Abstract
Purpose
This study investigates the feasibility of using 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) to predict the pCR (pathologic complete response) rate after neoadjuvant chemoradiotherapy (NCRT) in patients with locally advanced rectal cancer.
Methods
A total of 88 patients with locally advanced rectal cancer were retrospectively analyzed. All patients were treated with NCRT, followed by radical surgery, and 18F-FDG PET/CT was performed before and after NCRT. For a semiquantitative assessment, a volume of interest was drawn, including the whole tumor region, and the maximum SUV (SUVmax), SUVmax normalized to liver uptake (SLR), SUVmax normalized to blood pool uptake (SBR), the metabolic tumor volume at SUV 2.0 (MTV[2.0]), SUV 2.5 (MTV[2.5]), and SUV 3.0 (MTV[3.0]) were measured. In addition, their percentage changes after NCRT were assessed. The pCR was verified through a histologic examination of postsurgical specimens. A receiver operating characteristic curve analysis was conducted to predict the pCR by using these PET parameters.
Results
The pCR was predicted in 17 patients (19 %). The values of the area under the curve (AUC) for predicting the pCR were 0.774 for SUVmax after NCRT, 0.826 for SLR after NCRT, 0.815 for SBR after NCRT, 0.724 for MTV(2.5) after NCRT, 0.729 for the percentage change in SUVmax, 0.700 for the percentage change in SLR, and 0.749 for the percentage change in MTV (2.5). Among these PET parameters, SLR after NCRT showed the highest AUC value. The optimal criterion, sensitivity, specificity, and accuracy of SLR after NCRT for predicting the pCR were ≤1.41, 88 %, 65 %, and 68 %, respectively.
Conclusions
F-FDG PET was found to be useful for predicting the pCR after NCRT in patients with locally advanced rectal cancer. Among various PET parameters, SUVmax normalized to liver uptake after NCRT was the best predictor of the pCR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last decade, there has been a sharp increase in colorectal cancer in Korea [1]. This cancer is now the second most common cancer among men and the third most common cancer among women in the country [2]. The surgical resection of the tumor is the most important treatment method in colorectal cancer [3]. In locally advanced rectal cancer, neoadjuvant chemoradiotherapy (NCRT) increases the rate of tumor regression and sphincter preservation [4] and reduces the rate of local recurrence [5]. Therefore, NCRT followed by radical surgery has become the standard treatment method for patients with locally advanced rectal cancer [6].
Patients with the pathologic complete response (pCR) to NCRT generally show better long-term outcomes than those without [7], and only 15–30 % of patients with rectal cancer achieve pCR to NCRT [8, 9]. Although accurately predicting the pCR before radical surgery can facilitate the choice between sphincter-sparing surgery and aggressive surgery [10, 11], the histologic tumor response can be assessed only after surgical resection. Several imaging modalities, such as computed tomography (CT), magnetic resonance imaging (MRI), and endorectal ultrasound (EUS), have been used to predict responses to NCRT in rectal cancer, but these conventional types of morphological imaging modalities show limited accuracy—ranging from 30 % to 60 %—in terms of predicting the tumor response [12]. This limited accuracy is due to inflammation, edema, and fibrosis resulting from NCRT [13, 14].
18F-Fluorodeoxyglucose positron emission tomography (18F-FDG PET) can provide images of viable tumor tissue by reflecting glucose metabolism [15] and produce semiquantitative data [16]. Therefore, 18F-FDG PET has been widely used to diagnose, stage, restage, and monitor treatment responses in many types of malignancies [17, 18]. Many studies have reported the usefulness of 18F-FDG PET for predicting responses to neoadjuvant therapy in rectal cancer [12]. In general, these studies have used various metabolic parameters such as the maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), total lesion glycolysis (TLG), and changes in PET parameters [19–21], but optimal parameters for predicting the pCR have yet to be defined.
Several studies have reported the usefulness of SUVmax normalized to liver for a semiquantitative assessment of lesions [22–24]. This method can provide reliable data across diverse PET scanners [25] and improve accuracy for the characterization of tumors [26, 27]. Therefore, this study examines the usefulness of PET parameters normalized to liver obtained by 18F-FDG PET/CT before and after NCRT for predicting the pCR in patients with locally advanced rectal cancer.
Materials and Methods
Patient Population
18F-FDG PET/CT images and medical records of 162 consecutive patients with rectal cancer who received NCRT at the authors’ institute from June 2005 to March 2013 were retrospectively reviewed. In this study, patients were enrolled if they met all of the following inclusion criteria: (1) newly diagnosed and histologically proven rectal cancer, (2) the completion of chemotherapy with 5-fluorouracil and leucovorin [11], (3) the surgical resection of the rectal tumor after the completion of NCRT, and (4) 18F-FDG PET/CT before and after NCRT. As a result, those patients who did not undergo surgery (n = 19), those who had no 18F-FDG PET/CT before or after NCRT (n = 54), and a patient who received capecitabine as chemotherapy (n = 1) were excluded, and therefore a total of 88 patients were enrolled in the study.
The Institutional Review Board of the institute approved the study, and no informed consent was required.
18F-FDG PET/CT Acquisition
PET/CT images were acquired on the Biograph6 PET/CT scanner (Siemens Medical Solution, Knoxiville, TN, USA). All patients fasted for at least 6 h before the intravenous injection of 18F-FDG (7.4 MBq/kg of body weight). In patients with a blood glucose level above 7.2 mmol/l, the injection was delayed until the level decreased below 7.2 mmol/l, and the blood glucose level did not exceed 7.2 mmol/l at the time of the 18F-FDG injection in any patient. PET/CT imaging from the skull base to the upper thigh (five to six bed positions) started about 60 min after the injection of 18F-FDG. During the PET/CT scan, CT images with no intravenous iodinated contrast were acquired using a six-slice helical CT scanner (130 kVp, 30 mA, 0.6-s/CT rotation, pitch of 6). Then PET emission images were acquired over the corresponding area with a 16.2 cm axial field of view at 3.5 min per bed position. These CT images were used for attenuation correction, and image reconstruction was performed using a conventional iterative algorithm (ordered-subset expectation maximization, 2 iterations and 8 subsets).
Imaging Analysis and the Determination of Parameters
All PET/CT images were reviewed on e-soft workstations (Siemens Medical Systems, Iselin, NJ). An ellipsoid volume of interest including the whole rectal tumor was drawn by not including the adjacent urinary bladder potentially showing high 18F-FDG uptake. Then the SUVmax and MTV of each dataset were measured. In the case of no discernible focal uptake in the rectum after NCRT, a circular region of interest 2 cm in diameter was drawn at the site corresponding to rectal tumor on PET/CT before NCRT.
MTV was automatically calculated using thresholds of SUV 2.0, SUV 2.5, and SUV 3.0, and outcomes were designated MTV(2.0), MTV(2.5), and MTV(3.0), respectively [21, 28]. To normalize the FDG uptake of the rectal tumor, a circular region of interest (ROI) 3 cm in diameter was drawn in the right lobe of the liver showing homogeneous FDG uptake. In addition, a circular ROI 1 cm in diameter was also drawn in the lumen of descending thoracic aorta to measure FDG uptake of the blood pool [29]. Also, the mean SUV (SUVmean) of the liver and that of the blood pool were measured, respectively. Then the ratio of the SUVmax of the rectal tumor to the SUVmean of the liver (SLR) and the ratio of SUVmax of the rectal tumor to the SUVmean of blood pool (SBR) were calculated, respectively.
The SUVmax, SLR, SBR, and MTV of PET before NCRT were defined as SUV1, SLR1, SBR1, and MTV1, respectively, and the SUVmax, SLR, SBR, and MTV of PET after NCRT, as SUV2, SLR2, SBR2, and MTV2, respectively. Percentage changes in PET parameters were calculated as follows:
Pathologic Assessment
An experienced pathologist analyzed the surgical specimens containing the primary tumor area and the circumferential resection margin. Pathologic responses to NCRT were classified to two groups. The pCR group was defined as no residual malignant cell other than fibrosis in the surgical specimen (ypT0N0), and the non-pCR group, as any evidence of residual malignant cells in the surgical specimen [30]. According to tumor regression grade (TRG) by Mandard et al. [31], patients with TRG1 were classified as the pCR group, while patients with TRG2-5 were classified as the non-pCR group.
Statistical Analysis
The results are presented as frequency and percentages for categorical variables and the mean ± SD for continuous variables. The Mann–Whitney test was employed to compare PET parameters of the pCR and non-pCR groups. The paired samples t-test was used to compare the SUVmean of liver and blood pool before and after NCRT. The receiver operating characteristic (ROC) curve was analyzed to determine the ability of each parameter to predict the pCR. The pairwise comparisons of ROC curves for PET parameters were performed with the method of Hanley and McNeil [32]. The multi-ROC analysis was performed to determine whether combining PET parameters can improve the diagnostic performance for predicting the pCR [33].
All statistical analyses were conducted using MedCalc 13.0 (MedCalc Software, Belgium). Two-tailed p values of <0.05 were considered statistically significant.
Results
Patient Characteristics
Table 1 shows the characteristics of the 88 patients. The mean patient age was 59.2 ± 11.1, and 73 % were men. The mean distance from the anal verge to the tumor was 4.5 ± 2.7 cm. All patients received a total radiation dose of 5,040 cGy in 28 fractions. The mean interval from PET before NCRT to the initiation of NCRT was 11 ± 6.7 days, and that from the end of NCRT to PET after NCRT was 42.3 ± 7.8 days. The mean interval from the end of NCRT to surgery was 53.2 ± 9.0 days. Based on a histologic examination of surgical specimens, 17 (19 %) patients were classified as the pCR group, and 71 (81 %), as the non-pCR group. The mean values of SUVmean of liver before NCRT and after NCRT were 2.6 ± 0.4 and 2.6 ± 0.5, respectively. The mean values of SUVmean of blood pool before NCRT and after NCRT were 2.0 ± 0.3 and 1.9 ± 0.3, respectively. Also, there was no significant difference in the SUVmean of liver (p = 0.70) and the SUVmean of blood pool (p = 0.65) between before and after NCRT.
A total of 40 (45 %) patients had no discernible focal uptake in the rectum after NCRT, and the mean value of SUV2 measured at the corresponding site of the rectal tumor identified on PET before NCRT was 3.3 ± 1.2 in these patients.
Comparison of PET Parameters Between pCR and Non-pCR Groups
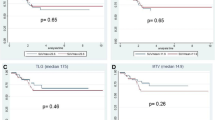
Figure 1 compares the PET parameters between the pCR and non-pCR groups. There were no significant differences in SUV1 (p = 0.89), SLR1 (p = 0.35), SBR1 (p = 0.33), and MTV1 (2.5) (p = 0.14) between the two groups. The pCR group showed significantly lower SUV2 (p = 0.0005), SLR2 (p < 0.0001), SBR2 (p = 0.0001), and MTV2 (2.5) (p = 0.0040) and significantly higher ΔSUV (p = 0.0035), ΔSLR (p = 0.010), ΔSBR (p = 0.044), and ΔMTV (2.5) (p = 0.0015) than the non-pCR group.
Changes of SUVmax (a), SLR (b), SBR (c), and MTV (2.5) (d) after NCRT. Dots indicate median values, and horizontal lines depict interquartile ranges. Red lines indicate the pCR group, and black lines, the non-pCR group. There were significant differences in ΔSUV (p = 0.0035), ΔSLR (p = 0.010), ΔSBR (p = 0.044), and ΔMTV (2.5) (p = 0.0015) between the pCR and non-pCR groups
ROC Curve Analysis for Predicting the pCR
The ability of the PET parameters to predict the pCR was calculated using their ROC curves (Fig. 2 and Table 2). Before NCRT, only MTV1 (2.0) was a significant predictor of the pCR (AUC, 0.645; p = 0.029). By contrast, SUV1 (AUC 0.510; p = 0.89), SLR1 (AUC 0.573; p = 0.35), SBR1 (AUC 0.581; p = 0.23), MTV1 (2.5) (AUC 0.617; p = 0.085), and MTV1 (3.0) (AUC 0.602; p = 0.13) were not significant predictors of the pCR. After NCRT, SUV2 (AUC 0.774; p < 0.0001), SLR2 (AUC 0.826; p < 0.0001), SBR2 (AUC 0.815; p < 0.0001), MTV2 (2.0) (AUC 0.724; p < 0.0001), MTV2 (2.5) (AUC 0.724; p < 0.0001), and MTV2 (3.0) (AUC 0.722; p < 0.0001) were significant predictors of the pCR. In terms of percentage changes in the PET parameters, ΔSUV (AUC 0.729; p = 0.0011), ΔSLR (AUC 0.701; p = 0.0060), ΔSBR (AUC 0.664; p = 0.025), ΔMTV (2.0) (AUC 0.687; p = 0.0055), ΔMTV (2.5) (AUC 0.749; p < 0.0001), and ΔMTV (3.0) (AUC 0.713; p = 0.0004) were significant predictors of the pCR. The comparisons of ROC curves for the PET parameters are summarized in Table 3. There were significant differences between SLR2 and SUV2 (p = 0.044), SLR2 and ΔSLR (p = 0.019), SLR2 and ΔSBR (p = 0.0068), SBR2 and ΔSLR (p = 0.0024), SBR2 and ΔSBR (p = 0.0047), ΔSUV and ΔSBR (p = 0.037), respectively. By contrast, there was no significant difference between other PET parameters. Among all PET parameters, SLR2 showed the highest AUC value for predicting the pCR. Based on the Yuden index and the ROC curve, the optimal criterion, sensitivity, specificity, and accuracy of SLR2 for predicting the pCR were ≤1.41, 88.2 %, 64.8 %, and 68.2 %, respectively.
For the combination of the PET parameters, we selected only SLR2 and ΔMTV (2.5), which showed the highest AUC among the PET parameters after NCRT and percentage changes of the PET parameters, respectively. Among all possible combinations in multi-ROC analysis, combined criterion of SLR2 ≤ 1.01 and ΔMTV (2.5) > 98.1 showed the highest sum of sensitivity and specificity (Fig. 3 in Supplementary Material online). With this criterion, the AUC, sensitivity, specificity, and accuracy for predicting the pCR were 0.759 (p = 0.0007), 58.8 %, 93.0 %, and 86.4 %, respectively. However, it showed no significant difference compared with SLR2 (p = 0.27) or ΔMTV (2.5) (p = 0.90). As a result, the combined PET parameters failed to improve the diagnostic performance for predicting the pCR (Fig. 4 in Supplementary Material online).
Discussion
There are two major findings in the current study. First, PET parameters obtained by 18F-FDG PET/CT before and after NCRT predicted the pCR in patients with locally advanced rectal cancer. Second, among the PET parameters, SLR2 was the most useful parameter for predicting the pCR.
Several imaging modalities have been used for predicting responses to neoadjuvant therapy in patients with rectal cancer, but limited accuracy has prevented the determination of changes in the surgical approach or additional therapy. EUS is an examiner-dependent modality [34], and the reported accuracy of EUS varies from 48 % to 62 % [35]. CT accuracy has been reported to range from 46 % to 65 % [36, 37], and MRI accuracy, from 47 % to 54 % [38–40]. Although the degree of accuracy varies according to the design and setting of the study, these morphological imaging modalities generally overstage the rectal tumor after neoadjuvant therapy [36, 40].
18F-FDG PET has been reported to be more accurate than morphological imaging modalities in predicting responses to neoadjuvant therapy in rectal cancer [37, 41]. Many studies have demonstrated the usefulness of 18F-FDG PET in terms of various metabolic parameters for predicting responses to NCRT in locally advanced rectal cancer [12]. The results of the present study are generally consistent with the findings of previous studies. However, the results are inconsistent in terms of accuracy and optimal cutoff values. These differences may be due the definition of endpoints. In the present study, endpoints were set as the pCR, not as the responder [19, 42, 43], because of the pCR is closely correlated with the local control and better prognosis [11, 44].
SUVmax is a widely used quantitative parameter of 18F-FDG PET, and other volume-based PET parameters such as MTV and TLG are dependent on SUVmax [45]. These PET parameters are limited in terms of a straightforward assessment of metabolic characteristics because various factors such as body weight, obesity, the blood glucose level, and the postinjection uptake time can affect SUVmax [46]. To overcome this problem, normalized SUVmax has been used, and the liver is widely used as a reference region [25]. Several studies have reported the usefulness of 18F-FDG PET for predicting treatment responses by using the tumor-to-liver ratio of FDG uptake in malignant patients [22, 23, 47]. In this study, SLR2 was the best predictor of the pCR. It is conceivable that normalized SUVmax is less affected by above-mentioned factors than other PET parameters. This suggests that SLR2 is not only useful for predicting the pCR but also applicable to various PET scanners.
Among the PET parameters before NCRT, only MTV1 (2.0) predicted the pCR in this study. MTV differs from other PET parameters in reflecting the tumor volume, which is an important independent factor in determining radiotherapy outcome [48]. In addition, MTV represents real tumor burden more accurately than the tumor volume measured by CT [49]. Therefore, the rectal tumor with a larger MTV1 can be more resistant to the standard radiotherapy.
The generally recommended interval between radiotherapy and restaging 18F-FDG PET is at least 3 months to avoid interference by radiation-induced inflammation [50]. However, it is practically inappropriate in a neoadjuvant setting. Many previous studies for rectal cancer performed restaging 18F-FDG PET about 4–6 weeks after neoadjuvant radiotherapy [12]. And there were several reports that inflammation was not a significant problem at this time [41, 51, 52]. In this study, mean interval from NCRT to restaging 18F-FDG PET was 6.1 ± 1.2 weeks, and the shortest interval was 4 weeks. Therefore, we postulated that radiation-induced inflammation had a minimal effect on restaging 18F-FDG PET in the current study.
Although the accuracy of SLR2 for predicting the pCR was superior to other PET parameters, SLR2 plays a limited role in clinical decision making because of its suboptimal accuracy. The gastrointestinal tract can show variable 18F-FDG uptake without pathologic condition [53]. For this reason, physiological 18F-FDG uptake in the rectum may mask residual malignant lesion. In addition, the metabolism of a shrunken rectal tumor after NCRT can be affected by the partial volume effect. These factors can cause false-positive for the pCR, and lead to the relative lower specificities of the PET parameters including SLR2. In this regard, a combination of 18F-FDG PET/CT and other morphological imaging modalities or biochemical markers is expected to improve its accuracy for predicting the pCR in rectal cancer [54, 55].
This study has several limitations. First, the study was designed as a retrospective observational study. Second, survival data on enrolled patients were not considered. Third, it was difficult to promptly apply the suggested optimal cutoff criteria for PET parameters in a clinical setting because they were based on a single institutional data set. Fourth, we did not correct the partial volume effect for the rectal tumor after NCRT.
Conclusions
The results suggest that SLR2 is more useful for predicting the pCR than PET parameters without liver normalization in patients with locally advanced rectal cancer. This new parameter may help clinicians plan surgery and adjuvant therapy.
References
Shin A, Kim KZ, Jung KW, Park S, Won YJ, Kim J, et al. Increasing trend of colorectal cancer incidence in Korea, 1999–2009. Cancer Res Treat. 2012;44(4):219–26.
Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: Incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45(1):1–14.
Kuhry E, Schwenk WF, Gaupset R, Romild U, Bonjer HJ. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev 2008;CD003432
Valentini V, Coco C, Cellini N, Picciocchi A, Fares MC, Rosetto ME, et al. Ten years of preoperative chemoradiation for extraperitoneal T3 rectal cancer: acute toxicity, tumor response, and sphincter preservation in three consecutive studies. Int J Radiat Oncol Biol Phys. 2001;51(2):371–83.
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40.
Eich HT, Stepien A, Zimmermann C, Hellmich M, Metzger R, Holscher A, et al. Neoadjuvant radiochemotherapy and surgery for advanced rectal cancer: prognostic significance of tumor regression. Strahlenther Onkol. 2011;187(4):225–30.
Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99(7):918–28.
Janssen MH, Ollers MC, Riedl RG, van den Bogaard J, Buijsen J, van Stiphout RG, et al. Accurate prediction of pathological rectal tumor response after two weeks of preoperative radiochemotherapy using (18)F-fluorodeoxyglucose-positron emission tomography-computed tomography imaging. Int J Radiat Oncol Biol Phys. 2010;77(2):392–9.
van Stiphout RG, Lammering G, Buijsen J, Janssen MH, Gambacorta MA, Slagmolen P, et al. Development and external validation of a predictive model for pathological complete response of rectal cancer patients including sequential PET-CT imaging. Radiother Oncol. 2011;98(1):126–33.
Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro Jr U, Silva e Sousa Jr AH, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240(4):711–7.
Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol. 2012;23(10):2479–516.
de Geus-Oei LF, Vriens D, van Laarhoven HW, van der Graaf WT, Oyen WJ. Monitoring and predicting response to therapy with 18F-FDG PET in colorectal cancer: a systematic review. J Nucl Med. 2009;50 Suppl 1:43S–54S.
Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging—a meta-analysis. Radiology. 2004;232(3):773–83.
Capirci C, Rubello D, Chierichetti F, Crepaldi G, Carpi A, Nicolini A, et al. Restaging after neoadjuvant chemoradiotherapy for rectal adenocarcinoma: role of F18-FDG PET. Biomed Pharmacother. 2004;58(8):451–7.
Kubota K. From tumor biology to clinical Pet: a review of positron emission tomography (PET) in oncology. Ann Nucl Med. 2001;15(6):471–86.
Weber WA, Ziegler SI, Thodtmann R, Hanauske AR, Schwaiger M. Reproducibility of metabolic measurements in malignant tumors using FDG PET. J Nucl Med. 1999;40(11):1771–7.
Boellaard R, O’Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010;37(1):181–200.
Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, et al. Recommendations on the use of 18 F-FDG PET in oncology. J Nucl Med. 2008;49(3):480–508.
Maffione AM, Ferretti A, Grassetto G, Bellan E, Capirci C, Chondrogiannis S, et al. Fifteen different 18F-FDG PET/CT qualitative and quantitative parameters investigated as pathological response predictors of locally advanced rectal cancer treated by neoadjuvant chemoradiation therapy. Eur J Nucl Med Mol Imaging. 2013;40(6):853–64.
Sun W, Xu J, Hu W, Zhang Z, Shen W. The role of sequential 18(F)-FDG PET/CT in predicting tumour response after preoperative chemoradiation for rectal cancer. Color Dis. 2013;15(5):e231–8.
Chennupati SK, Quon A, Kamaya A, Pai RK, La T, Krakow TE, et al. Positron emission tomography for predicting pathologic response after neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Am J Clin Oncol. 2012;35(4):334–9.
Findlay M, Young H, Cunningham D, Iveson A, Cronin B, Hickish T, et al. Noninvasive monitoring of tumor metabolism using fluorodeoxyglucose and positron emission tomography in colorectal cancer liver metastases: correlation with tumor response to fluorouracil. J Clin Oncol. 1996;14(3):700–8.
Flamen P, Van Cutsem E, Lerut A, Cambier JP, Haustermans K, Bormans G, et al. Positron emission tomography for assessment of the response to induction radiochemotherapy in locally advanced oesophageal cancer. Ann Oncol. 2002;13(3):361–8.
Lee JW, Paeng JC, Kang KW, Kwon HW, Suh KS, Chung JK, et al. Prediction of tumor recurrence by 18 F-FDG PET in liver transplantation for hepatocellular carcinoma. J Nucl Med. 2009;50(5):682–7.
Kamibayashi T, Tsuchida T, Demura Y, Tsujikawa T, Okazawa H, Kudoh T, et al. Reproducibility of semi-quantitative parameters in FDG-PET using two different PET scanners: influence of attenuation correction method and examination interval. Mol Imaging Biol. 2008;10(3):162–6.
Delbeke D, Martin WH, Sandler MP, Chapman WC, Wright Jr JK, Pinson CW. Evaluation of benign vs. malignant hepatic lesions with positron emission tomography. Arch Surg. 1998;133(5):510–5. discussion 5–6.
Blake MA, Slattery JM, Kalra MK, Halpern EF, Fischman AJ, Mueller PR, et al. Adrenal lesions: characterization with fused PET/CT image in patients with proved or suspected malignancy—initial experience. Radiology. 2006;238(3):970–7.
Lee JA. Segmentation of positron emission tomography images: some recommendations for target delineation in radiation oncology. Radiother Oncol. 2010;96(3):302–7.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S–50S.
Stipa F, Chessin DB, Shia J, Paty PB, Weiser M, Temple LK, et al. A pathologic complete response of rectal cancer to preoperative combined-modality therapy results in improved oncological outcome compared with those who achieve no downstaging on the basis of preoperative endorectal ultrasonography. Ann Surg Oncol. 2006;13(8):1047–53.
Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73(11):2680–6.
Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148(3):839–43.
Shultz EK. Multivariate receiver-operating characteristic curve analysis: prostate cancer screening as an example. Clin Chem. 1995;41(8 Pt 2):1248–55.
Orrom WJ, Wong WD, Rothenberger DA, Jensen LL, Goldberg SM. Endorectal ultrasound in the preoperativestaging of rectal tumors. A learning experience. Dis Colon Rectum. 1990;33(8):654–9.
Edelman BR, Weiser MR. Endorectal ultrasound: its role in the diagnosis and treatment of rectal cancer. Clin Colon Rectal Surg. 2008;21(3):167–77.
Huh JW, Park YA, Jung EJ, Lee KY, Sohn SK. Accuracy of endorectal ultrasonography and computed tomography for restaging rectal cancer after preoperative chemoradiation. J Am Coll Surg. 2008;207(1):7–12.
Denecke T, Rau B, Hoffmann KT, Hildebrandt B, Ruf J, Gutberlet M, et al. Comparison of CT, MRI and FDG-PET in response prediction of patients with locally advanced rectal cancer after multimodal preoperative therapy: is there a benefit in using functional imaging? Eur Radiol. 2005;15(8):1658–66.
Kuo LJ, Chern MC, Tsou MH, Liu MC, Jian JJ, Chen CM, et al. Interpretation of magnetic resonance imaging for locally advanced rectal carcinoma after preoperative chemoradiation therapy. Dis Colon Rectum. 2005;48(1):23–8.
Chen CC, Lee RC, Lin JK, Wang LW, Yang SH. How accurate is magnetic resonance imaging in restaging rectal cancer in patients receiving preoperative combined chemoradiotherapy? Dis Colon Rectum. 2005;48(4):722–8.
Hoffmann KT, Rau B, Wust P, Stroszczynski C, Hunerbein M, Schneider U, et al. Restaging of locally advanced carcinoma ofthe rectum with MR imaging after preoperative radio-chemotherapy plus regional hyperthermia. Strahlenther Onkol. 2002;178(7):386–92.
Amthauer H, Denecke T, Rau B, Hildebrandt B, Hunerbein M, Ruf J, et al. Response prediction by FDG-PET after neoadjuvant radiochemotherapy and combined regional hyperthermia of rectal cancer: correlation with endorectal ultrasound and histopathology. Eur J Nucl Med Mol Imaging. 2004;31(6):811–9.
Rosenberg R, Herrmann K, Gertler R, Kunzli B, Essler M, Lordick F, et al. The predictive value of metabolic response to preoperative radiochemotherapy in locally advanced rectal cancer measured by PET/CT. Int J Color Dis. 2009;24(2):191–200.
Capirci C, Rampin L, Erba PA, Galeotti F, Crepaldi G, Banti E, et al. Sequential FDG-PET/CT reliably predicts response of locally advanced rectal cancer to neo-adjuvant chemo-radiation therapy. Eur J Nucl Med Mol Imaging. 2007;34(10):1583–93.
Valentini V, Aristei C, Glimelius B, Minsky BD, Beets-Tan R, Borras JM, et al. Multidisciplinary RectalCancer Management: 2nd European Rectal Cancer Consensus Conference (EURECA-CC2). Radiother Oncol. 2009;92(2):148–63.
Moon SH, Hyun SH, Choi JY. Prognostic significance of volume-based PET parameters in cancer patients. Korean J Radiol. 2013;14(1):1–12.
Adams MC, Turkington TG, Wilson JM, Wong TZ. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am J Roentgenol. 2010;195(2):310–20.
Song MJ, Bae SH, Yoo Ie R, Park CH, Jang JW, Chun HJ, et al. Predictive value of (1)(8)F-fluorodeoxyglucose PET/CT for transarterial chemolipiodolization of hepatocellular carcinoma. World J Gastroenterol. 2012;18(25):3215–22.
Dubben HH, Thames HD, Beck-Bornholdt HP. Tumor volume: a basic and specific response predictor in radiotherapy. Radiother Oncol. 1998;47(2):167–74.
Zhu D, Ma T, Niu Z, Zheng J, Han A, Zhao S, et al. Prognostic significance of metabolic parameters measured by (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with small cell lung cancer. Lung Cancer. 2011;73(3):332–7.
Ziessman HA, O’Malley JP, Thrall JH. Nuclear medicine: the requisites. Elsevier Mosby, Philadelphia. 2013. pp. 232.
Guillem JG, Puig-La Calle Jr J, Akhurst T, Tickoo S, Ruo L, Minsky BD, et al. Prospective assessment of primary rectal cancer response to preoperative radiation and chemotherapy using 18-fluorodeoxyglucose positron emission tomography. Dis Colon Rectum. 2000;43(1):18–24.
Cascini GL, Avallone A, Delrio P, Guida C, Tatangelo F, Marone P, et al. 18F-FDG PET is an early predictor of pathologic tumor response to preoperative radiochemotherapy in locally advanced rectal cancer. J Nucl Med. 2006;47(8):1241–8.
Prabhakar HB, Sahani DV, Fischman AJ, Mueller PR, Blake MA. Bowel hot spots at PET-CT. Radiographics. 2007;27(1):145–59.
Kim JW, Kim HC, Park JW, Park SC, Sohn DK, Choi HS, et al. Predictive value of (18)FDG PET-CT for tumour response in patients with locally advanced rectal cancer treated by preoperative chemoradiotherapy. Int J Color Dis. 2013;28(9):1217–24.
Lambrecht M, Deroose C, Roels S, Vandecaveye V, Penninckx F, Sagaert X, et al. The use of FDG-PET/CT and diffusion-weighted magnetic resonance imaging for response prediction before, during and after preoperative chemoradiotherapy for rectal cancer. Acta Oncol. 2010;49(7):956–63.
Acknowledgments
This study was supported by the Establishment Center for PET Application Technology Development of the Korea Institute of Radiological and Medical Sciences (KIRAMS) and by a grant from the Ministry of Education, Science, and Technology (50441–2014).
Conflict of Interest
Jihyun Park, Kyoung Jin Chang, Young Seok Seo, Byung Hyun Byun, Joon Ho Choi, Hansol Moon, Ilhan Lim, Byung Il Kim, Chang Woon Choi, and Sang Moo Lim declare no conflict of interest.
Ethics Statement
This study was approved by the Institutional Review Board at Korea Cancer Center Hospital (IRB No. K-1403-002-032), and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Acquisition of informed consent was exempted by the board because of retrospective nature of the study. Details that might disclose the identity of the subjects were omitted.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, J., Chang, K.J., Seo, Y.S. et al. Tumor SUVmax Normalized to Liver Uptake on 18F-FDG PET/CT Predicts the Pathologic Complete Response After Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Nucl Med Mol Imaging 48, 295–302 (2014). https://doi.org/10.1007/s13139-014-0289-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-014-0289-x