Abstract
Purpose
Early detection of a response to neoadjuvant chemotherapy for locally advanced rectal cancer may spare patients from additional toxic but ineffective chemotherapy. Using 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET), we evaluated tumor response prospectively in the early course of preoperative chemotherapy.
Methods
The subjects were 15 patients who received neoadjuvant chemotherapy (XELOX or XELOX plus bevacizumab) for locally advanced rectal cancer. Patients underwent 18F-FDG PET before chemotherapy, at the end of the first cycle of chemotherapy, and before surgical resection. Magnetic resonance imaging (MRI) was performed before chemotherapy, after the second cycle of chemotherapy, and before resection. After resection, the SUVmax and diameter were compared and graded according to the tumor regression grade (TRG).
Results
The TRG was assessed as TRG1 in one patient, TRG2 in five patients, and TRG3 in nine patients. We divided the patients into two groups: non-responders (NR) included the TRG1 and TRG2 patients, and responders (R) included the TRG3 patients. The tumor size before surgery was significantly smaller in the R group than in the NR group. The SUVmax at the end of the first cycle of chemotherapy and before surgical resection was significantly lower in the R group than in the NR group.
Conclusion
Performing 18F-FDG PET at the end of the first cycle of chemotherapy allowed us to predict the pathological response of locally advanced rectal cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Local recurrence of rectal cancer is difficult to treat and often carries a dismal prognosis. In recent years, surgical and multimodal treatments have been combined to reduce locoregional recurrence. While total mesorectal excision (TME) has dramatically improved oncologic and functional outcomes following surgery for rectal cancer [1–3], the risk of local recurrence continues to threaten patients with locally advanced rectal cancer. As surgery alone is often not curative, preoperative treatment is required to achieve radial resection and to improve the local control rate [4]. A recent report reviewed the strategy of neoadjuvant treatment for locally advanced rectal cancer [5]. Neoadjuvant chemoradiotherapy (CRT) has become standard treatment for locally advanced rectal cancer to prevent local recurrence [6, 7]; however, the adverse effects of radiotherapy compromise the patient’s quality of life. Radiation enterocolitis, chronic cystitis, and sexual dysfunction have all been reported to be associated with CRT following TME resection [8–10]. The recent availability of new agents, including capecitabine, irinotecan, and oxaliplatin, has substantially expanded the options available for the management of recurrent and unresectable colorectal cancer. Several studies indicate that new chemotherapy regimens can omit radiotherapy from the neoadjuvant settings [11–14]. However, neoadjuvant therapy is not beneficial for all patients. The treatment response ranges from pathological complete response to resistance and favorable regression of advanced rectal cancer is not always achieved with neoadjuvant chemotherapy [11, 14]. Moreover, it is challenging to identify which patients have had no or minimal tumor response to neoadjuvant therapy before or during treatment.
The 18F-FDG PET scan is more accurate than any other technique for detecting residual tumors. Several studies have found that 18F-FDG PET performed after neoadjuvant CRT for rectal cancer can predict patient prognosis [15–17]. However, Leibold et al. reported that early response could not be detected by 18F-FDG PET during preoperative CRT [18]. Aiba et al. suggested that MRI scans before and after neoadjuvant chemotherapy can be helpful to predict treatment response. However, to our knowledge, there are no reports on the early detection of 18F-FDG PET response during neoadjuvant chemotherapy for locally advanced rectal cancer. There is a clear need for a reliable and noninvasive method for predicting treatment response. Thus, we conducted this study to investigate the use of 18F-FDG PET for predicting the pathological grade of rectal cancer.
Patients and methods
Patient selection
Patients with biopsy-proven locally advanced resectable rectal adenocarcinoma (T3 or T4 and N0-N2) diagnosed between 2011 and 2013, were enrolled in this study. Other eligibility criteria were as follows: the tumor was located within 12 cm of the anal verge (mid/lower rectum), as defined by colonoscopy; the patient was 75 years or younger at the time of enrollment; there was no severe impairment of major organ function, including the heart, liver, kidney, and lung; the performance status was 0–1 on the Eastern Cooperative Oncology Group (ECOG) scale; and the fasting blood sugar level did not exceed 150 mg/dl.
Written informed consent was obtained from all patients according to the study protocol. The protocol was approved previously by the institutional review board at the Osaka Rosai Hospital in Sakai, Japan. Table 1 summarizes the clinicopathological characteristics of the patients. The contributions to this study were as follows: conception and design, JN, JH, YO, HM, HY, IT, TM, RN, YD, and MM; patient recruitment, JN, JH, and RN; and analysis and interpretation of data, JN, JH, YO, HM, MU, NH, TH. All authors were involved in the preparation and revision of this report for submission.
Treatment protocols
XELOX consisted of a 2-h intravenous infusion of oxaliplatin, 130 mg/m2 on day 1, plus oral capecitabine, 1000 mg/m2 twice daily for 2 weeks of a 3-week cycle. In some patients, bevacizumab 7.5 mg/kg was administered as a 30- to 90-min intravenous infusion before oxaliplatin on day 1 of the 3-week cycle. Preoperative chemotherapy was continued for four cycles. For the bevacizumab-treated patients, bevacizumab was omitted from the last cycle. All patients completed the chemotherapy regimen without grade 4 side effects developing.
18F-FDG PET scan procedure and data interpretation
18F-FDG PET was performed before neoadjuvant chemotherapy (first PET), after one cycle of chemotherapy (second PET), and before surgery (third PET). After the patient had fasted for 5 h, 18F-FDG, 3.083 MBq/kg, was injected intravenously and images were obtained using a combined PET/CT scanner (SET-3000GCT/M, Shimazu, Kyoto, Japan). Image emission data were acquired over approximately 20 min. After attenuation corrections were performed on the obtained data, images were reconstructed using a dynamic row-action maximum likelihood algorithm. The reconstructed sectional images were evaluated visually and quantitatively using the maximum standardized uptake value (SUVmax) inside a volume of interest (VOI) on the lesion. SUVmax was calculated as follows: [(maximum activity in VOI)/(volume of VOI)]/[(injected FDG dose)/(patient weight)]. ΔSUVmax was calculated as follows: [SUVmax (the first PET) − SUVmax (the second or third PET)] × 100/[SUVmax (the first PET)].
MRI scan procedure
MRI scanning was performed before chemotherapy (first MRI), after the second cycle of chemotherapy (second MRI), and before surgery (third MRI). All MRI scans were reviewed by a radiologist (YO). T stage was assessed by MRI T2-weighted images. The longest diameter of the tumor was measured on MRI T2-weighted images.
Histology
The primary tumor and harvested lymph nodes were analyzed microscopically. Slices were stained with hematoxylin-eosin and an experienced pathologist (MH) reviewed all the patient specimens. Pathologic staging of the tumors was done according to the TNM system, as recommended by the AJCC Cancer Staging Manual, 7th edn. [19]. The chemotherapy response was evaluated using the tumor regression grade (TRG) system proposed by Rodel et al. [20] as follows: TRG 0, no regression; TRG 1, minor regression (dominant tumor mass with obvious fibrosis in 25 % or less of the tumor mass); TRG 2, moderate regression (dominant tumor mass with obvious fibrosis in 26–50 % of the tumor mass); TRG 3, good regression (dominant fibrosis outgrowing the tumor mass, representing more than 50 % tumor regression; and TRG 4, total regression (no visible tumor cells, only fibrotic mass).
Statistical analysis
Data are expressed as mean ± standard deviation (SD) for continuous variables and as numbers and percentages for categorical variables. The relationship between the pathologic response as assessed by TRG during or after chemotherapy and the clinical parameters in the responder and non-responder patient groups was established using Fisher’s test. The tumor size and SUVmax values were compared among the groups using the paired t test. p < 0.05 was considered significant.
Results
Primary tumor size
All patients completed the course of neoadjuvant chemotherapy following complete resection. The second MRI was performed 4–8 weeks (mean 6 weeks) after the first round of chemotherapy and the third MRI was performed 12–15 weeks after the first round of chemotherapy. The tumor size was 35–110 mm (median 58 mm) at the first MRI, 18–90 mm (median 35 mm) at the second MRI, and 15–46 mm (median 24 mm) at the third MRI (Fig. 1a). In all patients, the tumor size was smaller at the second and third MRI than at the first MRI.
Time course of SUVmax in the primary tumors
The first PET scan was performed 0–5 weeks (median 1.9 weeks) before chemotherapy was started. The second PET scan was performed 12–38 days (median 15 days) after the start day of the first course of chemotherapy. The third PET scan was performed 9–24 days (median 13 days) after the last chemotherapy was completed. There was a significant decrease in the SUVmax value in the second and third PET scans compared with the first PET scan (Fig. 1b). However, there was no significant decrease from the second to the third PET scans.
Histological chemotherapy response of the primary tumor
After resection, the histopathological response was evaluated according to the TRG. There was one patient with TRG1, five with TRG2, and nine with TRG3. Before chemotherapy, there were no significant correlations between the histological response and any clinicopathological characteristic, including age, sex, tumor size, CEA level, TNM stage, chemotherapy regimen, histological type, or SUVmax (Table 2). The final ypTNM staging was stage I in five patients, stage IIA in six patients, stage IIIA in one patient, and stage IIIB in two patients (supplementary Table 1).
MRI and PET scan results analyzed according to chemotherapy response
The patients were divided into two groups according to their response to chemotherapy. The non-responder (NR) group included TRG1 and TRG2 patients, and the responder (R) group included TRG3 patients. The tumor size on the first and second MRI was not significantly different between the two groups (Fig. 2a). However, the tumor size on the third MRI was significantly smaller in the R group than in the NR group (mean 22.2 vs. 35.0 mm, respectively; p = 0.017). We compared the chemotherapy response and SUVmax in the two groups (Fig. 2b). There was no significant difference in SUVmax between the groups before chemotherapy, but it was significantly greater in the NR group on the second PET (4.9 vs. 9.3; p = 0.023) and on the third PET (3.2 vs. 10.3; p = 0.007).
Tumor shrinkage and ΔSUVmax analyzed according to chemotherapy response
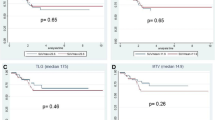
The tumor shrinkage ratio did not differ significantly between the two groups during chemotherapy (Fig. 3a); however, the tumor shrinkage ratio was significantly greater on the third MRI than on the second MRI (60.8 vs. 42.2 %; p = 0.046). The ΔSUVmax between the first and second PET scans and the first and third PET scans was compared in the two groups (Fig. 3b). The ΔSUVmax was significantly higher in the R group than in the NR group after the first round of chemotherapy (44.5 vs. 21.3 %; p = 0.047) and before resection (64.8 vs. 10.2 %; p = 0.004). After a median follow-up period of 32 months, all patients in the R group remained disease-free; however, two patients in the NR group showed metastatic progression, lung metastasis (DFS 8 months), and liver metastasis (DFS 11 months).
Box plots of tumor shrinkage ratio (%Δtumor size) (a) or reduction rate of SUVmax (%ΔSUVmax) (b) for each group. %Δtumor size was calculated as follows: [tumor size in baseline (the first MRI) − tumor size post chemotherapy (the second or third MRI)] × 100/[tumor size in baseline (the first MRI)]. %ΔSUVmax was calculated as follows: [SUVmax (the first PET) − SUVmax (the second or third PET)] × 100/[SUVmax (the first PET)]
Discussion
This study showed that 18F-FDG PET can predict chemotherapy response after the first chemotherapy course given to patients with locally advanced rectal cancer. In contrast, MRI imaging failed to predict early chemotherapy response.
Neoadjuvant CRT has proven successful for treating locally advanced rectal cancer. Preoperative CRT for rectal cancer has been reported to improve local control and to minimize treatment toxicity more effectively than postoperative CRT [21]. In Western countries, neoadjuvant CRT is used as a standard combined modality treatment for treating locally advanced rectal cancer [21]. XELOX treatment is superior to bolus 5-FU/LV (Mayo Clinic or Roswell Park) as adjuvant treatment, and XELOX chemotherapy has been shown to benefit patients with unresectable metastatic colorectal cancer [22, 23]. The advantages of XELOX or XELOX + BV treatment include that there is no need for a central venous port [24] and that it has been associated with lower rates of neutropenia than FOLFOX [25]. Several groups have looked at omitting radiotherapy from the treatment regimen for locally advanced rectal cancer [11–14]. Several studies of neoadjuvant chemotherapy without radiation for rectal cancer are planned or ongoing (NCT01650428, NCT01515787, and NCT01211210). Thus, preoperative multimodal treatments are still being developed. As for CRT and chemotherapy, tumor responses to neoadjuvant treatment vary considerably. Some patients experience serious side effects and not all patients benefit equally [26]. Moreover, pelvic radiotherapy has been reported to affect sexual function and cause urinary and fecal incontinence [27–29]. There is clinical interest in preventing side effects in patients who are not responding to neoadjuvant treatment.
In this study, the longest tumor diameter measured by MRI was significantly shorter in the R group than in the NR group before resection, whereas the change in diameter did not predict histological response at the end of two cycles of chemotherapy. The RECIST criteria are widely accepted, but the correlation between morphologic tumor response and patient outcome is weak [30]. Several studies have analyzed the tumor volume change on MRI after preoperative CRT as a parameter of treatment response [31–33]. Musino et al. reported that diffusion-weight MRI during preoperative CRT can be used to evaluate the early response of primary rectal tumors [34]. Nougaret et al. reported that MRI volumetry can predict the histological response after four cycles of FOLFOX plus irinotecan chemotherapy [35]. Recently, Aiba et al. reported the usefulness of the MRI calculated total volume before and after neoadjuvant chemotherapy [36]. These reports indicate that two or three dimensional volumes measured by diffusion-weight MRI can be used to predict neoadjuvant-therapy response. Thus, MRI might be a reliable tool for predicting the final clinical T and N stages.
Recent studies have looked at the use of 18F-FDG PET for the early prediction of neoadjuvant CRT response. In the early phase, 8–14 days after starting preoperative CRT, 18F-FDG PET does not predict the pathological response well enough to justify an early change in therapy [18]. However, 3 weeks after starting CRT, 18F-FDG PET is a reliable and accurate diagnostic tool for assessing response to neoadjuvant treatment [37]. These results indicate that there is an optimal time frame for evaluating treatment response; however, no studies have evaluated 18F-FDG PET as a tool for early prediction of the response to neoadjuvant chemotherapy. In this study, 18F-FDG PET scanning was done at the end of the first cycle of chemotherapy and in all except one patient, the SUVmax had decreased (range −31.6 to 61.7 %). These decreases continued to the time of the preoperative PET scan in the R group. In the NR group, a relapse, indicated by an increasing SUVmax, was noted in three of six patients. Comparing the tumor size as measured by MRI, the SUVmax was significantly decreased in the R group at the end of the first cycle of chemotherapy. Thus, we could detect the decrease in the SUVmax of the primary tumor early in treatment in the R group. Early identification of inadequate response to neoadjuvant treatment could spare the patients from the toxicity of ineffective treatment. If the decrease in the SUVmax was poor, neoadjuvant therapy could be changed to a more powerful chemotherapy regimen including targeted therapy or additional radiotherapy during the neoadjuvant chemotherapy. However, the true ability of the 18F-FDG PET scan to detect early tumor response cannot be confirmed based on the limited number of patients in this series, so further studies are warranted. While the median follow-up period was short, distant metastases developed in two patients from the NR group developed, but in none from the R group. These outcomes might suggest that the primary tumor response to neoadjuvant chemotherapy reflects the response to distant micrometastasis. From this viewpoint, 18F-FDG PET scan results at the end of the first cycle of chemotherapy could be a prognostic factor for locally advanced rectal cancer. Thus, when the SUVmax does not decrease, treatment for primary tumor or adjuvant chemotherapy should be changed to more powerful systemic chemotherapy.
In conclusion, our data show that the 18F-FDG PET scan may be a useful tool for predicting primary tumor early response to neoadjuvant chemotherapy.
References
Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1(8496):1479–82.
Hida J, Okuno K, Tokoro T. Distal dissection in total mesorectal excision, and preoperative chemoradiotherapy and lateral lymph node dissection for rectal cancer. Surg Today. 2014;44(12):2227–42.
MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341(8843):457–60.
Wong RK, Tandan V, De Silva S, Figueredo A. Pre-operative radiotherapy and curative surgery for the management of localized rectal carcinoma. Cochrane Database Syst Rev. 2007;2:CD002102.
Uehara K, Nagino M. Neoadjuvant treatment for locally advanced rectal cancer: a systematic review. Surg Today. 2015. doi:10.1007/s00595-015-1218-z.
Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–23.
Hospers GA, Punt CJ, Tesselaar ME, Cats A, Havenga K, Leer JW, et al. Preoperative chemoradiotherapy with capecitabine and oxaliplatin in locally advanced rectal cancer. A phase I-II multicenter study of the Dutch Colorectal Cancer Group. Ann Surg Oncol. 2007;14(10):2773–9.
van Duijvendijk P, Slors JF, Taat CW, van Tets WF, van Tienhoven G, Obertop H, et al. Prospective evaluation of anorectal function after total mesorectal excision for rectal carcinoma with or without preoperative radiotherapy. Am J Gastroenterol. 2002;97(9):2282–9.
Birgisson H, Pahlman L, Gunnarsson U, Glimelius B, Swedish Rectal Cancer Trial Group. Adverse effects of preoperative radiation therapy for rectal cancer: long-term follow-up of the Swedish Rectal Cancer Trial. J Clin Oncol. 2005;23(34):8697–705.
Peeters KC, van de Velde CJ, Leer JW, Martijn H, Junggeburt JM, Kranenbarg EK, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients—a Dutch colorectal cancer group study. J Clin Oncol. 2005;23(25):6199–206.
Hasegawa J, Nishimura J, Mizushima T, Miyake Y, Kim HM, Takemoto H, et al. Neoadjuvant capecitabine and oxaliplatin (XELOX) combined with bevacizumab for high-risk localized rectal cancer. Cancer Chemother Pharmacol. 2014;73(5):1079–87.
Ishii Y, Hasegawa H, Endo T, Okabayashi K, Ochiai H, Moritani K, et al. Medium-term results of neoadjuvant systemic chemotherapy using irinotecan, 5-fluorouracil, and leucovorin in patients with locally advanced rectal cancer. Eur J Surg Oncol. 2010;36(11):1061–5.
Schrag D, Weiser MR, Goodman KA, Gonen M, Hollywood E, Cercek A, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32(6):513–8.
Uehara K, Hiramatsu K, Maeda A, Sakamoto E, Inoue M, Kobayashi S, et al. Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor-risk rectal cancer: n-SOG 03 phase II trial. Jpn J Clin Oncol. 2013;43(10):964–71.
Capirci C, Rubello D, Chierichetti F, Crepaldi G, Fanti S, Mandoliti G, et al. Long-term prognostic value of 18F-FDG PET in patients with locally advanced rectal cancer previously treated with neoadjuvant radiochemotherapy. AJR Am J Roentgenol. 2006;187(2):W202–8.
Guillem JG, Moore HG, Akhurst T, Klimstra DS, Ruo L, Mazumdar M, et al. Sequential preoperative fluorodeoxyglucose-positron emission tomography assessment of response to preoperative chemoradiation: a means for determining longterm outcomes of rectal cancer. J Am Coll Surg. 2004;199(1):1–7.
Kalff V, Duong C, Drummond EG, Matthews JP, Hicks RJ. Findings on 18F-FDG PET scans after neoadjuvant chemoradiation provides prognostic stratification in patients with locally advanced rectal carcinoma subsequently treated by radical surgery. J Nucl Med. 2006;47(1):14–22.
Leibold T, Akhurst TJ, Chessin DB, Yeung HW, Macapinlac H, Shia J, et al. Evaluation of (1)(8)F-FDG-PET for early detection of suboptimal response of rectal cancer to preoperative chemoradiotherapy: a prospective analysis. Ann Surg Oncol. 2011;18(10):2783–9.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4.
Rodel C, Martus P, Papadoupolos T, Fuzesi L, Klimpfinger M, Fietkau R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23(34):8688–96.
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40.
Cassidy J, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26(12):2006–12.
Cassidy J, Tabernero J, Twelves C, Brunet R, Butts C, Conroy T, et al. XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol. 2004;22(11):2084–91.
Yoshida Y, Hoshino S, Aisu N, Naito M, Tanimura S, Mogi A, et al. Administration of chemotherapy via the median cubital vein without implantable central venous access ports: port-free chemotherapy for metastatic colorectal cancer patients. Int J Clin Oncol. 2015;20(2):332–7.
Yoshida Y, Hoshino S, Aisu N, Mogi A, Yamada T, Kojima D, et al. Can grade 2 neutropenia predict the risk of grade 3 neutropenia in metastatic colorectal cancer patients treated with chemotherapy? Support Care Cancer. 2015;23(6):1623–7.
Gosens MJ, Dresen RC, Rutten HJ, Nieuwenhuijzen GA, van der Laak JA, Martijn H, et al. Preoperative radiochemotherapy is successful also in patients with locally advanced rectal cancer who have intrinsically high apoptotic tumours. Ann Oncol. 2008;19(12):2026–32.
Lange MM, den Dulk M, Bossema ER, Maas CP, Peeters KC, Rutten HJ, et al. Risk factors for faecal incontinence after rectal cancer treatment. Br J Surg. 2007;94(10):1278–84.
Marijnen CA, van de Velde CJ, Putter H, van den Brink M, Maas CP, Martijn H, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2005;23(9):1847–58.
Pollack J, Holm T, Cedermark B, Holmstrom B, Mellgren A. Long-term effect of preoperative radiation therapy on anorectal function. Dis Colon Rectum. 2006;49(3):345–52.
Avril NE, Weber WA. Monitoring response to treatment in patients utilizing PET. Radiol Clin North Am. 2005;43(1):189–204.
Barbaro B, Fiorucci C, Tebala C, Valentini V, Gambacorta MA, Vecchio FM, et al. Locally advanced rectal cancer: MR imaging in prediction of response after preoperative chemotherapy and radiation therapy. Radiology. 2009;250(3):730–9.
Curvo-Semedo L, Lambregts DM, Maas M, Thywissen T, Mehsen RT, Lammering G, et al. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy–conventional MR volumetry versus diffusion-weighted MR imaging. Radiology. 2011;260(3):734–43.
Yeo SG, Kim DY, Kim TH, Chang HJ, Oh JH, Park W, et al. Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: long-term outcomes and prognostic significance of pathologic nodal status (KROG 09-01). Ann Surg. 2010;252(6):998–1004.
Musio D, De Felice F, Magnante AL, Ciolina M, De Cecco CN, Rengo M, et al. Diffusion-weighted magnetic resonance application in response prediction before, during, and after neoadjuvant radiochemotherapy in primary rectal cancer carcinoma. Biomed Res Int. 2013;2013:740195.
Nougaret S, Fujii S, Addley HC, Bibeau F, Pandey H, Mikhael H, et al. Neoadjuvant chemotherapy evaluation by MRI volumetry in rectal cancer followed by chemoradiation and total mesorectal excision: Initial experience. J Magn Reson Imaging. 2013;38(3):726–32.
Aiba T, Uehara K, Nihashi T, Tsuzuki T, Yatsuya H, Yoshioka Y, et al. MRI and FDG-PET for assessment of response to neoadjuvant chemotherapy in locally advanced rectal cancer. Ann Surg Oncol. 2014;21(6):1801–8.
Guerra L, Niespolo R, Di Pisa G, Ippolito D, De Ponti E, Terrevazzi S, et al. Change in glucose metabolism measured by 18F-FDG PET/CT as a predictor of histopathologic response to neoadjuvant treatment in rectal cancer. Abdom Imaging. 2011;36(1):38–45.
Acknowledgments
This study was supported by the Osaka Medical Research Foundation for Incurable Disease.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Nishimura, J., Hasegawa, J., Ogawa, Y. et al. 18F-Fluorodeoxyglucose positron emission tomography (18F-FDG PET) for the early detection of response to neoadjuvant chemotherapy for locally advanced rectal cancer. Surg Today 46, 1152–1158 (2016). https://doi.org/10.1007/s00595-015-1297-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-015-1297-x