Abstract
Habitat heterogeneity and wetland area play important roles in aquatic biodiversity; however, other factors are equally important in the composition and distribution of ecological communities. Over a 3-year period, including a year of drought, we demonstrate how beavers physically altered isolated shallow-water wetlands in Miquelon Lake Provincial Park, Canada, which then influenced aquatic invertebrates diversity and abundance of functional feeding groups and taxa. Digging channels by beavers extended aquatic habitats over 200 m into the upland zone and created unique aquatic habitats, which became hot-spots for predaceous aquatic invertebrates. Some taxa (e.g., Gerridae and Gyrinidae) were found exclusively in beaver ponds, while Culicidae were primarily in wetlands without beavers. Amphipoda were strongly associated with beaver ponds in drought and post-drought years. During extreme drought in 2009, species richness, diversity and abundance declined dramatically, but recovered quickly in 2010. Although species richness was associated with wetland area, increased niche availability through active maintenance of wetlands by beavers played an important role in aquatic invertebrate diversity and distribution. Understanding the role of common, but seldom surveyed within-wetland habitats in boreal wetlands expands our ability to understand aquatic biodiversity, the importance of habitat heterogeneity and the role of other taxa in species assemblages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In its early form, niche theory (Hutchinson 1957) suggested that habitat heterogeneity and consequently, niche partitioning, are main factors influencing ecological communities, and that niche availability and species diversity are positively associated. More recently, however, the concept of ecological niche construction (Odling-Smee et al. 1996) has been used to describe how organisms can create physical changes in the environment through ecosystem engineering (Jones et al. 1994; Wright and Jones 2006), which in turn creates new habitats for other species. This alteration of habitats allows taxa with very little niche overlap to interact indirectly through habitat modifications by one or both taxa (Stachowicz 2001).
However, a wide range of abiotic and biotic factors also characterize wetland habitats and can result in each wetland developing a distinct aquatic invertebrate community (Jeffries 2011) dominated by specific functional feeding groups (Cummins 1973; Merritt et al. 2008). Key factors are directly and indirectly hydrological in nature and are associated with hydroperiod (Jeffries 1994; de Szalay and Resh 1996; Gathman and Burton 2011), water chemistry (Angradi and Jicha 2010) and vegetation communities (de Szalay and Resh 1996; de Szalay and Cassidy 2001; Kratzer and Batzer 2007). Surrounding landscape (Batzer et al. 2004), specific structure and composition of aquatic vegetation (Christensen and Crumpton 2010; Gathman and Burton 2011), and the presence of woody debris (Braccia and Batzer 2001) are additional influences. Under particular conditions, these environmental factors can shape the structure of aquatic invertebrate communities (Van de Meutter et al. 2007) and the relative abundance of functional feeding groups (Hawkins and Sedell 1981). For example, such factors might affect nutrient and energy cycling of food web dynamics (Hann 1995) through an increase in habitat structural complexity (Van de Meutter et al. 2008).
While environmental factors can directly affect aquatic invertebrate diversity and abundance, fluctuations in natural abiotic and biotic factors often produce weak (less than 20 % of the variation), or even no response (Batzer et al. 2004). Resistance to variable habitat condition has been attributed to dominance of habitat generalists in these communities (Batzer et al. 2004). Evolutionary adaptations in these taxa such as resistance to desiccation (Batzer and Wissinger 1996) and passive or active dispersal (Van de Meutter et al. 2007) aid in survival during unpredictable fluctuation in wetland habitats (Bazzanti et al. 2010). Factors such as immigration, habitat suitability and reproductive rates also influence stability of such ecological communities (Kadman and Allouche 2007).
As ecosystem engineers, species such as beaver (Castor canadensis Kuhl) can also alter aquatic invertebrate communities (McDowell and Naiman 1986; France 1997). By creating and maintaining wetlands, beavers can impact temporal and spatial dynamics of pond hydrology (Westbrook et al. 2006; Hood and Bayley 2008) and composition and extent of riparian vegetation communities (Hood and Bayley 2009). In Ontario, Canada, France (1997) determined that addition of coarse woody debris and sediment by beavers increased species richness and abundance of some taxa of benthic invertebrates adjacent to beaver lodges. Likewise, McDowell and Naiman (1986) determined that the shift of lotic ecosystems to lentic environments by beaver impoundments changed the composition of functional feeding groups from collectors and predators to shredders and scrapers. However, little is known about aquatic invertebrate responses to physical alterations of wetlands through digging of channels and alteration of pond bottoms by beavers.
Because changes to pond structure, vegetation architecture, and hydrology are known to affect aquatic invertebrate diversity (McDowell and Naiman 1986; Heino 2000; Hornung and Foote 2006), activities of beavers in a wetland likely affect taxa richness and abundance at the microhabitat scale. Boreal wetlands of Miquelon Lake Provincial Park (MLPP) at the southern extent of the Cooking Lake Moraine (CLM) in east-central Alberta, Canada provide a unique site for examining relationships between aquatic invertebrates and beavers because of the lack of lotic systems in the park and the isolated nature of the wetlands. MLPP also has a well-established beaver population (Bromley and Hood 2013), which has extensively modified the landscape through the digging of channels, cutting of trees, and increasing the depth and extent of wetlands.
The purpose of our study is to examine how the modification of open-water wetlands by beavers (as an ecosystem engineer) influences the diversity, composition and within-pond distribution of aquatic invertebrates. We also investigate whether abiotic factors (i.e., wetland area, depth, precipitation and water chemistry) and biotic factors (i.e., habitat type and presence or absence of beavers) play a role in aquatic invertebrate assemblages. Specifically, our study tests the following hypotheses: (H1) that there will be habitat-specific species assemblages and functional feeding groups that are specific to wetlands with beavers, (H2) that aquatic invertebrate richness and abundance will be higher in wetlands with beavers than in those without beavers present, and (H3) that beaver-created features such as channels will provide more heterogeneous habitat and consequently, higher diversity and abundance of aquatic invertebrates than wetlands lacking beavers.
Methods
Study Area
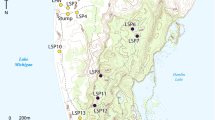
Miquelon Lake Provincial Park (MLPP; 53°21′N 112° 55′W) is at the southern extent of the Cooking Lake Moraine in east-central Alberta, Canada (Fig. 1). The CLM is an isolated pocket of the dry mixed-wood boreal region, surrounded by the Aspen Parkland ecotone (Achuff 1994). MLPP (~13 km2) consists of knob and kettle topography, which is characterized by isolated shallow open-water wetlands. With an average total annual precipitation of 457 mm (Environment Canada, http://www.weatheroffice.gc.ca), the park has a continental climate that is characterized by warm summers and cold winters. Total annual precipitation was measured using the hydrologic year (1 November to 31 October). Total precipitation differed among the three survey years. In the 2007 to 2008 (2008) hydrologic year, total precipitation was 327.9 mm. The 2008 to 2009 (2009) year had total precipitation of 224.7 mm, and the 2009 to 2010 (2010) year had total precipitation of 516.6 mm. Lower precipitation in 2009 resulted from a drought from July 2008 through 2009.
Common emergent vegetation in MLPP consists of cattail (Typha latifolia L.), celery-leaved buttercup (Ranunculus sceleratus L.), sedge (Carex spp.), creeping spike-rush (Eleocharis palustris L.), common great bulrush (Scirpus lacustris L.) and bulrush (Scirpus spp.). Apart from common duckweed (Lemna minor L.), star duckweed (L. trisulca L.) and pondweed (Potamogeton spp.), floating-leaved plants are uncommon. Hornwort (Ceratophyllum demersum L.) and spiked water milfoil (Myriophyllum spicatum L. var. exalbescens) are dominant submergent species.
Although game fish were present historically in the park, only brook stickleback (Culaea inconstans Kirtland) is still found in Miquelon Lake (Mitchell 1990). No fish were present in the wetlands used in this study, as determined by minnow trapping, over 5 years of aquatic surveys and visual observations for other ongoing research projects.
Despite their extirpation from much of the CLM during the 1800s (Hood and Bayley 2008), beaver populations have since recovered and almost all wetlands in the park have current or historical use by beaver (Bromley and Hood 2013). In 2008, beaver density in MLPP was four active lodges per km2 (Bromley and Hood 2013), but numbers have fluctuated since the 2009 drought. Trapping is limited to management purposes.
Streams in the park are rare and those that exist are intermittent; however, beaver channels play a similar role by connecting many wetlands. Unlike more lotic systems, beavers in MLPP do not build extensive dams to create wetland habitats, although small dams do exist at some sites. Instead, beavers appear to expand territory through extensive channel systems dug perpendicularly from the wetland edge to access adjacent wetlands or upland foraging areas. As well as being quite long (~100 to 200 m), these channels are often over 70 cm deep. Because beavers use channels to transport woody material back to their lodge, channel walls are quite steep and lack the gradual shorelines of other littoral habitats in the wetland.
We selected eight wetlands with active beaver colonies (“active” sites) and eight wetlands without recent beaver colonies (“inactive” sites). Wetlands were roughly comparable in size. Beaver activity was determined through winter lodge surveys using methods outlined in Hood and Bayley (2008) and Bromley and Hood (2013). We originally chose wetlands that consistently either had beavers present (“active”) or lacked beavers (“inactive”) since January 2008. We maintained the same selection criteria when we were required to choose replacement sites due to drought. Sixteen wetlands were surveyed in 2008, 14 in 2009 (three wetlands were not surveyed due to low water levels and one new wetland was added), and 15 in 2010 (two were still dry and the one added in 2009 was retained).
Pond Metrics and Water Chemistry Parameters
To obtain wetland areas (ha) for the original 16 study wetlands selected in 2008, we used an orthophoto with a 0.25 m resolution in an ArcMap 9.3 Geographic Information System (ESRI 2006). We could not obtain comparable images to conduct the same analyses for wetland areas in 2009 and 2010. In 2008, we also measured water depths (cm) with a folding ruler and hand-held sonar (calibrated to the ruler) along a 10 m × 10 m grid across the entire wetland. Water depths in beaver channels, which are maintained by beaver in active sites, were also measured. We were confident that our measurements were accurate to within 1 cm. Finally, we analyzed pond characteristics such as depth and area to determine differences due to beaver activities.
When collecting aquatic invertebrates, we also measured various environmental parameters including air and water surface temperature (°C), pH ( Hanna Instruments HI 98128), electrical conductivity (EC; Eutech Instrument ECTestr11), dissolved oxygen (LaMotte Dissolved Oxygen 5860 Kit), and water clarity (LaMotte Secchi Disk 0171-CL). These data were used in analyses to compare environmental conditions among years and between active and inactive ponds.
Aquatic Invertebrate Sampling
We sampled aquatic invertebrates over the 3-year period (2008, 2009, and 2010) at the active and inactive sites. Aquatic invertebrate surveys commenced shortly after ice-off each year to ensure a comparable sample of early spring aquatic invertebrates. Depending on ice-off dates and snow cover, survey times varied slightly from year to year, but all commenced by early to mid-May and were completed over a 2 to 3-week period before mid-June.
Invertebrate use of within-pond habitats were measured with five samples from vegetated-edge and open-water habitats and, after 2008, in beaver channels as well. At vegetated-edge habitats (VE) along the water’s edge, we took four samples at the four cardinal directions and then one sample at a randomly selected location chosen between 0 and 360°. In open-water habitats (OW) we randomly selected sample sites in the main body of each of the 16 wetlands (one sample at the pond center and four samples at each of the four cardinal directions). During these surveys, we noticed that beaver channels (BC) appeared to be distinct from the vegetated-edge or open-water habitats. We did not sample these areas in 2008, but in 2009 and 2010, we added five samples from beaver channels in the active and inactive wetlands. This addition produced a total of 15 samples per pond from all habitats, except in 2009, when we could not take the full complement of samples in channel and open-water habitats due to drought effects on water levels. Accordingly, for both 2009 and 2010 we sampled a total of six habitat combinations: 1) vegetated edge in active wetlands, 2) vegetated edge in inactive wetlands, 3) open water in active wetlands, 4) open water in inactive wetlands, 5) beaver channel in active wetlands, and 6) beaver channel in inactive wetlands. Beaver channels in inactive wetlands were remnants of historic beaver activity and were not currently maintained by beavers.
All aquatic invertebrate samples were obtained using a D-net (0.07 m2, 500 μm mesh). Each sample consisted of a 1-m sweep that entered the water column, lightly contacted the surface of the benthos (except in most open-water habitats) and then was pulled up through the water column back to the surface. In vegetated-edge habitats, the net contacted the submerged parts of the plants as well as the top of the benthos and water column. In most cases in open-water habitats, the bottom of the wetland was out-of-reach of the net and only the water column could be sampled. Sweep-net sampling is commonly used to sample for aquatic invertebrate diversity in lentic environments (Uzarski and Genet 2004; Kratzer and Batzer 2007; Alsfeld et al. 2009). Although this method is not designed to capture all invertebrates (Marglois et al. 2001; Alsfeld et al. 2009), it is an effective technique for capturing many taxa and results in the second highest Simpson’s index of equitability in many aquatic environments (Turner and Trexler 1997).
We brought each net to a field processing station at the edge of the wetland where the net contents were rinsed out with tap water into a white enamel pan (25 × 41 × 6 cm). All large plant material was rinsed and then removed, and we returned all tadpoles to the wetland. Remaining contents of the pan were sieved through a 500 μm mesh and we stored the contents in the mesh in a plastic container with 70 % ethanol solution. We included identification labels with the sample to ensure continuity during processing and storage.
In the laboratory, we sorted each sample using dissecting scopes (10× to 30×) or compound microscopes. The 2008 samples were sent to Dr. Heather Proctor’s lab at the Biological Sciences Department at the University of Alberta (Edmonton) for identification. We identified the 2009 and 2010 samples at our lab (Augustana Campus) with taxonomic (Clifford 1991; Thorp and Covich 2001) and photographic guides (courtesy of Dr. H. Proctor from the previous year’s samples). All specimens were identified to the lowest possible taxonomic level. For all years, we recorded total number of taxa and total number of individuals within each taxon relative to the habitat type and wetland status where they were found. Lastly, we grouped the taxa observed in each habitat type and wetland status into functional feeding groups (FFG) (Cummins 1973; Merritt et al. 2008). We also noted all taxa that were unique to a particular habitat type (i.e., VE, OW, or BC) and wetland status as defined by beaver activity (active or inactive). Because of dramatic differences in water levels from year to year, these differences were categorized by year and habitat type.
For each sampling year and each pond, we pooled the five samples from each within-wetland habitat type (VE, OW, or BC) and calculated taxa richness (# of species: S), taxa diversity (Shannon Index: H'; Shannon 1948), taxa evenness (Pielou’s evenness index: J' = H'/lnS; Pielou 1966), and density (per sample abundance) for the three habitat types. We then calculated these metrics for the wetland as a whole. These measures were also categorized by wetland status to assess the difference between active and inactive beaver sites.
Data Analyses
Wetland depth, open-water area and water chemistry parameters were compared using an analysis of variance (ANOVA; Statistica, v. 6, StatSoft Inc. 2003) with year, wetland status (active or inactive), and within-wetland habitat (VE, OW, BC) as factors and water chemistry parameters (i.e., pH, EC) as response variables. We used an independent t-test to assess differences in the open-water area of active and inactive ponds and then the water depth of active and inactive ponds.
We used a nested mixed-linear model design (IBM SPSS v.19) to determine whether measures of aquatic invertebrate biodiversity (S, H', J', and density) differed between active beaver wetlands and wetlands lacking beavers (inactive). We used the same method to assess the differences in these biodiversity measures among habitats within active and inactive wetlands (VE, OW, BC). For the former analysis, wetland status (active or inactive) was a fixed factor and individual wetlands were treated as a nested random factor in the analysis. For the latter analysis, both wetland status and habitat were fixed factors. Any significant differences were further tested using a Fisher LSD post hoc test. Prior to any analyses, we tested the data for normality using the Kolmogrov-Smirnov test and tested homogeneity of variances using the Browne and Forsythe test. Non-normal data were transformed and residuals were examined for significant differences. We also examined the relationship between wetland area and S, H', J', and density by using simple linear regression with pond area as the predictor variable and each of the diversity measures as the response variable. Significance level for all statistical analyses was set at α = 0.05.
To assess differences in the number of taxa in each FFG in each wetland status (active or inactive) and each habitat type within a wetland (VE, OW, BC), we used a Chi-squared (χ 2) test of homogeneity. Where significant differences existed, we analyzed the standardized residuals to assess which category (k) most influenced the outcome. Standardized residuals (c) allow differences to be represented by standard deviations from a hypothesized value (in this case, no difference between counts).
Finally, to identify differences in invertebrate assemblages by wetland status and habitat type, we applied a non-metric multidimensional scaling analysis (NMDS; McCune and Grace 2002; PC-ORD v. 6; McCune and Mefford 2011). We ordinated aquatic invertebrate communities using random starting configurations to identify spatial (i.e., VE, OW, BC habitats and wetland status) and environmental patterns (i.e., water chemistry, water depth, date, beaver presence) in the aquatic invertebrate communities. We selected the Sorenson (Bray-Curtis) similarity index to calculate a matrix of distances or similarities among taxa. To improve quality of community description, we eliminated taxa that occurred in <10 % of the samples. We also performed a log-normal transformation to normalize the data. In addition, we used 50 runs with real data and then performed a randomization (Monte Carlo) test using 200 runs with randomized data to assess the strength (stress) of the axes compared to the stress that would be expected by chance (McCune and Grace 2002). Initially, we performed NMDS ordinations on taxon- and site-specific matrices (wetland status and within-in wetland location) and then ran ordinations that included environmental variables in each pond (e.g., water depth, pH, electrical conductivity, date). Because of annual differences in wetland locations, beaver presence, precipitation and wetland conditions, each year were ordinated separately. Passive overlays of aquatic invertebrate responses were graphed with a joint plot cut-off value of r 2 = 0.2. We then used analysis of variance (ANOVA; Statistica, v. 6; StatSoft Inc. 2003) to examine the spatial and environmental variations in those taxa that appeared to have the most influence in the ordination. NMDS is more flexible than some ordination methods (McCune and Grace 2002) and is able to accommodate ecological community data sets where zero-counts are common (Clarke 1993).
Results
Wetland Metrics and Water Chemistry Parameters
Wetland areas in 2008 did not differ between sites with beavers and those without beavers (\( \overline{x} \) without beavers = 1.4 ha, s = 1.3 ha, \( \overline{x} \) with beavers = 1.6 ha, s = 1.0 ha; F 1,14 = 0.12, P = 0.73). No data were available for 2009 and 2010 to conduct similar analysis, but we observed variations in wetland areas due to the effects of drought in 2009 and higher than average precipitation in 2010.
In 2008, wetlands with beavers were deeper, on average, than wetlands without beavers (\( \overline{x} \) without beavers = 55.6 cm, s = 32.1 cm, \( \overline{x} \) with beavers = 65.2 cm, s = 43.1 cm; t 665 = 3.27, P = 0.001). Average water depth in the channels in wetlands with beavers was also deeper than average water depths in unmaintained channels in abandoned wetlands (\( \overline{x} \) without beavers = 19.0 cm, s = 7.7 cm, \( \overline{x} \) with beavers = 42.6 cm, s = 16.3 cm; t 161 = 6.66, P < 0.001).
Electrical conductivity (EC) in all wetlands was lower in 2008 (\( \overline{x} \) = 975 μS/L, SE = 114) than in 2009 (\( \overline{x} \) = 1668 μS/L, SE = 192) and 2010 (\( \overline{x} \) = 1617 μS/L, SE = 230; F 2,41 = 4.34, P = 0.02). The presence or absence of beavers did not affect EC regardless of year (F 1,41 = 0.18, P = 0.67). Water pH also did not differ among ponds, regardless of year (F 2,43 = 2.78, P = 0.07).
Habitat-Specific Taxa Assemblages
Over the 3 years, we collected and identified a total of 46 aquatic invertebrate taxa represented by more than 991,000 individuals. In all years, Daphnia spp. was the most abundant taxon and accounted for 91.5 % of the individuals in 2008, 97.9 % of the individuals in 2009, yet only 62 % of the individuals in 2010 (Cyclopoida was the second most abundant taxon in this year, at 34 %). Although often observed during field visits, larger Dytiscidae were not well-represented using sweep-net methodology.
Year-to-year differences were apparent in habitat-specific taxa assemblages (Supplementary Tables S1, and S2); however, Gerridae and Gyrinidae were unique to beaver channels in active wetlands in both 2009 and 2010. They comprised only a small percentage of the overall number of individuals though (Supplementary Table S2). Tabanidae larvae were unique to remnant beaver channels in inactive beaver wetlands for those same years, and were not found in our samples from VE and OW in 2008.
In all years, the predator (p) FFG had the greatest number of taxa regardless of the presence or absence of beavers (Supplementary Table S3). In 2009 and 2010, they were especially common in beaver channels of active wetlands, which drove within-wetland habitat differences (χ 2 = 8.49, df = 2, P = 0.0145 for 2009, and χ 2 = 7.85, df = 2, P = 0.0197 for 2010). The mean observed minus expected value for predators in 2009 and 2010 was 8.83. Chaoboridae larvae were the most abundant predator in all years. In all wetlands, they were the most abundant predator in the beaver channels and their numbers were lower in the other habitat types.
Collector-gatherers (cg) had the most taxa after predators, but again did not differ between active and inactive wetlands over the 3 years, except inactive wetlands in 2010, when they slightly exceeded predators by 3 % (Supplementary Table S3). Chironomidae were the most abundant collector-gatherers in 2009 and 2010, but Conchostraca were in high numbers (57 % of the sample) in 2008, especially in open-water habitats. Beaver channels and vegetated-edge habitats almost always had more FFGs than open-water habitats, although on some occasions open-water habitats had a slightly higher count than vegetated-edge habitats (Supplementary Table S3). No particular FFG accounted for this difference.
In 2009, within-wetland standardized residuals suggest that open-water habitats (c = −2.2) were important in the overall differences in the abundance of FFGs as indicated by the χ 2 value reported above. Beaver channels were second in importance at c = 1.6. In 2010, beaver channels alone were driving within-wetland differences in FFGs (c = 2.3), while open-water and vegetated-edge habitats were less important (c = −1.0 and c = −1.3, respectively).
Habitat-Specific Differences in Aquatic Invertebrate Richness, Diversity and Abundance
When 2009 and 2010 were compared without 2008 so beaver channels could be included in the analysis, taxa richness (S) was highest in beaver channels for both years combined (F 2,82 = 7.05, P = 0.002). Wetlands in 2010 had the highest H' (F 1,82 = 26.05, P < 0.001) and J' (F 1,82 = 27.67, P < 0.001); however, 2009 had the highest density (per sample abundance; F `1,82 = 27.66, P < 0.0001). When we removed beaver channels from the analysis to allow for even comparisons among all years, 2008 had the highest taxa richness (S) among the 3 years (F 2,85 = 8.79, P = 0.0003). In addition, vegetated-edge habitats had higher S than open-water habitats (F 1,85 = 12.95, P = 0.0008).
Wetlands in 2009 had the lowest taxa diversity (H') among all 3 years (F 2,85 = 9.45, P = 0.0002). Vegetated-edge habitat again had the highest diversity (H') among all habitat types (F 1,85 = 9.09, P = 0.003). There was no difference in diversity (H') between 2008 and 2010. Evenness (J') was also lowest in 2009 (F 2,85 = 9.80, P = 0.0001) and vegetated-edge habitat had higher J' than open-water habitats in all years (F 1,85 = 6.37, P = 0.001). Wetlands in 2009 had the highest total density (per sample abundance, F `1,85 = 14.07, P < 0.0001), although there was no significant difference among habitats.
When we analyzed data from 2008 alone, all measures of aquatic invertebrate species richness and abundance (S, H', J'), except density, were higher in vegetated-edge habitats than in open-water habitats, regardless of pond status (Table 1). Density (per sample abundance) was higher in open-water habitats, but when Daphnia spp. were removed from the analysis, there was no difference between the two habitats (F 1,30 = 0.581, P = 0.452). We noted a weak positive linear relationship between wetland area and species richness (R 2 = 0.29, P = 0.03), but found no obvious relationship between area and the other measures of diversity.
In 2009, species richness was higher in wetlands with beavers than those without beavers (F 1,32 = 4.853, P = 0.035). Regardless of beaver occupancy, species richness differed within-wetland habitat type (F 2,32 = 4.337, P = 0.022, Fig. 2). In particular, species richness in active beaver channels was higher than both open-water habitats and vegetated-edge habitats in wetlands without beavers (Fisher LSD, P = 0.007, and P = 0.02, respectively). Vegetated-edge habitats in active wetlands also had higher species richness than both open-water habitats and vegetated-edge habitats in wetlands without beavers (Fisher LSD, P = 0.02, and P = 0.04, respectively). Despite the trend towards lower species richness in abandoned beaver ponds, beaver channels in these wetlands supported higher species richness than open-water habitats in those same wetlands (Fisher LSD, P = 0.02). No other measures of species diversity or abundance differed among wetland types or habitat types.
Mean species richness (S) of aquatic invertebrates relative to wetland status (with beavers – A, or without beavers – I) and habitat type (beaver channel – BC, open water – OW, and vegetated edge – VE) in Miquelon Lake Provincial Park Canada, during the spring of 2009. Error bars represent 95 % confidence interval. Abbreviations match first letters of taxa listed in Supplementary Table S1
In 2010, species richness was highest in beaver channels (F 2,41 = 5.112, P = 0.01), but the presence or absence of beavers was not a significant factor (F 1,41 = 0.02, P = 0.89). Species richness in beaver channel habitats was higher than in open-water and vegetated-edge habitats (Fisher LSD, P = 0.006 and P = 0.01, respectively). As in 2009, no other measures of species diversity or abundance differed among wetland or habitat types.
Effects of Within-Pond Habitats and Environmental Variables on Aquatic Invertebrate Assemblages
Results of NMDS ordinations for species-habitat relationships varied for each year. In 2008, a three-dimensional solution was recommended (final stress: 13.15, P = 0.02) and there was a substantial overlap in aquatic invertebrate community composition. Axis 1, which represented 51 % of variation, indicated an association between Daphnia spp. and open-water habitats in wetlands with beavers (R = −0.91). Axis 2 accounted for 19 % of the variation and axis 3 represented 13 % of the variation. All other taxa were well within the area of overlap for active and inactive wetlands, but were associated with vegetated-edge habitats, which were represented by axis 2. Environmental variables (e.g., date, pH and electrical conductivity) were not specific to any axis in 2008 and did not differ with wetland status.
In 2009, a three-dimensional solution was also recommended. NMDS ordination resulted in axis 1 representing 58 % of the variation, axis 2 accounting for 24.8 % of the variation and axis 3 representing 8.5 % of the variation (final stress: 12.07, P = 0.0196). Hyalellidae, which were predominantly found in ponds with beavers, were associated with axis 1 (R = −0.85) and had little correspondence with the other two axes. Too few counts from inactive wetlands were available to perform an ANOVA on the data. Environmental variables (e.g., date, pH and electrical conductivity) were not specific to any axis in the NMDS ordination and did not differ with wetland status or habitat type.
NMDS plots for the 2010 data indicated overlap in most aquatic invertebrate assemblages regardless of the presence of beavers and the within-wetland habitats (final stress: 16.4, P = 0.0196); however, mosquito larvae (Culicidae) and water mites (Hydrachnidae) were positively associated with wetlands lacking beavers (R = 0.69 and R = 0.55; Supplementary Figure S1A). In addition, amphipods were positively associated with wetlands with beavers (R = 0.46). When Culicidae data were log-normal transformed and combined for 2008 and 2010 (none were found during the drought of 2009), they were strongly associated with sites lacking beavers (\( \overline{x} \) without beavers = 20.6, s = 46.3, \( \overline{x} \) with beavers = 6.3, s = 26.1; F 1,72 = 13.879, P = 0.0004). When tested against environmental variables, only pH had a consistent association with sites with beavers present, in 2010 (R = 0.68). When assessed using an independent t-test, this relationship was not significant (t 11 = 1.34, P = 0.21).
Discussion
Our study examines existing lentic systems that, although modified by beavers, were created mainly through geomorphologic processes. Wetlands in MLPP are remnants of a glacial retreat whereby large pieces of ice were left behind and formed kettle lakes and ponds within a morainal landscape (Bayrock and Hughes 1962). Where other studies examine the shift from lotic to lentic environments, we examine the changes beavers make to existing lentic wetlands and how those changes affect biodiversity. Hood and Bayley (2008) determined that during drought, active maintenance of ponds by beavers helps maintain open-water areas in ponds, which in turn provides habitat for other species. Our work further expands on these findings, by examining differences in aquatic invertebrate assemblages during pre-drought, drought and post-drought years in active and inactive beaver ponds.
We determined that wetlands that are actively maintained by beavers provide unique microhabitats for aquatic invertebrates. Long-term data from other studies indicate that wetland areas and the extent of groundwater are dramatically increased by beavers, even in non-riverine landscapes (Westbrook et al. 2006; Hood and Bayley 2008). In their study of ponds in Elk Island National Park, Canada, Hood and Bayley (2008) determined that ponds with beavers had nine times more open-water area than those same ponds when beavers were not present. Westbrook et al. (2006) determined that the presence of beaver impoundments (ponds) in riverine systems of Rocky Mountain National Park, USA had significant effects on groundwater recharge and the ability of the water table to withstand effects of drought.
In our study, water depths both in the main body and in beaver channels of active wetlands were significantly deeper than in wetlands that were even recently abandoned by beavers. It is likely that differences in water depths would result in greater volumes of water in active beaver ponds. Despite these differences, water depth initially did not appear to play a role in aquatic invertebrate diversity or distribution. When aquatic invertebrate density increased in 2009 as water levels were dropping during the drought, other measures of biodiversity (i.e., H', J', and S) decreased. The increase in aquatic invertebrate density was likely due to reduced habitat availability and use of remaining wetted areas as a type of refugia.
Water chemistry did not appear to differ in our wetlands relative to beaver activity; however, the drought in 2009 and the gradual recovery of water levels in 2010 influenced electrical conductivity. Prior to the drought EC was lower, but during and immediately following the drought EC was significantly higher, likely due to lower water levels and more concentrated ions in the water column. In 2010, NMDS ordination associated higher pH with wetlands with beavers, which might be attributed to increased digging activity by beavers in alkaline soils within MLPP. Regular disturbance of sediment could result in an increase in dissolved ions released from the wetland substrate. However, lack of similar results for other years when beavers were still actively modifying wetland bottoms and beaver channels suggests more research is needed.
Although wetland status (with and without beavers) influenced species assemblages, within-wetland habitats appeared to be more influential in most cases. The strongest trend, regardless of year, was higher species richness, diversity and evenness in vegetated-edge habitats compared to open-water habitats. This finding is similar to other studies that suggest that vegetation within ponds can affect the distribution and diversity of aquatic invertebrate communities (de Szalay and Resh 1996; Meyer et al. 2011). In their study, Meyer et al. (2011) determined that aquatic invertebrate diversity, abundance and biomass were consistently higher in vegetated-edge habitats than open-water habitats. Although we observed a tendency for species richness (S) and density to be higher in wetlands with active beaver populations, there was no significant difference from inactive ones.
The unique aspect of our study sites was that the amount of vegetated shoreline was dramatically increased by construction of channels by beavers. Despite the vegetated margins on beaver channels, channels have steeper sides than the pond edges and sometimes lack emergent or submerged aquatic vegetation typical of other littoral zones. The combination of emergent and submerged vegetation is often attributed to higher levels of diversity of aquatic invertebrates (Christensen and Crumpton 2010). Despite effects of vegetation on community assemblages (Pollack et al. 1988), several studies indicate that flooding regime has a greater influence on species diversity than vegetation alone, with density increasing during droughts and diversity increasing during periods of higher water levels (Jeffries 1994; Navarro-Llácer et al. 2010, but also see Gathman and Burton 2011). We also noted this pattern during our study.
Although island biogeography theory (MacArthur and Wilson 1963, 1967) would suggest that larger wetlands have higher biodiversity, only species richness in 2008 showed a weak, but significant relationship to wetland area. Our inability to repeat this same analysis for 2009 and 2010 makes it difficult to determine whether this trend would still hold in all 3 years. Beaver channels not only increase the perimeter of the wetland, they also increase open-water areas. Their steep sides and width (over 1 m in some cases) result in a deep channel with an open-water section.
Beavers construct channels to transport branches from upland areas into the main body of a pond, where they eat the material or incorporate it into food caches adjacent to their lodges. This transfer of terrestrial plant material into the wetland could import associated terrestrial invertebrates into the channels, which could increase availability of prey for aquatic invertebrates. Constant movement through these narrow channels also serves to push water along channels as beavers swim down them. This pulse of water could facilitate movement of nutrients, as would bioturbidation through disturbance of the substrate.
Although not uncommon aquatic taxa, in our study Gerridae and Gyrinidae were unique to beaver channels in wetlands with active beaver colonies, perhaps due to sampling bias in smaller areas. These two predator taxa contributed to the number of predators in beaver channels. In addition, Chaoboridae larvae were the most abundant predator in beaver channels in both 2009 and 2010, and Tabanidae larvae were unique to beaver channels in wetlands lacking beavers. Because the original beaver occupancy surveys in MLPP were conducted in early 2008 (Bromley and Hood 2013), long-term data needed to determine duration of occupancy or abandonment by beavers prior to our study are lacking. Even during this study, wetland occupancy by beavers was dynamic and re-colonization or abandonment of some sites was apparent during the study, especially during and following drought. With wetland abandonment, the lack of ongoing maintenance of channels by beavers resulted in a decrease in habitat for species that prefer these channel environments.
After predators, collector-gatherers (i.e., Chironomidae and Conchostraca) were the most common functional feeding group in all wetlands, regardless of beaver occupancy. This finding is similar to studies that examine changes in aquatic invertebrate assemblages before and after beavers impound streams, thereby changing lotic habitats to lentic habitats (McDowell and Naiman 1986; Collen and Gibson 2001). As with our study, other studies determined that within the collector functional feeding group, Chironomidae was one of the more dominant taxa in beaver impoundments (McDowell and Naiman 1986; Clifford et al. 1993; Marglois et al. 2001). McDowell and Naiman (1986) also determined that predators and collectors increased in importance over shredders and scrapers once an impoundment on a small stream was created by beavers.
Ongoing maintenance of wetlands by beavers might be important for certain species (e.g., Gerridae and Gyrinidae); however, these families are not adequately sampled with D-nets and might have been more readily caught in the confined area of a beaver channel. However, it appears the excavation of beaver channels and their regular use could provide important within-wetland habitats for some aquatic invertebrates. Beaver channels in particular were an important influence in the assemblage of functional feeding groups and served as potential “hunting hot-spots” for various predators. As such, actively maintained beaver channels contribute a unique niche that is not found in wetlands lacking beavers. Dominance of predators in actively maintained beaver channels also suggests that regular activity of beavers in these channels increases the importance of this habitat, not just the existence of the channel itself.
When we specifically examined species richness, diversity, abundance and density, precipitation appeared to be a driving factor in year-to-year differences for all measures. Dramatic decline in all measures except for density (per sample abundance) was likely caused by the drought of 2009. Decreased wetted area would logically result in higher densities of individuals that are restricted to remaining aquatic habitat within a wetland. Other measures of diversity might be lower because some species are capable of entering dormancy to resist desiccation (Batzer and Wissinger 1996). As is often seen with aquatic invertebrates, various measures of biodiversity can rebound once water levels rise (Gathman and Burton 2011). Such annual variation in water levels and beaver activity made it important to assess each year independently.
For all years, vegetated-edge habitats were important drivers of aquatic invertebrate diversity and abundance; however, once beaver channels were assessed as an additional habitat type in 2009 and 2010, they tended to play a more significant role regarding species richness. In addition, wetlands with beavers, especially during drought, tended to have higher species richness than wetlands without beavers. Other biodiversity measures (i.e., H', J', and per sample abundance) did not differ between wetlands with beavers and those without, or among within-wetland habitat types. This finding reinforces the need to utilize different biodiversity measures when assessing communities comprised of species of varying reproductive outputs and drought tolerance.
Although it is well-documented that beavers can affect aquatic invertebrate assemblages in lotic environments and lakes (McDowell and Naiman 1986; Clifford et al. 1993; France 1997; Butts 2001; Marglois et al. 2001), we could find no research on the effect on aquatic invertebrate assemblages of beaver occupancy in pre-existing lentic habitats. Despite unique habitats provided by beavers (e.g., channels, lodges, food caches), in most cases aquatic invertebrate assemblages overlapped with wetland occupancy by beavers and habitat type. However, certain species demonstrated more specific habitat preferences within our study sites.
Amphipoda (Hyalellidae in particular) were strongly associated with wetlands with beavers in 2009 and 2010. Mainly a collector-gatherer, amphipods feed on dead plant and animal material (Clifford 1991) and are in turn an important food source for some waterbirds (McParland and Paszkowski 2006). Naiman et al. (1984) established that beavers increase coarse particulate matter (e.g., by-products of plant material) and dissolved organic matter (e.g., through soil disturbance), which then provide additional food resources (e.g., fungal and microbial matter) for collectors. France (1997) also suggested that allochthonous inputs from beaver activities enhance aquatic food resources, which in turn enhance invertebrate populations supporting fish, mammals and waterfowl. In our study area, we observed increased gull and waterfowl feeding activity on ponds when amphipods were hatching.
In addition to amphipods, mosquitoes were strongly associated with wetlands lacking beavers in 2008 and 2010 (non-drought years). Butts (2001) also determined that establishment of beaver impoundments reduced previously high incidences of mosquitoes (Genus Aedes). Over time, however, Butts (2004) determined that other species of mosquitoes became established along the vegetated margins of older beaver ponds. It is possible that by maintaining deeper water habitats, beavers reduce the amount of habitat available for mosquito larvae. Also, increased number of predators in beaver ponds might help regulate numbers of mosquito larvae.
Although wetland area plays a role in some aspects of aquatic biodiversity, the addition of unique niches such as beaver channels is often overlooked. Yet channels are common features within some aquatic ecosystems and their exclusion has implications for studies examining aquatic invertebrate communities. For example, when sampling ponds for either environmental impact assessments or biodiversity studies, habitat classifications should differentiate beyond open-water and vegetated-edge habitats. Physical alterations and organic inputs by other species, including beavers, muskrats, and waterfowl, likely play a much larger role in aquatic ecology than is currently recognized. Our decision to expand our sampling design from two aquatic habitats in 2008 to include beaver channels as a third and unique habitat, resulted in greater insight and understanding of complex and dynamic aquatic ecosystems.
References
Achuff PL (1994) Natural regions, subregions and natural history themes of Alberta: a classification for protected areas management (updated and revised version). Prepared for Parks Services. Alberta Environment, Edmonton, 72 pp
Alsfeld AJ, Bowman JL, Deller-Jacobs A (2009) Effects of woody debris, microtopography, and organic matter amendments on the biotic community of constructed depressional wetlands. Biological Conservation 142:247–255
Angradi TR, Jicha TM (2010) Mesohabitat-specific macroinvertebrate assemblage responses to water quality variation in mid-continent (North America) great rivers. Ecological Indicators 10:943–954
Batzer DP, Wissinger SA (1996) Ecology of insect communities in nontidal wetlands. Annual Review of Entomology 41:75–100
Batzer DP, Palik BJ, Buech R (2004) Relationships between environmental characteristics and macroinvertebrate communities in seasonal woodland ponds of Minnesota. Journal of the North American Benthological Society 23(1):50–68
Bayrock LA, Hughes GM (1962) Surficial geology of the Edmonton District, Alberta. Earth Sciences Report 1962–06. Alberta Geological Survey, Edmonton
Bazzanti M, Coccia C, Dowgiallo MG (2010) Microdistribution of macroinvertebrates in a temporary pond of Central Italy: taxonomic and functional analyses. Limnologica 40(1):291–299
Braccia A, Batzer DP (2001) Invertebrates associated with woody debris in a Southeastern U.S. forested floodplain wetland. Wetlands 21(1):18–31
Bromley CK, Hood GA (2013) Beavers (Castor canadensis) facilitate early access by Canada geese (Branta canadensis) to nesting habitat and areas of open water in Canada’s boreal wetlands. Mammalian Biology 78:73–77
Butts WL (2001) Beaver ponds in upstate New York as a source of anthropophilic mosquitoes. Journal of the American Mosquito Control Association 17:85–86
Butts WL (2004) Changes in distribution and abundance of mosquito populations in an ecological research tract over a 35 year history. Journal of the American Mosquito Control Association 20:319–320
Christensen JR, Crumpton WG (2010) Wetland invertebrate community responses to varying emergent litter in a prairie pothole emergent marsh. Wetlands 30(6):1031–1043
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology 18:117–143
Clifford HF (1991) Aquatic invertebrates of Alberta. University of Alberta Press, Edmonton, 538 pp
Clifford HF, Wiley GM, Casey RJ (1993) Macroinvertebrates of a beaver-altered boreal stream in Alberta, Canada, with special reference to the fauna of dams. Canadian Journal of Zoology 71:1439–1447
Collen P, Gibson RJ (2001) The general ecology of beavers (Castor spp.) as related to their influence on stream ecosystems and riparian habitats and the subsequent effects on fish—a review. Reviews in Fish Biology and Fisheries 75:1009–1013
Cummins KW (1973) Trophic relations of aquatic insects. Annual Review of Entomology 18:183–206
de Szalay FA, Cassidy W (2001) Effects of muskrat (Ondatra zibethicus) lodge construction on invertebrate communities in a Great Lakes coastal wetland. American Midland Naturalist 146(2):300–310
de Szalay FA, Resh VH (1996) Spatial and temporal variability of trophic relationships among aquatic macroinvertebrates in a seasonal marsh. Wetlands 16(4):458–466
ESRI (2006) Environmental Research Institute, Inc., Redlands, CA, USA
France RL (1997) The importance of beaver lodges in structuring littoral communities in boreal headwater lakes. Canadian Journal of Zoology 75:1009–1013
Gathman JP, Burton TM (2011) A Great Lakes coastal wetland invertebrate community gradient: relative influence of flooding regime and vegetation zonation. Wetlands 31:329–341
Hann BJ (1995) Nektonic macroinvertebrates in a wetland pond (Crescent Pond, Delta Marsh, Manitoba). UFS (Delta Marsh) Annual Report 30:68–77
Hawkins CP, Sedell JR (1981) Longitudinal and seasonal changes in functional organization of macroinvertebrate communities in four Oregon streams. Ecology 62(2):387–397
Heino J (2000) Lentic macroinvertebrate assemblage structure along gradients in spatial heterogeneity, habitat size and water chemistry. Hydrobiologia 418(1):229–242
Hood GA, Bayley SE (2008) Beaver (Castor canadensis) mitigate the effects of climate on the area of open water in boreal wetlands in western Canada. Biological Conservation 141:556–567
Hood GA, Bayley SE (2009) A comparison of riparian plant community response to herbivory by beaver (Castor canadensis) and ungulates in Canada’s boreal mixed-wood forest. Forest Ecology and Management 258:1979–1989
Hornung JP, Foote AL (2006) Aquatic invertebrate responses to fish presence and vegetation complexity in western boreal wetlands, with implications for waterbird productivity. Wetlands 26(1):1–12
Hutchinson GE (1957) Concluding remarks. Cold Spring Harbor Symposium on Quantitative Biology 22:415–427
IBM, SPSS Software (2010) IBM SPSS statistics version 19. Armonk, New York
Jeffries M (1994) Invertebrate communities and turnover in wetland ponds affected by drought. Freshwater Biology 32:603–612
Jeffries M (2011) The temporal dynamics of temporary pond macroinvertebrate communities over a 10-year period. Hydrobiologia 661(1):391–405
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. Oikos 69:373–386
Kadman R, Allouche O (2007) Integrating the effects of area, isolation, and habitat heterogeneity on species diversity: a unification of island biogeography and niches theories. American Midland Naturalist 170(3):443–454
Kratzer EB, Batzer DP (2007) Spatial and temporal variation in aquatic macroinvertebrates in the Okefenokee Swamp, Georgia, USA. Wetlands 27(1):127–140
MacArthur RH, Wilson EO (1963) An equilibrium theory of insular biogeography. Evolution 17:273–387
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton
Marglois BE, Raesly RL, Shumway DL (2001) The effects of beaver-created wetlands on the benthic macroinvertebrate assemblages of two Appalachian streams. Wetlands 21(4):554–563
McCune B, Grace JB (2002) Analysis of ecological communities. MjM Software Design, Gleneden Beach
McCune B, Mefford MJ (2011) PC-ORD multivariate analysis of ecological data. Version 6. MjM Software, Glenedon Beach
McDowell DM, Naiman RJ (1986) Structure and function of a benthic invertebrate stream community as influenced by beaver (Castor canadensis). Oecologia 68:481–489
McParland CE, Paszkowski CA (2006) Effects of small-bodied fish on invertebrate prey and foraging patterns of waterbirds in Aspen Parkland wetlands. Limnology and Aquatic Birds 567:43–55
Merritt RW, Cummins KW, Berg MB (eds) (2008) An introduction to aquatic insects of North America, 4th edn. Kendall/Hunt Publishing Company, Dubuque
Meyer CK, Peterson SD, Whiles MR (2011) Quantitative assessment of yield, precision, and cost-effectiveness of three wetland invertebrate sampling techniques. Wetlands 31:101–112
Mitchell PA (1990) Miquelon Lake. In: Mitchell PA, Prepas EE (eds) Atlas of Alberta Lakes. University of Alberta Press, Edmonton
Naiman RJ, McDowell DM, Farr BS (1984) The influence of beaver (Castor canadensis) on the production dynamics of aquatic insects. Verhandlungen der Internationalen Vereinigung für Theoretische und Angewandte Limnologie 22:1801–1810
Navarro-Llácer C, Baeza D, de las Heras J (2010) Assessment of regulated rivers with indices based on macroinvertebrates, fish and riparian forest in the southeast of Spain. Ecological Indicators 10:935–942
Odling-Smee FJ, Laland KN, Feldman MW (1996) Niche construction. American Naturalist 147:641–648
Pielou EC (1966) The measurement of diversity in different types of biological collections. Journal of Theoretical Biology 13:131–144
Pollack MM, Naiman RJ, Hanley TA (1988) Plant species richness in riparian wetlands—a test of biodiversity theory. Ecology 79:94–105
Shannon CE (1948) A mathematical theory of communication. Bell System Technical Journal 27:379–423, And 623–656
Stachowicz JJ (2001) Mutualism, facilitation, and the structure of ecological communities. BioScience 51(3):235–246
StatSoft, Inc. (2003) Statistica version 6. Tulsa, Oklahoma
Thorp JP, Covich AP (2001) Ecology and classification of North American freshwater invertebrates. Academic Press/Harcourt Brace, Inc, San Diego
Turner AM, Trexler JC (1997) Sampling aquatic invertebrates from marshes: evaluating the options. Journal of the North American Benthological Society 16(3):694–709
Uzarski DG, Genet JA (2004) Invertebrate habitat use in relation to fetch and plant zonation in northern Lake Huron coastal wetlands. Aquatic Ecosystem Health and Management 7(2):249–267
Van de Meutter F, De Meester L, Stoks R (2007) Metacommunity structure of pond macro invertebrates: effects of dispersal mode and generation time. Ecology 88(7):1687–1695
Van de Meutter F, Cottenie K, De Meester L (2008) Exploring differences in macroinvertebrate communities from emergent, floating-leaved and submersed vegetation in shallow ponds. Fundamental and Applied Limnology 173(1):47–57
Westbrook CJ, Cooper DJ, Baker BW (2006) Beaver dams and overbank floods influence groundwater-surface water interactions of a Rocky Mountain riparian area. Water Resources Research 42, W06406. doi:10.1029/2005WR004560
Wright JP, Jones CG (2006) The concept of organisms as ecosystem engineers ten years on: progress, limitations, and challenges. BioScience 56:203–209
Acknowledgments
We are grateful for financial assistance from the Beaver Hills Initiative, Alberta Sport, Recreation, Parks and Wildlife Foundation, the Augustana Faculty of the University of Alberta, Alberta Tourism Parks and Recreation, and Dr. Suzanne Bayley. In addition, we are thankful for the field and lab assistance of Dr. Heather Proctor, Nils Anderson, Nicole Madu, Tim Nelner, Curtis Stratmoen, Stephen Olson, Makrina Scott, and Dustin Rahib and the statistical advice of Drs. Rebecca Rooney and David Locky. Finally, we thank Dee Patriquin and the anonymous reviewers, and the Associate Editor whose comments and advice helped to make this a stronger manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
(DOC 129 kb)
Table S2
(DOC 44 kb)
Table S3
(DOC 52 kb)
Supplementary Figure S1
(JPEG 115 kb)
Rights and permissions
About this article
Cite this article
Hood, G.A., Larson, D.G. Beaver-Created Habitat Heterogeneity Influences Aquatic Invertebrate Assemblages in Boreal Canada. Wetlands 34, 19–29 (2014). https://doi.org/10.1007/s13157-013-0476-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-013-0476-z