosystems. J Appl Ecol 52:1528–1537
Morin PJ (1986) Interactions between intraspecific competition and predation in an amphibian predator-prey system. Ecology 67:713–720
Pechmann JHK, Estes RA, Scott DE, Gibbons JW (2001) Amphibian colonization and use of ponds created for trial mitigation of wetland loss. Wetlands 21:93–111
Peig J, Green AJ (2009) New perspective for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118:1883–1891
Peig J, Green AJ (2010) The paradigm of body condition: a critical reappraisal of current methods based on mass and length. Funct Ecol 24:1323–1332
Petranka JW, Hopey ME, Jennings BT, Baird SD, Boone SJ (1994) Breeding habitat segregation of wood frogs and American toads: the role of interspecific tadpole predation and adult choice. Copeia 1994:691–697
Pope KL, Matthews KR (2002) Influence of anuran prey on the condition and distribution of Rana mucosa in the Sierra Nevada. Herpetologica 58:354–363
Richter SC, Drayer AN, Strong JR, Kross CS, Miller DL, Gray MJ (2013) High prevalence of ranavirus infection in permanent constructed wetlands in Eastern Kentucky, USA. Herpetol Rev 44:464–466
Rodolf VHW, Rödel MO (2005) Oviposition site selection in a complex and variable environment: the role of habitat quality and conspecific cues. Oecologia 142:316–325
Rowe JC, Garcia TS (2014) Impacts of wetland restoration efforts on an amphibian assemblage in a multi-invader community. Wetlands 34:141–153
Rubbo MJ, Kiesecker JM (2005) Amphibian breeding distribution in an urbanized landscape. Conserv Biol 19:504–511
Rubbo MJ, Shea K, Kiesecker JM (2006) The influence of multi-stage predation on population growth and the distribution of the pond-breeding salamander, Ambystoma jeffersonium. Can J Zool 84:449–458
Semlitsch RD (2000) Principles for management of aquatic-breeding amphibians. J Wildl Manag 64:615–631
Shulse CD, Semlitsch RD, Trauth KM, Williams AD (2010) Influences of design and landscape placement parameters on amphibian abundance in constructed wetlands. Wetlands 30:915–928
Strand JA, Weisner SEB (2013) Effects of wetland construction on nitrogen transport and species richness in the agricultural landscape – experiences from Sweden. Ecol Eng. 56:14–25
Sztatecsny M, Gallauner A, Klotz L, Baierl A, Schabersberger R (2013) The presence of common frogs (Rana temporaria) increases the body condition of syntopic alpine newts (Ichthyosaura alpestris) in oligotrophic high-altitude ponds: benefits of high-energy prey in a low-productivity habitat. Ann Zoologicwe Fenn. 54:209–215
Vasconcelos D, Calhoun AJK (2006) Monitoring created seasonal pools for functional success: a six-year case study of amphibian responses, Sears Island, Maine, USA. Wetlands 26:992–1003
Vitousek PM (1994) Beyond global warming: ecology and global change. Ecology 75:1861–1876
Walls SC, Williams MG (2001) The effect of community composition on persistence of prey with their predators in an assemblage of wetland-breeding amphibians. Oecologica 128:134–141
Wang Y, Li Y (2009) Habitat selection by the introduced American bullfrog (Lithobates catesbeianus) on Daishan Island, China. J Herpetol 43:205–211
Wellborn GA, Skelly DK, Werner EE (1996) Mechanisms creating community structure across a freshwater habitat gradient. Annu Rev Ecol Syst 27:337–363
Acknowledgments
We thank David Brown, Melissa Pilgrim, Brad Ruhfel, and two anonymous reviewers for comments on an earlier draft of our manuscript. Field assistance was provided by John Bourne, Mackenzie Bramlett, Kristin Hinkson, Sherrie Lundsford, Logan Phelps, Luke Romance, and Jennifer Strong. This research was partially funded by the Kentucky Academy of Science’s Marcia Athey Fund and Eastern Kentucky University’s Department of Biological Sciences. Research was approved by the EKU Institutional Animal Care and Use Committee (protocol #05-2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kross, C.S., Richter, S.C. Species Interactions in Constructed Wetlands Result in Population Sinks for Wood Frogs (Lithobates sylvaticus) while Benefitting Eastern Newts (Notophthalmus viridescens). Wetlands 36, 385–393 (2016). https://doi.org/10.1007/s13157-016-0751-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-016-0751-x