Abstract
In this paper, we describe the influence of flooding on nutrient conditions and phytoplankton Chl-a concentrations in a hydrologically dynamic Danube floodplain in North–Eastern Croatia. Based on hydrological typology, the connectivity gradient extends from the main river stream (Eupotamal) to the backwater channels and side-arms (Parapotamal) to the edge of the floodplain (Paleopotamal). A spatial gradient of phytoplankton Chl-a occurred during low water levels, while uniformity of Chl-a across wetland habitats was observed during high water levels. Nutrient enrichment assays indicated that inorganic nutrients in Eupotamal waters (N/P ratio 16–22) could support a higher growth rate of the assay alga Chlorella kessleri, while potential N limitation was found in Parapotamal and Paleopotamal waters (both with N/P ratios < 14). Spatial expansion of N limitation toward the main river was observed as inundation continued. Lateral flood pulse connectivity along with the duration of inundation period affects the input and the retention of nutrients, especially of nitrogen. Understanding of the relationship between spatial (location of the floodplain waterbodies regarding the river) and temporal (hydrological connectivity between the river and its floodplain) dimensions contribute to the importance of healthy floodplains for the maintenance of water quality and environmental conditions in the Danube River.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Algal biomass and overall ecosystem productivity may be controlled by both nutrient composition and nutrient availability. In freshwaters, phosphorus (P) has been regarded as the most probable limiting nutrient for phytoplankton growth (Wetzel 2001), but co-limitation by P and nitrogen (N) is not uncommon (Elser et al. 1990; Jannson et al. 1996). Nitrate is the most common dissolved nitrogen form in rivers and agricultural runoff and it is often used to assess water quality and ecosystem ecological integrity (Smith et al. 2007). For example, in the Danube floodplain restoration project in Austria, nitrate is used as a kind of “hydrological tracer” (Tockner et al. 1998). Although nutrient limitation of algal growth is common in aquatic ecosystems, it is difficult to carry out its quantification. Evaluating the growth response of algae after nutrient additions in laboratory conditions makes quantification of nutrient limitation possible and comparable across studies (Downing et al. 1999; Horvatić et al. 2006; Peršić et al. 2009). The use of green algae Chlorella kessleri in bioassay approaches is suitable since it belongs to a group of ubiquitous species. It is a unicellular alga whose growth can be monitored accurately and rapidly and is amenable to laboratory cultivation (Lukavský 1992). Also, the algal growth potential of C. kessleri varies widely for various freshwater environments (Peršić et al. 2005; Horvatić et al. 2006; Peršić et al. 2009).
Floodplain-river ecosystems are natural fragmented systems with periodic hydrological connections (Thoms et al. 2005). The overall value of floodplain production is difficult to assess and is likely to vary greatly depending on the variations of river flow and flood pulses (Schemel et al. 2004). Physical and chemical conditions within the floodplain are affected by the size and position of floodplain waterbodies and their hydrological connectivity with the parent river (Junk and Furch 1993; Junk and Wantzen 2004). Hydrological connectivity greatly contributes to the exchange of nutrients and organic matter between the river and its floodplain (Tockner et al. 1999) and plays a crucial role for species that require different habitat conditions during their life-cycle (Amoros and Bornette 2002). Spatial heterogeneity of water chemistry and phytoplankton biomass appears with decreasing water levels and is accelerated by the subsequent surface separation of water bodies (Pithart et al. 2007). Therefore, spatial variability of limnological characteristics is one of the main features of river-floodplain system and is largely determined by the fluctuations in water level. However, considerably less is known about spatial patterns of floodplain productivity and what controls these patterns (Ahearn et al. 2006).
Therefore, riparian floodplains are among the most valuable, but unfortunately also the most degraded ecosystems in Europe. Based on the World Wide Fund for Nature (WWF 1999) for the Danube River Pollution Reduction Program, 80–90% of the original floodplain area in the Danube basin has been lost. The remaining areas along the Danube River are characterized by reduced hydrological exchange between the main river channel and its floodplain area. In fact, one of the last and the largest transboundary floodplains in the Middle Danube consists of a natural complex of more than 70,000 ha and embraces three protected areas: the Kopački Rit Nature Park in Croatia, the Danube-Drava National Park in Hungary, and the Gornje Podunavlje Special Nature Reserve in Serbia.
The main objective of this study was to describe and explain the effect of hydrological connectivity with the parent river on algal growth (chlorophyll-a concentrations) and nutrient concentrations in a river-floodplain system. We intended to identify spatial variability of potential nutrient limitation in the Danube floodplain in relation to hydrological connectivity. To determine the limiting nutrients of algal growth in different floodplain waterbodies, we used nutrient enrichment assays in laboratory conditions. The importance of measuring algal growth potential in nutrient enrichment assays is the differentiation between nutrients determined by chemical analysis and nutrients that are actually available for algal growth.
Methods
Study Area and Hydrological Classification of the Floodplain Waters
The Danube River is the second longest river in Europe with a length of 2,778 km and watershed that forms the largest international river basin (817,000 km2). It rises in Germany and flows through ten countries (Germany, Austria, the Slovak Republic, Hungary, Croatia, Serbia, Bulgaria, Romania, Moldova and Ukraine) to its mouth on the Black Sea. The present study was conducted in a 37 km long river-floodplain section of the Danube River in North–Eastern Croatia (r. km 1,426–1,389). In the investigated section of the Danube River the altitude ranges from 78.08 m (Aljmaš) to 80.64 m above sea level (Batina/Bezdan). Its width here is about 250–880 m, the depth of the bed when water is in the middle is 3–6 m and the oscillation of water level over last 50 years is from 78.70 to 87.08 m asl with the range of 8.38 m (Tadić et al. 2003, Maričić 2005). Land configuration and low slope (0.055‰) are typical characteristics of lowland fluvial rivers and provide adequate conditions for meandering. The average current velocity in this river section is about 0.4 m s−1. The Danube discharge is mainly influenced by alpine flow conditions and peaks in spring and early summer due to snowmelt. The average discharge at Batina/Bezdan gauge is 2268 m3 s−1 (the mean flow of 30 years course), with the mean minimum and mean maximum values varying between 742 and 8360 m3 s−1 (Maričić 2005). Hydrological measurements for this study were conducted on a daily basis at the Apatin gauging station (r. km 1404) and were provided by Croatian Water Management Authority.
The Danube floodplain area in North–East Croatia covers approx. 53,000 ha, of which the protected area of the Nature Park Kopački Rit includes 23,000 ha and is a worldwide known wetland in the region of Slavonia and Baranja, Croatia. The Nature Park Kopački Rit is situated at the confluence of the Drava and Danube Rivers (45° 37′ 51″ N, 18° 53′ 31″ E), which forms the inland delta where the rivers meet. An inland delta is not known among other European rivers and it makes this area globally important (Schwarz 2005). The aquatic environments of the Kopački Rit floodplain include floodplain lakes, occasionally flooded areas and recesses with flowing water, as well as relict oxbow lakes and backwater channels (Schwarz 2005). The Nature Park Kopački Rit has been selected as the Ramsar Area protected by the Ramsar Convention (Convention on internationally important wetland areas) and included in the Important Bird Area (IBA) list. Nevertheless, the Nature Park Kopački Rit has recently been subjected to some degradation and therefore included on the ‘Montreux record’ - the list of designated Ramsar sites where substantial changes in ecological character have occurred. Also, a recent war left behind nearly 3,000 ha of floodplain contaminated with mines and unexploded ordnance.
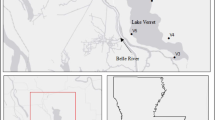
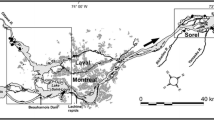
In our investigation 13 sampling sites were established. Three sites are located along the main river channel and 10 sites are located in the lateral dimension from the main river stream towards the floodplain (Fig. 1, Table 1). Along the main river channel three sampling sites (within the same water body) were chosen along the main transport ways of Danube floodwater into and out of the investigated floodplain. Based on hydrological connectivity among the chosen sites and the river, we have distinguished five types of aquatic habitats along the flooding gradient (Table 1). The typology was made according to existing approaches of Ward and Tockner (2001), Ward et al. (2002) and Schwarz (2005). Similar approaches are regularly used in Danube River catchments (Šporka 1998; Illyová and Némethová 2005; Chovanec et al. 2006; Schwarz 2006; Ot’ahel’ová et al. 2007). The sites within the floodplain were chosen based on the connectivity patterns of a river-floodplain system. The water flow in the Kopački Rit floodplain is directed from the north to the south towards the lower part of the floodplain. To the north of the present park's boundaries lay important, but still unprotected parts of the Danube floodplain (Fig. 1) that extend to the Danube-Drava National Park in Hungary (Schwarz 2005).
The map of the investigated area. The study sites are shown, coding as in Table 1
Analytical Methods
Water samples were collected monthly from March to July 2004. Water temperature (WT, °C), water depth (WD, m), Secchi depth (SD, m), pH, and conductivity (EC, μS cm−1) were measured at each site. Dissolved oxygen (DO, mg l−1) was determined using the Winkler method (APHA 1995). Samples for chemical and phytoplankton pigment analysis were collected in plastic bottles previously rinsed with sampling water, stored in containers with ice and analyzed the same day. The following nutrients were assayed: ammonium (NH4, mg N l−1); nitrate (NO3, mg N l−1); nitrite (NO2, mg N l−1); Kjeldahl nitrogen (mg N l−1); total nitrogen (TN, mg N l−1); orthophosphate (o-PO4, mg P l−1); and total phosphorus (TP, mg P l−1), using standard methods (APHA 1995). Water samples (0.5–1 l) for phytoplankton pigment analyses were filtered through Whatman GF/C filters, extracted with acetone and were kept for 2–4 h in darkness in a refrigerator at 4°C (Komárková 1989). Chlorophyll concentrations (Chl-a, Chl-b and Chl-c) of the prepared samples were determined spectrophotometrically and calculated according to equations of Strickland and Parsons (1968). To reveal how habitat types respond to physical disturbance (flooding), spatial heterogeneity was indicated by site-specific coefficients of variation (CV) of phytoplankton Chl-a and N-NO3 concentrations based on mean monthly level. Variance rather than mean environmental value is a valuable parameter for understanding how patterns and processes are linked in floodplain systems (Palmer et al. 1997; Tockner et al. 2000).
Homogeneity of variances was tested using Levene’s test and data, apart from pH values, were log transformed, if necessary, to reduce heterogeneity of variances. Significant differences in water quality parameters among the investigated sites were calculated by one-way analysis of variance (ANOVA) and Tukey Unequal N HSD tests. Relationships among physical and chemical parameters were determined by Pearson correlation. Regression analysis was used to model Danube water level through time (separately for each month). A significance level of p < 0.05 was used for all tests. Statistical analysis of data was performed using Statistica for Windows 7.0 (StatSoft, Inc. 2005).
Algal Bioassays
Algal growth potential was evaluated in microplates with a suspended culture of Chlorella kessleri Fott et Nov. strain LARG/1 by the laboratory miniaturized growth bioassay method according to Lukavský (1992) and Horvatić et al. (2006). Cultures were incubated in the Bold’s basal medium (BBM) in a 25°C controlled temperature room and illuminated by fluorescent tubes (Tungsram, Hungary) that provided continuous daylight (PAR, 400–700 nm) at approximately 140 μmol photon m−2 s−1. Due to prior uptake and possible storage of nutrients, it was necessary to starve C. kessleri cells before experimental use (Lukavský 1992; Horvatić et al. 2006). If algae are cultivated in dilute media, the amount of growth from nutrient carry-over in subsequent test waters is negligible (Shoaf 1978). The algal culture in its exponential phase was washed from the nutrient medium with sterile distilled water and cultivated for 3–5 days in sterile distilled water. The algal cell density in this solution was determined using Bürker-Türk counting chamber (Karl Hecht KG, Sondheim, Germany) under a light microscope (Axiovert 25, Carl Zeiss, Inc., Göttingen, Germany). The initial cell density in the inoculum solution was 8 × 105 cells ml−1. Water samples from the investigated sites were filtered through Whatman GF/C glass fibre filters to eliminate particles. Bioassays were carried out in polystyrene 96-well microplates (TPP, Switzerland) with well volume of 300 μl. The miniaturized growth bioassays were conducted with six pseudo replicates of control samples (250 μl tested water sample from each site and month + 50 μl algal inoculums in distilled H2O), N enriched samples (250 μl tested water sample + 50 μl algal inoculums with added nutrient N as KNO3 in final concentration of 0.16 g l−1), and P enriched samples (250 μl tested water sample + 50 μl algal inoculums with added P as K2HPO4 in final concentration of 0.02 g l−1). High concentrations of nutrients ensured that enrichments would not be depleted during the experiment (Horvatić et al. 2006). Prior to adding algal inoculum solution, the uncovered microplates with water samples from the investigated sites were exposed to UV light for sterilization. The microplates were covered with lids, placed in a glass incubation chamber and exposed to cultivation conditions (temperature of 25°C, continuous daylight (PAR, 400–700 nm) at approximately 140 μmol photon m−2 s−1, and CO2 ca. 2% v/v). The growth of C. kessleri was determined by measuring the optical density at 750 nm every day, for approximately 14 days (until the stationary phase of growth), using an automated microplate reader (Multiskan MS, Labsystem, Finland) controlled by GENESIS II software (Windows™ Based Microplate Software). The results were plotted as growth curves. The average specific growth rate for exponentially growing culture of C. kessleri (μ day−1) was quantitatively determined from the measured absorbance using the exponential model y = ea+μx (where y = the population estimate, x = time, a = integration constant and μ = slope or growth rate) during its exponential growth period (up to the 7th day). Growth rate was calculated from six parallels, with respect to time (for each day of exponential phase). The difference in the average specific growth rate of C. kessleri in the enriched (μE) and control water samples (μC) is expressed as the degree of algal response to nutrient enrichment (Δμ) was calculated using the method in Downing et al. (1999). The bioassay experiments were used to determine whether nutrient addition stimulates C. kessleri growth relative to the control and thereby ascertain the limiting nutrient in the original water sample. The differences between treatments and the control growth with respect to time were tested with ANOVA. Dunett's test was used as a post hoc test to identify treatment growth rates that were significantly different from the control growth rate. If no significant difference in the C. kessleri growth rate was determined between the control and the treatments - no limitation was inferred. An increase in the growth rate of C. kessleri following the addition of N or P alone was inferred as N or P limitation. To test statistically significant differences between habitat types, a one-way ANOVA design was used with Tukey's HSD post hoc test.
Results
Hydrology
Spring and early summer flooding of the Danube floodplain started at the end of March and lasted until the beginning of August 2004. The flooding had two flood pulses. The first flood pulse started at the end of March and the second one started in June, both with amplitude of 2.4 m, and total duration of 55 and 74 days (Fig. 2). When the Danube water level (Dwl) reaches 2.5 m above the Apatin gauge zero point (1404 r. km), floodwaters enter channels that fill the lakes of the floodplain. Furthermore, when the Danube water level reaches 4 m, water in the floodplain channels starts to overflow into surrounding lowland areas.
Mean monthly water levels with average daily course in 2004 of the Danube River, 1404 r. km (in m above Apatin station gauge zero point). Horizontal line represents the threshold level of the Danube floodwaters flow into/out of the floodplains of Nature Park Kopački Rit. The hydrograph data were provided by Croatian Water Management Authority
An overbank flooding of the Danube floodplain in March was caused by the rapid increase in the Danube water level when Dwl ranged from 1.05 to 4.94 m above gauge zero point (mean 2.40 m, with coefficient of variation 60%). Linear regression analysis also indicated ascending water level of the Danube in March (R 2 = 0.84, F = 70.2, p < 0.001). On the other hand, a slow decrease in the water level resulted in the receding of floodwaters to channels and lakes by the end of April (descending water level with R 2 = 0.87, F = 88.6, p < 0.001) and Dwl ranged from 2.85 to 4.58 m above gauge zero point, mean 3.56 m with CV = 14%. During May, water levels stagnated (R 2 = 0.28, F = 2.4, p > 0.05) ranging from 2.28 to 3.28 m above gauge zero point, with mean water level 2.86 m and CV = 10%, while another rapid increase in water level (from 2.50 to 4.88 m above gauge zero point, mean 4.0 m, CV = 19%) caused an overbank flooding in June (ascending water level, R 2 = 0.53, F = 11.1, p = 0.002). Finally, by the end of July floodwaters receded to floodplain channels and lakes and the main river channel (descending water level (R 2 = 0.91, F = 147.3, p < 0.001) ranging from 2.5 to 4.22 m above gauge zero point, mean 3.3 m and CV = 17%).
Physical-Chemical Characteristics and Phytoplankton Chl-a Concentrations
Table 2 summarizes the mean values and ranges of physical and chemical parameters as well as of phytoplankton Chl-a at five investigated habitat types across the river-floodplain transect. Nitrogen concentrations (N-NO3, TN) were significantly higher (Tukey HSD, p < 0.05) in Eupotamal waters (the Danube River and its side-arms) than in the Parapotamal A, Parapotamal B, and Paleopotamal waters (floodplain). Habitat types significantly differentiated also in WD, SD, DO, EC, Kjeldahl nitrogen, and N/P ratios (Table 2). Although phosphate concentrations did not differ significantly among different floodplain habitat types, they reflected the same decreasing spatial trend.
A wide range of parameter values were found at all sites during the investigation period. For example, minimal values of DO concentration (from 1.3 to 1.4 mg O2 l−1) and WT (from 6 to 7.5°C) were measured in March at all sites. Dissolved oxygen concentration increased rapidly after the first flood pulse in April and stayed high during May when maximal values were determined at all habitat types (from 13.5 to 17.7 mg O2 l−1). A similar pattern was found for the N-NO3 and TN concentrations.
A strong positive correlation occurred between Danube water level (Dwl) and N-NO3 concentrations (r = 0.82, p < 0.001), as well as between Dwl and TN (r = 0.82, p < 0.001). Also, a significant positive correlation occurred between Dwl and EC (r = 0.38, p = 0.008), as well as between Dwl and DO (r = 0.50, p < 0.001). On the other hand, a significant negative correlation was determined between Dwl and phytoplankton Chl-a concentrations (r = -0.57, p < 0.001).
To reveal how habitat type responded to physical disturbance (flooding), habitat heterogeneity was measured as the coefficients of variation (CV) of phytoplankton Chl-a and N-NO3 concentrations (Fig. 3a and b). The CV of phytoplankton Chl-a (CV = 87%) peaked at stagnating water level in May (Fig. 3a). In May, maximal values of phytoplankton Chl-a concentration were found in the Eupotamal waters (Danube River and its side-arms), while a decrease of phytoplankton Chl-a concentration was observed in lateral dimension from the main stream (Eupotamal) towards the floodplain (Parapotamal and Paleopotamal, Fig. 4a). On the other hand, uniformity of river-floodplain habitat types with low CV was observed during ascending water level in March (CV = 24%), and in June (CV = 22%). In March, an increase of phytoplankton Chl-a concentration was determined in the lateral dimension from the main stream of the Danube (Eupotamal A) towards the marginal part of the floodplain (Paleopotamal) (Fig. 4a). In addition, high concentrations of phytoplankton Chl-b and Chl-c were determined in March (at the beginning of an early spring inundation period) at all investigated habitat types (from 9.4 to 18.5 μg l−1 Chl-b and from 25.3 to 45.2 μg l−1 Chl-c, Table 2).
a Phytoplankton Chl-a concentrations (mean values ± standard deviations) and habitat heterogeneity measured as the coefficient of variation of Chl-a concentrations; b N-NO3 concentrations and habitat heterogeneity measured as the coefficient of variation of N-NO3 concentrations across different mean monthly water level of the Danube River during the study (March-July 2004), number of replicates per sampling date: 13
Mean values ± standard deviations of phytoplankton Chl-a concentrations (a) and mean values ± standard deviations of N-NO3 concentrations (b) during the inundation period (horizontal axis represents months indicated by numbers 3,5,7,4,6 for March, May, July, April, June, respectively, and mean monthly water level for each month) at five investigated river-floodplain habitat types
Unlike phytoplankton Chl-a, spatial heterogeneity of N-NO3 concentrations increased significantly with increasing water level (Fig. 3b). At the beginning of the flood period in March 2004 (mean Danube water level 2.4 m), nitrate concentrations were uniformly low in all habitat types (CV = 12%). A concentration gradient (CV = 36%) first formed after the first flood pulse in March (Figs. 3b and 4b). By the end of April, as floodwaters receded to floodplain channels and lakes, mean nitrate concentrations differed by a factor of 2 between Eupotamal A (2549.1 μg N l−1) and Paleopotamal waters (1256.7 μg N l−1). The highest spatial heterogeneity (CV = 41%) for nitrates was determined during the highest mean monthly water level of the River Danube in June (Fig. 3b), with mean nitrate concentrations in Eupotamal A waters of 1764.4 μg N l−1 and in Paleopotamal waters of 789.3 μg N l−1 (Fig. 4b).
Nutrient Enrichment Bioassay
The algal growth potential in original water samples from the investigated sites of the river-floodplain system was investigated up to the stationary phase of C. kessleri growth, which was usually established by the 14th day of incubation. The average specific growth rate of C. kessleri was the highest in the control water samples of Eupotamal waters (μ = 0.337 d−1 in Eupotamal A, μ = 0.356 d−1 in Eupotamal B) (Fig. 5). A decreasing trend in the growth rate of C. kessleri was observed from the main river channel towards the floodplain. This pattern was similar to that of nitrate concentrations and the N/P ratio (Fig. 5, Table 2).
A quantitative index of nutrient limitation was calculated from the difference in the growth rate of the control samples and the growth rate of treatment samples (N, P) and was expressed as the degree of nutrient limitation (Fig. 6). A significant degree of N limitation (Tukey HSD, p < 0.01) occurred in water samples from Parapotamal A (ΔN = 0.180 d−1), Parapotamal B (ΔN = 0.206 d−1), and Paleopotamal (ΔN = 0.213 d−1) habitat types. A significant degree of P limitation (ΔP = 0.116 d−1) was only determined in water samples from Parapotamal A habitat type (Fig. 6). Table 3 summarizes the main findings of the nutrient enrichment assay. A significant N limitation was determined at all sites of the Paleopotamal habitat type over the entire 5 month inundation period.
Mean, mean ± standard error (box) and mean ± standard deviation (whisker) values of the degree of nutrient limitation (Δ N d-1, Δ P d-1) for the growth rate of C. kessleri in the water samples from each habitat type (number of samples per habitat type: 10–20). “*” and “**” represents statistically significant nutrient limitation at p < 0.05 and p < 0.01, respectively
Discussion
The Effect of Hydrological Connectivity on Nutrients and Phytoplankton Chl-a Concentrations
The characteristic ecology of floodplain channels and lakes in the investigated river-floodplain area strongly relates to variations in frequency and duration of inundation. Based on hydrological records, high water levels in the Danube floodplain of Kopački Rit are characteristic for spring (March/April) and the beginning of summer (June/July) (Mihaljević et al. 1999). Spring and early summer flooding started at the end of March and lasted until August 2004. Without these natural flood disturbances, the floodplains of the Danube River have a tendency towards geographical and temporal uniformity with a resulting reduction in biodiversity (Tockner et al. 1998; Ward and Tockner 2001). Based on hydrological typology, the connectivity gradient extends from the main river stream (Eupotamal A) to the edge of the floodplain (Paleopotamal). Among the investigated sites, a gradient was observed in physical and chemical conditions from the main channel (Eupotamal A) towards the marginal part of the floodplain (Paleopotamal).
At the beginning of the inundation period when phytoplankton Chl-a concentrations were high, the average oxygen saturation indicated under-saturation. The possible continuous input and decomposition of allochthonous organic matter could have had an influence on decreased oxygen concentrations, even in periods of autotrophy. These results emphasize the general heterotrophic conditions that prevailed at the investigated habitat types. Moreover, Palijan and Fuks (2006) found a higher number of oligotrophic and eutrophic bacteria in the surface waters of the Csonakut Channel and Lake Sakadaš (in this paper classified as Parapotamal and Paleopotamal waters) during March 2004, thus indicating heterotrophic conditions. Therefore, the low nitrogen concentrations occurring in March could be the consequence of nutrient uptake by phytoplankton (Hein et al. 2003) or bacterioplankton (Palijan and Fuks 2006).
Chlorophyll-a concentration was inversely related to the mean monthly water level. March, which included both the pre-flood low flow and the rising limb of the hydrograph, had the lowest mean flow during the study and also had the highest mean Chl-a concentration. The high March Chl-a concentration was consistent with an annual spring phytoplankton bloom (Vidaković et al. 2008). The March bloom was dominated by diatoms (Mihaljević et al. 2009), which is consistent with the high Chl-a, Chl-b, and Chl-c concentrations. The highest mean monthly flow during the study occurred in June, when Chl-a concentration was lowest. The June sampling occurred 3 weeks after the start of flooding and, because the floodplain retention capacity was already filled, the June flood pulse had less effect on connectivity and water exchange. That could explain why habitat heterogeneity concerning nitrogen (high CV) was highest during that time. The March bloom and the low June Chl-a concentrations were consistent across sites, as indicated by the accompanying low Chl-a coefficients of variation (low CV).
The stagnating water flow occurred in May and is consistent with high spatial heterogeneity in phytoplankton Chl-a (high CV). At that time, the clear water phase occurred in Paleopotamal waters (Mihaljević et al. 2009), in contrast to the high planktonic production phase in Eupotamal waters. The maximal development of phytoplankton in floodplain waters occurred after floods (Mihaljević et al. 2009). Therefore, phytoplankton responded slower to the reduction in water flow than water chemistry. According to Tockner et al. (2000), the relationship between habitat heterogeneity and the river stage, which has a major influence on biodiversity patterns, is a functional characteristic of a river-floodplain system.
Throughout the study nitrate concentrations decreased with the distance from the main river channel. This gradual decrease towards the floodplain could have been a result of N uptake by macrophytes, algae, or bacteria (Hamilton and Lewis 1987; Knowlton and Jones 1997; Unrein 2002; Olde Venterink et al. 2003). Since the highest N-NO3 concentrations occurred in Eupotamal waters (River Danube and its active side-arms), it seems that the floodwaters of the Danube River are the source of nutrients, especially nitrogen. High amounts of nitrogen brought by the overbank flooding waters or sediment resuspension related to the inflow of flooding waters, could have supported the abundant macrophyte development recorded during the study period (Vidaković and Bogut 2007; Čerba et al. 2009; Bogut et al. 2010).
Spatial Distribution of Nutrient Limitation
Understanding the nutrients essential for phytoplankton growth is important in the successful control of eutrophication (Smith et al. 1999). Such control efforts must be directed toward the limiting nutrient(s), which promote algal growth. The limiting nutrient can be determined using different methods, for example, by measuring ambient nutrient concentration and nutrient ratios based on intracellular nutrient concentrations (Redfield 1958; Järvinen et al. 1999) or by enrichment assays (Levine and Whalen 2001; Dzialowski et al. 2005; Horvatić et al. 2006; Peršić et al. 2009). Using a single-species assay may or may not reflect nutrient limitation of the entire phytoplankton community, because a single species may not always be representative of algae in the investigated waters and may respond to nutrients differently from the complete community. For example, freshwater cyanobacteria often dominate at N/P ratios from 5 to 10, while a high N/P ratio (> 29) stimulates growth of green algae (Schindler 1977; Smith 1982). In our study, the growth rate of green algae C. kessleri increased when the N/P ratio was greater than 14 (mass ratio) observed in Eupotamal waters.
In our enrichment assays, the highest specific growth rate of C. kessleri in water from Eupotamal habitats and the gradual decrease in the growth rate of C. kessleri in waters from the main river channel towards the floodplain are consistent with physical and chemical features of the river-floodplain system. Inorganic nutrients present in Eupotamal waters were sufficient to support the higher growth rate of C. kessleri. Potential N limitation of the growth rate of C. kessleri was determined in Parapotamal and Paleopotamal waters that had N/P ratio < 14. Furthermore, a spatial expansion of N limitation was observed as inundation continued. For example, in March, N limitation was determined only in Paleopotamal waters, while at the end of the inundation period N limitation was determined along the lateral dimension from the main stream of the Danube River (Eupotamal A) towards the edge of floodplain (Paleopotamal). As explained earlier, the water level during sampling in June was high but the hydrological action was probably low. Therefore, Danube water was not entering the floodplain. This could have enabled biota to deplete nitrate imported during the flooding making the floodplain N limited.
The abiotic and biotic characteristics (e.g., N-NO3 concentrations, lower transparency, higher Chl-a concentrations) of the Danube River and its active side-arms (Eupotamal waters) suggest that the light limitation is a more probable growth regulator than nutrient limitation. As reported by Hamilton and Lewis (1990) and Knowlton and Jones (1997), light limitation of algal growth prevails in floodplain waters with high connectivity. On the other hand, higher transparency, lower depth, and lower N-NO3 concentrations in Parapotamal and Paleopotamal waters are consistent with potential N limitation of algal growth. Therefore, the long inundation periods along with the pulsing connectivity controls the input of nutrients in particular nitrates and greatly influences spatial heterogeneity (Amoros and Bornette 2002). Distribution of nutrients in different habitat types of the Danube River floodplain strongly depends on the retention time of the floodwaters and nutrient uptake by phytoplankton, bacterioplankton, and macrophytes. Therefore, this approach to the typology of Danube River floodplain habitat types should be further developed using other biological, geomorphologic, and hydrodynamic parameters, in addition to hydrological connectivity.
In conclusion, nutrient availability decreased with the hydrological connectivity gradient. Furthermore, a spatial gradient of phytoplankton Chl-a was observed during stagnating water flow, while uniformity of Chl-a among habitat types followed the increase of water level. Consequently, the increase in the degree of N limitation was observed from the Eupotamal waters towards the edge of floodplain and N limitation expanded spatially as inundation continued. According to Thoms et al. (2005), for nutrient dynamics in river-floodplain systems the most important factors are the location of the floodplain waterbodies regarding the river (spatial dimension) and its hydrological connectivity (temporal dimension).
References
Ahearn DS, Viers JH, Mount JF, Dahlgren RA (2006) Priming the productivity pump: flood pulse driven trends in suspended algal biomass distribution across a restored floodplain. Freshwater Biology 51:1417–1433
APHA (1995) Standard methods for examination of water and wastewater, 16th edn. American Public Health Association, Washington, DC
Amoros C, Bornette G (2002) Connectivity and biocomplexity in waterbodies of riverine floodplains. Freshwater Biology 47:517–539
Bogut I, Čerba D, Vidaković J, Gvozdić V (2010) Interactions of weed-bed invertebrates and Ceratophyllum demersum L. stands in a floodplain lake. Biologia 65:113–121
Chovanec A, Waringer J, Straif M, Graf W, Reckendorfer W, Waringer-Löschenkohl, Weidbacher H, Schultz H (2006) The Floodplain Index - a new approach for assessing the ecological status of river/floodplain-systems according to the EU Water Framework Directive. In: Proceedings 36th International Conference of IAD. Austrian Committee Danube Research/IAD, Vienna. ISBN 13: 978-3-9500723-2-7. pp 23–26
Čerba D, Bogut I, Vidaković J, Palijan G (2009) Invertebrates in Myriophyllum spicatum L. stands in Lake Sakadaš, Croatia. Ekologia - Ecology 28:94–105
Downing JA, Osenberg CW, Sarnelle O (1999) Metaanalysis of marine nutrient enrichment experiments: variation in the magnitude of nutrient limitation. Ecology 80:1157–1167
Dzialowski AR, Wang SH, Lim NC, Spotts WW, Huggins DG (2005) Nutrient limitation of phytoplankton growth in central plains reservoirs, USA. Journal of Plankton Research 27:587–595
Elser JJ, Marzolf ER, Goldman CR (1990) Phosphorus and nitrogen limitation of phytoplankton growth in the freshwaters of North America: A review and critique of experimental enrichments. Canadian Journal of Fisheries and Aquatic Sciences 47:1468–1477
Hamilton SK, Lewis WM (1987) Causes of seasonality in the chemistry of a lake on the Orinoco River floodplain, Venezuela. Limnology and Oceanography 32:1277–1290
Hamilton SK, Lewis WM (1990) Basin morphology in relation to chemical and ecological characteristics of lakes on the Orinoco River floodplain, Venezuela. Archives of Hydrobiology 119:393–425
Hein T, Baranyi C, Herndl G, Wanek W, Schiemer F (2003) Allochthonous and autochthonous particulate organic matter in floodplains of the River Danube: the importance of hydrological connectivity. Freshwater Biology 48:220–232
Horvatić J, Peršić V, Mihaljević M (2006) Bioassay method in evaluation of trophic conditions and nutrient limitation in the Danube wetland waters (1388–1426 r. km). Hydrobiologia 563:453–463
Illyová M, Némethová D (2005) Long-term changes in cladoceran assemblages in the Danube floodplain area (Slovak–Hungarian stretch). Limnologica 35:274–282
Jannson M, Blomqvist P, Jonsson A, Bergstrom AK (1996) Nutrient limitation of bacterioplankton, autotrophic and mixotropic phytoplankton, and heterotrophic nanoflagellates in Lake Ortrasket. Limnology and Oceanography 41:1552–1559
Järvinen M, Salonen K, Sarvala J, Vuorio K, Virtanen A (1999) The stoichiometry of particulate nutrients in Lake Tanganyika - implications for nutrient limitation of phytoplankton. Hydrobiologia 407:81–88
Junk WJ, Furch K (1993) A general review of tropical South American floodplains. Wetlands Ecology and Management 2:231–238
Junk WJ, Wantzen KM (2004) The flood pulse concept: new aspects, approaches and applications - an update. In: Proceedings of the Second International Symposium on the Management of Large Rivers for Fisheries Volume II. Welcomme RL and Petr T, Eds., FAO Regional Office for Asia and the Pacific, Bangkok, Thailand, RAP Publication 2004/17 pp 117–149
Knowlton MF, Jones JR (1997) Trophic status of Missouri River floodplain lakes in relation to basin type and connectivity. Wetlands 17:468–475
Komárková J (1989) Primárni produkce řas ve slatkovodních ekosysteméch. In: Dykyová D (ed) Metody studia ecosystémů. Academia Praha, Praha, pp 330–347
Levine MA, Whalen SC (2001) Nutrient limitation of phytoplankton production in Alaskan Arctic foothill lakes. Hydrobiologia 455:189–201
Lukavský J (1992) The evaluation of algal growth potential (AGP) and toxicity of water by miniaturized growth bioassay. Water Research 26:1409–1413
Maričić S (2005) Analyses of one of the rare natural retention in the Middle Danube. In: Proceedings of the Ninth International Symposium on Water Management and Hydraulic Engineering. Vienna, Austria, pp. 383–395
Mihaljević M, Getz D, Tadić Z, Živanović B, Gucunski D, Topić J, Kalinović I, Mikuska J (1999) Kopački Rit. Research Survey and Bibliography (Kopački Rit. Pregled istraživanja i bibliografija). Croatian Academy of. Arts and Sciences, Zagreb
Mihaljević M, Stević F, Horvatić J, Hackenberger Kutuzović B (2009) Dual impact of the flood pulses on the phytoplankton assemblages in a Danubian floodplain lake (Kopački Rit Nature Park, Croatia). Hydrobiologia 618:77–88
Olde Venterink H, Wiegman F, der Lee GEM, Vermaat JE (2003) Role of active floodplains for nutrient retention in the river Rhine. Journal of Environmental Quality 32:1430–1435
Ot’ahel’ová H, Valachovič M, Hrivnák R (2007) The impact of environmental factors on the distribution pattern of aquatic plants along the Danube River corridor (Slovakia). Limnologica 37:290–302
Palijan G, Fuks D (2006) Alteration of factors affecting bacterioplankton abundance in the Danube River floodplain (Kopački Rit, Croatia). Hydrobiologia 560:405–415
Palmer MA, Hakenkamp ACC, Nelson-Baker K (1997) Ecological heterogeneity in streams: why variance matters. Journal of the North American Benthological Society 16:189–202
Peršić V, Horvatić J, Mihaljević M (2005) Bioassay method in the trophic evaluation of a wetland area-case study in the Danubian region (1426–1388 rkm). Periodicum Biologorum 107:293–298
Peršić V, Horvatić J, Has-Schön E, Bogut I (2009) Changes in N and P limitation induced by water level fluctuations in Nature Park Kopački Rit (Croatia): nutrient enrichment bioassay. Aquatic Ecology 43:27–36
Pithart D, Pichlová R, Bílý M, Hrbáček J, Novotná K, Pechar L (2007) Spatial and temporal diversity of small shallow waters in river Lužnice floodplain. Hydrobiologia 584:265–275
Redfield A (1958) The biological control of chemical factors in the environment. American Scientist 46:205–221
Schemel LE, Sommer TR, Müller-Solger AB, Harrell WC (2004) Hydrologic variability, water chemistry, and phytoplankton biomass in a large floodplain of the Sacramento River, CA, U.S.A. Hydrobiologia 513:129–139
Schindler DW (1977) Evolution of phosphorus limitation in lakes. Science 195:260–262
Schwarz U (2005) Landscape ecological characterisation and typology of riparian and fluvial landforms of the Kopački Rit (In German: Landschaftsökologische Charakterisierung des Kopački Rit unter besonderer erücksichtigung von Flusslandschaftsformen sowie deren Genese und Typologie). Dissertation at the Institute of Geography, University of Vienna
Schwarz U (2006) Genesis and typology of riparian and fluvial landforms of the Kopački Rit within the Danube floodplain corridor in Croatia and Serbia. In: Proceedings 36th International Conference of IAD. Austrian Committee Danube Research/IAD, Vienna. ISBN 13: 978-3-9500723-2-7. pp 318–321
Shoaf WT (1978) Use of algal assays to determine the potential for phytoplankton growth. Hydrological Sciences Journal 23:439–444
Smith VH (1982) The nitrogen and phosphorus dependence of algal biomass in lakes: An empirical and theoretical analysis. Limnology and Oceanography 27:1101–1112
Smith VH, Tilman GD, Nekola JC (1999) Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environmental Pollution 100:179–196
Smith AJ, Bode RW, Kleppel GS (2007) A nutrient biotic index (NBI) for use with benthic macroinvertebrate communities. Ecological Indicators 7:371–386
Strickland JDH, Parsons TR (1968) A practical handbook of sea water analysis. Fisheries Research Board of Canada, Ottawa, Canada 168:1–311
Šporka F (1998) The typology of floodplain water bodies of the Middle Danube (Slovakia) on the basis of the superficial polychaete and oligochaete fauna. Hydrobiologia 386:55–62
Tadić Z, Bonacci O, Radeljak I, Tadić L (2003): Water regime of Kopački Rit Nature Park. In: Proceedings of 3 rd Croatian conference on water, Osijek, pp 941–950
Thoms MC, Southwell M, McGinness HM (2005) Floodplain-river ecosystems: Fragmentation and water resources development. Geomorphology 71:126–138
Tockner K, Schiemer F, Ward JV (1998) Conservation by restoration: the management concept for a river-floodplain system on the Danube River in Austria. Aquatic Conservation: Marine and Freshwater Ecosystems 8:71–86
Tockner K, Pennetzdorfer D, Reiner N, Schiemer F, Ward JV (1999) Hydrological connectivity, and the exchange of organic matter and nutrients in a dynamic river-floodplain system (Danube, Austria). Freshwater Biology 41:521–535
Tockner K, Malard F, Ward JV (2000) An extension of the flood pulse concept. Hydrological Process 14:2861–2883
Unrein F (2002) Changes in phytoplankton community along a transversal section of the Lower Parana floodplain, Argentina. Hydrobiologia 468:123–134
Vidaković J, Bogut I (2007) Periphyton nematode assemblages in association with Myriophyllum spicatum L. in Lake Sakadaš, Croatia. Russian Journal of Nematology 15:79–88
Vidaković J, Bogut I, Mihaljević M, Palijan G, Čerba D, Čačić Lj, Stević F, Zahirović Ž, Galir A (2008) A review of systematic hydrobilogical research in the Nature Park Kopački Rit in the period 1997–2007. Hrvatske vode 16:259–270
Ward JV, Tockner K (2001) Biodiversity: towards a unifying theme for river ecology. Freshwater Biology 46:807–819
Ward JV, Tockner K, Arscott DB, Clarett C (2002) Riverine landscape diversity. Freshwater Biology 47:517–539
Wetzel RG (2001) Limnology. Lake and River Ecosystems, 3rd edn. Academic, San Diego
WWF (1999) Danube Pollution Reduction Programme (DPRP) UNDP/GEF. Evaluation of Wetlands and floodplain areas in the Danube River Basin. WWF Danube-Carpathian Programme and WWF-Floodplain Institute (WWF-Germany)
Acknowledgments
We thank the technical staff at the Nature Park Kopački Rit in Osijek for their assistance with the field surveys. We thank Jadranka Zlomislić (Professor of English Language, Faculty of Philosophy in Osijek) for help with a language check what improved the clarity of our presentation, and two anonymous reviewers for their careful read and thoughtful comments which improved this paper. This research was supported in part by the Croatian Ministry of Science, Education and Sports (Project number 0122023).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peršić, V., Horvatić, J. Spatial Distribution of Nutrient Limitation in the Danube River Floodplain in Relation to Hydrological Connectivity. Wetlands 31, 933–944 (2011). https://doi.org/10.1007/s13157-011-0208-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-011-0208-1