Abstract
Simplification of communities is a common consequence of anthropogenic modification. However, the prevalence and mechanisms of biotic homogenization among wetland systems require further examination. Biota of wetlands in the North American Prairie Pothole Region are adapted to high spatial and temporal variability in ponded-water duration and salinity. Recent climate change, however, has resulted in decreased hydrologic variability. Land-use changes have exacerbated this loss of variability. We used aquatic-macroinvertebrate data from 16 prairie-pothole wetlands sampled between 1992 and 2015 to explore homogenization of wetland communities. Macroinvertebrate communities of small wetlands that continued to cycle between wet and dry phases experienced greater turnover and supported unique taxa compared to larger wetlands that shifted towards less dynamic permanently ponded, lake-like regimes. Temporal turnover in beta-diversity was lowest in these permanently ponded wetlands. Additionally, wetlands that shifted to permanently ponded regimes also experienced a shift from palustrine to lacustrine communities. While increased pond permanence can increase species and overall beta-diversity in local areas previously lacking lake communities, homogenization of wetland communities at a larger, landscape scale can result in an overall loss of biodiversity as the diverse communities of many wetland systems become increasingly similar to those of lakes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, freshwater ecosystems are experiencing widespread habitat degradation and biodiversity loss (Dudgeon et al., 2006; Reid et al., 2018). Compared to taxa in surrounding terrestrial communities, freshwater taxa are perhaps the most imperiled biota (Sala et al., 2000; Reid et al., 2018). Not only are freshwater taxa being lost at alarming rates globally, regionally many aquatic ecosystems and their biotic communities are becoming increasingly similar or homogenous (Rahel, 2002; Petsch, 2016). Olden (2008) defined biotic homogenization as “the process by which species invasions and extinctions increase the genetic, taxonomic or functional similarity of two or more locations over a specified time interval.” In many cases, endemic species in an ecosystem are replaced by non-endemic invaders, often as a result of anthropogenic modifications to the environment (McKinney & Lockwood, 1999). Biotic homogenization often results in a loss of ecosystem function and, in turn, ecosystem health (Kinzig et al., 2001; Cardinale et al., 2002; Vaughn, 2010). Since increased similarity implies a decrease in variability, biotic homogenization is often detected as temporal decreases in beta-diversity (Olden & Rooney, 2006; Olden et al., 2018). While many of the foundational papers exploring evidence and mechanisms for biotic homogenization have been rooted in the aquatic sciences, these investigations are largely limited to fish and plant communities in riverine and lacustrine environments (Olden, 2006). The prevalence of and mechanisms for biotic homogenization in wetland ecosystems remain relatively unexplored. Therefore, a better understanding of biotic homogenization in wetland ecosystems is needed to contribute to landscape-level biodiversity conservation efforts.
In the context of biotic homogenization, previous investigations on the influence of climate and land-use driven modifications to wetlands have been limited to examination of plant communities (Houlahan & Findlay, 2004; Aronson & Galatowitsch, 2008; Spyreas et al., 2010). Little is known about how widespread spatial and temporal changes in wetland hydrology influences aquatic animal diversity, especially aquatic macroinvertebrates. One possible explanation for this lack of information is that there are very few studies on aquatic-macroinvertebrate communities that both span time periods long enough to detect a biotic homogenization trend and have sufficient taxonomic resolution needed to detect fine-scale community shifts. Another possible explanation is that wetland aquatic macroinvertebrates are highly adaptable to exist in these highly variable environments and are often dominated by generalists, making them resistant to hydrologic and anthropogenic changes (Batzer, 2013; Janke et al., 2019).
Decreased spatial and temporal variability of hydrological regimes and increased ponded-water connectivity are potential mechanisms promoting biotic homogenization. However, increased ponded-water extent, duration, and depth combined with decreased dissolved-ion concentrations can also make systems less deterministic (Daniel et al., 2019). Chase (2007) found that increasing hydroperiod and therefore reducing determinism in aquatic systems leads to increased species diversity, which results in an increase in biotic filtering (competition/predation), stochastic processes, and subsequent beta diversity. In such cases, increased biodiversity as a result of greater stochasticity is predicted to increase overall productivity (Chase, 2010). Potentially this shift towards a more stochastic assembly might be the key to understanding why very few aquatic-macroinvertebrate studies have identified land-use, salinity, or water depth as a significant driver of species richness or beta-diversity (reviewed in Batzer, 2013). While biotic homogenization in its strictest sense is limited to an overall decrease in beta-diversity over time, it can also be tied to the replacement of endemic taxa with non-endemic taxa. This taxa replacement can at times increase both alpha and beta-diversity in individual wetlands (Olden & Rooney, 2006).
We contend that wetland ecosystems provide an opportunity to gain useful insights into mechanisms driving biotic homogenization and the susceptibility and resistance of systems to these drivers. In order to explore biotic homogenization in the context of wetland aquatic-macroinvertebrate communities, we developed three hypotheses that relate biotic homogenization to hydrologic variability in prairie-pothole wetlands: (1) reduced spatial variability of wetland hydrologic regimes over time will lead to a decrease in mean annual between-wetland beta diversity, (2) reduced temporal variability in an individual wetland’s hydrological regime will decrease mean between-year beta-diversity in an individual wetland over time, and (3) a reduction in wetlands that frequently transition from ponded to dry will result in a loss of unique macroinvertebrate communities adapted to these conditions. We examined these hypotheses using hydrologic and aquatic-macroinvertebrate data collected over a 24-year period from the Cottonwood Lake Study Area (CLSA), an area of the North American Prairie Pothole Region (PPR) in east central North Dakota. The CLSA is the most extensively studied and monitored wetland complex in the PPR. As typical for the region, wetlands in this complex have shifted in recent decades towards longer hydroperiods, with the larger wetlands becoming less dynamic and lake-like over the past two decades (McKenna et al., 2017).
Study area
The PPR is a highly cultivated landscape dominated by agriculture making it one of the largest and most human-modified, wetland-dominated regions on Earth (van der Valk, 2005). The PPR consists of approximately 777,000 km2 of prairie-wetland mosaic spanning north and west from northwest Iowa through the Dakotas and into central Alberta (Smith et al., 1964). Depressional, prairie-pothole wetlands (hereafter referred to simply as “prairie potholes”) are the predominant wetland type occurring in the PPR. Prairie potholes can exhibit high spatial and temporal environmental variation in ponded-water extent, duration, depth, and salinity. The high variation is mostly attributable to the position of wetlands along elevation, groundwater, and geologic spatial gradients; and temporal gradients dominated by high inter-annual variation in precipitation (Euliss et al., 2004). Like many wetland systems, prairie potholes support unique biotic communities due to their hydrologic and geochemical dynamics (Euliss et al., 1999). Historically, biotic variation was maintained through high spatio-temporal variability in precipitation and temperature, in combination with the presence of numerous, heterogenous, wetland basins on the PPR landscape (Winter & Rosenberry, 1998; Anteau et al., 2016). However, contemporary changes in regional climate and land-use have been shown to alter the spatial and temporal hydrologic variability of these systems (Mushet et al., 2015; Cressey et al., 2016; McKenna et al., 2017).
An estimated 60–65% of the historically present wetland basins in the PPR have been lost due to anthropogenic modifications, e.g., ditching, filling (Dahl, 2014). Of these wetlands, the smaller, temporarily and seasonally ponded wetlands have been preferentially lost (Kahara et al., 2009; Serran & Creed, 2015; Van Meter & Basu, 2015). The loss of smaller wetlands on the landscape often consolidates runoff into the remaining wetlands which, in turn, decreases the number of small wetlands on the PPR landscape while simultaneously increasing the pond duration and size of wetlands that remain, resulting in decreased dynamics in their response to climate variability (Anteau, 2012; McCauley et al., 2015). The effect of modified water flow paths can be exacerbated by shifts in climate, especially during periods of above or below average precipitation. Beginning in 1993, the southern portion of the PPR has experienced an extended, multi-decade, wet period (Ballard et al., 2014; McKenna et al., 2017). These wet conditions extended north into the Canadian portion of the PPR starting in 2005 (Hayashi et al., 2016). The increased precipitation has resulted in cascading effects resulting in a shift towards more permanently ponded, connected, and lake-like wetland hydrological regimes (Mushet et al., 2015; Cressey et al., 2016; Vanderhoof & Alexander, 2016; Vanderhoof et al., 2016; McKenna et al., 2017). Under these conditions ponded-water variability and dissolved-ion concentrations can become homogenized by shifting towards larger and fresher ponds during wet periods (Mushet et al., 2015; Leibowitz et al., 2016; Cressey et al., 2016). The current shift towards more permanently ponded and fresher wetlands in the PPR provides a model to examine how decreased hydrologic variability influences biotic homogenization.
The CLSA is part of a complex of U.S Fish and Wildlife Service managed Waterfowl Production Areas located on the eastern edge of the Missouri Coteau in Stutsman County, North Dakota (Fig. 1). Surface and groundwater hydrology of the sixteen wetlands forming the CLSA complex have been continually monitored since 1979. The wetlands forming the CLSA wetland complex were initially classified in 1967 into permanently (P) and temporarily (T) ponded groupings. Using this original 1967 classification (Stewart & Kantrud, 1967), half (N = 8) of the wetlands were identified as permanently ponded and half (N = 8) as temporarily ponded. However, under the later Stewart & Kantrud (1971) classification system, the CLSA wetlands designated as permanently ponded contain both semi-permanently (N = 7) and permanently ponded (N = 1) wetlands (Class IV and V, respectively). Similarly, the wetlands originally classified as temporarily ponded contain both temporarily (N = 2) and seasonally ponded (N = 6) wetlands (Class II and III, respectively). Additionally, inter-annual changes in precipitation have influenced surface-water connections and extent of ponded water, which in turn significantly altered ponded-water permanence (Leibowitz et al., 2016; McKenna et al., 2017).
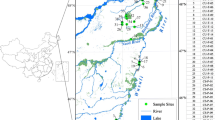
Map of prairie-pothole wetlands in the Cottonwood Lake Study Area, Stutsman County, North Dakota (from McKenna et al. 2017). Wetland P-11 (not shown) is located 3.2 km west of the core study area depicted here
For this study, we used the original temporary (T) and permanent (P) wetland classifications since they separate the wetlands that were permanently ponded throughout the time frame of the study (P-Wetlands) from those that cycled between ponded and dry (T-Wetlands). Two of the wetlands originally classified as P-Wetlands (P-3, P-8) lose water to surface outlets, which limit maximum water depths, so we added in a third classification called open-basin wetland (OB-Wetland). Since the two OB-Wetlands are depth limited, they are typically shallower than other P-Wetlands that have greater ponded-water storage capacity during wet periods that allows for emergent vegetation to occur over a greater proportion of their area compared to other P-Wetlands (Fig. 2). The wetlands in the CLSA are embedded within a prairie-grassland (i.e., non-cropland) landscape and are relatively untouched by anthropogenic landscape alterations. The CLSA wetlands have also remained mostly absent of sustained fish communities during the duration of this study, apart from fathead minnows (Pimephales promelas) and yellow perch (Perca flavescens) being introduced into “P-11” in 2014 and persisting in subsequent years, and occasional, but temporary, invasions of fathead minnows into wetland P8. Thus, the CLSA wetland complex for the most part provides an ideal system to explore potential environmental mechanisms of biotic homogenization in prairie potholes without the need to disentangle the influence landscape modifications or fish.
a Maximum observed annual ponded-water depths (m), b maximum ponded-water extent (%), c and open-water area (%) for sixteen Cottonwood Lake Study Area wetlands sampled from 1979 to 2015. For each plot, green squares represent temporarily ponded wetlands (T-Wetlands), blue triangles represent permanently ponded wetlands with closed basins (P-Wetlands), and orange circles represent permanently ponded wetlands with open basins (OB-Wetland). The solid lines represent fitted means for wetlands by hydrological regime. The gray-shaded areas surrounding each regression line represent the 95% confidence intervals. The black dashed lines indicate the transition for three different time periods; the “pre-wetting period” (1979–1992), the “filling period” (1993–1999), and the “post-filling period” (2000–2015)
Data
To evaluate temporal patterns in beta-diversity and potential drivers of biotic homogenization, we used a combination of available biotic and abiotic CLSA datasets. All long-term wetland monitoring data from the CLSA are openly available from the U.S. Geological Survey through the Missouri Coteau Wetland Ecosystem Observatory at https://www.sciencebase.gov/catalog/item/52f0ffd9e4b0f941aa181fc6. We used the following datasets for this study: aquatic-macroinvertebrate counts (Mushet et al., 2017a), wetland stage (ponded-water elevation; Mushet et al., 2016), and vegetation-zone area (Mushet et al., 2017b). Although several of the data sets from CLSA have been collected since 1979, we restricted most of our analyses to data from collected from 1992 to 2015, the period that coincides with the availability of aquatic-macroinvertebrate data.
Environmental variables
To provide context to general temporal trends in hydrologic variability observed in CLSA wetlands, we indexed mean annual pond duration in each basin with measured water depths (m), the proportion of the wetland basin that was ponded with water, and the proportion of the wetland that classified as open water. Water depths were manually recorded from staff gauges that were installed within the ponded portion of each CLSA wetland. To quantify the proportion of a wetland basin that was ponded, we used each wetland’s maximum water-surface elevation for each year and overlaid the elevation as a plane onto a digital elevation model (DEM) of the wetland complex (Mushet et al., 2017b) using ArcGIS. From this overlay, we calculated the area of the water-surface-elevation plane as a proportion of the area of a water-surface elevation plane from the date when the pond was at its maximum pool level for the period of record (1979–2015). We used wetland vegetation-zone delineations from each CLSA wetland (Mushet et al., 2017b) to quantify the proportion of the basin classified as open water. Wetland vegetation zones (wet meadow, shallow marsh, deep marsh, and open water) were delineated annually for all CLSA wetlands using aerial photographs of each wetland acquired during mid-summer using a digital camera at altitudes of photography ranging from 300 to 1,500 m above-ground-level. Aerial photographs were georeferenced using computer databases and major vegetative zones delineated using Mapping and Image Processing Software (MIPS). From these data, we calculated the proportion of the wetland that was identified as open water, i.e., the total area of open water divided by the total area of all wetland zones. We plotted each wetland’s depth, the proportion of the basin that was ponded, and proportion of the basin that was open water over time (1979–2015). We incorporated a smoothing line with shaded 95% confidence intervals through the mean values for wetlands by hydrological regime. Using patterns observed in these plots, we developed a hydroclimatic period classification highlighting three different periods: (1) the “pre-wetting period” (1979–1992), (2) the “filling period” (1993–1999), and (3) the “post-filling period” (2000–2015). The classification was based on the observed hydrologic variability. Between 1979 and 1992, CLSA wetlands cycled between being ponded and being dry; from 1993 to 1999, most wetland ponds were in a period of consistent filling until their water levels peaked in 1999; and from 2000 to 2015, the T-Wetlands continued to frequently alternate between ponded and dry while the P-Wetlands and OB-Wetlands maintained high, ponded-water levels (Fig. 2).
Biotic variables
We used aquatic-macroinvertebrate taxonomic composition and diversity to evaluate biotic homogenization. Compared to other wetland fauna, aquatic macroinvertebrates are abundant and speciose (Batzer & Wissinger, 1996). Aquatic macroinvertebrates have been monitored at CLSA since 1992. Recorded data include counts and biomass by taxa (lowest feasible resolution; typically, genus). Aquatic macroinvertebrates were sampled at CLSA using unbaited, vertically oriented, funnel traps (Swanson, 1978). Samples were collected monthly from random locations within each vegetation zone along three transects in each wetland from April to September. The number of vegetation zones present in a wetland varied by wetland and year, therefore the number of funnel traps used during a sampling occasion also varied by wetland and year. Since we used funnel-type activity traps the more benthic aquatic macroinvertebrates are likely underrepresented in the samples. Collected samples were stored in ethyl alcohol (80%) and processed in a U.S. Geological Survey laboratory in Jamestown, North Dakota. For our analyses, we calculated mean count for each unique taxonomical group for each wetland by year. We summarized the CLSA aquatic-macroinvertebrate data by each unique taxon’s mean abundance for each funnel trap used per wetland per year. The resulting dataset consisted of rows identifying the wetland and year sampled and columns indicating the mean abundance of each unique aquatic-macroinvertebrate taxon observed during the whole study. We then created a presence–absence matrix from the summarized aquatic-macroinvertebrate count data to reduce the influence of relative abundances that might result from an uneven sampling bias.
Statistical analysis
All statistical analyses were completed in R v. 3.5.2 (R Core Team, 2015). To visualize how water depths and vegetation-zone compositions of CLSA wetlands have changed over time (1979–2015) in response to climatological conditions, we plotted water depth (m), proportion of each wetland basin ponded with water, and proportion of each wetland that was in an open-water state. We investigated biotic homogenization of aquatic macroinvertebrates by exploring different measures of beta-diversity at different spatial scales over time.
Analysis of aquatic-macroinvertebrate biotic homogenization
The most common way to detect evidence for biotic homogenization is through quantifications of beta-diversity (Olden & Rooney, 2006). As different metrics of beta-diversity may capture different aspects of similarity between communities, we used multiple approaches for assessing beta-diversity to evaluate both turnover of aquatic-macroinvertebrate communities, total variation, and compositional differences of individual wetlands. We calculated Sorensen Index-based measures of beta-diversity on our presence/absence matrix to calculate beta-diversity. Biotic homogenization has been shown to occur at the local scale (individual wetland) at which beta-diversity in the form of temporal turnover is reduced over time, and at the complex scale at which between-site variation is reduced over time (Lambdon et al., 2008; Legendre & Cáceres, 2013). For this study, we compared within-wetland beta-diversity by comparing mean annual changes in beta-diversity between years, and we compared complex-scale beta-diversity by comparing mean annual beta-diversity between the 16 CLSA wetlands per year over the duration of the study (1992–2015). For each metric quantifying a measure of beta-diversity that could be compared by wetland regime, we ran an ANOVA model to identify differences in beta-diversity among hydroclimatic periods and hydrologic regimes. Hydroclimatic period was a categorical variable that grouped observations into the “filling period” and “post-filling period” periods described above. The “pre-wetting” period was not used as a categorical variable for beta-diversity type analysis since we did not have aquatic-macroinvertebrate data for years prior to 1992.
Our first form of biotic homogenization explored was the simplification of between-wetland communities. We analyzed how mean beta-diversity varied annually among CLSA wetlands using the beta.multi function and Sorensen Index-based dissimilarities derived from our presence–absence matrix using the ‘betapart’ package (Baslega et al., 2018). This approach calculates three multi-site dissimilarity coefficients: (1) Sorensen’s coefficient, a measure of overall between-wetland beta-diversity per year, which is partitioned to calculate (2) Simpson’s coefficient, a measure of turnover without the influence of richness differences, and (3) the nestedness coefficient, a measure of the nestedness of a site resulting from between-wetland richness differences (Baslega, 2010). We compared each year’s mean beta-diversity, turnover, and nestedness using data from all CLSA wetlands. Since overall between-wetland beta-diversity would be expected to decrease as the number of wetlands used in the analysis are reduced, we chose not to compare total beta-diversity by hydroclimatic period.
Our second form of biotic homogenization explored was within-wetland biotic homogenization over time, i.e., the reduction of between-year beta-diversity in aquatic-macroinvertebrate communities for each individual wetland. We analyzed within-wetland homogenization using the beta.temp function from the ‘betapart’ package (Baslega et al., 2018). Using Sorensen Index-based dissimilarities on our presence/absence matric, we partitioned between-year mean annual beta-diversity for each sampled wetland into three beta-diversity coefficients: (1) Sorensen’s coefficient for total between-year beta-diversity, (2) Simpson’s coefficient, for between-year turnover, and (3) the nestedness coefficient, for the between-year nestedness. For this analysis, we removed the sampling period 1992 since only two wetlands contained water during this exceptionally dry year. We plotted total beta-diversity, turnover, and nestedness over the 23-year period. Using an ANOVA, we then compared the average between-year total beta-diversity, turnover, and nestedness for all CLSA wetlands by regime (T-Wetlands, P-Wetlands, OB-Wetlands).
To better understand the potential patterns of an individual wetland’s contribution to diversity, we portioned our overall spatio-temporal beta-diversity into individual points in time. To do this, we used Local Contributions to Beta Diversity (LCBD) analyses (Legendre & Cáceres, 2013) to identify which wetland-by-year combination contributed significantly to either degraded or enhanced diversity. The indices derived from LCBD analysis are comparative indicators of how compositional uniqueness of taxa of sites by year contribute to overall beta-diversity (Legendre & Cáceres, 2013). The LCBD values are the squared distances of a site to the data centroid (Legendre, 2014). We used the beta.div function in the ‘adespatial’ package (Dray et al., 2016) using Sorensen Index-based dissimilarities to compute LCBD scores for each wetland and used a permutation test based on 1,000 permutations to identify which site by-year aquatic-macroinvertebrate communities were significantly different in taxonomical composition to the mean. We used an ANOVA to test if mean LCBD scores were significantly different depending on hydrologic regime and hydroclimatic period.
One of the common patterns in biotic homogenization is the replacement of specialist or endemic taxa with more ubiquitous, and sometimes, non-native taxa. Even though our understanding of what taxa are generalist or non-native to prairie potholes is limited, the replacement of taxa resulting in differing aquatic-macroinvertebrate communities over time can reveal patterns that are potentially relevant to the process of biotic homogenization. Thus, in addition to analysis of beta-diversity we explored potential compositional shifts of aquatic-macroinvertebrate taxa over time by analyzing how specific taxa compositions have changed. We used non-metric multidimensional scaling (NMDS) to graphically display changes in CLSA wetland macroinvertebrate community composition for the different hydrologic regimes and hydroclimatic periods. Our NMDS model was created using the metamds function in the ‘vegan’ package (Oksanen et al., 2019), in which we used Sorensen Index-based dissimilarities on our aquatic-macroinvertebrate presence/absence matrix. We plotted each wetland by years’ location on the first two NMDS axes and highlighted both the hydrologic regime and hydroclimatic period to visually observe their aquatic-macroinvertebrate communities in two-dimensional space. To evaluate whether aquatic-macroinvertebrate community compositions differed by hydrologic regime or hydroclimatic period, we ran a PERMANOVA using the Adonis function in the ‘vegan’ package (Oksanen et al., 2019).
In addition to the NMDS model, we used a hierarchical clustering approach to create groups of sample units (communities) of similar species composition to compare their distributions by wetland regime category over time. Wetlands were clustered by species composition using the Ward clustering technique. We created a distance matrix based on Sorensen Index-based dissimilarities with the vegdist function in the ‘vegan’ package using the aquatic-macroinvertebrate presence/absence matrix for the years 1992 to 2015 and used the hclust function in the ‘stats’ package to create a cluster dendrogram (Murtagh & Legendre, 2014). The cluster dendrogram was then pruned into groups, with number of groups being selected by the combination of how many sample units a cluster and identifying unique branches from the dendrogram while keeping a limited number of unique clusters. We used the Indval function from the ‘labdsv’ package (Roberts & Roberts, 2016) to run an indicator species analysis and detect taxa that are significantly associated with the selected clusters. We then plotted the number of wetlands belonging to each wetland by regime category over time.
Results
Environmental changes: potential drivers of biotic homogenization
As previously shown in McKenna et al. (2017), we found CLSA wetlands shifted towards having increased extent, depth, and open water after 1993 compared to the period between 1979 and 1993 (Fig. 2). The combination of year, wetland regime, and time period all significantly influenced variation in ponded-water depth, percentage of the wetland basin that was ponded, and the percentage of the wetland delineated as open water (Supplemental File, Tables 1, 2, 3). The percentage of a basin that was ponded and the percentage of a basin that was delineated as open water for T- P-, and OB-Wetlands all had dynamic and temporally coherent oscillations of wetting and drying periods (Fig. 2). However, post-1993 water levels in T-wetlands continued to cycle between wet and dry, while in P-Wetlands water levels increased and vegetation-zone extent decreased and then remained relatively stable over time (Fig. 2). The depth-constrained OB-Wetlands typically had less open water due to increased area for emergent vegetation to establish and were very stable in the amount of open water during the “filling period” and “post-filling period” periods compared to both T- and P-Wetlands. The primary difference in hydrologic conditions between the “filling period” and “post-filling period” was that T-Wetlands continued to cycle between ponded and dry, while P- and OB-Wetlands remained permanently ponded in more lake-like conditions (Fig. 2).
Total beta-diversity trends
Our analysis of total Sorensen Index-based dissimilarity between each wetland at a given year did not indicate a consistent increase or decrease of mean total beta-diversity over time (Fig. 3). The between-wetland dissimilarity values were typically high (> 0.7) throughout the duration of the study (Supplemental File, Table 4). Throughout the analyzed time period, most of the beta-diversity was a product of turnover of aquatic-macroinvertebrate taxa between wetlands (Fig. 3). The “filling period” between 1993 and 1999 actually had lower mean annual between-wetland beta-diversity when compared to the “post-filling period” period that occurred between 2000 and 2015. This result was unsurprising considering all the wetlands were dry during a portion of the year 1992 and rapidly begin to fill during the following 6 years (Fig. 2) when they were likely being colonized by the many of the same aquatic macroinvertebrates that were either rapid dispersers or can withstand periodic drying events. During the “post-filling period” period, there was greater overall variability in mean total annual beta-diversity as well as the nestedness as turnover components of the overall beta-diversity (Fig. 3). While P-Wetlands and OB-Wetlands had reduced hydrologic variability during the “post-filling period” period, they also exhibited very different temporal hydrologic characteristics than the T-Wetlands during this period compared to the “filling period.” Therefore, this greater difference in hydrologic characteristics between hydrologic regimes during the “post-filling period” period could have been a mechanism for overall greater beta-diversity in the CLSA complex.
Mean Annual between-wetland-by-year Sorensen Index-based dissimilarities derived from aquatic-macroinvertebrate presences and absences for all 16 sampled Cottonwood Lake Study Area Wetlands beginning in 1993 and ending in 2015. Each unique color by shape combination connected by a solid line represents a different component of beta-diversity; (1) the green squares represent the proportion of mean annual beta-diversity that was attributed to turnover (Simpson’s Coefficient), (2) the red circles represent the proportion of mean annual beta-diversity that was attributed to nestedness (Nestedness Coefficient), and the black triangles represent the mean between-wetland total beta-diversity value per year (Sorensen’s Coefficient). The vertically oriented black dashed line indicates the transition from the “filling period” (1993–1999) to the “post-filling period” (2000–2015) on the x-axis
Temporal turnover of beta-diversity
Overall, our analysis within-wetland, between-year beta-diversity did not reveal any linear patterns of consistent increases or decreases of total beta-diversity, turnover, or nestedness over time (Fig. 4). Like the between-wetland analysis of beta-diversity, the within-wetland beta-diversity was mostly driven by changes in turnover compared to nestedness (Fig. 4a, b). However, overall variance of total beta-diversity was significantly different for wetlands in the “filling period” and “post-filling period” period (Fig. 4c, Table 1). The wetlands with the greatest amount of hydrologic variability over time (i.e., T-Wetlands) did have significantly higher amounts of between-year beta-diversity (Fig. 4c, Table 1). Therefore, we cannot completely reject or support our hypothesis that reduced hydrologic variability in an individual wetland over time would lead to reduce between-year beta-diversity in an individual wetland over time.
Mean annual between-year Sorensen Index-based dissimilarities based on aquatic-macroinvertebrate presences and absences for all 16 sampled Cottonwood Lake Study Area Wetlands beginning in 1993 and ending in 2015. Each plot represents a different component of between-year within-wetland beta-diversity; a the proportion of mean annual between-year, within-wetland total beta-diversity that was attributed to turnover (Simpson’s Coefficient), b the proportion of mean annual between-year, within-wetland total beta-diversity that was attributed to nestedness (Nestedness Coefficient), and c the mean annual between-year, within-wetland beta-diversity value (Sorensen’s Coefficient). The solid lines represent fitted means for wetlands by hydrological regime. The gray-shaded areas represent 95% confidence intervals for each fitted regression line. The black dashed line indicates the transition from the “filling period” (1993–1999) to the “post-filling period” (2000–2015)
Contribution of individual wetlands to beta diversity
Our analysis of temporal variability of an individual wetland’s LCBD was to complement our overall between-wetland mean annual beta-diversity analysis by partitioning the beta-diversity into individual wetlands. The LCBD analysis did reveal temporal patterns in each wetland’s unique contribution to beta-diversity (Fig. 5; Table 2; Supplemental File, Fig. 1). During the “filling period,” T-Wetlands declined in mean LCBD scores, while P-Wetland and OB-Wetlands remained fairly constant (Fig. 5). Then in the “post-filling period” period T-Wetlands and P-Wetlands showed a hump-shaped mean in LCBD scores where the gradually increased and decreased, while the OB-Wetlands remained fairly constant. Our ANOVA model indicated that variation of LCBD scores differed by hydrologic regime, and that hydrologic regimes differed in LCBD score variation in the different hydroclimatic periods (Table 2). We found that when ponded, the T-Wetlands were more likely to host significantly unique taxonomical community compositions. Our LCBD values per wetland-by-year plot revealed that after the year 2000, T-Wetlands were more likely to have LCBD values that were statistically greater than the mean (P Value < 0.05), indicating aquatic-macroinvertebrate communities in these wetlands were more distinct in taxonomical composition relative to the “average” aquatic-macroinvertebrate composition over the duration of the study. The P-Wetlands appear to increase in LCBD values between 1995 and 2005 but decrease from 2005 to 2015 (Fig. 5).
Temporal trends of Sorensen Index dissimilarity based Local Contributions to Beta Diversity (LCBD) values derived from the presence/absence-based aquatic-macroinvertebrate compositions for Cottonwood Lake Study Area wetland (n = 16) by year (n = 23) combinations plotted from 1993 to 2015. The y-axis represents the LCBD values, which are the partitioned amount of beta-diversity that a single wetland in a single year contributes to the overall beta-diversity observed in the study. Green points represent temporarily ponded wetlands (T-Wetlands), blue points represent permanently ponded wetlands with closed basins (P-Wetlands), and orange points represent permanently ponded wetlands with an open basin (OB-Wetland). Wetland-by-year combinations with LCBD values that were significantly greater than mean LCBD values (alpha < 0.05) are indicated by solid asterisks. Sites with LCBD values not significantly greater than overall mean LCBD values are indicated by solid circles. The solid lines represent fitted mean LCBD values for wetlands by hydrological regime. The gray-shaded areas represent 95% confidence intervals for each fitted regression line. The black dashed line indicates the transition from the “filling period” (1993–1999) to the “post-filling period” (2000–2015)
Aquatic-macroinvertebrate community shifts over time
Our NMDS analysis based on Sorensen’s Index-based dissimilarities of aquatic-macroinvertebrate compositions using our presence–absence matrix yielded a three-dimensional solution with a stress of 0.15. Since most of the variation is found in the first two axis, we only plotted site scores on the first two axis (Fig. 6). The NMDS plot and complementary PERMANOVA showed wetlands varied by regime and hydroclimatic period, and that hydrologic regime explained the greatest amount of variation in the Sorensen’s Index-based dissimilarity matric (Fig. 6, Table 3), Typically, T-Wetlands were ordinated towards the left, OB-Wetlands towards the center, and P-Wetlands towards the right on Axis 1. There was a lot of overlap among wetlands of different regime categories during the early “filling period” period, and reduced overlap during the “post-filling” period when a majority of the P-Wetlands shifted to the right side of the Axis 1 and towards the bottom of Axis 2, indicating a shift in aquatic-macroinvertebrate composition (Fig. 6).
Non-metric multidimensional scaling ordination (k = 3, stress = 0.15) analyzed from Sorensen’s Index-based dissimilarities of presence/absence-based aquatic-macroinvertebrate taxonomic compositions for Cottonwood Lake Study Area wetland (n = 16) by year (n = 23) combinations. Hydrologic regime by hydroclimatic period categories was indicated by unique color by shape combinations. Open-basin wetlands (OB-Wetlands) during the “filling period” (1993–1999) are indicated by golden circles, open-basin wetlands during the “post-filling period” (2000–2015) are indicated by dark orange circles, permanently ponded wetlands (P-Wetlands) during the “filling period” (1993–1999) are indicated by light-blue squares, permanently ponded wetlands during the “post-filling period” (2000–2015) are indicated by dark blue squares, temporarily ponded wetland (T-Wetlands) during the “filling period” (1993–1999) are indicated by light-green triangles, and temporarily ponded wetlands during the “post-filling period” (2000–2015) are indicated by forest-green triangles
Our cluster analysis revealed three clusters that were visually divergent in aquatic-macroinvertebrate community composition and evenness of sample units (Fig. 7, Clusters 1–3, N = 163, 84, and 96, respectively). Indicator species analysis revealed that each of the three clusters had unique, statistically significant, indicator taxa with indicator values greater than or equal to 0.4 (0–1 scale, Table 4). The indicator values for cluster 1 were highest for the mosquito genera Culiseta and Aedes, the moss bladder snail (Aplexa hypnorum), Eubranchipus bundyi (a fairy shrimp), and Eubranchipus ornatus (a fairy shrimp), which are all typically associated with wetlands with shorter hydroperiods (Den Hartog & De Wolf 1962; Cvancara, 1983; Chase & Knight, 2003; Silver et al., 2012; Libonatti & Ruta, 2018). Cluster number 2 had the greatest number of significant indicator taxa, most of which are indicative of clear, highly vegetated waters (Table 4; Cvancara, 1983; Thorp & Covich, 2009). The significant indicator taxa for cluster number 3 were Dasycorixa rawsoni (a water boatman), Physa gyrina (pond snail), and the family Sphaeriidae (fingernail clams), which are all typically associated with semi-permanent or permanently ponded environments (Cvancara, 1983; Thorp & Covich, 2009).
Dendrogram representation of Ward’s method of cluster analysis of Sorensen’s Index dissimilarities of presence/absence-based aquatic-macroinvertebrate taxonomic compositions for Cottonwood Lake Study Area wetland (n = 16) by year (n = 23) combinations. The dendrogram was pruned to three unique clusters that are indicated by differently colored branches. Cluster number 1 (n = 163) is represented by green branches, cluster 2 (n = 84) by red branches, and cluster 3 (n = 96) by blue branches
Our plots detailing the number of wetlands by regime belonging to each cluster revealed that the first cluster was associated primarily with T-Wetlands and OB-Wetlands, while cluster two had a high frequency of occurrence in all regime categories until the year 2002, when only T-Wetlands and OB-Wetlands were grouped into the cluster (Fig. 8). The P-Wetlands changed in cluster representation over time, during the first two years, most of the P-Wetlands shifted from cluster 1 to cluster 2, and by 2002 all had shifted to cluster 3 and remained there throughout the remainder of the study (Fig. 8). The only P-Wetland that occurred in cluster 3 prior to 1999 was P-11, the only CLSA wetland originally classified as a Class V, permanently ponded wetland. The T-Wetlands and OB-Wetlands often cycled between being part of clusters 1 and 2, except from 2002–2004 when one of the T-Wetlands (T-3) belonged to cluster number 3 (Fig. 8). This is also the period when ponded water from one of the P-Wetlands (P-1) was merged with T-3. Over time P-Wetlands experienced a shift to more permanent and stable water regimes that was reflected by an increase in their frequency in cluster membership associated with lacustrine communities (Figs. 2, 8). During the early portion of the study, except for Wetland P-11, P-Wetland aquatic-macroinvertebrate communities were grouped into cluster 1 much like the T-Wetlands and OB-Wetlands. However, they shifted from cluster 1 to cluster 2 in 1995 and remained there until 2001, and then shifted to cluster 3 in 2002 (Fig. 8).
Scatterplot indicating the number of Cottonwood Lake Study Area wetland (n = 16) by year (n = 23) combinations belonging to a cluster 1, b, cluster 2, and c cluster 3 of Ward’s method of cluster analysis of Sorensen’s Index dissimilarities of presence/absence-based aquatic-macroinvertebrate taxonomic compositions during the years 1992 to 2015. Each unique point by color combination represents the hydrological regime category for each wetland. The black dashed line indicates the transition from the “filling period” (1993–1999) to the “post-filling period” (2000–2015)
Discussion
We found that within the relatively undisturbed confines of the CLSA, which hosts a variety of different sized wetland basins, there was no long-term pattern of reduced beta-diversity. However, if beta-diversity and hydrologic variability patterns observed at the CLSA are comparable to the surrounding more modified portions of the prairie-pothole region, there might be a greater concern for biotic homogenization on the landscape. We found that the T-Wetlands had more temporally variable hydrologic regimes, more temporal turnover of beta-diversity, greater contributions to overall beta-diversity during the study and occupied a larger but unique gradient of our NMDS ordination compared to the more hydrologically stable P-Wetlands and OB-Wetlands. Considering that approximately 60–65% of prairie potholes have been drained (Dahl, 2014), and a disproportionate number of those wetlands are smaller more temporarily ponded basins (Serran & Creed, 2015), then the assumed loss of these smaller wetlands would result in a loss of unique aquatic-macroinvertebrate communities and subsequent decreases in total beta-diversity from portions of the landscape. Since all closed basin P-Wetlands shifted to more lake-like systems during this study and T-Wetlands continued to cycle between ponded and dry, the aquatic-macroinvertebrate community type we categorized as typically associated with temporarily ponded or shallow, highly vegetated wetlands was no longer present during the latter portion of the study (Fig. 8). In addition to the loss of smaller wetland basins, many PPR studies have also indicated that semi-permanent type wetlands are shifting to deeper, lake-like wetlands throughout a large portion of the PPR (Ballard et al., 2014; Cressey et al., 2016; Hayashi et al., 2016). Our cluster frequency analysis and NMDS analysis indicate that this hydrologic shift towards more lacustrine water regimes could potentially result in a shift in aquatic-macroinvertebrate communities in these wetlands.
Even though the 25-year dataset used to evaluate this temporal trend contains a longer temporal gradient than any other known aquatic-macroinvertebrate study conducted in prairie-pothole wetlands, it begins in a year in which most of the wetland ponds were completely dry and then immediately transitioned to a period of deluge (Winter & Rosenberry, 1998). The deluge caused the wetlands to rapidly increase in depth in the following five years before stabilizing at higher levels than previously present (Fig. 2). This does not capture the historical dynamic temporal variability of hydrological conditions in P-Wetlands, in which there were more frequent hydrological fluctuations. As a result, we cannot conclusively compare aquatic macroinvertebrates during historical, and arguably more “normal,” hydrological regimes to their current state.
While we hypothesized that both between-wetland beta-diversity and between-year beta-diversity in individual wetlands would decrease when hydrologic characteristics between wetlands and between years become less variable, we observed a trend where P-Wetland exhibited the most beta-diversity during the early portion of the “post-filling period” period (2000–2006). We hypothesize that this might indicate a shift towards increases in stochastic assembly processes and biotic interactions that are intrinsic to each wetland and that can increase overall compositional dissimilarity, even in seemingly homogenous habitats. For example, many of the P-Wetlands had high turnover in the years 2001 and 2002 (Fig. 4). We hypothesize this change may be due to an iridovirus outbreak that decimated tiger salamander (Ambystoma mavortium) populations at CLSA in 2000 (Dooley, 2001). Tiger salamanders are the native top aquatic predators in these more permanently ponded systems and their presence can alter the abundance and composition of aquatic macroinvertebrates (Holomuzki et al., 1994; Benoy, 2008). Their abundances rapidly increased post-1993 and peaked in the year 2000 (Mushet & Solensky, 2018). When the predatory salamander population collapsed following the viral outbreak in 2000, it allowed many aquatic-macroinvertebrate populations to increase. This time frame also coincides with when P-Wetlands had the highest LCBD values (Fig. 5; Supplemental File, Fig. 1), indicating that beta-diversity in these wetlands was highest during those years.
While we were not surprised by the results of T-Wetlands having typically higher LCBD scores when ponded, the ecological mechanisms behind these results are less understood. When relating the results to hydrologic variability, the continuation of dynamic water regimes and fluctuating environmental conditions have likely made T-Wetland aquatic-macroinvertebrate communities less susceptible to biotic homogenization. T-Wetlands did show a mean increase in ponded water, but they continued to fluctuate between wetting and drying phases, which resulted in fluctuations in the amount of the basin that was in open water vs. a vegetated marsh state. The frequent drying of T-Wetlands can result in periodic reshuffling of aquatic-macroinvertebrate communities which would result in higher beta-diversity and resulting between-wetland differentiation due to ecological drift. Another not mutually exclusive hypothesis is that the more temporally homogenous hydrologic characteristics in P-Wetlands and OB-Wetlands result in different in taxonomic compositions of macroinvertebrate communities between the more permanently ponded hydrologic regimes and the more temporarily ponded regimes. This is because there is likely a subset of taxa that either require period drying to complete their life-cycle so they only occurred in wetlands that periodically dry up and another subset of taxa that cannot tolerate desiccation (Gleason & Rooney, 2018). This is supported by the patterns in our NMDS ordination (Fig. 6) and cluster representation (Fig. 8, Table 4). Using natural history characteristics of indicator taxa from the first cluster, we can arguably categorize this cluster as the temporarily ponded wetland macroinvertebrate communities. Mosquitos typically have short larval life cycles and flying adults can colonize and disperse to newly ponded wetlands; and their survival in wetlands that dry periodically is typically much higher than in those that remain ponded (Chase & Knight, 2003). The moss bladder snail (Aplexa hypnorum) is typically only found in temporarily ponded wetlands; and the eggs and larval stages of this snail are freeze-and-desiccation tolerant (Den Hartog & De Wolf, 1962). The knobbedlip fairy shrimp (Eubranchipus bundyi) deposits eggs in dry wetland basins and will rapidly hatch after seven days post-thaw in the spring, allowing them to persist under very limited ponding periods (Dabron, 1976). Cluster number two contains a diverse combination of insects and crustaceans that can occupy a wide gradient of hydrologic regimes, and many of these taxa are associated with emergent or submergent vegetation characteristic of semi-permanent wetlands (Thorp & Covich, 2009). Cluster 3 captures the more permanently ponded or lake-like wetland macroinvertebrate community. The strongest indicator was Dasycorixa rawsoni (a water boatman). This species is associated with permanently ponded waterbodies and were first observed in the CLSA in 1998 and 1999. They subsequently became extremely numerous in relative abundance in the following years (Hanson et al., 2003; Mushet et al., 2018).
Importance of temporarily ponded wetland contributions to biodiversity
There has been an observed disproportionate amount of smaller temporarily ponded wetlands that have lost hydrologic function on the landscape (Serran & Creed, 2015; Van Meter & Basu, 2015). There has also been a potential loss of the unique aquatic macroinvertebrates associated with these systems. This potential loss of specialist taxa is concerning due to the widespread loss of temporary wetlands on the landscape and their continued susceptibility to climate change, drainage, and filling with sediments (Serran & Creed, 2015; Creed et al., 2017). The loss of temporally ponded wetlands has been an identified threat to fairy shrimp (Anostraca) in wetland systems across the United States (Angeler et al., 2008). Fairy shrimp are important to breeding waterfowl in the region, especially during early spring since when they are among the first available invertebrates for consumption by arriving migratory waterfowl (Eldridge, 1990). A gadwall (Mareca strepera) diet study conducted in central North Dakota found that fairy shrimp comprised 2/3 of the pre-egg-laying diet and 33% of the full breeding-season diet of gadwall hens (Serie & Swanson, 1976). This preference was hypothesized to be a result of the high quantities and diversity of amino acids found in Anostracans (Serie & Swanson, 1976).
Importance of permanently ponded wetland contributions to biodiversity
When compared to wetlands of different hydrologic regimes, permanently ponded wetlands also contain uniquely adapted aquatic-macroinvertebrate community compositions (Figs. 6, 8). During drought events that cause smaller wetland basins to dry these systems provide refugia to organisms that are not adapted to desiccation (Mushet et al., 2013). While larger more permanently ponded wetlands are less vulnerable to drainage, outside of protected areas such as the CLSA, many are still vulnerable to anthropogenic degradation such as increased nutrients inputs, chemicals, and sedimentation due to runoff (Gleason & Euliss, 1998; Guntenspergen et al., 2002; Main et al., 2014). The contemporary hydrologic shift towards deeper and less saline systems (Mushet et al., 2015; Cressey et al., 2016) is believed to have facilitated an increase in the distribution of fish in previously fishless habitats which in turn alter the resident biotic communities (Zimmer et al., 2001, 2002; Hanson et al., 2005; Wiltermuth, 2014; McLean et al., 2016a). The invasion of fish into prairie potholes has had a negative impact on aquatic macroinvertebrates in larger wetlands (Anteau & Afton, 2008; Anteau et al., 2011; Wiltermuth 2014; McLean et al., 2016b). In addition to fish directly altering aquatic-macroinvertebrate abundance and distribution through competition and predation, the reduction in macroinvertebrates that feed on algae can promote algal blooms, causing a regime shift from a clear submergent vegetation state to a turbid eutrophic state, thus reducing aquatic-macroinvertebrate habitat and trophic complexity (Hanson & Butler, 1994; Scheffer & Jeppesen, 2007). However, our understanding of taxa that were historically present in prairie potholes compared to more lacustrine systems of the PPR is limited by our lack of long-term distributional data for prairie-pothole biota.
Conclusions
Prairie-pothole wetlands occur in a highly modified landscape and are susceptible to land-use and climate changes. Historically, wetlands in the region were subjected to highly variable environmental conditions. In recent years, land-use modifications and climate change have resulted in less spatial and temporal variation in wetland ecosystems. These characteristics have likely facilitated some form of biotic homogenization; however, our ability to detect homogenization at larger spatial and temporal scales is challenged, as there are few long-term biomonitoring efforts in the region. We provided examples of how aquatic-macroinvertebrate dynamics differ by ponded-water regimes. More information is needed to determine which prairie-pothole species are susceptible to replacement and their preferred habitats. It may also be important to understand the resiliency of the native biotic communities if prairie-pothole ponded-water dynamics were to return to their historical state. Since the macroinvertebrate communities of wetlands that typically dry appear to be more resistant to biotic homogenization, long-term maintenance of biodiversity in prairie-pothole complexes would require a priority being placed on the conservation and restoration of wetlands with temporally ponded and seasonally ponded water regimes on the PPR landscape.
References
Angeler, D. G., O. Viedma, S. Sánchez-Carrillo & M. Alvarez-Cobelas, 2008. Conservation issues of temporary wetland Branchiopoda (Anostraca, Notostraca: Crustacea) in a semiarid agricultural landscape: What spatial scales are relevant? Biological Conservation 141: 1224–1234.
Anteau, M. J., 2012. Do interactions of land use and climate affect productivity of waterbirds and Prairie-Pothole wetlands? Wetlands 32: 1–9.
Anteau, M. J. & A. D. Afton, 2008. Amphipod densities and indices of wetland quality across the upper-Midwest, USA. Wetlands 28: 184–196.
Anteau, M. J., A. D. Afton, A. C. Anteau & E. B. Moser, 2011. Fish and land use influence Gammarus lacustris and Hyalella azteca (Amphipoda) densities in large wetlands across the upper Midwest. Hydrobiologia 664: 69–80.
Anteau, M. J., M. T. Wiltermuth, M. P. van der Burg & A. T. Pearse, 2016. Prerequisites for understanding climate-change impacts on northern Prairie wetlands. Wetlands 36: 299–307.
Aronson, M. F. J. & S. Galatowitsch, 2008. Long-term vegetation development of restored prairie pothole wetlands. Wetlands 28: 883–895.
Ballard, T., R. Seager, J. E. Smerdon, B. I. Cook, A. J. Ray, B. Rajagopalan, Y. Kushnir, J. Nakamura & N. Henderson, 2014. Hydroclimate variability and change in the Prairie Pothole Region, the “Duck Factory” of North America. Earth Interactions 18(14): 1–28.
Baselga, A., 2010. Partitioning the turnover and nestedness components of beta diversity. Global Ecology and Biogeography 19: 134–143.
Baselga, A., D. Orme, S. Villeger, J. De Bortoli, F. Leprieur & M. A. Baselga, 2018. Package ‘betapart’.
Batzer, D. P., 2013. The seemingly intractable ecological responses of invertebrates in North American wetlands: a review. Wetlands 33: 1–15.
Batzer, D. P. & S. A. Wissinger, 1996. Ecology of insect communities in nontidal wetlands. Annual Review of Entomology 41: 75–100.
Benoy, G. A., 2008. Tiger salamanders in prairie potholes: a fish in amphibian’s garments? Wetlands 28: 464–472.
Cardinale, B. J., M. A. Palmer & S. L. Collins, 2002. Species diversity enhances ecosystem functioning through interspeci c facilitation. Nature 415: 426–429.
Chase, J. M., 2007. Drought mediates the importance of stochastic community assembly. Proceedings of the National Academy of Sciences United States of America 104: 17430–17434.
Chase, J. M., 2010. Stochastic community assembly causes higher biodiversity in more productive environments. Science 328: 1388–1391.
Chase, J. M. & T. M. Knight, 2003. Drought-induced mosquito outbreaks in wetlands. Ecology Letters 6: 1017–1024.
Creed, I. F., C. R. Lane, J. N. Serran, L. C. Alexander, N. B. Basu, A. J. K. Calhoun, J. R. Christensen, M. J. Cohen, C. Craft, E. D’Amico, E. DeKeyser, L. Fowler, H. E. Golden, J. W. Jawitz, P. Kalla, L. K. Kirkman, M. Lang, S. G. Leibowitz, D. B. Lewis, J. Marton, D. L. McLaughlin, H. Raanan-Kiperwas, M. C. Rains, K. C. Rains & L. Smith, 2017. Enhancing protection for vulnerable waters. Nature Geoscience 10: 809–815.
Cressey, R. L., J. E. Austin & J. D. Stafford, 2016. Three responses of wetland conditions to climatic extremes in the Prairie Pothole Region. Wetlands 36: 357–370.
Cvancara, A. M., 1983. Aquatic Mollusks of North Dakota. North Dakota Geological Survey.
Dahl, T. E., 2014. Status and Trends of Prairie Wetlands in the United States 1997 to 2009. Department of Interior, Fish and Wildlife Service, Ecological Services, Washington D.C: 67.
Daniel, J., J. E. Gleason, K. Cottenie & R. C. Rooney, 2019. Stochastic and deterministic processes drive wetland community assembly across a gradient of environmental filtering. Oikos. https://doi.org/10.1111/oik.05987.
Dabron, G. R., 1976. The life cycle of Eubranchipus bundyi (Forbes) (Crustacea: Anostraca) in a temporary vernal pond of Alberta. Canadian Journal of Zoology 54: 193–201.
Den Hartog, C. & L. De Wolf, 1962. The life cycle of the water snail Aplexa hypnorum. Basteria 26: 61–72.
Dooley, E. E., 2001. Virus targets amphibians. Environmental Health Perspectives 109: A16.
Dray, S., G. Blanchet, D. Borcard, G. Guenard, T. Jombart, G. Larocque, P. Legendre, N. Madi & H. H. Wagner, 2016. Adespatial: Multivariate Multiscale Spatial Analysis. R package version 0.0–4.
Dudgeon, D., A. H. Arthington, M. O. Gessner, Z. Kawabata, D. J. Knowler, C. Lévêque, R. J. Naiman, A. H. Prieur-Richard, D. Soto, M. L. J. Stiassny & C. A. Sullivan, 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews 81: 163–182.
Eldridge, J., 1990. 13.3.3. Aquatic Invertebrates Important for Waterfowl Production. Waterfowl Management Handbook, https://digitalcommons.unl.edu/icwdmwfm/16.
Euliss, N. H., D. A. Wrubleski & D. M. Mushet, 1999. Wetlands of the Prairie Pothole Region: Invertebrate Species Composition, Ecology, and Management. Springer, New York: 471–514.
Euliss, N. H., J. W. LaBaugh, L. H. Fredrickson, D. M. Mushet, M. K. Laubhan, G. A. Swanson, T. C. Winter, D. O. Rosenberry & R. D. Nelson, 2004. The wetland continuum: a conceptual framework for interpreting biological studies. Wetlands 24: 448–458.
Gleason, R. A. & N. H. Euliss Jr., 1998. Sedimentation of prairie wetlands. Great Plains Research 8: 97–112.
Gleason, J. E. & R. C. Rooney, 2018. Pond permanence is a key determinant of aquatic macroinvertebrate community structure in wetlands. Freshwater Biology 63: 264–277.
Guntenspergen, G. R., S. A. Peterson, S. G. Leibowitz & L. M. Cowardin, 2002. Indicators of wetland condition for the Prairie Pothole Region of the United States. Environmental Monitoring and Assessment 78(3): 229–252.
Hanson, M. A., K. D. Zimmer, M. G. Butler, B. A. Tangen, B. R. Herwig & N. H. Euliss, 2005. Biotic interactions as determinants of ecosystem structure in prairie wetlands: an example using fish. Wetlands 25: 764–775.
Hanson, M. A. & M. G. Butler, 1994. Responses of plankton, turbidity, and macrophytes to biomanipulation in a shallow prairie lake. Canadian Journal of Fisheries and Aquatic Sciences 51: 1180–1188.
Hanson, B. A., N. H. Euliss Jr., D. M. Mushet & S. W. Chorda III, 2003. First record of Dasycorixa rawsoni (Hemiptera: Corixidae) in the United States. Entomological News 114: 235–236.
Hayashi, M., G. van der Kamp & D. O. Rosenberry, 2016. Hydrology of Prairie wetlands: understanding the integrated surface-water and groundwater processes. Wetlands 36: 237–254.
Holomuzki, J. R., J. P. Collins & P. E. Brunkow, 1994. Trophic control of fishless ponds by tiger salamander larvae. Oikos 71: 55–64.
Houlahan, J. E. & C. S. Findlay, 2004. Effect of invasive plant species on temperate wetland plant diversity. Conservation Biology 18: 1132–1138.
Janke, A. K., M. J. Anteau & J. D. Stafford, 2019. Prairie wetlands confer consistent migrant refueling conditions across a gradient of agricultural land use intensities. Biological Conservation 229: 99–112.
Kahara, S. N., R. M. Mockler, K. F. Higgins, S. R. Chipps & R. R. Johnson, 2009. Spatiotemporal patterns of wetland occurrence in the Prairie Pothole Region of eastern South Dakota. Wetlands 29: 678–689.
Kinzig, A. P., S. Pacala & D. Tilman, 2001. The Functional Consequences of Biodiversity: Empirical Progress and Theoretical Extensions. Princeton University Press, Princeton.
Lambdon, P. W., F. Lloret & P. E. Hulme, 2008. Do nonnative species invasions lead to biotic homogenization at small scales? The similarity and functional diversity of habitats compared for alien and native components of Mediterranean floras. Diversity and Distributions 14: 774–785.
Legendre, P., 2014. Interpreting the replacement and richness difference components of beta diversity. Global Ecology Biogeography 23: 1324–1334.
Legendre, P. & M. Cáceres, 2013. Beta diversity as the variance of community data: dissimilarity coefficients and partitioning. Ecology Letters 16: 951–963.
Leibowitz, S. G., D. M. Mushet & W. E. Newton, 2016. Intermittent surface water connectivity: fill and spill vs. fill and merge dynamics. Fill and Merge Dynamics. Wetlands 36: 323–342.
Libonatti, M. L. & R. Ruta, 2018. Family scirtidae. In Thorp and Covich’s Freshwater Invertebrates. Academic Press, Cambridge: 599–603.
Main, A. R., J. V. Headley, K. M. Peru, N. L. Michel, A. J. Cessna & C. A. Morrissey, 2014. Widespread use and frequent detection of neonicotinoid insecticides in wetlands of Canada’s Prairie Pothole Region. PLoS ONE 9(3): e92821.
McCauley, L. A., M. J. Anteau, M. Post van der Burg & M. T. Wiltermuth, 2015. Land use and wetland drainage affect water levels and dynamics of remaining wetlands. Ecosphere 6: 92.
McKenna, O. P., D. M. Mushet, D. O. Rosenberry & J. W. LaBaugh, 2017. Evidence for a climate-induced ecohydrological state shift in wetland ecosystems of the southern Prairie Pothole Region. Climatic Change 145: 273–287.
McKinney, M. L. & J. L. Lockwood, 1999. Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends in Ecology & Evolution 14: 450–453.
McLean, K. I., D. M. Mushet, D. A. Renton & C. A. Stockwell, 2016a. Aquatic macroinvertebrate communities of prairie-pothole wetlands and lakes under a changed climate. Wetlands 36: 423–435.
McLean, K. I., D. M. Mushet & C. A. Stockwell, 2016b. From “Duck Factory” to “Fish Factory”: climate induced changes in vertebrate communities of Prairie Pothole wetlands and small lakes. Wetlands 36: 407–421.
Murtagh, F. & P. Legendre, 2014. Ward’s hierarchical agglomerative clustering method: which algorithms implement Ward’s criterion? Journal of classification 31: 274–295.
Mushet, D. M. & M. J. Solensky, 2018. Cottonwood Lake Study Area—Amphibians. U.S. Geological Survey Data Release. https://doi.org/10.5066/F7X9297Q.
Mushet, D. M., N. H. Euliss Jr. & C. A. Stockwell, 2013. Complex spatial dynamics maintain northern leopard frog genetic diversity in a temporally varying landscape. Herpetological Conservation and Biology 8: 163–175.
Mushet, D. M., M. B. Goldhaber, C. T. Mills, K. I. McLean, V. M. Aparicio, R. B. McCleskey, J. M. Holloway & C. A. Stockwell, 2015. Chemical and Biotic Characteristics of Prairie Lakes and Large Wetlands in South-Central North Dakota: Effects of a Changing Climate. US Geological Survey.
Mushet, D. M., D. O. Rosenberry, N. H. Euliss Jr. & M. J. Solensky, 2016. Cottonwood Lake study area-Water surface elevations. U.S. Geological Survey Data Release. https://doi.org/10.5066/F7707ZJ6.
Mushet, D.M., N.H. Euliss Jr. & M.J. Solensky, 2017a. Cottonwood Lake Study Area—Invertebrate Counts. U.S. Geological Survey Data Release. https://doi.org/10.5066/F7BK1B77.
Mushet, D. M., N. H. Euliss, Jr., B. A. Hanson, J. L. Neau, R. B. Bryant & S. F. Erickson, 2017b. Cottonwood Lake Study Area-Wetland Vegetation Zones: U.S. Geological Survey Data Release. https://doi.org/10.5066/F7J101DQ.
Mushet, D. M., O. P. McKenna, J. W. LaBaugh, N. H. Euliss & D. O. Rosenberry, 2018. Accommodating state shifts within the conceptual framework of the wetland continuum. Wetlands 38: 647–651.
Oksanen, J., G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs & H. Wagner, 2019. vegan: Community Ecology Package. R package version 2.5-4. https://CRAN.R-project.org/package=vegan.
Olden, J. D., 2006. Biotic homogenization: a new research agenda for conservation biogeography. Journal of Biogeography 33: 2027–2039.
Olden, J. D, 2008. Biotic Homogenization. In: Encyclopedia of Life (ELS). John Wiley & Sons, Ltd, Chichester.
Olden, J. D. & T. P. Rooney, 2006. On defining and quantifying biotic homogenization. Global Ecology and Biogeography 15: 113–120.
Olden, J. D., L. Comte & X. Giam, 2018. The Homogocene: a research prospectus for the study of biotic homogenisation. NeoBiota 37: 23.
Petsch, D. K., 2016. Causes and consequences of biotic homogenization in freshwater ecosystems. International Review of Hydrobiology 101: 113–122.
R Core Team, 2015. R: a language and environment for statistical computing. R foundation for statistical computing, Vienna.
Rahel, F. J., 2002. Homogenization of freshwater faunas. Annual Review of Ecology and Systematics 33: 291–315.
Reid, A. J., A. K. Carlson, I. F. Creed, E. J. Eliason, P. A. Gell, P. T. Johnson, K. A. Kidd, T. J. MacCormack, J. D. Olden, S. J. Ormerod, J. P. Smol, W. W. Taylor, K. Tockner, J. C. Vermaire, D. Dudgeon & S. J. Cooke, 2018. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biological Reviews 94: 849–873.
Roberts, D. W. & M. D. W. Roberts, 2016. Package ‘labdsv.’ Ordination and Multivariate.
Sala, O. E., F. S. Chapin, J. J. Armesto, E. Berlow, J. Bloomfield, R. Dirzo, E. Huber-Sanwald, L. F. Huenneke, R. B. Jackson, A. Kinzig, R. Leemans, D. M. Lodge, H. A. Mooney, M. Oesterheld, N. L. Poff, M. T. Sykes, B. H. Walker, M. Walker & D. H. Wall, 2000. Global biodiversity scenarios for the year 2100. Science 287: 1770–1774.
Scheffer, M. & E. Jeppesen, 2007. Regime shifts in shallow lakes. Ecosystems 10: 1–3.
Serie, J. R. & G. A. Swanson, 1976. Feeding ecology of breeding gadwalls on saline wetlands. The Journal of Wildlife Management 40: 69–81.
Serran, J. N. & I. F. Creed, 2015. New mapping techniques to estimate the preferential loss of small wetlands on prairie landscapes. Hydrology Processes 30: 396–409.
Silver, C. A., S. M. Vamosi & S. E. Bayley, 2012. Temporary and permanent wetland macroinvertebrate communities: phylogenetic structure through time. Acta Oecologica 39: 1–10.
Smith, A. G., J. H. Stoudt & J. B. Gollop, 1964. Prairie Potholes and Marshes. Waterfowl Tomorrow. United States Fish and Wildlife Service, Washington, DC: 39–50.
Spyreas, G., B. W. Wilm, A. E. Plocher, D. M. Ketzner, J. W. Matthews, J. L. Ellis & E. J. Heske, 2010. Biological consequences of invasion by reed canary grass (Phalaris arundinacea). Biological Invasions 12: 1253–1267.
Stewart, R. E. & H. A. Kantrud, 1967. Proposed Classification of Potholes in the Glaciated Prairie Region. Northern Prairie Wildlife Research Center, Jamestown.
Stewart, R. E. & H. A. Kantrud, 1971. Classification of natural ponds and lakes in the glaciated prairie region. US Fish and Wildlife Service, Bureau of Sport Fisheries and Wildlife, Washington, DC.
Swanson, G. A., 1978. Funnel trap for collecting littoral aquatic invertebrates. The Progressive Fish-Culturist 40: 73.
Thorp, J. H. & A. P. Covich, 2009. Ecology and Classification of North American Freshwater Invertebrates. Academic Press, New York.
Vanderhoof, M. K. & L. C. Alexander, 2016. The role of lake expansion in altering the wetland landscape of the Prairie Pothole Region, United States. Wetlands 36: 309–321.
Vanderhoof, M. K., L. C. Alexander & M. J. Todd, 2016. Temporal and spatial patterns of wetland extent influence variability of surface water connectivity in the Prairie Pothole Region, United States. Landscape Ecology 31: 805–824.
van der Valk, A. G., 2005. Prairie potholes of North America. In Keddy, P. A. & L. H. Fraser (eds), The world’s largest wetlands: ecology and conservation. Cambridge University Press, Cambridge: 415–445.
Van Meter, K. J. & N. B. Basu, 2015. Signatures of human impact: size distributions and spatial organization of wetlands in the Prairie Pothole landscape. Ecological Applications 25: 451–465.
Vaughn, C. C., 2010. Biodiversity losses and ecosystem function in freshwaters: emerging conclusions and research directions. BioScience 60: 25–35.
Wiltermuth, M. T., 2014. Influences of climate variability and landscape modifications on water dynamics, community structure, and amphipod populations in large prairie wetlands: Implications for waterbird conservation. PhD Thesis, North Dakota State University.
Winter, T. C. & D. O. Rosenberry, 1998. Hydrology of Prairie Pothole Wetlands during Drought and Deluge: a 17-year study of the cottonwood lake wetland complex in North Dakota in the perspective of longer term measured and proxy hydrological records. Climatic Change 40: 189–209.
Zimmer, K. D., M. A. Hanson & M. G. Butler, 2001. Effects of fathead minnow colonization and removal on a prairie wetland ecosystem. Ecosystems 4: 346–357.
Zimmer, K. D., M. A. Hanson & M. G. Butler, 2002. Effects of fathead minnows and restoration on prairie wetland ecosystems. Freshwater Biology 47: 2071–2086.
Acknowledgements
We thank Darold Batzer and two anonymous individuals for providing their constructive reviews of our manuscript. Data from Cottonwood Lake Study Area wetlands are publicly available through the Missouri Coteau Wetland Ecosystem Observatory (https://www.sciencebase.gov/catalog/item/52f0ffd9e4b0f941aa181fc6) and maintained through funding received from the U.S. Geological Survey’s Land Change Science Research and Development Program. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. While the aquatic homogenocoene is not an official epoch recognized by the USGS, we use it here to be consistent with the other papers in this special issue of Hydrobiologia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: André A. Padial, Julian D. Olden & Jean R. S. Vitule / The Aquatic Homogenocene
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McLean, K.I., Mushet, D.M., Sweetman, J.N. et al. Invertebrate communities of Prairie-Pothole wetlands in the age of the aquatic Homogenocene. Hydrobiologia 847, 3773–3793 (2020). https://doi.org/10.1007/s10750-019-04154-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-04154-4