Abstract
The success of restoration in attaining wildlife conservation goals can be strongly dependent on both site-scale and landscape-scale habitat characteristics, particularly for species with complex life cycles. Wetland management activities typically target plant communities, and bottom-up responses in higher trophic levels may be dependent on spatially explicit habitat use. We surveyed plant and amphibian assemblages at 26 sites enrolled in the Wetlands Reserve Program (WRP) in the Willamette Valley, Oregon to determine the relative influence of plant management, non-native species, and surrounding landscape on amphibian counts across multiple life history stages. Explanatory variables negatively associated with native anuran counts included percent invasive plant cover, non-native fish presence, invasive bullfrog counts, and area of urban land cover. In addition, native anurans were positively associated with WRP site age, suggesting that the benefits of restored wetlands may increase over time. This study emphasized the importance of adaptive approaches to maintaining diverse communities in restored habitats by considering impacts of synergistic stressors in a multi-invader context. Although invasive plant management provided indirect benefits to native amphibians, the most effective way to enhance native amphibian populations may be through eliminating the strong top-down forces exerted by non-native vertebrates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wetland habitats in the United States have incurred significant losses in total area through agricultural and urban development, as well as hydrological modifications (Dahl 1990). Consequently, a disproportionately high number of obligate wetland species are listed as threatened or endangered (46 %; Boylan and MacLean 1997 in Whigham 1999), contributing to simplified community structure and compromised ecosystem function (Gibbs 2001). A renewed appreciation of the ecological benefits provided by wetlands has prompted federal and state administrative policies to direct funding toward wetland creation, preservation, and restoration programs (Vottler and Muir 1996; Dahl 2006). The Wetlands Reserve Program (WRP), administered through the United States Department of Agriculture’s Natural Resource Conservation Service (NRCS), is a voluntary project aimed at mitigating wetland loss by providing technical and financial support to landowners wishing to restore wetlands on agricultural land (NRCS 2011). Over 1 million hectares of land are enrolled in the WRP with the objective of enhancing wetland function and restoring vitality of agricultural lands (NRCS 2012).
One goal of the WRP is to provide habitat for wetland-dependent fauna (NRCS 2012). Restoration success in achieving wildlife conservation goals is typically evaluated using hydrologic and vegetative criteria, with the assumption that faunal establishment is linked to floral establishment (Petranka et al. 2003; Gray and Teels 2006). The WRP prioritizes benefits for migratory birds (Gray and Teels 2006), and considerable information is available about the program’s contribution to waterfowl conservation (King et al. 2006). However, relatively few studies have quantified the effects of plant management on other wetland-associated vertebrates (Petranka et al. 2003; King et al. 2006). Lentic-breeding amphibian species are experiencing global declines and are an excellent focal group in which to study the effects of wetland restoration. Amphibians are frequently cited as indicators of environmental quality as they possess a unique life history which exposes them to both aquatic and terrestrial habitats (Blaustein and Kiesecker 2002; Waddle et al. 2012).
The ability of amphibians to successfully establish and persist in restored wetlands is influenced by wetland-specific (site-scale) and landscape-scale habitat variables. Within breeding ponds, amphibians face abiotic stressors (e.g., wetland desiccation, chemical contaminants, habitat disturbance) and biotic stressors (e.g., native and invasive competitors and predators) (Blaustein and Kiesecker 2002). They are also particularly sensitive to surrounding landscape composition because of annual breeding migrations (Semlitsch 1998). Isolated breeding ponds imbedded in fragmented landscapes can become population sinks if dispersers experience high mortality (Rothermel 2004). Both local habitat quality and regional land use are potentially strong predictors of long-term amphibian diversity and abundance at restored WRP sites. Further, the relative importance of local and regional factors may depend on ontogeny, with survival in the aquatic life stages (eggs and larvae) regulated by within-pond processes and survival in the terrestrial life stages (juveniles and adults) regulated by both within-pond and surrounding landscape processes (Wilbur 1980; Sztatecsny et al. 2004; Van Buskirk 2005).
Invasive species can directly and indirectly impact amphibian abundance and diversity at restored wetlands (Ricciardi and MacIsaac 2011). Many studies have examined the effects of a single invader on native amphibians, but it is exceedingly challenging to disentangle community-level impacts (Preston et al. 2012). The presence of multiple invaders occupying a range of trophic levels can produce additive effects on native communities when interactions between invaders are facilitative (i.e., invasional meltdown; Simberloff and Von Holle 1999). Alternatively, the impact of invasive species on amphibians may be mediated by habitat characteristics such as the physical structure of emergent vegetation (Kiesecker et al. 2001; Porej and Hetherington 2005). Invasive plants can reduce the quality of amphibian breeding habitats (Brown et al. 2006; Davis et al. 2012), but may also provide refuge to diffuse antagonistic interactions (Hartel et al. 2007; Janssen et al. 2007; Watling et al. 2011). Thus, management of invasive plants can potentially have unintended negative impacts on native amphibian communities. Further, habitat disturbance caused by restoration actions may initially enhance invasion potential for exotic vertebrate competitors and predators of native amphibians (Shea and Chesson 2002). Harmful invasive species that commonly co-occur in lentic, freshwater WRP habitats in the western United States are reed canarygrass (Phalaris arundinacea), the American bullfrog (Lithobates catesbeianus), and non-native fish (bluegill [Lepomis macrochirus], largemouth bass [Micropterus salmoides], and western mosquitofish [Gambusia affinis]). These taxa are capable of reducing native amphibian abundance and diversity by altering ecosystem function and/or dynamics of biotic interactions, and their impacts may be mediated directly or indirectly via habitat restoration.
Our study objective was to determine whether native amphibian diversity and abundance (counts) at WRP sites in the Willamette Valley, OR could be predicted by invasive plant management, the presence of non-native vertebrates (American bullfrogs and fish), and regional landscape quality. We hypothesized that active management would extend positive benefits to the plant community via reduced invasive cover which in turn would translate to greater counts of native amphibians and reduced counts of invasive bullfrogs. However, we expected biotic interactions with non-native fish and bullfrogs to be the strongest determinants of native amphibian counts, with site-scale variables having a greater effect on the response for premetamorphic stages (eggs and larvae) and landscape-scale variables having a greater effect on the response for postmetamorphic stages (juveniles and adults). To address these hypotheses, we explored (1) whether active management is effective at reducing invasive plant species and increasing plant diversity, (2) whether invasive plant cover predicts amphibian counts and diversity given other habitat covariates, and (3) the relative importance of site-scale and landscape-scale variables in predicting stage-specific amphibian counts. These aims highlighted how current invasive plant management strategies applied within the WRP contribute to the program’s wildlife habitat restoration goals for amphibians in the presence of complex trophic interactions.
Methods
Research was conducted in Oregon’s Willamette Valley where approximately 43 % of upland habitat has been converted for agriculture (Baker et al. 2004) and 57 % of emergent wetlands have been lost within the last century (Morlan 2000). These losses parallel the Oregon state listing of 24 % of wetland-dependent amphibians as imperiled in conservation status rank (Morlan 2000).
Survey Design
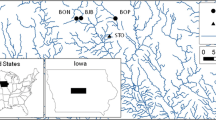
We selected 26 WRP sites located between Portland, OR (ca. 45° 28′ 56.81″; elevation 47 m MSL) and Eugene, OR (ca. 44° 11′ 4.69″; elevation 100 m MSL) containing freshwater lentic wetlands lacking permanent fluvial inputs based on (1) landowner permission to access site, (2) hydroperiod (both seasonal and permanent wetlands likely to remain inundated until the end of the study period in August), and (3) spatial independence (Fig. 1). At sites where multiple wetlands were present, a single water body was randomly selected. All study sites were separated by a distance of at least 2.5 km to limit potential for dispersal by individual amphibians between populations (Petranka et al. 2007). Sites ranged in age from 5 to 15 yrs (\( \overline{x} \) = 9.81 ± 3.36 yrs) since enrollment in the WRP, and wetlands retained in the study ranged in size from 0.08 ha to 14.7 ha (\( \overline{x} \) = 2.8 ± 0.6 ha) prior to any natural or mechanical drawdown. We categorized each wetland based on management intensity as passively managed (N = 8; received no management or only minimal intervention through hydraulic modifications) or actively managed (N = 18; intensive management activities were applied to >50 % of the wetland area at least twice in the past 3 years) based on information from landowners and NRCS restoration technicians (Kross et al. 2008; Evans-Peters et al. 2012). In addition to management intensity (MGMT; passive or active), additional information obtained through landowner and NRCS communications included WRP age since enrollment (AGE; yrs) and wetland hydroperiod (HYDRO; seasonal or permanent). Seasonal wetlands were typically dry by late summer, while permanent wetlands never fully dried.
Each site was visited once within each of three sample periods in 2011 (March–May, May–July, and July–August). Amphibian count data were collected during each sampling period, while data on plant species composition were collected only during the May–July sampling period. During each site visit, we recorded two site-scale physical habitat characteristics: water temperature (WATEMP; °C at 10 cm depth 1 m from waterline, averaged over three sampling periods) and log-transformed wetland area (AREA; calculated in MapSource and Google Earth version 6.2 from on-the-ground waterline delineation using a Garmin GPS unit). We also recorded information on non-native fish presence (FISH; absent or present, verified via landowner/NRCS communications and minnow trapping during each sampling period).
We incorporated six landscape-scale coverage variables using data layers developed by the USGS Gap Analysis Program (USGS 2011) and The Wetlands Conservancy (TWC 2009) in a Geographic Information System (GIS; ESRI ArcMap version 10.0). We created 1,000 m buffers (Lehtinen et al. 1999) around the 26 wetland study sites and calculated area (m2) of forest cover (FOR1000), urban land (URB1000), and wetlands (WET1000) from converted polygons within the buffer.
Plant Community Sampling
Plant surveys were conducted at each site once during peak growing season (May 12–July 13, 2011). We demarcated 30 sampling points (1 m2 quadrats) distributed evenly among ten transect belts (5 m long) spaced at equal intervals around the entire wetland perimeter (modified from Mueller-Dombois and Ellenberg 1974). The three sampling quadrats in each belt were located in three habitat zones: shore (within 3 m upland of waterline), waterline, and shallow water (<1 m water depth) zone. For each zone, we estimated % cover of plants, bare ground, and open water to the nearest 5 % (Baines et al. 1994). Plants were identified to the lowest taxonomic group possible (usually species) and assigned to the categories of invasive or native to Oregon in order to produce a variable for mean site-level percent invasive plant cover (INVCOV).
Amphibian Community Sampling
Native and invasive amphibian species count data were estimated at each site during the three sampling periods. Sampling periods were defined to maximize detection of all life stages of amphibians between the breeding and emergence periods. We conducted 30 min time-restricted amphibian searches following standard breeding pond visual encounter survey (VES) protocol along a curvilinear wetland perimeter transect (Crump and Scott 1994; Olson et al. 1997). Starting from a random point, the observer walked clockwise along the waterline and systematically searched within 1 m of either side of the path. The observer spent an equal proportion of time searching the waterline, the shallow water zone (1 m out from waterline), and the shore zone (1 m upland from waterline) (Crump and Scott 1994). Counts of amphibians by life-stage were recorded for all individuals encountered. VESs were supplemented with D-frame dipnetting for species/life stages unobservable from the surface (e.g., salamander larvae). Dipnet sweeps were taken at 5 min intervals over the course of the VES resulting in six dipnetting events for each sampling period. Each sweep was standardized to cover a length of 1 m in the shallow water zone (Crump and Scott 1994). Catch per dipnet sweep was added to the visual encounter survey count data for each site visit since dipnet sweeps yielded detections of species and life stages that were not detected in VES.
Statistical Analysis
Plant Community
Plant diversity was characterized using taxon richness (Whittaker 1972), Simpson’s diversity (Simpson 1949), and gamma diversity (Whittaker 1972). Simpson’s diversity (D) was the sum of the relative abundance (count) of each taxon following:
where p i equals the proportion of individuals of the ith taxon for an open community. Gamma diversity described the number of unique taxa present at a study site compared to all study sites combined (Magurran 2004).
We used linear models (R version 2.15.2; R Core Team 2012) to identify management and/or habitat variables that significantly influenced plant diversity in the wetland basin unit. Response variables for the analysis included plant taxon richness (log-transformed), Simpson’s diversity (cube-transformed), and mean percent (\( \overline{x} \)%) invasive plant cover. Our model set (for each response) contained all combinations of explanatory variables of MGMT, AGE, and HYDRO without interaction terms, as well as the null intercept-only model. These three predictors were selected based on a priori hypotheses about their roles in regulating the plant community (Table 1). We ranked competing models in the set using Akaike’s information criterion corrected for small sample sizes (AIC C ) in R packages bbmle and AICcmodavg (Akaike 1973; Hurvich and Tsai 1989). Models with a ∆AIC C < 2.0 from the top-ranked model were considered competitive (Burnham and Anderson 2002). Model weights (w i ) represented the relative support for the model given the data, and parameter estimates with confidence intervals were used to determine the direction and strength of the effects.
Anuran Counts
Amphibian count data were analyzed using generalized linear models (GLMs) in R packages bbmle and AICcmodavg for the three most common anuran species encountered: Pacific chorus frog (Pseudacris regilla), northern red-legged frog (Rana aurora), and American bullfrog. Species count data were recorded as the highest encounter during any one sampling period for each life stage (egg, larvae, juvenile, and adult) to limit the potential for multiple counts of the same individual over the survey season (Denton and Richter 2013). Since the biphasic, aquatic-terrestrial life cycle of amphibians may expose them to different stressors over ontogeny, we analyzed premetamorphic and postmetamorphic counts separately.
Independent variables considered in GLMs were bullfrog count (LICA; log-transformed and averaged over three sampling periods), AGE, INVCOV, FISH, HYDRO, and URB1000 (log-transformed). Pairwise combinations of these predictor variables were assessed for multicollinearity, and since Pearson coefficients were r < 0.70 (Shulse et al. 2010), all predictors were retained in the initial pool of variables. For each anuran response variable, we developed a set of 16 empirical candidate models based on a priori hypotheses of important ecological interactions informed through a literature review (Table 2, Online Resource 1). These candidate models were limited to fewer than two predictors to prevent overfitting. Residuals indicated overdispersion (Hoef and Boveng 2007), thus GLMs were fit using a negative binomial error distribution with log link function:
Candidate models were compared using QAIC C values, model weights (w i ), and maximum log-likelihood ratio statistics (LL) (Johnson and Omland 2004). Since the overdispersion coefficient (\( \widehat{c} \)) was greater than 1, we ranked models with QAIC C (as opposed to AIC C ) following:
where L is the maximum likelihood estimate for the model and k is the number of fitted parameters (Symonds and Moussalli 2010). All models with ∆QAIC C < 2.0 were considered competitive and were retained following examination of diagnostic plots for fit (Burnham and Anderson 2002). However, if an additional parameter with minimal explanatory power was added to a model within the competitive set and did not improve the model’s maximum log-likelihood, the model with the additional parameter was considered uninformative (Burnham and Anderson 2002). We assessed the relative strength of the variables included in these models from parameter estimates and 95 % confidence intervals.
Amphibian Community Analysis
Statistical analyses for the entire amphibian assemblage, including rare species, were non-parametric. Response variables included counts of eggs, larvae, juveniles, and adults (averaged over three sampling periods) for all amphibian species/life stages detected in surveys. Site-scale covariates included AGE, INVCOV, FISH, WATEMP, HYDRO, and AREA (log-transformed), while landscape-scale covariates (coverage within a 1,000 m radius from the site; log-transformed) included FOR1000, WET1000, and URB1000.
Non-metric multidimensional scaling (NMS; Mather 1976) was performed in the vegan package in R to describe important patterns in species composition by ordinating the 26 sample units in amphibian species space (average count for each species and life stage for each site). The ordination was overlain with a joint plot to display the strongest correlations between the environmental variables and the ordination axes based on the Pearson’s r statistic. The r 2 values represented the correlation between the ordination distance and the distance in original space. NMS was conducted using a random starting configuration with the Sørensen (Bray-Curtis) distance measure. Amphibian count data were relativized by species maximum to rescale and equalize the influence of disproportionately abundant species and life stages. To facilitate detection of relevant relationships between community composition and habitat variables with minimal accumulation of noise, we considered removing rare species. Six species/life stage combinations never occurred in the matrix, and upon comparing the cumulative variance of column (species) sums prior to the adjustment and after the adjustment, we determined that it was appropriate to remove these rare individuals from subsequent analyses. NMS was followed by a multi-response permutation procedure (MRPP; Mielke 1984) executed in R to compare differences in amphibian species composition between categorical grouping variables (FISH, HYDRO, and MGMT). MRPP is a nonparametric procedure for testing whether there is a significant difference between two or more groups of sample units by comparing within-group and between-group Sørensen (Bray-Curtis) dissimilarity matrices, weighted by group size (n) (Mielke 1984).
Results
Plant Community
A total of 96 plant taxa were present at the 26 wetland sites sampled (87 at actively managed wetlands and 42 at passively managed wetlands), with a mean plant taxon richness of 11.8 (95 % confidence interval [CI] = 9.22 to 14.32) per site (Table 3, Online Resource 2). Native plants having the highest mean percent coverage were spikerush (Eleocharis spp.; \( \overline{x} \) % = 11.0, 95 % CI = 7.15 to 14.87), cattail (Typha latifolia; \( \overline{x} \) % = 4.9, 95 % CI = 1.92 to 7.84), and American water plantain (Alisma subcordatum; \( \overline{x} \) % = 2.4, 95 % CI = 0.37 to 4.51), and invasive plants having the highest coverage were reed canarygrass (Phalaris arundinacea; \( \overline{x} \) % = 15.6, 95 % CI = 7.78 to 23.32), meadow foxtail (Alopecurus pratensis; \( \overline{x} \) % = 3.4, 95 % CI = 0.50 to 6.36), and water smartweed (Polygonum amphibium; \( \overline{x} \) % = 2.0, 95 % CI = −0.46 to 4.35). Total invasive cover and reed canarygrass cover at the sites were highly correlated (r = 0.704, p < 0.001), and invasive cover at the study sites was highly dominated by reed canarygrass.
Plant taxon richness, Simpson’s diversity, and gamma diversity were higher at actively managed sites (Table 3). The models ranked as best by AIC C indicated that management intensity most adequately explained the variation for Simpson’s plant diversity and % invasive cover (Table 4). HYDRO was included along with MGMT in a competitive model predicting Simpson’s plant diversity, but its parameter estimate did not have a significant effect on the slope of the response (Table 4). Simpson’s diversity was higher (βMGMT = 0.56, 95 % CI = 0.21 to 0.70) and % invasive cover was lower (βMGMT = −16.21, 95 % CI = −30.03 to −2.39) at actively managed sites (Table 4). Percent vegetative cover (Welch’s two-sample t-test; t(22.78) = 1.45, p = 0.16) and % bare ground (t(20.79) = 0.87, p = 0.40) were not significantly different between actively and passively managed sites.
Amphibian Community
All six amphibian species inhabiting the Willamette Valley were encountered during surveys: Pacific chorus frog, northern red-legged frog, rough-skinned newt (Taricha granulosa), northwestern salamander (Ambystoma gracile), long-toed salamander (Ambystoma macrodactylum), and American bullfrog. Amphibian diversity was similar between actively and passively managed sites under multiple metrics (Table 3). Chorus frogs were the most common species, occupying all 26 survey sites, followed by bullfrogs, which occurred at 20 (76.9 %) sites (Fig. 2a). Northwestern salamanders and long-toed salamanders were rarely detected, each occurring at only three (11.5 %) sites (Fig. 2a). The northern red-legged frog, a threatened species, was present at 13 (50.0 %) sites and occurred most frequently at seasonal, fishless wetlands (Fig. 2b). Native amphibians occupied fishless sites more often than sites containing non-native fish, except for rough-skinned newts, which were most common at fish-bearing permanent sites (Fig. 2b). The invasive bullfrog also occurred most frequently at sites with non-native fish (Fig. 2b). Most amphibians were more common at actively managed wetlands as opposed to passively managed wetlands, apart from long-toed salamanders, which were detected at two (25.0 %) passively managed sites and one (5.6 %) actively managed site (Fig. 2c).
Bar plots depicting the percentage of sites occupied for all six amphibian species detected from visual encounter surveys and dipnet sampling: a the percentage of all 26 sites occupied by amphibians, b the percentage of sites occupied, categorized by fish presence and hydroperiod, and c the percentage of sites occupied, categorized by management regime
WRP management characteristics such as AGE, INVCOV, and HYDRO commonly influenced anuran counts. AGE was included in the top-ranked models for premetamorphic life stages of Pacific chorus frog and postmetamorphic life stages of northern red-legged frog, having a positive effect on the slope of the response given habitat covariates (Table 5). The best model for postmetamorphic red-legged frogs and competitive models for postmetamorphic chorus frogs indicated a negative association between counts and INVCOV (Table 5). Non-native species consistently appeared in the highest-ranked models for native anurans. Postmetamorphic chorus frogs and red-legged frogs were negatively associated with LICA given covariates (Table 5). FISH had a stronger parameter effect on the slope of the native species’ responses than LICA when included in models, and FISH was the only informative variable included in top models for premetamorphic red-legged frogs (Table 5). Native anuran counts were consistently lower whereas bullfrog counts were higher when non-native fish were present (Table 5). URB1000 had a negative effect on postmetamorphic chorus frog counts and occurred in models alongside site-level covariates of LICA or FISH (Table 5). No models were informative for premetamorphic nor postmetamorphic bullfrogs, since the null models were competitive in the set, and all parameter effects had 95 % confidence intervals crossing zero.
The NMS ordination converged after 20 iterations at a 2-dimensional solution to represent the relationship between species counts and wetland sample units, with final stress of 0.202 and instability of 0.00 (p = 0.020, R 2 = 0.748). The strongest (p < 0.05) quantitative habitat vectors related to amphibian species composition were HYDRO (r 2 = 0.377, p = 0.005) and FISH (r 2 = 0.317, p = 0.014). Bullfrogs were associated with FISH and HYDRO along Axis 1 (egg: r = 0.942, r 2 = 0.299, p = 0.013; juvenile: r = 0.612, r 2 = 0.336, p = 0.006; adult: r = 0.844, r 2 = 0.287, p = 0.018), whereas native amphibians were negatively associated (Fig. 3). Chorus frog larvae (Axis 1 r = −0.999, r 2 = 0.348, p = 0.002) and long-toed salamander larvae (Axis 1 r = −0.976, r 2 = 0.272, p = 0.001) were strongly negatively associated with FISH and HYDRO, while other native species and life stages were not significantly correlated to the ordination axes (all p > 0.05) (Fig. 3). Multi response permutation procedure (MRPP) results indicated that there were significant differences in amphibian species composition between sample units categorized by FISH (non-native fish absent [N = 12] vs. non-native fish present [N = 14]; A = 0.020, p = 0.041) and HYDRO (permanent [N = 10] vs. seasonal [N = 16]; A = 0.033, p = 0.002), but not MGMT (active [N = 18] vs. passive [N = 8]; A = −0.006, p = 0.77).
Non-metric multidimensional scaling (NMS) plot of the ordination of sample units in species space overlain with a joint plot showing relationships with the strongest environmental gradients along axes 1 and 2. Only species and environmental vectors that that are strongly (p < 0.05) correlated to the ordination axes are shown. Sites that are clustered near each other have lower Sørensen distances and thus more similar species composition. Species/life stage relationships are denoted by labeled black dots. Shaded boxes distinguish presence/absence of non-native fish at a wetland site. Significant differences in species composition occurred between wetlands categorized by fish presence (MRPP; A = 0.020, p = 0.041)
Discussion
Active vegetation management at WRP sites in the Willamette Valley, Oregon is effective at reducing unwanted invasive plant species and increasing plant diversity (also see Evans-Peters et al. 2012). Although amphibian diversity and community composition did not differ between actively and passively managed WRP sites, the impact of management on the wetland plant community indirectly transcended to the native amphibians through the effect on invasive plant cover. Postmetamorphic life stages of Pacific chorus frog and northern red-legged frogs were negatively associated with percent invasive plant cover, and active management reduces the cover of invasive wetland plants. Conversely, neither premetamorphic nor postmetamorphic stages of bullfrogs showed a relationship with percent invasive cover. Reed canarygrass, which dominated the invasive plant community, may provide unsuitable egg deposition substrate for native amphibians because of its thick culm (Watson et al. 2000). Larval amphibian mortality may increase in wetlands choked by dense reed canarygrass cover due to the accumulation of toxic alkaloids and excessive organic input resulting in anoxic conditions (Rittenhouse 2011). These negative effects on early life stages may be reflected in postmetamorphic life stages as decreased adult recruitment. Further, movements of postmetamorphic stages of relatively small-bodied native anurans (as opposed to heavier, large-bodied bullfrogs) may be impeded by the dense above ground biomass and tangled rhizomatous mats formed by reed canarygrass. Results of our models indicate that bullfrogs may be more tolerant of invasive vegetation at WRP sites. Thus, management actions that reduce the cover of invasive reed canarygrass (and other invasive plants) could improve habitat quality for native amphibians, especially for postmetamorphic chorus frogs and red-legged frogs.
Premetamorphic chorus frogs and postmetamorphic red-legged frogs were positively associated with the additional management variable of WRP site age. The relationship between WRP age and native anuran counts suggests the potential for temporally-explicit recolonization following habitat alteration. The benefits of restored wetlands for native amphibians may increase over time corresponding to vegetative succession and system stability. Bullfrog counts were not associated with WRP age, possibly because bullfrogs often readily colonize and are fairly tolerant of bare-ground habitats characteristic of initial phases of restoration (Porej and Hetherington 2005). Antagonistic encounters between bullfrogs and native species may be influenced by priority effects in timing of colonization and occur more frequently in newly created or restored wetlands, leading to the exclusion of natives. Many studies have found that interactions between bullfrogs or non-native fish and native amphibians are highly context-dependent and mediated by habitat quality (Hayes and Jennings 1986; Adams 1999; Pearl et al. 2005; Adams et al. 2011). For this reason, dense emergent vegetation occurring at later stages of restoration could provide important refuge for native amphibian species (e.g., Kiesecker et al. 2001).
Species-specific and ontogeny-specific differences in native anuran associations with invasive vertebrates were detected. Bullfrog count was a negative predictor of postmetamorphic chorus frog and red-legged frog counts, however non-native fish had the strongest negative relationship with native anuran counts and occurred in top models for all life stages. While bullfrogs have been implicated in the decline of red-legged frogs in the Willamette Valley (Nussbaum et al. 1983), several studies describe behaviors in native anurans that suggest they are adapting to the presence of bullfrogs. In fact, Hayes and Jennings (1986) reasoned that non-native fish—not bullfrogs—are the strongest factor contributing to ranid frog declines. Kiesecker and Blaustein (1997) found that red-legged frog larvae from populations syntopic with bullfrogs exhibited antipredator behaviors (e.g., reduced activity levels and increased refuge use) when exposed to bullfrog chemical cues. However, experimental studies have documented reduced red-legged frog and chorus frog activity levels, development rates, and survivorship due to exploitative competition from bullfrogs (Kupferberg 1997; Kiesecker et al. 2001). Our study only detected negative effects of bullfrogs on postmetamorphic phases, and this is likely due to asymmetric phenologies of native anurans and invasive bullfrogs. The majority of the chorus frog and red-legged frog postmetamorphic detections were of juveniles, with emergence events corresponding with bullfrog breeding season and an elevated likelihood of encounter. Further information is needed on the long-term dynamics of coexisting native and invasive populations, especially as bullfrogs are increasingly common in seasonal ponds throughout the Willamette Valley (Cook 2011; Cook et al. 2013).
The strongest structuring components of amphibian community composition were the presence of non-native fish and wetland hydrology; management regime did not directly influence the species composition of the amphibian assemblage. Ordination of amphibian communities with respect to hydroperiod and fish revealed a contrast between native amphibian and bullfrog associations. Native amphibians—especially aquatic larval stages of long-toed salamanders (rarely detected), red-legged frogs, and chorus frogs—were negatively associated with non-native fish and permanent hydroperiods while most life stages of bullfrogs were positively associated. Invasive bullfrogs have a larval period that typically extends beyond 1 year, so it follows that permanent water bodies will enhance successful development to metamorphosis and subsequent natal pond returns (Boone et al. 2004). Long-toed salamanders, chorus frogs, and red-legged frogs, however, commonly metamorphose within one season in the Pacific Northwest (Jones et al. 2005), and larvae may be afforded greater protection from vertebrate predators in seasonal wetlands (Skelly 1996). NMS allowed us to explore stage-specific responses to habitat and management variables that were not detected through modeling since we did not incorporate hydroperiod into a priori GLMs.
Consistent with previous studies (e.g., Pearl et al. 2005), we found reduced native amphibian occurrence (especially for rarely-detected long-toed salamanders and northwestern salamanders) but increased invasive bullfrog occurrence at sites inhabited by non-native fish. The rough-skinned newt, however, was an anomaly among native amphibian species, occurring most commonly at sites with non-native fish. This species is highly toxic and unpalatable to many predators (Brodie 1968), offering an explanation for its association with fish. The most frequently encountered species of fish in this study were largemouth bass and bluegill, which coevolved with bullfrogs in their native eastern range (Adams et al. 2003). Amphibians native to the Willamette Valley evolved in the absence of these novel predators; as such, they may not possess innate or learned antipredator behaviors to respond appropriately to risk (Pearl et al. 2005; Garcia et al. 2012). In addition, bullfrogs can be facilitated by bluegill, which reduce densities of aeschnid dragonflies that commonly prey on bullfrog larvae (Werner and McPeek 1994; Adams et al. 2003). Invasional meltdown (Simberloff and Von Holle 1999) between fish and bullfrogs is known to produce intensified direct and indirect impacts on native amphibians (e.g., Kiesecker and Blaustein 1998).
Urban land cover was negatively associated with counts of postmetamorphic chorus frogs, but was not associated with patterns of community composition that included rare species and life stages. The relative importance of urban land cover varied among species and life stages; this may reflect differences in habitat requirements/specialization, dispersal distance, reproductive potential, and physical tolerances (Cushman 2006). Postmetamorphic chorus frogs are dependent on upland terrestrial habitats and migrate to aquatic breeding sites once winter rains begin (Nussbaum et al. 1983; Bulger et al. 2003). Thus, high quality, connected matrix habitat consisting of forest buffers, wetlands, and minimal human disturbance is expected to facilitate chorus frog dispersal. In contrast to chorus frogs, red-legged frogs and bullfrogs were unaffected by urban cover. These species have relatively large dispersal distances compared to the chorus frog (Smith and Green 2005), and thus may be less constrained by landscape composition immediately surrounding a central wetland. Also, bullfrogs are tolerant of human modified habitats and chemical contaminants (Smith et al. 2004; Boone et al. 2007), and may even experience competitive release in urban landscapes which are sub-optimal for native amphibians. An additional variable describing regional agricultural land cover would have provided further insight into the anthropogenic pressures faced by native amphibians at these sites.
An inherent constraint to the interpretation of multi-species abundance or occupancy models is biased count data resulting from imperfect detection. This problem may be especially apparent in herpetological studies, since detection rates may differ as a function of habitat covariates, and species may vary in cryptic color patterns, activity levels, and breeding phenologies (Mazerolle et al. 2007). For these reasons, we used count in place of abundance to emphasize the estimate of a true population value. Every effort was made to select sampling periods based on the most likely detection window for each species in the assemblage, but observations may have been biased toward late-season breeders (e.g., chorus frogs, red-legged frogs, rough-skinned newts, and bullfrogs) over early-season breeders (e.g., long-toed salamanders and northwestern salamanders). In addition, our survey methodology may have yielded higher detectability to conspicuous anurans (chorus frogs, red-legged frogs, and bullfrogs), breeding adult rough-skinned newts, and larvae of all species, as opposed to adults of fossorial mole salamanders (long-toed salamanders and northwestern salamanders). Although the potential for detection bias is worth noting, we opted not to use occupancy models because binary presence-absence data provides less ecological information (Welsh et al. 2013). Ideally, we would have used abundance models accounting for detectability in open populations, however at present these models only exist for single-species analyses (e.g., Royle et al. 2007) or multi-species closed populations (e.g., Waddle et al. 2010). Since amphibian breeding seasons are characterized by emigration and immigration events, the assumption of a closed population for which to estimate species-specific detectability through mark-recapture techniques would be unrealistic for our system. Further, recent simulation data suggests that occupancy models correcting for detection probabilities can have similar bias and even greater estimate variance compared to unadjusted models (Welsh et al. 2013).
Our results emphasize the importance of accounting for both site-scale and landscape-scale conditions in conservation planning, especially for species that utilize aquatic and terrestrial habitats throughout their annual cycle. By including inter-patch landscape structure as a scoring criteria for WRP wetland locations, practitioners can better provide for the requirements of native amphibians, increase the persistence of viable breeding populations (Lehtinen et al. 1999), and minimize the risk of creating population sinks or ecological traps (Shulse et al. 2010). At the site-scale, invasive species, especially fish, strongly influenced amphibian assemblages. In the Willamette Valley, where flooding events allow for widespread movement of aquatic organisms, it is imperative that managers incorporate landscape-scale dynamics into adaptive strategies. Efforts to restore local and regional habitat quality (e.g., removing invasive plant species, creating vegetative buffers, and reducing human impacts) may benefit native amphibians by indirectly contributing to the resistance of vertebrate invasions (Adams and Pearl 2007). However, the most effective way to enhance native amphibian populations may be through focusing wetland creation in habitats resilient to or removed from non-native vertebrates.
In multi-trophic invaded systems, complex species interactions make management outcomes on wildlife difficult to predict. This study illustrates that strong top-down forces exerted by non-native vertebrate species can be primary regulators of native amphibian abundance and diversity. Restoration ecology currently focuses on bottom-up effects of invasive plant management on biodiversity, presenting the need for a paradigm shift which also considers higher-order interactions within novel systems.
References
Adams M, Pearl C (2007) Problems and opportunities managing invasive bullfrogs: is there any hope? Biological Invaders in Inland Waters: Profiles, Distribution, and Threats 2:679–693
Adams MJ (1999) Correlated factors in amphibian decline: exotic species and habitat change in western Washington. Journal of Wildlife Management 63:1162–1171
Adams MJ, Pearl CA, Bury RB (2003) Indirect facilitation of an anuran invasion by non-native fishes. Ecology Letters 6:343–351
Adams MJ, Pearl CA, Galvan S, Mccreary B (2011) Non-native species impacts on pond occupancy by an anuran. Journal of Wildlife Management 75:30–35
Akaike H (1973) Information theory as an extension of the maximum likelihood principle. In: Petrov BN, Csaki F (eds) Second International Symposium on Information Theory. Akademiai Kiado, Budapest, pp 267–281
Baines D, Sage RB, Baines MM (1994) The implications of red deer grazing to ground vegetation and invertebrate communities of Scottish native pinewoods. Journal of Applied Ecology 31:776–783
Babbitt KJ (2005) The relative importance of wetland size and hydroperiod for amphibians in southern New Hampshire, USA. Wetlands Ecology and Management 13:269–279
Baker JP, Hulse DW, Gregory SV et al (2004) Alternative futures for the Willamette River basin, Oregon. Ecological Applications 14:313–324
Blaustein AR, Kiesecker JM (2002) Complexity in conservation: lessons from the global decline of amphibian populations. Ecology Letters 5:597–608
Boers AM, Veltman RLD, Zedler JB (2007) Typha x glauca dominance and extended hydroperiod constrain restoration of wetland diversity. Ecological Engineering 29:232–244
Boone MD, Little EE, Semlitsch RD, Fox SF (2004) Overwintered bullfrog tadpoles negatively affect salamanders and anurans in native amphibian communities. Copeia 2004:683–690
Boone MD, Semlitsch RD, Little EE, Doyle MC (2007) Multiple stressors in amphibian communities: effects of chemical contamination, bullfrogs, and fish. Ecological Applications 17:291–301
Brodie ED Jr (1968) Investigations on the skin toxin of the adult rough-skinned newt, Taricha granulosa. Copeia 1968(2):307–313
Brown CJ, Blossey B, Maerz JC, Joule SJ (2006) Invasive plant and experimental venue affect tadpole performance. Biological Invasions 8:327–338
Bulger JB, Scott NJ Jr, Seymour RB (2003) Terrestrial activity and conservation of adult California red-legged frogs (Rana aurora draytonii) in coastal forests and grasslands. Biological Conservation 110:85–95
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Cook MT (2011) Invasive bullfrog use of novel ephemeral habitats in the Pacific Northwest. Thesis, Oregon State University, Corvallis, USA
Cook MT, Heppell SS, Garcia TS (2013) Invasive bullfrog larvae lack developmental plasticity to changing hydroperiods. The Journal of Wildlife Management 77:655–662
Crump ML, Scott NJ Jr (1994) Visual encounter surveys. In: Heyer WR, Donnelly MA, McDiarmid RW, Hayek LC, Foster MS (eds) Measuring and monitoring biological diversity: standard methods for amphibians. Smithsonian Institutional Press, Washington, D.C., pp 84–91
Cushman SA (2006) Effects of habitat loss and fragmentation on amphibians: a review and prospectus. Biological Conservation 128:231–240
Dahl TE (1990) Wetlands Losses in the United States 1780’s to 1980’s. US Department of the Interior, Fish and Wildlife Service, Washington
Dahl TE (2006) Status and trends of wetlands in the conterminous United States 1998 to 2004. U.S. Department of the Interior; Fish and Wildlife Service, Washington
Davis MJ, Purrenhage JL, Boone MD (2012) Elucidating predator–prey interactions using aquatic microcosms: complex effects of a crayfish predator, vegetation, and atrazine on tadpole survival and behavior. Journal of Herpetology 46:527–534
Denton RD, Richter SC (2013) Amphibian communities in natural and constructed ridge top wetlands with implications for wetland construction. Journal of Wildlife Management 77(5):886–896
Evans-Peters GR, Dugger BD, Petrie MJ (2012) Plant community composition and waterfowl food production on Wetland Reserve Program easements compared to those on managed public lands in Western Oregon and Washington. Wetlands 32:391–399
Garcia TS, Thurman LL, Rowe JC, Selego S (2012) Antipredator behavior of the invasive American bullfrog (Lithobates catesbeianus) in a novel environment. Ethology 118:1–9
Gibbs JP (2001) Wetland loss and biodiversity conservation. Conservation Biology 14:314–317
Gray RL, Teels BM (2006) Wildlife and fish conservation through the Farm Bill. Wildlife Society Bulletin 34:90–913
Hartel T, Nemes S, Cogălniceanu D, Öllerer K, Schweiger O, Moga CI, Demeter L (2007) The effect of fish and aquatic habitat complexity on amphibians. Hydrobiologia 583:173–182
Hayes MP, Jennings MR (1986) Decline of ranid frog species in western North America: are bullfrogs (Rana catesbeiana) responsible? Journal of Herpetology 20(4):490–509
Hoef JMV, Boveng PL (2007) Quasi-poisson vs. negative binomial regression: how should we model overdispersed count data? Ecology 88:2766–2772
Hurvich CM, Tsai CL (1989) Regression and time series model selection in small samples. Biometrika 76:297–307
Janssen A, Sabelis MW, Magalhães S, Montserrat M, Van der Hammen T (2007) Habitat structure affects intraguild predation. Ecology 88:2713–2719
Johnson JB, Omland KS (2004) Model selection in ecology and evolution. Trends in Ecology & Evolution 19:101–108
Jones L, Leonard W, Olson D (2005) Amphibians of the Pacific Northwest. Seattle Audobon Society, Seattle
Kiesecker JM, Blaustein AR (1997) Population differences in responses of red-legged frogs (Rana aurora) to introduced bullfrogs. Ecology 78(6):1752–1760
Kiesecker JM, Blaustein AR (1998) Effects of introduced bullfrogs and smallmouth bass on microhabitat use, growth, and survival of native red-legged frogs (Rana aurora). Conservation Biology 12:776–787
Kiesecker JM, Blaustein AR, Miller CL (2001) Potential mechanisms underlying the displacement of native red-legged frogs by introduced bullfrogs. Ecology 82:1964–1970
King SL, Twedt DJ, Wilson RR (2006) The role of the Wetland Reserve Program in conservation efforts in the Mississippi River Alluvial Valley. Wildlife Society Bulletin 34:914–920
Kross J, Kaminski RM, Reinecke KJ, Penny EJ, Pearse AT (2008) Moist-soil seed abundance in managed wetlands in the Mississippi Alluvial Valley. Journal of Wildlife Management 72:707–714
Kupferberg SJ (1997) Bullfrog (Rana catesbeiana) invasion of a California river: the role of larval competition. Ecology 78(6):1736–1751
Larson DL, Anderson PJ, Newton W (2001) Alien plant invasion in mixed-grass prairie: effects of vegetation type and anthropogenic disturbance. Ecological Applications 11:128–141
Lehtinen RM, Galatowitsch SM, Tester JR (1999) Consequences of habitat loss and fragmentation for wetland amphibian assemblages. Wetlands 19:1–12
Magurran AE (2004) Measuring biological diversity. Blackwell, Oxford
Mather PM (1976) Computational methods of multivariate analysis in physical geography. Wiley, London
Mazerolle MJ, Bailey LL, Kendall WL, Royle JA, Converse SJ, Nichols JD (2007) Making great leaps forward in herpetology: accounting for detectability in field studies. Journal of Herpetology 41:672–689
Mielke PW Jr (1984) Meteorological applications of permutation techniques based on distance functions. Handbook of Statistics 4:813–830
Miller RC, Zedler JB (2003) Responses of native and invasive wetland plants to hydroperiod and water depth. Plant Ecology 167:57–69
Morlan JC (2000) Summary of current status and health of Oregon’s freshwater wetlands. Oregon State of the Environment Report. Oregon Press Board 45–52
Mueller-Dombois D, Ellenberg H (1974) Aims and methods of vegetation ecology. Wiley, New York
[NRCS 2011] Natural Resource Conservation Service (2011) Restoring America’s wetlands: a private lands conservation success story. http://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb1045079.pdf. Accessed 12 Dec 2011
[NRCS 2012] Natural Resources Conservation Service (2012) Conservation Programs. Wetlands Reserve Program. http://www.nrcs.usda.gov/programs/wrp/.html. Accessed 4 Jan 2012
Nussbaum RA, Brodie ED Jr, Storm RM (1983) Amphibians and reptiles of the Pacific Northwest. University of Idaho Press, Moscow
Olson DH, Leonard WP, Bury RB (eds) (1997) Sampling amphibians in lentic habitats. Society for Northwestern Vertebrate Biology, Olympia
Pearl CA, Adams MJ, Leuthold N, Bury RB (2005) Amphibian occurrence and aquatic invaders in a changing landscape: implications for wetland mitigation in the Willamette Valley, Oregon, USA. Wetlands 25:76–88
Petranka JW, Harp EM, Holbrook CT, Hamel JA (2007) Long-term persistence of amphibian populations in a restored wetland complex. Biological Conservation 138:371–380
Petranka JW, Kennedy CA, Murray SS (2003) Response of amphibians to restoration of a southern Appalachian wetland: a long-term analysis of community dynamics. Wetlands 23:1030–1042
Porej D, Hetherington TE (2005) Designing wetlands for amphibians: the importance of predatory fish and shallow littoral zones in structuring of amphibian communities. Wetlands Ecology and Management 13:445–455
Preston DL, Henderson JS, Johnson PTJ (2012) Community ecology of invasions: direct and indirect effects of multiple invasive species on aquatic communities. Ecology 93:1254–1261
R Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rejmánek M (2000) Invasive plants: approaches and predictions. Austral Ecology 25:497–506
Ricciardi A, MacIsaac HJ (2011) Impacts of biological invasions on freshwater ecosystems. Fifty years of invasion ecology: the legacy of Charles Elton. Wiley-Blackwell, West Sussex
Riley SPD, Busteed GT, Kats LB, et al. (2005) Effects of urbanization on the distribution and abundance of amphibians and invasive species in southern California streams. Conservation Biology 19:1894–1907
Rittenhouse TAG (2011) Anuran larval habitat quality when reed canary grass is present in wetlands. Journal of Herpetology 45:491–496
Rothermel BB (2004) Migratory success of juveniles: a potential constraint on connectivity for pond-breeding amphibians. Ecological Applications 14:1535–1546
Royle JA, Kéry M, Gautier R, Schmid H (2007) Hierarchical spatial models of abundance and occurrence from imperfect survey data. Ecological Monographs 77:465–481
Semlitsch RD (1998) Biological delineation of terrestrial buffer zones for pond-breeding salamanders. Conservation Biology 12:1113–1119
Shea K, Chesson P (2002) Community ecology theory as a framework for biological invasions. Trends in Ecology & Evolution 17:170–176
Shulse CD, Semlitsch RD, Trauth KM, Williams AD (2010) Influences of design and landscape placement parameters on amphibian abundance in constructed wetlands. Wetlands 30:915–928
Simberloff D, Von Holle B (1999) Positive interactions of nonindigenous species: invasional meltdown? Biological Invasions 1:21–32
Simpson EH (1949) Measurement of diversity. Nature 163:688
Skelly DK (1996) Pond drying, predators, and the distribution of Pseudacris tadpoles. Copeia 1996(3):599–605
Smith GR, Vaala DA, Dingfelder HA, Temple KG (2004) Effects of nitrite on bullfrog (Rana catesbeiana) tadpoles from central Ohio, USA. Bulletin of Environmental Contamination and Toxicology 72:1012–1016
Smith MA, Green DM (2005) Dispersal and the metapopulation paradigm in amphibian ecology and conservation: are all amphibian populations metapopulations? Ecography 28:100–128
Summers EA (2010) Evaluating ecological restoration in Tennessee hardwood bottomland forests. Thesis, University of Tennessee, Knoxville, USA
Symonds MRE, Moussalli A (2010) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behavioral Ecology and Sociobiology 65:13–21
Sztatecsny M, Jehle R, Schmidt B, Arntzen JW (2004) The abundance of premetamorphic newts (Triturus cristatus, T. marmoratus) as a function of habitat determinants: an a priori model selection approach. Herpetological Journal 14:89–97
[TWC 2009] Oregon Natural Heritage Information Center and The Wetlands Conservancy (2009) Oregon Wetlands Cover. http://oregonexplorer.info. Accessed 10 Jan 2012
[USGS 2011] US Geological Survey, Gap Analysis Program (GAP) (2011) National Land Cover, Version 2. http://gapanalysis.usgs.gov/data. Accessed 11 Jan 2012
Van Buskirk J (2005) Local and landscape influence on amphibian occurrence and abundance. Ecology 86:1936–1947
Vottler TH, Muir TA (1996) Wetland protection legislation. Water-Supply Paper, United States Geological Survey, Washington
Waddle JH, Dorazio RM, Walls SC, Rice KG, Beauchamp J, Schuman MJ, Mazzotti FJ (2010) A new parameterization for estimating co-occurrence of interacting species. Ecological Applications 20:1467–1475
Waddle JH, Glorioso BM, Faulkner SP (2012) A quantitative assessment of the conservation benefits of the Wetlands Reserve Program to amphibians. Restoration Ecology 21:1–7
Watling JI, Hickman CR, Orrock JL (2011) Predators and invasive plants affect performance of amphibian larvae. Oikos 120:735–739
Watson JW, McAllister KR, Pierce DJ, Alvarado A (2000) Ecology of a remnant population of Oregon spotted frogs (Rana pretiosa) in Thurston County, Washington. Final Report. Washington Department of Fish and Wildlife, Olympia
Welsh AH, Lindenmayer DB, Donnelly CF (2013) Fitting and interpreting occupancy models. PLoS ONE 8(1):e52015. doi:10.1371/journal.pone.0052015
Werner EE, McPeek MA (1994) Direct and indirect effects of predators on two anuran species along an environmental gradient. Ecology 75(5):1368–1382
Whigham DF (1999) Ecological issues related to wetland preservation, restoration, creation and assessment. Science of the Total Environment 240:31–40
Whittaker RH (1972) Evolution and measurement of species diversity. Taxon 21:213–251
Wilbur HM (1980) Complex life cycles. Annual review of Ecology and Systematics 11:67–93
Acknowledgments
We are grateful for the support from a diverse group of collaborators and for funding from Oregon State University, The Wildlife Society, and private donors. The WRP landowners were imperative for the success of this project—without their generosity in providing access to their private lands, this study would not have been possible. D. Aleshire (NRCS) and J. Jebousek (United States Fish and Wildlife Service) offered critical logistical and technical assistance, as did personnel from other partners, including The Nature Conservancy and Oregon Metro. We greatly appreciate the efforts of field assistants who accompanied us in the field for long, arduous hours. We are obliged to recognize two anonymous reviewers and the associate editor who provided constructive comments that improved an earlier version of this manuscript. We also thank L. Ganio, B. Dugger, S. Chan, A. Herlihy, B. McCune, G. Evans-Peters, and D. Olson for contributing their wealth of expertise to various aspects of this study. Finally, A.R. Wallace provided inspiration.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Online Resource 1
(PDF 197 kb)
Online Resource 2
(PDF 346 kb)
Rights and permissions
About this article
Cite this article
Rowe, J.C., Garcia, T.S. Impacts of Wetland Restoration Efforts on an Amphibian Assemblage in a Multi-invader Community. Wetlands 34, 141–153 (2014). https://doi.org/10.1007/s13157-013-0492-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-013-0492-z