Abstract
Loss of wetlands worldwide has necessitated the creation of wetlands to counteract declines of fauna associated with these habitats. Ephemeral wetlands have been disproportionally lost and hydrology of ephemeral wetlands is challenging to restore. Created wetlands with water control structures may be a viable option. In Western Kentucky, we surveyed three ephemeral wetland types [managed open canopy (MOC), unmanaged open canopy (UMOC), and unmanaged closed canopy (UMCC); managed = created wetlands with water control structures] to estimate amphibian richness and occupancy among wetlands, and estimated abundance of three locally common species: Southern Leopard Frog (Lithobates sphenocephalus), Spotted Salamander (Ambystoma maculatum), and Crawfish Frog (L. areolatus). In addition, we quantified physical characteristics and water quality among wetland types. Managed Open Canopy wetlands had a greater percent of submergent vegetation than both UMCC and UMOC wetlands, shallower depth at 1.0 m from the wetted wetland edge than UMOC wetlands, and were larger than UMCC wetlands. Mean predicted amphibian species richness and occupancy was highest at larger wetlands (0.15–0.78 ha). Occupancy of three common species was not influenced by management. Estimated abundance of L. areolatus, a species of conservation concern, was higher at MOC wetlands, and conversely, A. maculatum abundance was highest at UMCC wetlands. Larger wetlands had higher estimated abundances of L. areolatus and L. sphenocephalus. Our results suggest that created, open canopy wetlands managed for hydroperiod have similar species richness to unmanaged ephemeral wetlands. Furthermore, these managed wetlands provide habitat for a species of concern in Kentucky (i.e., L. areolatus).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High rates of global historical wetland loss (> 50%; Meyers 1997) has increased efforts for wetland creation and management. Since the advent of Ducks Unlimited in 1937, wetlands have been created and managed for game species (e.g., duck, deer, turkey; Biebighauser 2011); however, recently the emphasis has shifted to non-game species (Stahlschmidt et al. 2012; Calhoun et al. 2014). Specifically, wetland creation for amphibians has been heavily advocated in recent years due to widespread population declines (Brown et al. 2012). Investigations have focused on wetland design (Snodgrass et al. 2000; Porej and Hetherington 2005; Shulse et al. 2010; Drayer and Richter 2016; Rothenberger et al. 2019), placement in the landscape (Houlahan and Findlay 2003; Rannap et al. 2009; Rothenberger et al. 2019), amphibian community dynamics (Porej and Hetherington 2005; Shulse et al. 2010; Denton and Richter 2013; Drayer and Richter 2016), and creating habitats for species of conservation concern (Ammon et al. 2003; Ashpole et al. 2018; Magnus and Rannap 2019). Yet, follow-up monitoring has indicated various levels of success regarding how well populations and communities establish (Brown et al. 2012; Calhoun et al. 2014; Rothenberger et al. 2019). A central finding from previous studies indicates that hydroperiod, or the length of time a wetland is inundated, can strongly influence species richness in created wetlands (i.e., Snodgrass et al. 2000; Babbit 2005; Denton and Richter 2013; Drayer and Richter 2016).
Historically, wetlands with ephemeral hydroperiods (i.e., dry completely at least once annually) were common in the conterminous United States (Tiner 2003). These habitats are vital for maintaining amphibian diversity (Kirkman et al. 1999; Calhoun et al. 2017) as predators are excluded by seasonal drying (Porej and Hetherington 2005; Kross and Richter 2016; Toledo 2005), a process essential for successful reproduction of many amphibian species (Kross and Richter 2016). Furthermore, ephemeral wetlands can produce considerable amphibian biomass (159 kg/ha/year; Gibbons et al. 2006), which can bolster amphibian population sizes while providing an important ecological link between aquatic and terrestrial systems (Regester and Whiles 2006; Capps et al. 2015). Yet, managing and creating ephemeral wetlands can present challenges. Short-duration hydroperiods are difficult to mimic and wetland creation and restoration efforts seldom produce hydrologically dynamic, and thus ecologically, functioning ephemeral wetlands (Gamble and Mitsch 2009; Moreno-Mateos et al. 2012; Calhoun et al. 2014; but see Strain et al 2017; Rothenberger et al. 2019). However, where managers are available on site, wetlands with water control structures can produce seasonal hydroperiods (i.e., ephemeral wetlands) by adjusting water levels in response to rain events or time of year (Galatowitsch and Van der Valk 1994; Baecher et al. 2018). This management strategy is an important conservation tool for species of concern that rely on ephemeral wetlands.
The Mississippi Embayment in Western Kentucky, a northern extension of the Southeastern Coastal Plain region known for its amphibian diversity, contains 34 amphibian species. This region is bounded by the Mississippi River, Ohio River, and Tennessee River and is characterized by alluvial deposits and loess. The Mississippi Embayment contains the highest concentration of wetlands and fertile soil in the state; much of Kentucky’s historic wetland loss (81%; Dahl 1990) occurred in this region due to conversion of wetlands to agricultural land. This region of Kentucky is an Amphibian Conservation Area for the state as it hosts several amphibian species of state conservation concern, including Mole Salamander (Ambystoma talpoideum), Lesser Siren (Siren intermedia), Green Treefrog (Hyla cinerea), and Crawfish Frog (Lithobates areolatus), a species considered near threatened by the International Union for Conservation of Nature (Hammerson and Parris 2004; Kentucky’s Comprehensive Wildlife Conservation Strategy 2013). To support amphibian diversity and overcome historical wetland loss in Western Kentucky, creation of wetlands with a variety of hydroperiods and, in particular, ephemeral wetlands is essential. Although created wetlands have been established in Western Kentucky since at least 1998 (Dahl 2006, 2011), to our knowledge, no study has evaluated created, ephemeral wetlands in this region with respect to physical wetland characteristics or amphibian use of created wetlands with water control structures.
Wetlands with water control structures were created from 2002 to 2016 at West Kentucky Wildlife Management Area (WKWMA) as part of a management plan for amphibians and waterfowl (Fig. 1). Comparing these created, ephemeral wetlands with water control structures to unmanaged (water control structures absent) ephemeral wetlands, our objectives were to: (1) characterize differences in physical wetland characteristics and water quality parameters across wetland management category, (2) analyze amphibian species richness and occupancy among wetland management categories, canopy cover and wetland size, and (3) estimate abundance of three locally common amphibian species: Southern Leopard Frog (L. sphenocephalus), Spotted Salamander (Ambystoma maculatum), and L. areolatus relating to wetland management category and wetland size.
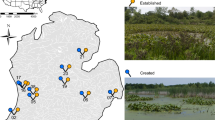
Map of study site: West Kentucky Wildlife Management Area (WKWMA), McCracken County, Kentucky, USA with wetland management types: Unmanaged Open Canopy (UMOC) represented by open circles, Unmanaged Closed Canopy (UMCC) represented by open triangles, and Managed Open Canopy (MOC) represented by open crosses. Inset depicts the state of Kentucky with McCracken County in dark gray
Methods
Study Site
West Kentucky Wildlife Management Area is located in McCracken County, KY adjacent to the Ohio River in the Mississippi Embayment physiographic province (Fig. 1). The Management area encompasses 2630 hectares (ha) and is owned and leased by Kentucky Department of Fish and Wildlife Resources. Over 100 wetlands occur on the site (Price and Kreher 2016). These wetlands are of unknown origin; most naturally occur on the site, but a few wetlands were likely created for livestock or other purposes prior to 1980 (T. Kreher pers. comm). Regardless, these wetlands do not have water control structures. Several wetlands, built from 2002 to 2016, are actively managed for hydroperiod with water control structures. These managed wetlands were created to provide habitat for amphibians and migrating birds (Price and Kreher 2016). At these managed wetlands, water control structures are used to slowly draw down water from May through July; these ponds are generally dry from late July through early October. The uplands surrounding the managed wetlands are maintained as grassland and have been planted with game forage plant species including Eastern Gamagrass (Tripsacum dactyloides), Orchardgrass (Dactylis glomerata), Red Clover (Trifolium pretense), and Redtop (Agrostis gigantea), while unmanaged wetlands have grassland species that recruited naturally.
Physical wetland characteristics and water quality
We examined habitat conditions at 30 ephemeral wetlands. Prior to sampling, we grouped the wetlands into three management categories. Wetland management categories include both level of management (i.e. water control structures present/absent; managed = water control structure present) and canopy cover class (i.e. open/closed) resulting in seven managed open canopy (MOC), seven unmanaged open canopy wetlands (UMOC), and 16 unmanaged closed canopy wetlands (UMCC) (Fig. 1). Managed wetlands ranged in age from one to 15 years. Wetlands in the unmanaged categories were ephemeral wetlands identified by Price and Kreher (2016) with unknown origins (i.e. present on WKWMA prior to 1980 with no known records of creation) and may be created or natural wetlands lacking water control structures. Percent canopy cover was determined for each wetland using a spherical densiometer (Forestry Suppliers, Jackson, MS, USA; Price and Kreher 2016). Wetlands were considered closed canopy if they had 95–100% canopy closure and were considered open canopy if they had 40% or less canopy closure; all sampled wetlands were above 95% or below 40% canopy cover. We used 2014 National Agriculture Imagery Program (NAIP) aerial imagery, acquired during the agricultural growing season, in a GIS (ArcMap 10.3, ESRI; Price and Kreher 2016) and, for wetlands constructed after 2014, we imported georeferenced Google Earth Imagery (Google Earth 2015, 2019). We used these layers to digitize maximum wetland surface area (i.e. area inside perimeter of wetland basin visible in aerial imagery) polygons and used the calculate geometry tool within the polygon layer to determine maximum surface area of each wetland. Percent vegetation (emergent or submergent) and wetland littoral depth measurements were recorded at 0.5 m and 1.0 m from the wetted wetland edge from May 1 to 10, 2017.
We also assessed water chemistry at the 30 wetlands. Water samples (250 ml) were collected from May 15 to 17, 2017 and placed on ice in accordance with standard sampling protocol. Water samples were analyzed for concentrations of total organic carbon (TOC), pH, alkalinity, chloride (Cl), sulfate (SO4), nitrate (NO3-N), ammonium (NH4-N), calcium (Ca), magnesium (Mg), potassium (K), sodium (Na), nitrite (NO2-N), iron (Fe), manganese (Mn), total suspended solids (TSS), and specific conductance (Cond). Water quality sampling, preservation, and analytic protocols were performed in accordance with standard methods (Greenberg et al. 1992). The Forest Hydrology Lab at the University of Kentucky conducted all water quality analyses.
To assess differences in water quality and wetland characteristics based on wetland management category, we used a non-parametric Kruskal–Wallis test with a Bonferroni correction in SPSS (SPSS 24, IBM Corp). Significant differences in mean rank, detected by the Kruskal–Wallis test (Kruskal and Wallis 1952), were further analyzed using Dunn-Bonferroni pairwise comparison tests (Dunn 1964).
Amphibian surveys
We used quantitative larval amphibian surveys (i.e. dipnet sweeps; Skelly and Richardson 2010) to assess amphibian communities at the 30 sites from May 1, 2017 through July 12, 2017 capturing the known larval window in Kentucky for all wetland breeding amphibian species expected at WKWMA (Fig. 2). Before each survey we recorded variables expected to influence detection including water temperature (with a digital thermometer) and day of year. At each wetland, dipnet surveys were conducted on five occasions. The number of dipnet sweeps per wetland per visit were determined based on square meter surface area of the wetland (1 sweep per 25 m2) at time of sampling (as per Shulse 2010). Captured larvae were counted and classified to species. We released all individuals at their capture location after counts were recorded.
Known periods of larval amphibian presence in wetlands for species present in McCracken Co., Kentucky by calendar date (pers. com. J. MacGregor). Asterisks indicate presumed larval windows due to limited data availability for the species in Kentucky. Note: Siren intermedia adults are aquatic and available for capture year-round. Black dotted lines indicate amphibian sampling window
Species richness analysis
To estimate amphibian community responses to site specific covariates (wetland area and management category) and sampling covariates (date and water temperature), we used a multi-species hierarchical Bayesian community occupancy model (Zipkin et al. 2009; Hunt et al. 2013). This approach incorporates species and assemblage-level covariate effects into the same modeling framework, allowing species-specific estimation of occurrence and detection probabilities along with site-specific estimates of species richness (Dorazio and Royle 2005; Zipkin et al. 2009). Using this modeling approach, individual species-level estimates are a combination of the single species and the average estimate of those parameters for the entire community (Pacifici et al. 2014), thus individual parameter estimates, particularly for rare species, are more precise and less likely to be biased (Sauer and Link 2002). Specifically, estimates for data-poor species with few detections are more precise because they can borrow information from data-rich species, or those with many detections (Pacifici et al. 2014). Notably, borrowing information may only be appropriate if the species that are sharing information have some degree of relatedness (Pacifici et al. 2014), whether ecological, functional, or behavioral. In this study, all species were sampled as larvae, and thus all individuals occur in ponds during the same time and are similarly detectable using our capture methods.
We generated species-specific observance matrices for five sampling occasions at each site, where detection was represented as 1, and non-detection as 0. We let \({z}_{i,j}\) denote true occupancy status such that \({z}_{i,j}=1\) if species i occupies site j, otherwise \({z}_{i,j}=0\). The occupancy state is considered a Bernoulli random variable, \({z}_{i,j}\sim Bern({\Psi }_{i,j})\), where \({\Psi }_{i,j}\) is the probability that species i occupies site j. Similarly, we modeled species detection as a Bernoulli random variable: \({y}_{i,j,k}\sim Bern({p}_{i,j,k}*{z}_{i,j})\), where \({y}_{i,j,k}\) is 1 if species i is detected at site j during survey k, or 0 otherwise and \({p}_{i,j,k}\) is the probability that species i is detected at site j during survey k.
We related species-specific amphibian covariate parameters (α and β values, described below) and occupancy and detection probabilities (Ψij and Θijk respectively) with the model below.
We modeled detection probabilities for each species with the following equation, within the model described above:
Parameters α2–α3 are interpreted as contrasts of the categorical predictor variable “management category” (i.e., UMOC and MOC) with "UMCC" as the reference category. The ui parameter is the mean community response (across species) to each α parameter listed above. For example, uα1 is the mean community response to the Area covariate. Continuous covariates (i.e., Area, Day of Year, and Water Temperature) were centered and scaled (i.e., [site’s Area value − mean]/SD). We used uninformative priors for the hyper-parameters (i.e., U(-3 to 3) for μα and μβ parameters and U(0, 5) for all σ parameters; species-specific model coefficients were truncated at ± 5 from μ to avoid traps).
We estimated species richness at sampled sites by summing indicator variables for estimated occupancy for each species at each site and simulated species richness at hypothetical sites with wetland area ranging from 0.01 to 0.8 hectares for each model iteration to generate a posterior predictive distribution for species richness as a function of wetland size. For species richness and abundance modeling, we fit models using WinBUGS (Spiegelhalter et al. 2003) called from R (3.4.4) (R Core Team 2018) and executed using the R2WinBUGS (Sturtz et al. 2005) package. We implemented these models in a Bayesian framework using Markov chain Monte Carlo (MCMC) sampling in WinBUGS to generate samples from the posterior distribution (Lunn et al. 2000). For the species richness model, we used 3 Markov chains, each of length 100,000; the first 70,000 were removed as burn-in, and remainder were thinned by a factor of 3. Across the three chains, this provided 30,000 samples to approximate posterior summary statistics for each model parameter including mean, standard deviation, and 2.5% and 97.5% percentiles of the distribution, which represent 95% Bayesian credible intervals. For species richness and abundance modeling (below) we assessed model convergence via the Gelman-Rubin diagnostic and a visual inspection of chains, with both measures indicating a reasonable assumption of convergence (i.e., for all monitored parameters, the Gelman-Rubin statistic value was at or below 1.02; Gelman and Rubin 1992).
Abundance analysis
We used a binomial mixture model (Royle 2004) to examine effects of site-specific covariates (wetland surface area and wetland management category) and sampling covariates (Day of Year and Water Temperature) expected to influence L. areolatus, L. sphenocephalus, and A. maculatum abundance estimates. We conducted five replicate count surveys at 30 spatially distinct sites (i) during temporally indexed surveys (j), denoted as cij (Royle and Dorazio 2008). Under this framework, counts were modeled as independent outcomes of binomial sampling with index Ni and detection probability pj. Abundances (λ) at the local level were modelled with a Poisson distribution, and heterogeneity in abundance among populations due to site-specific covariates (xi) were modelled using a Poisson-regression formulation of local mean abundances, given by log(λi) = β0 + β1xi. Sources of heterogeneity in detection were identified by modelling associations between sampling covariates and pi such that logit(pij) = α0 + α1xij. See Price et al. (2013) for further model description.
We organized count data by site and survey and specified species abundance with the model below.
Heterogeneity in detection probability was modelled with the following equation included within the model described above:
Parameters α2–α3 are interpreted as contrasts of the categorical predictor variable “management category” (i.e., UMOC and MOC) with “UMCC” as the reference category. Continuous covariates (i.e., Area, Day of Year, and Water Temperature) were centered and scaled (i.e., [site’s Area value − mean]/SD). We used uninformative priors; specifically, we assumed β0, β1, β2, β3 ~ N (0,10), α0 ~ N (0, 1.62) and α1, α2, ~ N (0,10). The α0 prior approximates a U (0,1) prior for expit(α0), where expit represents the inverse logit function (i.e., exp(α)/(1 + exp(α)). We used 3 Markov chains, each of length 150,000; the first 100,000 were removed as burn-in, and remainder were thinned by a factor of 3. Across the three chains, this provided 50,000 samples to approximate posterior summary statistics for each model parameter including mean, standard deviation, and 2.5% and 97.5% percentiles of the distribution, which represent 95% Bayesian credible intervals.
Results
Physical wetland characteristics and water quality
Three wetland characteristics differed by wetland category, percent submergent vegetation (χ2 (2) = 15.78, p < 0.001), wetland depth at 1.0 m from wetted wetland edge (χ2 (2) = 6.28, p < 0.04), and wetland surface area (χ2 (2) = 9.11, p < 0.01). MOC wetlands had a greater percent submergent vegetation (10.14 ± 4.13) than both UMCC and UMOC wetlands, shallower depth at 1.0 m from the wetted wetland edge (189.48 ± 13.85 mm) than UMOC wetlands, and larger wetland surface area (mean = 0.32 ha, SE = ± 0.10) than UMCC wetlands (Table 1). Of the sixteen water quality parameters tested, eight were different among wetland management categories, including: conductivity (χ2 (2) = 11.67, p < 0.01), TOC (χ2 (2) = 10.18, p < 0.01), PO4 (χ2 (2) = 9.10, p = 0.01), alkalinity (χ2 (2) = 9.63, p < 0.01), Ca (χ2 (2) = 10.18, p < 0.01), Mg (χ2 (2) = 10.61, p < 0.01), K (χ2 (2) = 9.39, p < 0.01), and NO2 (χ2 (2) = 6.58, p = 0.04); UMCC wetlands had the highest concentrations of six of these parameters (Table 2).
Species richness, occupancy, and detection
Among our 30 sampled wetlands, we observed 13 amphibian species including three species of conservation concern, A. talpoideum (41 total captures; 3 UMCC, 2 UMOC; 0.02–0.15 ha), H. cinerea (16 total captures; 2 MOC, 1 UMCC; 0.15–0.78 ha), and L. areolatus (286 total captures; 7 MOC, 9 UMCC, 4 UMOC; 0.01–0.78 ha) (Table 3). Amphibian species richness was positively associated with wetland size (Fig. 3). More specifically, assuming average values of other site and sampling covariates, predicted species richness per site increased from a median of 6 species (95% CI 3–9) at the smallest wetlands (< 0.01 ha) to 11 species (95% CI 7–13; Fig. 3) at the largest wetlands (0.78 ha). When examining amphibians as an assemblage, the mean wetland surface area coefficient estimate was positive (μα1: 0.83; 95% CI 0.20–1.62), indicating support for a positive relationship between mean occupancy probability and increasing wetland size (up to 0.78 ha; Fig. 3). There was no difference in estimated species richness among wetland management categories (mean UMCC = 6.44; 95% CI 5.44–7.44, UMOC = 6.86; 95% CI 5.00–7.57, MOC = 7.43; 95% CI 5.86–8.57). Likewise, we did not detect clear evidence for a positive or negative relationship between occupancy probability and wetland management category across the amphibian assemblage (UMCC mean 53.4%, 95% CI 24.5–80.8%; UMOC 47.6%, 95% CI 16.5–81.2%, MOC 42.7%, 95% CI 12.3–79.9%).
Relationship between wetland surface area (hectares) and median estimated species richness of amphibians in Western Kentucky, USA. Solid lines represent the posterior median and dashed lines represent the 95% predictive interval of species richness at hypothetical sites. Circles are site-specific mean richness estimates
Across amphibian species, we estimated a positive association between mean occupancy probability and wetland size, but the magnitude of the relationship varied among species (Fig. 4). Green Frog (L. clamitans), Southern Leopard Frog (L. sphenocephalus), and Upland Chorus Frog (Pseudacris feriarum) had high occupancy probability across all wetlands. Three species, Cope’s Gray Treefrog (Hyla chrysoscelis), Eastern Cricket Frog (Acris crepitans), and American Bullfrog (L. catesbeianus) only attained high mean occupancy probability (> 75%) at relatively large wetlands (> 0.3 ha; Fig. 4). Likewise, we estimated a positive association between mean occupancy and wetland size for two species of conservation concern, L. areolatus and H. cinerea. Mean occupancy probability of several species, American Toad (Anaxyrus americanus), H. cinerea, A. talpoideum, Small-Mouthed Salamander (A. texanum), and Tiger Salamander (A. tigrinum), was low across all sites (0.13–0.22; Table 3). Although A. talpoideum mean occupancy was low, precluding a conclusive association with wetland size, it is notable that 33 of the 41 captured individuals were in wetlands ≥ 0.15 ha. For locally common species, L. areolatus, L. sphenocephalus, and A. maculatum, estimated mean occupancy was not influenced by management category. More specifically, credible intervals were relatively wide around estimates, and mean occupancy varied little among species (79.4 to 89.80% at UMCC; 60.1 to 85.8% at UMOC; 44.1 to 83.4% at MOC wetlands; Fig. 5).
Relationship between wetland size and mean occupancy probability for thirteen amphibian species in Western Kentucky, USA: Green Frog (Lithobates clamitans), Southern Leopard Frog (L. sphenocephalus), Upland Chorus Frog (Pseudacris feriarum), Crawfish Frog (L. areolatus), Cope’s Gray Treefrog (Hyla chrysoscelis), Eastern Cricket Frog (Acris crepitans), American Bullfrog (L. catesbeianus), Spotted Salamander (Ambystoma maculatum), Green Treefrog (H. cinerea), Small-Mouthed Salamander (A. texanum), American Toad (Anaxyrus americanus), Mole Salamander (A. talpoideum), and Eastern Tiger Salamander (A. tigrinum). Credible intervals are omitted for clarity, and asterisks indicate species for which the Area parameter estimate (α1i) did not overlap zero
Mean detection probabilities varied from 13% for A. tigrinum to 96% for L. clamitans; (Table 3). The estimated assemblage response to the Day of Year covariate indicated that detection probability was greater when sampling occurred at later dates (β1 = 0.24; 95% CI 0.02–0.49; Fig. 6). Species most strongly influenced by increasing Day of Year included A. maculatum (β1 = 0.29; 95% CI 0.01–0.70) and H. chrysoscelis (β1 = 0.42; 95% CI 0.08–1.13). Four species, including L. areolatus, were not influenced by date and had consistently high (> 70%) estimated detection probabilities throughout the entire sampling period (Fig. 6). We did not detect clear evidence for a positive or negative influence of Water Temperature on detection probability of the amphibian assemblage.
Relationship between date and mean detection probability for thirteen amphibian species at wetlands in Western Kentucky, USA: Green Frog (Lithobates clamitans), Southern Leopard Frog (L. sphenocephalus), Upland Chorus Frog (Pseudacris feriarum), Crawfish Frog (L. areolatus), Cope’s Gray Treefrog (Hyla chrysoscelis), Eastern Cricket Frog (Acris crepitans), American Bullfrog (L. catesbeianus), Spotted Salamander (Ambystoma maculatum), Green Treefrog (H. cinerea), Small-Mouthed Salamander (A. texanum), American Toad (Anaxyrus americanus), Mole Salamander (A. talpoideum), and Eastern Tiger Salamander (A. tigrinum). Credible intervals are omitted for clarity, and asterisks indicate species for which the Area parameter estimate (α1i) did not overlap zero
Estimated abundance of locally common species
The estimated abundance of three locally common species varied across wetland management categories and with wetland surface area. Lithobates areolatus were most abundant in MOC wetlands (β3 = 1.55, 95% CI 1.11–2.01; mean 46.29, 95% CI 20.84–105.11; Fig. 7), but were also positively associated with UMCC wetlands (β0 = 2.194, 95% CI 1.48–3.07), and showed no relationship with UMOC wetlands (β2 = − 0.45, 95% CI − 1.14 to 0.19). In contrast, estimated abundances of A. maculatum were greatest in UMCC wetlands (β0 = 2.58, 95% CI 2.37–2.79; mean 13.23, 95% CI 10.67–16.22; Fig. 7) and negatively associated with UMOC wetlands (β2 = − 1.51, 95% CI − 2.04 to − 1.03). Lithobates sphenocephalus were not influenced by wetland management category, and mean abundance estimates were similar across wetland types (mean 8.24 to 15.51; Fig. 7). Wetlands with a greater surface area had higher estimated abundances of L. areolatus (β1 = 0.62, 95% CI 0.52–0.73) and L. sphenocephalus (β1 = 0.61, 95% CI 0.46–0.78; Fig. 8).
Relationship between wetland surface area (hectares) and mean estimated amphibian abundance [Crawfish Frog (Lithobates areolatus), Southern Leopard Frog (L. sphenocephalus), and Spotted Salamander (Ambystoma maculatum)]. Solid lines represent the mean and dashed lines represent the 95% credible interval
Discussion
Creating wetlands that function as natural habitats can be challenging because physical wetland characteristics are difficult to replicate and play a significant role in governing the amphibian species that use these habitats (Calhoun et al. 2014). Our comparison between managed and unmanaged ephemeral wetlands identified differences in physical wetland characteristics and water quality. Amphibian species richness and occupancy did not vary among managed and unmanaged wetlands and the overall amphibian community responded positively to larger wetlands. Although estimated occupancy of the three locally common species varied little among management categories, estimated abundances varied by management category and wetland surface area. Our research suggests wetlands 0.15–0.78 ha are important conservation tools for the overall amphibian community in Western Kentucky as wetlands in this size range support three species of conservation concern and greater than half of the predicted amphibian species richness in this study. In addition, we found larger (0.15–0.78 ha), created MOC wetlands to be a viable option for augmenting local populations of L. areolatus, a species of conservation concern.
Physical wetland characteristics influence chemical and biological processes within wetlands (Pollock et al. 1995). When compared to unmanaged wetlands, managed ephemeral wetlands located at WKWMA were characterized by a large wetland surface area, open canopy, submergent vegetation, and shallow wetland littoral depth. We observed a marked increase in aquatic macrophytes within our open canopy wetlands, MOC and UMOC, when compared with our closed canopy wetlands, UMCC. Large wetlands with maintained open canopy increase availability of solar radiation reaching the water surface (Shoemaker et al. 2005), boosting primary productivity (Skelly et al. 2002) leading to increased macrophytes (i.e. submergent vegetation), known to decrease conductivity (as observed in MOC and UMOC wetlands), increase dissolved oxygen, and create pockets of cooler water temperatures below, and warmer temperatures above submerged plants (Brix 1994; Sharip et al. 2012). Our MOC wetlands had the shallowest littoral depth compared with UMCC and UMOC wetlands and shallow littoral depth is linked to increases in water temperature of at least 2ºC compared with adjacent open water (Sharip et al. 2012). Hydroperiod fluctuations, characteristic of ephemeral wetlands, can also drive variability in water temperature, chemical and biological processes, availability of nutrients, and substrate exposure (Bidwell 2013; Gołdyn et al. 2015). Water temperature fluctuations initiate microbial activity within these systems (Kadlec 1999), prompting changes in water chemistry (Bodelier and Dedysh 2013). Given the variability in water quality in ephemeral wetland systems and our limited sample, water chemistry results for WKWMA wetlands should be considered tenuous. Nevertheless, we recorded different water quality values in UMCC sites compared with UMOC and MOC sites. We suspect these values are the result of allochthonous leaf litter input (Palik et al. 2006) at these closed canopy sites; however, the water quality parameter values for all WKWMA wetlands, UMCC, UMOC, and MOC, were all within the normal range for isolated, ephemeral wetlands (Brodman et al. 2003; Wingham and Jordan 2003).
We found overall estimated amphibian occupancy and species richness increased as wetland surface area increased. Wetland surface area has been positively correlated with species richness, particularly in ephemeral (Babbitt 2005) and open canopy wetlands (Werner et al. 2007). Research by Semlitsch et al. (2015) found the highest amphibian species richness in wetland sizes similar to those in our study (0.1 ha to 1.0 ha). However, this wetland size effect may be limited to wetlands between 0.1 and 1.0 ha as Snodgrass et al. (2000) found no relationship with species richness and wetland surface area in wetlands > 1.0 hectare in size. Semlitsch et al. (2015) suggest this intermediate wetland size effect on amphibian species richness is likely due to the threshold between increased drying pressure in small wetlands and increased predation pressure (i.e. fish, invertebrates) present in large wetlands. Thus, in our study, wetlands closer to 1.0 ha represent ephemeral wetlands that contain enough water to prevent drying too early for successful amphibian development but are not large enough for predator establishment. In addition, surface area of ephemeral wetlands may be particularly important for amphibian larvae because smaller wetlands may have less thermal (Lucas and Reynolds 1967) and structural refugia (Kopp et al. 2006) for developing larvae during the drying cycle. Our study indicates that occupancy probability of five species (A. crepitans, H. chrysoscelis, H. cinerea, L. areolatus, and L. catesbeianus) is higher at larger wetlands (up to 0.78 ha), as did Shulse et al. (2010) and Zipkin et al. (2012). Increased occupancy at larger ephemeral wetlands is likely related to the natural history of these species. Acris crepitans, H. cinerea, L. areolatus, and L. catesbeianus are frequently found in larger bodies of water (i.e., lakes; flooded fields) and H. chrysoscelis are habitat generalists and can be found in a variety of habitats (Dodd 2013). While our results indicate a positive association of the amphibian community with wetland size, it is important to note this association for some species could be driven by our choice to use a community model, specifically for species with limited capture data (i.e. A. tigrinum, A. texanum, A. talpoideum, A. americanus, H. cinerea, and L. catesbeianus). Using this modeling approach incorporates partial pooling to generate occupancy probabilities for each species, and rare species will tend toward the mean (Pacifici et al. 2014; Sauer and Link 2002; Zipkin et al. 2009, 2012). However, Zipkin et al. (2009, 2012, 2020) highlight the advantage of using the full community, given hierarchical model limitations, as rather than losing valuable information by removing rare species due to lack of data, these species can be included leading to more robust community conservation and management decisions.
Three species were common across all wetlands and permitted a more in-depth examination of variation in abundance. In accordance with each species’ preferred habitats and natural history, abundance varied by wetland management category. Lithobates areolatus and A. maculatum are known obligates of open canopy wetlands (Heemeyer et al. 2012; Dodd 2013) and closed canopy wetlands (Petranka 1998), respectively, and L. sphenocephalus was not partial to just one management category as expected for a habitat generalist (Dodd 2013). This suggests that although maintaining closed canopy wetlands is important for habitat specialists like A. maculatum (Calhoun et al. 2014), open canopy wetlands may benefit a wider range of species and can increase local amphibian diversity (Werner et al. 2007; Skelly et al. 2014). Open canopy wetlands benefit developing amphibian larvae by supporting increased vegetative growth (Skelly et al. 2002) necessary for predator avoidance (Kopp et al. 2006); notably we observed abundant submergent vegetation within MOC and UMOC wetlands. Further, open canopy wetlands tend to have higher water temperatures resulting in faster larval growth rates (Skelly 2005). Finally, the ability to regulate water level within these MOC wetlands allows managers to maintain ideal hydroperiod length during variable weather conditions, thus reducing predator populations (Porej and Hetherington 2005; Toledo 2005; Kross and Richter 2016) and maintaining local amphibian diversity (Kirkman et al. 1999; Calhoun et al. 2017).
While it is important to note that our study is limited to Western Kentucky and one amphibian breeding season, results indicate that created wetlands with water control structures can enhance local L. areolatus populations, as has been found for other amphibian species of conservation concern (i.e. L. luteiventris—Ammon et al. 2003; Spea intermontana—Ashpole et al. 2018; Triturus cristatus, Pelobates fuscus—Magnus and Rannap 2019). We found L. areolatus mean occupancy estimates to be higher than other parts of the species' range (Williams et al. 2012; Baecher et al. 2018) and abundance was highest in MOC wetlands suggesting that creation of large (0.15–0.78 ha), open canopy wetlands with shallow littoral depths and water control structures may be an important management strategy for this species. Baecher et al. (2018) corroborate this finding in Arkansas where L. areolatus calling intensity was greater at created wetlands with water control structures surrounded by restored native tallgrass prairie. Reduced abundances of L. sphenocephalus and A. maculatum in MOC wetlands are likely beneficial to persistence of L. areolatus in these habitats. Parris and Semlitsch (1998) found L. areolatus larval performance decreased alongside interspecific competition with other Lithobates sp., including competition with L. sphenocephalus. In addition, recently metamorphosed L. sphenocephalus leaving the wetlands may out-compete L. areolatus for food resources. For example, Crawford et al. (2009) found significant overlap in metamorph diet between the two species, and where they co-exist, significantly lower volumes of prey in L. areolatus stomach contents compared to L. sphenocephalus.
Managed open canopy wetlands (0.15–0.78 ha) with water control structures provide habitat for a wide variety of amphibians and function to augment local populations of amphibian species of conservation concern (i.e. L. areolatus) dependent on ephemeral wetland hydrology in Western Kentucky. Water control structures, while management intensive, provide an avenue for manipulating hydroperiod length, which is the most difficult component of natural wetlands to replicate (Calhoun et al. 2014). In addition, our findings corroborate previous research on wetland management that suggests wetland design features (i.e. wetland surface area, canopy cover) play a key role in facilitating increased species richness and abundance. Creating a matrix of wetlands with differing attributes (i.e. closed canopy cover, wetlands < 0.15 ha, etc.) is imperative for addressing local amphibian species declines (Petranka et al. 2007; Magnus and Rannap 2019), and large (0.15–0.78 ha) open canopy wetlands with water control structures should be considered a viable option.
References
Ammon EM, Gorley CR, Wilson KW, Ross DA, Peterson CR (2003) Advances in habitat restoration for the Columbia spotted frog (Rana luteiventris): a case study from the Provo River population. In: Proceedings: California riparian systems: processes and floodplains management, ecology and restoration. Riparian Habitat Joint Venture and Western Section of the Wildlife Society, Sacramento, CA, pp 348–356
Ashpole S, Bishop C, Murphy S (2018) Reconnecting amphibian habitat through small pond construction and enhancement, South Okanagan River Valley, British Columbia, Canada. Diversity 10:108
Babbitt KJ (2005) The relative importance of wetland size and hydroperiod for amphibians in southern New Hampshire, USA. Wetl Ecol Manage 13:269–279
Baecher JA, Vogrinc PN, Guzy JC, Kross CS, Willson JD (2018) Herpetofaunal communities in restored and unrestored remnant tallgrass prairie and associated wetlands in northwest Arkansas, USA. Wetlands 38:157–168
Biebighauser TR (2011) Wetland restoration and construction: a technical guide. Upper Susquehanna Coalition, Owego
Bidwell JR (2013) Physical and chemical monitoring of wetland water. In: Anderson JT, Davis CA (eds) Wetland techniques. Springer, Dordrecht, pp 325–353
Bodelier P, Dedysh SN (2013) Microbiology of wetlands. Front Microbiol 4:79
Brix H (1994) Functions of macrophytes in constructed wetlands. Water Sci Technol 29:71–78
Brodman R, Ogger J, Bogard T, Long AJ, Pulver RA, Mancuso K, Falk D (2003) Multivariate analyses of the influences of water chemistry and habitat parameters on the abundances of pond-breeding amphibians. J Freshw Ecol 18:425–436. https://doi.org/10.1080/02705060.2003.9663978
Brown DJ, Street GM, Nairn RW, Forstner MR (2012) A place to call home: amphibian use of created and restored wetlands. Int J Ecol. https://doi.org/10.1155/2012989872
Calhoun AJ, Arrigoni J, Brooks RP, Hunter ML, Richter SC (2014) Creating successful vernal pools: a literature review and advice for practitioners. Wetlands 34:1027–1038
Calhoun AJ, Mushet DM, Bell KP, Boix D, Fitzsimons JA, Isselin-Nondedeu F (2017) Temporary wetlands: challenges and solutions to conserving a ‘disappearing’ ecosystem. Biol Conserv 211:3–11
Capps KA, Berven KA, Tiegs SD (2015) Modelling nutrient transport and transformation by pool-breeding amphibians in forested landscapes using a 21-year dataset. Freshw Biol 60:500–511
Crawford JA, Shepard DB, Conner CA (2009) Diet composition and overlap between recently metamorphosed Lithobates areolatus and Lithobates sphenocephalus: implications for a frog of conservation concern. Copeia 2009:642–646
Dahl TE (1990) Wetlands losses in the United States 1780’s to 1980’s. U.S Department of the Interior, Fish and Wildlife Service, Washington, D.C., p 13
Dahl TE (2006) Status and trends of wetlands in the conterminous United States 1998 to 2004. U.S Department of the Interior, Fish and Wildlife Service, Washington, D.C., p 112
Dahl TE (2011) Status and trends of wetlands in the conterminous United States 2004 to 2009. U.S Department of the Interior, Fish and Wildlife Service, Washington, D.C., p 108
Denton RD, Richter SC (2013) Amphibian communities in natural and constructed ridge top wetlands with implications for wetland construction. J Wildl Manag 77:886–889
Dodd CK (2013) Frogs of the United States and Canada, vol 2. JHU Press, Baltimore
Dorazio RM, Royle JA (2005) Estimating size and composition of biological communities by modeling the occurrence of species. J Am Stat Assoc 100:389–398
Drayer AN, Richter SC (2016) Physical wetland characteristics influence amphibian community composition differently in constructed wetlands and natural wetlands. Ecol Eng 93:166–174
Dunn OJ (1964) Multiple comparisons using rank sums. Technometrics 6:241–252
Galatowitsch SM, Van der Valk AG (1994) Restoring prairie wetlands: an ecological approach. Iowa State University Press, Iowa
Gamble DL, Mitsch WJ (2009) Hydroperiods of created and natural vernal pools in central Ohio: a comparison of depth and duration of inundation. Wetl Ecol Manage 17:385–395
Gelman A, Rubin DB (1992) Inference from iterative simulation using multiple sequences. Stat Sci 7:457–472
Gibbons JW, Winne CT, Scott DE et al (2006) Remarkable amphibian biomass and abundance in an isolated wetland: implications for wetland conservation. Conserv Biol 20:1457–1465
Gołdyn B, Kowalczewska-Madura K, Celewicz-Gołdyn S (2015) Drought and deluge: Influence of environmental factors on water quality of kettle holes in two subsequent years with different precipitation. Limnologica 54:14–22
Google Earth (2015) https://www.google.com/earth/ Imagery. Accessed 5 Aug 2015
Google Earth (2019) https://www.google.com/earth/ Imagery. Accessed 29 Jul 2019
Greenberg AE, Clesceri LS, Eaton AD (1992) Standard methods for the examination of water and wastewater, 18th edn. American Public Health Association, Washington, DC
Hammerson G, Parris M (2004) Lithobates areolatus. The IUCN Red List of Threatened Species. https://doi.org/10.2305/IUCN.UK.2004.RLTS.T58546A11799946.en. Accessed 02 Jul 2019
Heemeyer JL, Williams PJ, Lannoo MJ (2012) Obligate crayfish burrow use and core habitat requirements of Crawfish Frogs. J Wildl Manag 76:1081–1091
Houlahan JE, Findlay CS (2003) The effects of adjacent land use on wetland amphibian species richness and community composition. Can J Fish Aquat Sci 60:1078–1094
Hunt SD, Guzy JC, Price SJ, Halstead BJ, Eskew EA, Dorcas ME (2013) Responses of riparian reptile communities to damming and urbanization. Biol Conserv 157:277–284
Kirkman LK, Golladay SW, Laclaire L, Sutter R (1999) Biodiversity in southeastern, seasonally ponded, isolated wetlands: management and policy perspectives for research and conservation. J North Am Benthol Soc 18:553–562
Kadlec RH (1999) Chemical, physical and biological cycles in treatment wetlands. Water Sci Technol 40:37–44
Kentucky’s Comprehensive Wildlife Conservation Strategy (2013) Kentucky Department of Fish and Wildlife Resources, #1 Sportsman's Lane, Frankfort, Kentucky 40601. https://fw.ky.gov/WAP/Pages/Default.aspx (Date updated 2/5/2013)
Kopp K, Wachlevski M, Eterovick PC (2006) Environmental complexity reduces tadpole predation by water bugs. Can J Zool 84:136–140
Kross CS, Richter SC (2016) Species interactions in constructed wetlands result in population sinks for wood frogs (Lithobates sylvaticus) while benefitting eastern newts (Notophthalmus viridescens). Wetlands 36:385–393
Kruskal WH, Wallis WA (1952) Use of ranks in one-criterion variance analysis. J Am Stat Assoc 47:583–621
Lucas EA, Reynolds WA (1967) Temperature selection by amphibian larvae. Physiol Zool 40:159–171
Lunn DJ, Thomas A, Best N, Spiegelhalter D (2000) WinBUGS-a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 10:325–337
Magnus R, Rannap R (2019) Pond construction for threatened amphibians is an important conservation tool, even in landscapes with extant natural water bodies. Wetl Ecol Manage 27:323–341
Moreno-Mateos D, Power ME, Comín FA, Yockteng R (2012) Structural and functional loss in restored wetland ecosystems. PLoS Biol 10(1):e1001247
Myers N (1997) The rich diversity of biodiversity issues. In: Reaka-Kudla ML, Wilson DE, Wilson EO (eds) Biodiversity II: understanding and protecting our biological resources. Joseph Henry Press, Washington, D.C., pp 128–129
Pacifici K, Zipkin EF, Collazo JA, Irizarry JI, DeWan A (2014) Guidelines for a priori grouping of species in hierarchical community models. Ecol Evol 4:877–888
Palik B, Batzer DP, Kern C (2006) Upland forest linkages to seasonal wetlands: litter flux, processing, and food quality. Ecosystems 9:142–151
Parris MJ, Semlitsch RD (1998) Asymmetric competition in larval amphibian communities: conservation implications for the Northern Crawfish Frog, Lithobates areolatus circulosa. Oecologia 116:219–226
Petranka JW (1998) Ambystoma maculatum. Salamanders of the United States and Canada. Smithsonian Institution Press, Washington, DC, pp 77–87
Petranka JW, Harp EM, Holbrook CT, Hamel JA (2007) Long-term persistence of amphibian populations in a restored wetland complex. Biol Conserv 138:371–380
Pollock MM, Naiman RJ, Erickson HE, Johnston CA, Pastor J, Pinay G (1995) Beaver as engineers: influences on biotic and abiotic characteristics of drainage basins. Linking species & ecosystems. Springer, Boston, MA, pp 117–126
Porej D, Hetherington TE (2005) Designing wetlands for amphibians: the importance of predatory fish and shallow littoral zones in structuring of amphibian communities. Wetl Ecol Manage 13:445–455
Price SJ, Guzy JC, Witzcak L, Dorcas ME (2013) Do ponds on golf courses provide suitable habitat for wetland-dependent animals? An assessment of turtle abundances. J Herpetol 47:243–250
Price SJ, Kreher T (2016) Amphibian habitat assessment at the Paducah Gaseous Diffusion Plant and the West Kentucky State Wildlife Management Area. Prepared for University of Kentucky Center for Applied Energy Research, Kentucky Research Consortium for Energy and Environment. UK/KRCEE Doc# P27.16.2015
R Development Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rannap R, Lohmus A, Briggs L (2009) Restoring ponds for amphibians: a success story. Hydrobiologia 634:87–95
Regester KJ, Whiles MR (2006) Decomposition rates of salamander (Ambystoma maculatum) life stages and associated energy and nutrient fluxes in ponds and adjacent forest in southern Illinois. Copeia 2006:640–649
Rothenberger MB, Vera MK, Germanoski D, Ramirez E (2019) Comparing amphibian habitat quality and functional success among natural, restored, and created vernal pools. Restor Ecol 27:881–891
Royle JA (2004) N-mixture models for estimating population size from spatially replicated counts. Biometrics 60:108–115
Royle JA, Dorazio RM (2008) Hierarchical modeling and inference in ecology: the analysis of data from populations, metapopulations and communities. Academic Press, Cambridge
Sauer JR, Link WA (2002) Hierarchical modeling of population stability and species group attributes from survey data. Ecology 83:1743–1751
Semlitsch RD, Peterman WE, Anderson TL, Drake DL, Ousterhout BH (2015) Intermediate pond sizes contain the highest density, richness, and diversity of pond-breeding amphibians. PLoS ONE 10(4):e0123055
Sharip Z, Hipsey MR, Schooler SS, Hobbs RJ (2012) Physical circulation and spatial exchange dynamics in a shallow floodplain wetland. International Journal of Design & Nature and Ecodynamics 7:274–291
Shoemaker WB, Sumner DM, Castillo A (2005) Estimating changes in heat energy stored within a column of wetland surface water and factors controlling their importance in the surface energy budget. Water Resour Res 41:10
Shulse CD, Semlitsch RD, Trauth KM, Williams AD (2010) Influences of design and landscape placement parameters on amphibian abundance in constructed wetlands. Wetlands 30:915–928
Skelly DK, Freidenburg LK, Kiesecker JM (2002) Forest canopy and the performance of larval amphibians. Ecology 83:983–992
Skelly DK, Halverson MA, Freidenburg LK, Urban MC (2005) Canopy closure and amphibian diversity in forested wetlands. Wetl Ecol Manage 13:261–268
Skelly DK, Bolden SR, Freidenburg LK (2014) Experimental canopy removal enhances diversity of vernal pond amphibians. Ecol Appl 24:340–345
Skelly DK, Richardson JL (2010) Larval sampling. In: Dodd CK (ed) Amphibian ecology and conservation: a handbook of techniques. Oxford University Press, Oxford, pp 55–70
Snodgrass JW, Komoroski MJ, Bryan AL, Burger J (2000) Relationships among isolated wetland size, hydroperiod, and amphibian species richness: implications for wetland regulations. Conserv Biol 14:414–419
Spiegelhalter DJ, Thomas A, Best N, Lunn D (2003) WinBUGS Version 1.4. MRC Biostatistics Unit, Cambridge
Stahlschmidt P, Pätzold A, Ressl L, Schulz R, Brühl CA (2012) Constructed wetlands support bats in agricultural landscapes. Basic Appl Ecol 13:196–203
Strain GF, Turk PJ, Helmick J, Anderson JT (2017) Amphibian reproductive success as a gauge of functional equivalency of created wetlands in the Central Appalachians. Wildl Res 44:354–364
Sturtz S, Ligges U, Gelman AE (2005) R2WinBUGS: a package for running WinBUGS from R.
Tiner RW (2003) Estimated extent of geographically isolated wetlands in selected areas of the United States. Wetlands 23:636
Toledo LF (2005) Predation of juvenile and adult anurans by invertebrates: current knowledge and perspectives. Herpetol Rev 36:395–399
Werner EE, Skelly DK, Relyea RA, Yurewicz KL (2007) Amphibian species richness across environmental gradients. Oikos 116:1697–1712
Williams PJ, Robb JR, Karns DR (2012) Occupancy dynamics of breeding Crawfish Frogs in southeastern Indiana. Wildl Soc Bull 36:350–357
Whigham DF, Jordan TE (2003) Isolated wetlands and water quality. Wetlands 23:541–549
Zipkin EF, DeWan A, Royle AJ (2009) Impacts of forest fragmentation on species richness: a hierarchical approach to community modelling. J Appl Ecol 46:815–822
Zipkin EF, Grant EHC, Fagan WF (2012) Evaluating the predictive abilities of community occupancy models using AUC while accounting for imperfect detection. Ecol Appl 22:1962–1972
Zipkin EF, DiRenzo GV, Ray JM, Rossman S, Lips KR (2020) Tropical snake diversity collapses after widespread amphibian loss. Science 367:814–816
Acknowledgements
We thank Tina Marshall, Marshall County High School (MCHS), MCHS Environmental Science and AP Physics students, Michaela Lambert, Jonathan Matthews, Rachel Pagano, Wendy Leuenberger, and Allison Davis for field assistance. Steve Hampson from the University of Kentucky (UK) Kentucky Research Consortium for Energy and Environment (KRCEE) Center for Applied Energy Research provided logistical support. Site management information was provided by Tim Kreher of Kentucky Fish and Wildlife Conservation Commission (KDFWR). Research collection permits (SC1711110, SC1811095) were provided by KDFWR. Funding was provided by KRCEE and the United States Department of Energy Portsmouth Paducah Project Office, United States Department of Agriculture McIntire-Stennis Cooperative Forestry Research Program (accession number 1001968), and UK Department of Forestry and Natural Resources. This research was approved under University of Kentucky Institutional Animal Care and Use Committee protocol (2013-1073).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Drayer, A.N., Guzy, J.C., Caro, R. et al. Created wetlands managed for hydroperiod provide habitat for amphibians in Western Kentucky, USA. Wetlands Ecol Manage 28, 543–558 (2020). https://doi.org/10.1007/s11273-020-09730-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-020-09730-3