Abstract
Because wetland restoration projects are becoming more common and are expensive, it is important to evaluate their success. Evaluation studies common use measurements of soils, vegetation, hydrology and wildlife to evaluate the success of wetland restoration. In contrast, the diversity of macrobenthos and their relationships with environmental factors are often neglected. To better understand the success of wetland restoration, we examined the abundance and diversity of macrobenthos in different stages of a freshwater wetland restoration project in the Yellow River Delta in China, with reference to environmental factors that might explain macrobenthic patterns. Macrobenthic species richness and density were greater in the oldest restoration area versus the younger and no-treatment areas. Macrobenthic biomass, however, was greatest in the no-treatment area. The oldest restoration area had deeper water levels, lower salinities, softer and wetter soils, and higher soil organic, nitrogen and carbon contents, and these variables largely distinguished the macrobenthic samples in a CCA analysis. A combination of landscape position and recovery time (time since the restoration was implemented) likely explains the abiotic differences among restoration areas. We recommend an adaptive management strategy, guided by long-term monitoring and experiments, to improve the success of this and other wetland restoration projects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estuarine wetlands occur in places where rivers merge into the open seas (Mitsch and Gosselink 2007), and are characterized by a gradient of fresh, brackish and salt marshes from the upper to the lower parts of the system (Batzer and Sharitz 2006). Estuarine wetland ecosystems are valued for their multiple functions in biodiversity conservation, sea defense, carbon storage and support for life in the adjoining ecosystems (Millenium Ecosystem Assessment 2005; Boon et al. 2014). Estuarine wetlands, however, are often disturbed by anthropogenic stressors. In particular, coastal wetlands are often lost completely due to land reclamation (De Paz et al. 2008; Bulleri and Chapman 2010; Golubkov and Alimov 2010; Warwick and Somerfield 2010; Bai et al. 2013; Sukumaran et al. 2013; Ling et al. 2014; Wang et al. 2014).
One response to the widespread loss of coastal wetlands has been increased interest in wetland restoration and creation (Cui et al. 2009; Niu et al. 2011; Barbier 2013; Martinez-Martinez et al. 2014; Rowe and Garcia 2014). Many restoration and creation projects have been implemented, but the value of the projects is often difficult to assess (Broome 1990; Josselyn et al. 1990; Broome and Craft 2000). Many projects lack any type of monitoring to evaluate success, and others have very limited monitoring, typically focused on short-term measurements of vegetation (Mitsch and Wilson 1996).
Ideally, monitoring would focus not just on vegetation but also on macrobenthos (Dou et al. 2014). Macrobenthos are a key constituent of wetland ecological systems, and provide a variety of ecosystem functions including promoting biogeochemistry cycling, processing matter deposition, and providing food for birds and fishes (Herman et al. 1999; Kristensen et al. 2014; Pinto et al. 2014; Schlacher et al. 2014). In addition, macrobenthos are sensitive indicators of environmental quality, with community structure varying as a function of water depth (Conlan et al. 2008), sediment characteristics (Picanço et al. 2014), and salinity (González-Ortegón et al. 2015). Fully assessing the success of a project would also include examining additional taxa and ecosystem functions (Benayas et al. 2009).
Other issues with many restoration projects are that monitoring does not last long enough to ensure that wetlands are sustainable, and adaptive management changes are not used to address inadequate outcomes (Broome and Craft 2000; Moreno-Mateos et al. 2012). As a result, we simply do not know if many restoration projects have been effective (Mitsch and Wilson 1996).
To address the utility of monitoring macrobenthos in addition to vegetation in evaluating restoration success, we studied a large wetland restoration project located on the Yellow River Delta in China. Wetlands in the area were degraded in 1996 when they were cut off from their historical water supply (Cui et al. 2009). The two phases of restoration, totaling 9350 ha, sought to reverse this process by delivering freshwater from the Yellow River into the degraded area. To date, studies of the restoration have focused only on changes in water, soil, and vegetation (Tang et al. 2006; Cui et al. 2009). We sought to build on this past work by (1) evaluating restoration outcomes in the different stages of the project by measuring macrobenthos in addition to vegetation and edaphic variables, (2) examining relationships between macrobenthos and environmental factors to better understand the factors determining macrobenthic community structure in this area, and (3) proposing adaptive management strategies based on our findings to improve restoration outcomes in this and other projects.
Materials and Method
Study Area
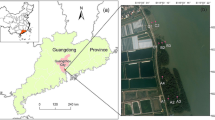
The Yellow River Delta is located north of Shandong Province, China at 118°33′E–119°20′E; 37°35′N–38°12′N, where the Yellow River flows into the Bohai Sea (Fig. 1). The region has a temperate and semi-humid continental monsoon climate with irregularly semidiurnal tide. The average annual temperature is 12.1 °C, with 196 frostless days and average annual precipitation and evaporation of 551.6 mm and 1962 mm, respectively (He et al. 2009; Cui et al. 2011). The Yellow River Delta Natural Nature Reserve was established by the Chinese government in 1992 to protect the wetland ecosystem because the delta plays an important role in diversity conservation. Periodic course changes of the Yellow River have resulted in changes in the landscape and the degradation of some wetlands (Zhang and Wang 2008). In particular, some freshwater wetlands in the Yellow River Delta that were previously dominated by Phragmites australis degenerated as a result of the scarcity of freshwater when the course of the Yellow River was artificially changed in 1996 (Cui et al. 2009). In June 2002, a wetland restoration project (the “Five Acres Wetland”) covering 2650 ha was initiated in the core zone of the Da Wenliu Management Station. In 2006, another wetland restoration project (the “Ten Acres Wetland”) covering 6700 ha was initiated adjacent to the first project. The two projects are located 4 km from the south of the current path of the Yellow River and 15 km east from the river entrance to the Bohai Sea, with the geographic center at N 37°45′48.4″ and E 119°03′07″. The restored locations lie in a low plain that inclines to the sea at a slope of 0.1 ‰ to 0.2 ‰.
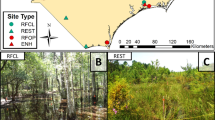
Study area and sampling plots: (a) location of Yellow River Delta Natural Nature Reserve in China, (b) study area within the Yellow River Delta, and (c) closeup of study area and sampling plots. R2002 represents the first wetland restoration project which was initiated in 2002 and is referred to as the Five Acres Wetland. Thirty sample plots (F1-F30) were located in R2002. R2006 represents the second wetland restoration project initiated in 2006, referred to as the Ten Acres Wetland. Sixteen sample plots (T1-T16) were located in R2006. R0 represents un-manipulated wetland. Six sample plots (N1-N6) were located in R0. In each section of the wetland, sample plots were set at 0.5 km to 1.0 km intervals. Figure 1 (d), (e), and (f) illustrate the main vegetation patterns of R2002, R2006 and R0, respectively. R2002 is dominated by Phragmites australis and areas of open water. R2006 is dominated by large areas of open water with some vegetation cover and mudflats. R0 is dominated by large areas of mudflat and small patches of Tamarix chinensis
The approach to wetland restoration in both projects used water management measures such as dikes, control breaks, and pumps to increase freshwater delivery during the dry season and storage, with the goal of generating large stands of Phragmites australis and areas of open water suitable for waterfowl (Li et al. 2011). Apart from natural rainfall, almost all the water required by the wetland restoration is pumped from the Yellow River. After 10 years, plant communities dominated by Phragmites australis have re-established in the Five Acres Wetland (Fig. 1d). Six years after the second restoration project began, the habitat that was historically intertidal mudflat is now dominated by large areas of open water with some vegetation cover and mudflats (Fig. 1e). An adjacent area that has not been restored (R0) is currently dominated by large areas of intertidal mudflat and small patches of Tamarix chinensis.
Sample Collection and Identification
Macrobenthos
To document the composition of macrobenthos, we sampled a total of 52 plots in R2002, R2006 and R0 (Fig. 1c). We collected a three rectangular sediment samples (33 cm long × 30 cm wide × 20 cm deep) in each of the plots in the autumn of 2012 using a stainless steel can-corer. We passed each sample through a 0.45 mm sieve, and the macrobenthos retained on the sieve were fixed in a 4 % buffered formalin. All macrobenthos were identified (in most cases to species level) under a dissecting microscope, counted, and weighed.
Environmental Factors
To identify factors that might explain macrobenthos community structure, we also sampled biotic and abiotic variables at each plot. We recorded the identify of all vascular plant species present and their coverage (visually estimated) in three replicate quadrants (1 m × 1 m) for each sample plot. We harvested above-ground biomass from a 50 cm × 50 cm quadrat centered in each 1 × 1 m quadrat, and measured below-ground biomass collected from three replicate soil cores (10 cm in diameter × 20 cm in depth) located adjacent to each 1 × 1 m plot. Above- and below-ground biomasses was air dried for 28 days and weighed.
To characterize abiotic conditions, we measured water depth and soil salinity, hardness, pH, moisture content, bulk density, porosity, organic matter, total nitrogen, and total carbon in each plot. Water depth was measured using a ruler. Soil hardness was measured using a soil penetrometer. We collected three replicate soil cores (5.05 cm in diameter × 5 cm in depth) in each sample plot to determine soil salinity, pH, moisture content, bulk density, porosity, organic matter, total carbon, and total nitrogen. Soil cores were weighed and dried in an oven (60 °C, 48 h), and reweighed to determine moisture content, bulk density, and porosity. Soil pH was measured on a 1:5 dry soil: water mixture, and pore water salinity using the rehydration method (Pennings et al. 2003; Cui et al. 2011). Soil total carbon (TC) and nitrogen (TN) were analyzed by TOC analyzer (TOC-V, Japan) and continuous-flow analysis instrument (AA3, Europe), respectively. Soil organic matter (SOM) was determined by Walkley-Black Method following Sikora and Moore (2014).
Data Analysis
We first compared macrobenthos, vegetation and abiotic variables among the three sampling areas using Analysis of Variance (ANOVA). Data were transformed (log10(x + 1) or square-root) when necessary. Plant species richness (S) and plant Shannon diversity (H’) did not meet the normality assumption of ANOVA even after transformation, so these variables were analyzed with non-parametric Kruskall-Wallis tests. These analyses were conducted using SPSS 20.0.
Shannon diversity (H’) and Pielou evenness (E) were calculated as:
where S is the total number of species in a sample plot, and Ci and C are the numbers of individual of species i and all species in the sample plot, respectively.
Second, we used multivariate approaches to identify relationships between macrobenthos (abundance of each species) and abiotic factors (water depth and soil salinity, pH, moisture content, bulk density, porosity, organic matter, total carbon, and total nitrogen). Preliminary analysis with detrended correspondence analysis (DCA) indicated that it was appropriate to analyze the dataset using unimodal methods such as canonical correspondence analyses (CCA) because the largest value of gradient length was larger than 4.0 (Lepš and Šmilauer 2003). CCA is a mature unimodal method that has been used in the field of Ecology since 1986 (ter Braak 1986). CCA relates species composition directly to environmental variables, performs well even with noise, and has become one of the most popular methods in gradient analyses (Palmer 1993; McCune et al. 2002; ter Braak 2014). We analyzed inter-species distances using biplot scaling. Prior to CCA, data were transformed as described above when necessary. CCA was performed using Canoco for Windows 4.5 (Braak and Šmilauer 2002).
Results
Macrobenthos and Biotic Factors
A total of 39 different macrobenthic species were recorded in the study plots (Table 1). The species were distributed across 2 phyla, 7 classes, and 25 families. We found 32, 7, and 5 species in R2002, R2006, and R0, respectively.
Species richness (S) of macrobenthos in the sample plots was twice as great in R2002 as in R0, and was lowest in R2006 (Fig. 2). Similarly, macrobenthos density was three times as high in R2002 as in R2006 and R0. Macrobenthos biomass did not follow the same pattern. Biomass was greatest in R0, and extremely low in R2006. Shannon diversity (H’) and Pielou evenness (E) of macrobenthos were higher in R2002 and R0 versus R2006.
Macrobenthos species richness (S), density, biomass, H’ and E in the three wetlands. Data are means ± 1 SE (n = 30, 16 and 6, in R2002, R2006 and R0, respectively). All ANOVA tests were significant (P < 0.05 in each case). Bars sharing a letter are not significantly different from one another (P > 0.05)
A total of 21 different plant species were recorded in the study plots. The dominant species in R2002 was Phragmites australis. Twenty other species were present at lower densities: Apocynum venetum, Artemisia carvifolia, Artemisia scoparia, Cynanchum chinense, Descurainia sophia, Echinochloa crusgali, Glycine soja, Imperata cylindrical, Ixeridium chinense, Limonium sinense, Melilotus officinalis, Mulgedium tataricum, Myriophyllum spicatum, Setaria glauca, Setaria viridis, Sonchus arvensis, Sonchus oleraceus, Triarrhena sacchariflora, Typha minima and Typha angustata. The study plots in R2006 and R0 did not contain any vegetation, although we observed small patches of vegetation in between plots (Fig. 1). Consequently, vegetation coverage, above- and below-ground biomass, S, H’, and E were higher in R2002 than in R2006 and R0 (Fig. 3).
Plant coverage, above- and below-ground biomass, S, H’ and E in the three wetlands. Data are means ± 1 SE (n = 30, 16 and 6, in R2002, R2006 and R0, respectively). ANOVA results are indicated above the groups of bars. The data on plant species richness and Shannon diversity did not meet the normality assumptions of ANOVA, therefore, these variables were compared using a non-parametric Kruskall-Wallis test. Results showed that species richness and Shannon diversity of plant also differed among the three wetlands (P < 0.05 in both cases)
Abiotic Factors
Abiotic factors differed between R2002, R2006, and R0 (Table 2, supporting information Figure S1). The R2002 wetland had deeper water levels, lower porewater salinities, softer and wetter soils, and higher soil organic, nitrogen and carbon contents than the other two wetlands. In addition, the R0 wetland had lower water levels and drier, saltier soils than the R2006 wetland. Soil pH, bulk density and porosity did not differ between wetlands.
Relationships Between Macrobenthos and Abiotic Factors
The first and second axes of the CCA ordination explained 27.6 and 17.7 %, respectively, of the variation (Fig. 4). The first CCA axis was positively correlated with soil hardness (0.50, P < 0.01), bulk density (0.42, P < 0.01), pH (0.55, P < 0.01), and soil salinity (0.89, P < 0.01), and negatively correlated with water depth (−0.49, P < 0.01), moisture content (−0.52, P < 0.01), porosity (−0.42, P < 0.01), and organic matter (−0.31, P < 0.05). The second CCA axis was positively correlated with soil hardness (0.28, P < 0.05), porosity (0.48, P < 0.01), organic matter (0.46, P < 0.01), total nitrogen (0.59, P < 0.01) and total carbon (0.49, P < 0.01), and negatively correlated with water depth (−0.41, P < 0.01), bulk density (−0.48, P < 0.01) and pH (−0.42, P < 0.01). Macrobenthic samples from R2002, R2006, and R0 were distributed in different ways across these environment gradients. Samples from R2002 were mainly located on the left side of the first axis, indicating that they were associated with high water depth, moisture content, organic matter, total nitrogen, and total carbon, and low salinity and hardness. Samples from R2006 were mainly located on the right side of the first axis, indicating that they were associated with low water depth, moisture content, organic matter, total nitrogen and total carbon, and high salinity and hardness. Samples from R0 were mainly distributed in the lower right quadrant of the biplot which is associated with low water depth and moisture content and high salinity. The various macrobenthos species were mainly distributed in the center and right of the biplot.
CCA biplot showing the relationships between macrobenthic communities and abiotic factors in the three wetlands. The arrows indicate environmental factors, the length of each arrow represents the strength of the relationship between the environmental variable and the distribution of macrobenthic species. Abbreviations: Pt, soil total porosity percentage; OM, soil organic matter; TC, soil total carbon; TN, soil total nitrogen. Open triangles indicate the location of each macrobenthos species; open circles: samples in R2002, open stars: samples in R2006; and crosses: samples in R0
Discussion
Has this wetland restoration project been successful in reducing wetland loss in the Yellow River Delta and recovering wetland ecological structure? R2002, which is located farthest from the coast, is now a densely-vegetated freshwater wetland with low salinity, dominated by Phragmites australis and supporting a rich diversity of macrobenthos. In contrast, R2006, which is closer to the coast, is mainly covered by open water, with sparse vegetation, and supports relatively few macrobenthic species. Nevertheless, although R2006 does not support abundant vegetation and macrobenthos, it does provide a large area used by migratory waterbirds (Li et al. 2011). Thus, whether R2006 is considered a success or a failure might depend on the variables considered. The restoration project, however, did not have quantifiable restoration targets (Shan 2007) beyond the goals of creating freshwater wetland and waterfowl habitat, and there is little information on comparable pristine systems in the area; thus, we cannot rigorously evaluate project success based on a priori criteria. We can, however, examine the different communities created in the two phases of the project versus the un-manipulated area. This comparison indicates strong temporal and abiotic controls on the management outcomes, and suggests how future projects could alter management approaches depending on the abiotic conditions and project goals.
Determinants of Macrobenthos Diversity Pattern
Macrobenthos are an important food source for waterbirds (Choi et al. 2014); thus, the effects of different management strategies on macrobenthic communities are likely to affect suitability of the wetland for waterbirds. Macrobenthic communities were the most diverse, and supported the highest number of individuals in R2002, where insects were a major component of the community; however, macrobenthic biomass was greatest in R0, where polychaetes and molluscs dominated the community. R2006 had very meagre macrobenthic communities characterized by low richness, low density, and extremely low biomass. We identified three main factors that appear to be structuring the macrobenthos and vegetation in the three wetlands: landscape position, recovery time, and consequent physical conditions.
Landscape Position
The three studied wetlands lie along a gradient from landward to seaward. This created a pre-existing salinity gradient that undoubtedly had a strong influence on macrobenthic communities (Adnitt et al. 2013). Dike construction and irrigation with freshwater strongly affected the pre-existing gradient, and further affected the diversity patterns of macrobenthos in R2002 and R2006. However, management efforts did not cause abiotic conditions in R2006 to converge with conditions in R2002. Instead, almost all abiotic conditions in R2006 remain intermediate between conditions in R2002 and R0. To some extent, this may be due to the longer history of management in R2002 than R2006 (see following paragraph), but it also likely reflects the underlying abiotic gradient driven by landscape position.
Recovery Time
In addition to supporting very limited macrobenthic communities, R2006 also has very low plant coverage, richness and biomass. Part of the explanation for this may be that less time has elapsed since restoration in R2006 versus R2002. It takes time for a wetland to equilibrate to new management conditions, and the time period required is not always easy to determine (Edwards and Proffitt 2003; Keeley et al. 2014). The recovery time period differ for different goals, that is, vegetation, macrobenthos, waterbirds and other wildlife may have different recovery time periods (Mitsch and Wilson 1996; Craft et al. 1999; Simenstad et al. 2006; Burger 2008). Long term monitoring, however, can assess when variables of interest appear to stabilize. The vegetation in R2002 was monitored by Cui et al. (2009) from 2001 (before the restoration project began) through 2007, and by us in 2012. Results showed that plant species richness increased by 50 % within 1 year after restoration from 8 species in 2001 to 14 in 2003 (1 year after the 2002 restoration), and then began to plateau, with a further increase of 28 % over the following 4 years (to 18 in 2007), and a subsequent increase of only 17 % over the next 4 years (to 21 in 2012). Thus, most of the vegetation recovery in R2002 occurred within 5 years, whereas R2006 shows very little vegetation growth even after 6 years. Therefore, it seems unlikely that recovery time alone can explain the limited macrobenthic and vegetative communities in R2006.
One difference between R2006 and R2002 is that R2006 was likely saltier to begin with due to its landscape position. If so, it may take a longer time period to observe full restoration in R2006. This possibility could be evaluated with long term monitoring of R2006 over the coming years. Alternatively, the management conditions may need to change, perhaps with increased delivery of freshwater, to produce conditions similar to those in R2002. Another possibility is that there is a recruitment bottleneck in R2006, in which case restoration might be accelerated if desired species were manually introduced (Batzer and Sharitz 2006). This possibility could be tested with transplants of various species into R2006.
Physical Conditions
Different macrobenthos species occur under different environmental conditions (Leibold and McPeek 2006). As discussed above, physical conditions suitable to freshwater species (Zedler 1983) have become more prevalent with the inflow of freshwater into R2002 (Cui et al. 2009), but likely also are a function of pre-existing differences between wetlands due to landscape position, and the length of time during which management has occurred. Regardless of the ultimate causes of the abiotic conditions, the strong abiotic gradients across the study area allow us to identify abiotic factors such as water depth and soil salinity (Sharma et al. 2011; Nishijima et al. 2013) that likely are important drivers of macrobenthic community structure.
Generally, water depth is closely related to wetland topography (Carter 1996). In this project however, water depth was further modified by diking and freshwater delivery. In previous studies, water depth strongly affected macrobenthic communities (Ysebaert et al. 2003; Carvalho et al. 2012; Pratt et al. 2014). In this study, water depth decreased in the three wetlands from landward to seaward, and macrobenthic samples sorted in the CCA analysis such that they were correlated with water depth.
Soil salinity is closely related to freshwater inflow (Mitsch and Gosselink 2007). In our study, salinity increased across the three wetlands from landward to seaward. Moreover, the deliberate irrigation of R2002 and R2006 with freshwater undoubtedly helped flush salts out of the soils. In the CCA, soil salinity was strongly correlated with CCA axis 1, and was strongly correlated with macrobenthic community composition. Achieving low salinity values, as was done with R2002, is probably necessary for the area to be dominated by freshwater wetlands and macrobenthos such as insects that are typical of freshwater wetlands. Although salinities in R2006 are fairly low, they may not be quite fresh enough, in combination with the lower water depth, to be suitable for Phragmites and the other plant species that now occur in R2002, or for the associated insect species. At the same time, conditions in R2006 are probably too fresh to allow survival of the marine species found in R0 (Torres et al. 2006).
A number of other abiotic variables differed among the wetlands and correlated with macrobenthic community composition in the CCA analysis. Soil hardness was lower in R2002 than that in R2006. This was probably mostly due to the flooded conditions, but may also have been affected by macrobenthos loosening the soil (Sassa et al. 2013). Moisture content was significantly higher in R2002 than in R2006 and R0, probably because R2002 was flooded more deeply with standing water. Organic matter, total nitrogen and total carbon were highest in R2002. These variables are often correlated with primary productivity (Batjes 2014; Ryals et al. 2014), and in this case the differences are probably explained by the high biomass of vegetation in R2002 which contributed organic matter, nitrogen and carbon to the soils (Stauffer and Brooks 1997). Although a correlative study like this one cannot rigorously determine which of these variables were more important in determining macrobenthic community structure, our impression and past work suggests that water depth and salinity were the most important variables to the macrobenthos (Verschuren et al. 2000; Batzer and Sharitz 2006; Waterkeyn et al. 2008).
Management Implications
The purpose of this wetland restoration project was to reduce the wetland loss in the Yellow River Delta and to recover its basic ecological structure and functions. Thus far, management has primarily focused on the reestablishment of vegetation. Vegetation coverage is one useful criterion of the success of wetland restoration, but not necessarily the most effective (López-Rosas et al. 2013). Macrobenthos are also key elements in wetland food webs and provide important insights into the health of wetland ecosystems (González-Ortegón et al. 2015), and populations at higher trophic levels such as birds indicate that a complete food web is present. Ideally, multiple taxa, including vegetation, macrobenthos and waterbirds, would be monitored to evaluate the success of a restoration project (Dahm et al. 1995; DeAngelis et al. 1998; Armitage et al. 2014).
This wetland restoration project was successful in developing dense vegetation and diverse macrobenthic assemblages in one wetland (R2002), but not in another (R2006). This indicates that a new phase of adaptive management is needed for the R2006 wetland. As discussed above, there are three possible reasons why the ecological communities in the R2006 wetland do not resemble those in the R2002 wetland: insufficient time, lack of immigration, and salinities that are too high. We think that the “insufficient time” hypothesis is unlikely to be correct; however, it would be possible to continue monitoring for additional years before making any other changes to allow more time for ecological communities to develop. At the same time, managers could test the “lack of immigration” hypothesis by experimentally transplanting a variety of plant and macrobenthic species into R2006. If these transplants fail to establish, it will suggest that the initial management protocol was not sufficient to create suitable abiotic conditions in R2006, and that managers should consider experimentally increasing freshwater delivery to R2006 for several years in order to assess the effects of increased freshwater supply on abiotic conditions and ecological communities. At the same time, managers should bear in mind that, because R2006 is closer to the ocean than R2002, it may be difficult to reduce salinities in R2006 to the levels that have been reached in R2002. Thus, it may be unrealistic to expect the same restoration outcomes in R2006 as in R2002.
Finally, we emphasize the importance of long-term monitoring (Borja et al. 2010) to estimate fully the changes in the ecosystem of the restored wetlands. For this project, only vegetation data were collected before the project began, limiting our ability to assess the effects of management on macrobenthos and birds. In addition, many restoration projects are only monitored for a few years. Without long-term monitoring, however, it is impossible to determine whether the restored systems are sustainable (Moreno-Mateos et al. 2012). Properly determining success may require a decade or more of monitoring (Caldwell et al. 2011). The monitoring program should be reviewed periodically and revised if needed to ensure that the most important variables are being measured (Lindenmayer and Likens 2009). Long-term monitoring and establishing the relationships between organisms and environmental factors can provide a scientific foundation for attaining consensus with regard to the solutions to management problems.
Conclusions
We examined the abundance and diversity of macrobenthos in different stages of a wetland restoration project, with reference to environmental factors that might explain macrobenthic patterns. We found that water depth, soil salinity, soil hardness, moisture content, organic matter, total nitrogen and total carbon all correlated with macrobenthic community patterns; however, we believe that water depth and salinity are the most important. The first phase of the restoration appears to have been more successful than the second. We recommend an adaptive management strategy, guided by experiments, to improve the success of the second phase. We also recommend long-term monitoring, including pre-project sampling, to evaluate the long-term success and sustainability of the restored wetlands. In order to ensure the best success and full value of future restoration projects in China or throughout the world, adequate monitoring and adaptive management should be included in the project design from the beginning.
References
Adnitt C, Brew D, Cottle R, Hardwick M, John S, Leggett D, McNulty S, Meaakins N, Staniland R (2013) Saltmarsh management manual. Environment Agency, Bristol
Armitage AR, Ho CK, Madrid EN, Bell MT, Quigg A (2014) The influence of habitat construction technique on the ecological characteristics of a restored brackish marsh. Ecol Eng 62:33–42
Bai J, Xiao R, Zhang K, Gao H, Cui B, Liu X (2013) Soil organic carbon as affected by land use in young and old reclaimed regions of a coastal estuary wetland, China. Soil Use Manag 29(1):57–64
Barbier EB (2013) Valuing ecosystem services for Coastal Wetland protection and restoration: progress and challenges. Resources 2(3):213–230
Batjes NH (2014) Total carbon and nitrogen in the soils of the world. Eur J Soil Sci 65(1):10–21
Batzer DP, Sharitz RR (Eds.) (2006) Ecology of freshwater and estuarine wetlands. Univ of California Press
Benayas JMR, Newton AC, Diaz A, Bullock JM (2009) Enhancement of biodiversity and ecosystem services by ecological restoration: a meta-analysis. Science 325(5944):1121–1124
Boon PI, Allen T, Carr G, Frood D, Harty C, Mcmahon A, Mathews S, Rosengren N, Sinclair S, White M, Yugovic J (2014) Coastal wetlands of Victoria, south-eastern Australia: providing the inventory and condition information needed for their effective management and conservation. Aquatic Conservation: Marine and Freshwater Ecosystems
Borja Á, Dauer DM, Elliott M, Simenstad CA (2010) Medium-and long-term recovery of estuarine and coastal ecosystems: patterns, rates and restoration effectiveness. Estuar Coasts 33(6):1249–1260
Braak CT, Šmilauer P (2002) CANOCO reference manual and CanoDraw for Windows user’s guide: software for canonical community ordination (version 4.5). Section on Permutation Methods. Microcomputer Power, Ithaca, New York
Broome SW (1990) Creation and restoration of tidal wetlands of the southeastern United States, vol 37. Island Press, Washington
Broome SW and Craft CB (2000) Tidal salt marsh restoration, creation, and mitigation. Pages 939–959 Reclamation of drastically disturbed lands. American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, Madison
Bulleri F, Chapman MG (2010) The introduction of coastal infrastructure as a driver of change in marine environments. J Appl Ecol 47(1):26–35
Burger J (2008) Environmental management: integrating ecological evaluation, remediation, restoration, natural resource damage assessment and long-term stewardship on contaminated lands. Sci Total Environ 400(1):6–19
Caldwell PV, Vepraskas MJ, Gregory JD, Skaggs RW, Huffman RL (2011) Linking plant ecology and long-term hydrology to improve wetland restoration success. Trans ASABE 54(6):2129–2137
Carter V (1996) Wetland hydrology, water quality, and associated functions. National Water Summary on Wetland Resources, 35–48
Carvalho S, Cunha MR, Pereira F, Pousão-Ferreira P, Santos MN, Gaspar MB (2012) The effect of depth and sediment type on the spatial distribution of shallow soft-bottom amphipods along the southern Portuguese coast. Helgol Mar Res 66(4):489–501
Choi CY, Battley PF, Potter MA, Ma Z, Liu W (2014). Factors affecting the distribution patterns of benthic invertebrates at a major shorebird staging site in the Yellow Sea, China. Wetlands, 1–12
Conlan K, Aitken A, Hendrycks E, McClelland C, Melling H (2008) Distribution patterns of Canadian Beaufort shelf macrobenthos. J Mar Syst 74(3):864–886
Craft C, Reader J, Sacco JN, Broome SW (1999) Twenty-five years of ecosystem development of constructed Spartina alterniflora (Loisel) marshes. Ecol Appl 9(4):1405–1419
Cui BS, Yang QC, Yang ZF, Zhang KJ (2009) Evaluating the ecological performance of wetland restoration in the Yellow River Delta, China. Ecol Eng 35(7):1090–1103
Cui BS, He Q, An Y (2011) Community structure and abiotic determinants of salt marsh plant zonation vary across topographic gradients. Estuar Coasts 34(3):459–469
Dahm CN, Cummins KW, Valett HM, Coleman RL (1995) An ecosystem view of the restoration of the Kissimmee River. Restor Ecol 3:225–238
de Paz L, Neto JM, Marques JC, Laborda AJ (2008) Response of intertidal macrobenthic communities to long term human induced changes in the Eo estuary (Asturias, Spain): implications for environmental management. Mar Environ Res 66(2):288–299
DeAngelis DL, Gross LJ, Huston MA, Wolff WF, Fleming DM, Comiskey EJ, Sylvester SM (1998) Landscape modeling for Everglades ecosystem restoration. Ecosystems 1:64–75
Dou P, Cui B, Xie T, Dong D, Gu B (2014) Macrobenthos diversity response to hydrological connectivity gradient. Wetlands, 1–11
Edwards KR, Proffitt CE (2003) Comparison of wetland structural characteristics between created and natural salt marshes in southwest Louisiana, USA. Wetlands 23(2):344–356
Golubkov S, Alimov A (2010) Ecosystem changes in the Neva Estuary (Baltic Sea): Natural dynamics or response to anthropogenic impacts? Mar Pollut Bull 61(4):198–204
González-Ortegón E, Baldó F, Arias A, Cuesta JA, Fernández-Delgado C, Vilas C, Drake P (2015) Freshwater scarcity effects on the aquatic macrofauna of a European Mediterranean-climate estuary. Sci Total Environ 503:213–221
He Q, Cui B, Cai Y, Deng J, Sun T, Yang Z (2009) What confines an annual plant to two separate zones along coastal topographic gradients? Hydrobiologia 630(1):327–340
Herman PMJ, Middelburg JJ, van de Koppel J, Heip CHR (1999) Ecology of estuarine macrobenthos. Adv Ecol Res 29:195–240
Josselyn M, Zedler J, Griswold T (1990) Wetland mitigation along the Pacific coast of the United States. In: Kusler JA, Kentula ME (eds) Wetland creation and restoration: the status of the science. Island Press, Washington, pp 3–36
Keeley NB, Macleod CK, Hopkins GA, Forrest BM (2014) Spatial and temporal dynamics in macrobenthos during recovery from salmon farm induced organic enrichment: when is recovery complete? Mar Pollut Bull 80(1):250–262
Kristensen E, Delefosse M, Quintana CO, Flindt MR, Valdemarsen T (2014) Influence of benthic macrofauna community shifts on ecosystem functioning in shallow estuaries. Mar Ecosyst Ecol 1:41
Leibold MA, McPeek MA (2006) Coexistence of the niche and neutral perspectives in community ecology. Ecology 87(6):1399–1410
Lepš J, Smilauer P (2003) Multivariate Analysis of Ecological Data Using CANOCO. Cambridge University Press, Cambridge
Li D, Chen S, Guan L, Lloyd H, Liu Y, Lv J, Zhang Z (2011) Patterns of waterbird community composition across a natural and restored wetland landscape mosaic, Yellow River Delta, China. Estuar Coast Shelf Sci 91(2):325–332
Lindenmayer DB, Likens GE (2009) Adaptive monitoring: a new paradigm for long-term research and monitoring. Trends Ecol Evol 24(9):482–486
Ling J, Wu ML, Chen YF, Zhang YY, Dong JD (2014) Identification of spatial and temporal patterns of Coastal Waters in Sanya Bay, South China Sea by Chemometrics. J Environ Inform 23(1):37–43
López-Rosas H, Moreno-Casasola P, López-Barrera F, Sánchez-Higueredo LE, Espejel-González VE, Vázquez J (2013) Interdune Wetland Restoration in Central Veracruz, Mexico: Plant Diversity Recovery Mediated by the Hydroperiod. In Restoration of Coastal Dunes (pp. 255–269). Springer, Berlin Heidelberg
Martinez-Martinez E, Nejadhashemi AP, Woznicki SA, Love BJ (2014) Modeling the hydrological significance of wetland restoration scenarios. J Environ Manag 133:121–134
McCune B, Grace JB, Urban DL (2002) Analysis of ecological communities (Vol. 28). Gleneden Beach, OR: MjM software design
Millenium Ecosystem Assessment (2005) Ecosystems and human well-being: wetlands and water synthesis. World Resources Institute, Washington
Mitsch WJ, Gosselink JG (2007) Wetlands. Hoboken
Mitsch WJ, Wilson RF (1996) Improving the success of wetland creation and restoration with know-how, time, and self-design. Ecological applications, 77–83
Moreno-Mateos D, Power ME, Comín FA, Yockteng R (2012) Structural and functional loss in restored wetland ecosystems. PLoS Biol 10(1):e1001247
Nishijima W, Nakano Y, Nakai S, Okuda T, Imai T, Okada M (2013) Impact of flood events on macrobenthic community structure on an intertidal flat developing in the Ohta River Estuary. Mar Pollut Bull 74(1):364–373
Niu Z, Zhang H, Gong P (2011) More protection for China’s wetlands. Nature 471(7338):305
Palmer MW (1993) Putting things in even better order: the advantages of canonical correspondence analysis. Ecology 74(8):2215–2230
Pennings SC, Selig ER, Houser LT, Bertness MD (2003) Geographic variation in positive and negative interactions among salt marsh plants. Ecology 84(6):1527–1538
Picanço TC, Almeida CMR, Antunes C, Reis PA (2014) Influence of abiotic characteristics of sediments on the macrobenthic community structure of Minho estuary saltmarsh. Limnetica 33(1):73–88
Pinto R, de Jonge VN, Marques JC (2014) Linking biodiversity indicators, ecosystem functioning, provision of services and human well-being in estuarine systems: application of a conceptual framework. Ecol Indic 36:644–655
Pratt DR, Lohrer AM, Pilditch CA, Thrush SF (2014) Changes in ecosystem function across sedimentary gradients in estuaries. Ecosystems 17(1):182–194
Rowe JC, Garcia TS (2014) Impacts of wetland restoration efforts on an amphibian assemblage in a multi-invader community. Wetlands 34(1):141–153
Ryals R, Hartman MD, Parton WJ, DeLonge MS, Silver WL (2014). Long-term climate change mitigation potential with organic matter management on grasslands. Ecological Applications
Sassa S, Watabe Y, Yang S, Kuwae T (2013) Ecological geotechnics: role of waterfront geoenvironment as habitats in the activities of crabs, bivalves, and birds for biodiversity restoration. Soils Found 53(2):246–258
Schlacher TA, Meager JJ, Nielsen T (2014) Habitat selection in birds feeding on ocean shores: landscape effects are important in the choice of foraging sites by oystercatchers. Mar Ecol 35(1):67–76
Shan K (2007) Theory, methodology and practices of wetland ecological restoration in Yellow River Delta Nature Reserve. Wetl Sci Manag 3(4):16–20
Sharma J, Baguley J, Bluhm BA, Rowe G (2011) Do meio-and macrobenthic nematodes differ in community composition and body weight trends with depth? PLoS One 6(1):e14491
Sikora FJ, Moore KP (2014) Soil test methods from the Southeastern United States
Simenstad C, Reed D, Ford M (2006) When is restoration not?: Incorporating landscape-scale processes to restore self-sustaining ecosystems in coastal wetland restoration. Ecol Eng 26(1):27–39
Stauffer AL, Brooks RP (1997) Plant and soil responses to salvaged marsh surface and organic matter amendments at a created wetland in central Pennsylvania. Wetlands 17(1):90–105
Sukumaran S, Vijapure T, Mulik J, Rokade MA, Gajbhiye SN (2013) Macrobenthos in anthropogenically influenced zones of a coralline marine protected area in the Gulf of Kachchh, India. J Sea Res 76:39–49
Tang N, Cui BS, Zhao XS (2006) The restoration of reed (Phragmites australis) wetland in the Yellow River Delta. Acta Ecol Sin 26(8):2617–2624
ter Braak CJF (1986) Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67:1167–1179
ter Braak CJF (2014) History of canonical correspondence analysis. Visualization and Verbalization of Data, 61
Torres G, Anger K, Giménez L (2006) Effects of reduced salinities on metamorphosis of a freshwater-tolerant sesarmid crab, Armases roberti: is upstream migration in the megalopa stage constrained by increasing osmotic stress? J Exp Mar Biol Ecol 338(1):134–139
Verschuren D, Tibby J, Sabbe K, Roberts N (2000) Effects of depth, salinity, and substrate on the invertebrate community of a fluctuating tropical lake. Ecology 81(1):164–182
Wang LZ, Twang YF, Wang L, Wang GQ (2014) Pollutant flushing characterizations of stormwater runoff and their correlation with land use in a rapidly urbanizing watershed. J Environ Inform 23(1):44–54
Warwick RM, Somerfield PJ (2010) The structure and functioning of the benthic macrofauna of the Bristol Channel and Severn Estuary, with predicted effects of a tidal barrage. Mar Pollut Bull 61(1):92–99
Waterkeyn A, Grillas P, Vanschoenwinkel B, Brendonck L (2008) Invertebrate community patterns in Mediterranean temporary wetlands along hydroperiod and salinity gradients. Freshw Biol 53(9):1808–1822
Ysebaert T, Herman PMJ, Meire P, Craeymeersch J, Verbeek H, Heip CHR (2003) Large-scale spatial patterns in estuaries: estuarine macrobenthic communities in the Schelde estuary, NW Europe. Estuar Coast Shelf Sci 57(1):335–355
Zedler JB (1983) Freshwater impacts in normally hypersaline marshes. Estuaries 6(4):346–355
Zhang GS, Wang RQ (2008) Research on dynamic monitoring of ecological environment in modern Yellow River Delta. China Environ Sci Chin Ed 28(4):380–384
Acknowledgments
We are grateful for the attentive experimental guidance of Qiang He. We thank Xiaojing Shao, Menglu Zhang, Fengju Li, Yali Tong and Yongyan Liu for their invaluable laboratory assistance; Zishan Yu and Chuanping Yang for the identification of macrobenthos; and Yueliang Liu and Pengfei Guo for granting access to the study areas. Steven Pennings assisted with the English. We thank China Scholarship Council. This research was funded by National Key Basic Research Program of China (2013CB430406), China National Funds for Distinguished Young Scientists (51125035), National Science Foundation for Innovative Research Group (51121003).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 2064 kb)
Rights and permissions
About this article
Cite this article
Li, S., Cui, B., Xie, T. et al. Diversity Pattern of Macrobenthos Associated with Different Stages of Wetland Restoration in the Yellow River Delta. Wetlands 36 (Suppl 1), 57–67 (2016). https://doi.org/10.1007/s13157-015-0641-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-015-0641-7