Abstract
Members of Cnidaria Medusozoa are known for their wide morphological variation, which is expressed on many different levels, especially in different phases of the life cycle. Difficulties in interpreting morphological variations have posed many taxonomic problems, since intraspecific morphological variations are often misinterpreted as interspecific variations and vice-versa, hampering species delimitation. This study reviews the patterns of morphological variation in the Medusozoa, to evaluate how different interpretations of the levels of variation may influence the understanding of the patterns of diversification in the group. Additionally, we provide an estimate of the cryptic diversity in the Hydrozoa, based on COI sequences deposited in GenBank. Morphological variations frequently overlap between microevolutionary and macroevolutionary scales, contributing to misinterpretations of the different levels of variation. In addition, most of the cryptic diversity described so far for the Medusozoa is a result of previously overlooked morphological differences, and there is still great potential for discovering cryptic lineages in the Hydrozoa. We provide evidence that the number of species in the Medusozoa is misestimated and emphasize the necessity of examining different levels of morphological variations when studying species boundaries, in order to avoid generalizations and misinterpretations of morphological characters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Following the fundamental work of Darwin (1859), understanding the expression of variation in nature has become essential for the study of evolution, since variation is the basis for evolutionary change. The interpretation of variation, however, has changed in recent years to incorporate phenotypic (developmental) plasticity, in addition to genetic diversity, as important drivers of evolutionary change (West-Eberhard 1989, 2003, 2005; Price et al. 2003; Schlichting 2004; Pigliucci 2007; Pfennig et al. 2010). A major concept is that selection acts on phenotypes and, consequently, phenotypic variation is selectable variation, whether or not it is initially associated with genetic variation (West-Eberhard 1989, 2003, 2005). Phenotypic plasticity can, therefore, contribute to microevolutionary and macroevolutionary processes, emphasizing the importance of the study of variation at different evolutionary levels.
Cnidaria are known for their great morphological variation (e.g., De Weerdt 1981; Silveira and Migotto 1991; Dawson 2005a; Griffith and Newberry 2008; Forsman et al. 2009; Menezes et al. 2013; Ong et al. 2013). Modular growth, characteristic of the polyp stage of many cnidarians, enables a wide variability of colony form through increased regenerative capacity and varying growth rates, branching, number of hydranths, and annulations, contributing to morphological variation in response to differences in environmental conditions (Hughes 1989; Gili and Hughes 1995; Marfenin 1997). The expression of alternative life cycle stages in Medusozoa (Marques and Collins 2004) is another important source of variation in this group. At the microevolutionary level, intraspecific variation in life cycles (e.g., Stefani 1959; Bouillon et al. 1991) has been suggested to have a genetic basis, by means of a switch mechanism responsible for the expression of alternative phenotypes in accordance with environmental cues (West-Eberhard 1986; Boero and Sarà 1987; Boero and Bouillon 1989; Cornelius 1990a; Boero et al. 1997; Bavestrello et al. 2000). At the macroevolutionary level, consecutive suppression and reexpression of the medusa stage during the evolutionary history of the group account for the interspecific remarkable diversity of life cycles (e.g., Boero and Sarà 1987; Boero et al. 1992; Cornelius 1992; Boero and Bouillon 1993, 1994; Piraino et al. 1996; Holst et al. 2007; Miranda et al. 2010; Straehler-Pohl and Jarms 2011). The origin of a medusa in the lineage leading to the Medusozoa extends the levels of variation in this group and reiterates the characteristic modular developmental basis of Cnidaria, contributing to its widely known phenotypic plasticity (see West-Eberhard 2003; Table 1).
Difficulties in interpreting the morphological variations of Medusozoa have led to many taxonomic problems and are still a source of disagreement among taxonomists on the importance of morphological characters used to diagnose species (e.g. Boschma 1948; De Weerdt 1984 [Hydrozoa, Milleporidae]; Gershwin 2001; Dawson 2003 [Scyphozoa, Aurelia]; Cornelius 1982, 1990b; Cunha et al. 2015 [Hydrozoa, Campanulariidae]; Hirano 1997; Miranda et al. 2009 [Staurozoa, Haliclystus]; Miglietta et al. 2009 [Hydrozoa, Hydractiniidae]). These disagreements occur mainly because intraspecific morphological variations are often misinterpreted as interspecific variations or vice-versa, and consequently, the diversity of the group is frequently misestimated. In many taxa that show some degree of developmental plasticity, intraspecific variation of adaptive traits often parallels interspecific variation (e.g., Badyaev and Foresman 2000; West-Eberhard 2003; Gomez-Mestre and Buchholz 2006). For taxonomy, the definition of species’ diagnostic characters may be confounded by these factors if different levels of variation are not initially considered. For this reason, the variation of morphological diagnostic characters should be carefully examined, and generalizations should be treated with caution.
Considering the phenotypic plasticity of Medusozoa and its importance in the evolutionary history of the group, in this study we reviewed the patterns of morphological variation known for Medusozoa. We specifically evaluated how different interpretations of the levels of morphological variation may influence the understanding of the patterns of diversification of the group. We offered an overview of the different levels of morphological variation in Medusozoa based on information from the literature and unpublished results, including morphological and genetic data. In addition, we present an analysis based on data from GenBank in order to provide an estimate of the potential cryptic diversity in Hydrozoa.
Material and methods
Levels of morphological variation in Medusozoa were assessed by compiling information from the literature on intraspecific and interspecific variation, as well as the occurrence of cryptic species. Additionally, the range of variation of several morphological characters on a case study with Orthopyxis sargassicola (Nutting, 1915) were evaluated (Online Resource 1) in order to illustrate some particular points in the discussion regarding intraspecific variation (comparison between intracolony and population-level variation). This species was chosen because of the large availability of data from different populations in Brazil, which were for the most part included in the molecular phylogeny of the genus Orthopyxis previously published (Cunha et al. 2015).
In order to estimate the potential cryptic diversity in Hydrozoa based on genetic data, we compiled information on COI haplotype sequences deposited in GenBank from several species (Online Resource 2). Genetic distance among haplotypes was calculated as the inverse of the minimum value of similarity recorded by pairwise comparisons of the sequences of each species, using BLAST (Altschul et al. 1990). We also calculated the geographical distance among haplotypes based on geographical coordinate information provided with the sequences metadata. When geographical coordinates were not provided, they were estimated based on the name of the sampling location. Species were then classified in accordance with their life cycles (holoplanktonic/pleustonic or benthic/meroplanktonic) based on literature information. With this data, we fitted a Linear Model (LM) including geographical distance and life cycle strategy as explanatory variables in relation to genetic distance among haplotypes, using R programming language (R Core Team 2015). Continuous variables were log10(x + 1) transformed to meet the assumptions of the LM. All data for each species included in this analysis are available in Online Resource 2.
Microevolutionary morphological variation: intraspecific variation
Intraspecific variation is the variation found within a species, including not only variation between conspecific populations but also individual variation, such as ontogenetic variation and polymorphisms (see Mayr 1973). Intraspecific variation may result from phenotypic plasticity when more than one phenotypic alternative is produced in response to environmental factors (West-Eberhard 1989, 2003). Genotype-specific alternatives are usually referred to polymorphisms (Mayr 1973; West-Eberhard 1989). The term polymorphism, however, has been used in many different contexts, sometimes including aspects of phenotypic plasticity (see Clark 1976 and discussion by West-Eberhard 2003: 378). In the case of marine colonial organisms, polymorphisms are defined as discontinuous variations in the morphology of zooids within a colony (Boardman and Cheetham 1973; Harvell 1994). They are considered an important evolutionary innovation in the Hydrozoa, which evolved independently in multiple lineages within the Hydroidolina (Cartwright and Nawrocki 2010; Maronna et al. 2016).
The extent of functional specialization of polyps varies among species but generally involves feeding (gastrozooids), reproduction (gonozooids), and defense (dactylozooids) (Millard 1975; Bouillon et al. 2004; Mills et al. 2007). Additional types of polymorphisms are found in particular groups (e.g., Namikawa et al. 1992; Gravier-Bonnet 2004, 2008), reaching their highest complexity in siphonophores (Pugh 1999; Bouillon et al. 2004; Dunn and Wagner 2006). Being distinctive features, many polymorphisms (and their absence) are important diagnostic characters (e.g., Calder 1988; Boero et al. 1998; Boero et al. 2000; Schuchert 2008 [Hydractiniidae, Milleporidae, Porpitidae, Zancleidae]; Calder 1997; Gravier-Bonnet 2004 [Plumulariidae]). However, their use as diagnostic characters of supraspecific taxa may cause some taxonomic inconsistencies, since their occurrence varies among species (e.g., Clava and Hydractiniidae, Boero et al. 1998; Schuchert 2004).
Another source of morphological variation is related to ontogeny. Many species, especially in the medusa stage, have been described based on ontogenetic differences (Mayer 1910; Bouillon and Boero 2000). This type of variation may be responsible for considerable differences in bell size and shape or in the number of tentacles and statocysts, and for the appearance or disappearance of morphological characters during development (Russell 1953; Zamponi and Girola 1989; Cornelius 1990b; Lindner and Migotto 2002; Widmer 2004). In the polyp stage of colonial species, such as many hydrozoans, ontogenetic variation is responsible for changes in zooid morphology during its development (Boardman and Cheetham 1973). Variations in internode length, number and orientation of branches are typical ontogenetic changes found in polyps within a colony, particularly in species with upright colonies (e.g., Cornelius 1975, 1990b; Kosevich 2006). Developmental changes may continue throughout the colony’s life because of its characteristic modular growth and may affect many levels of colony organization (Hughes 1989, 2005; Marfenin 1997). Differences associated with colony development (growth and senescence), such as hydranth budding and stolonal growth, branching and regression are usually referred to astogeny (Boardman and Cheetham 1973; Hughes 1989) and may be responsible for spatial and temporal changes in colony morphology (Braverman 1974; McFadden et al. 1984; Vogt et al. 2011). Although they appear during the course of development, variations in colony morphology may also result from variations in physiological processes (Dudgeon and Buss 1996; Vogt et al. 2008; Bumann and Buss 2008) and environmental factors (e.g., Vogt et al. 2011; Miglietta and Cunningham 2012).
Intraspecific variation may be triggered by many different factors, especially in different populations. Notably, individual morphological variation may sometimes parallel variations found among populations. For instance, experiments with replicated and transplanted colonies of Millepora spp. and Bougainvillia muscus (Allman, 1863) have shown that colony growth patterns change from branched forms to more robust, solid forms with variations in water flow (De Weerdt 1981; Griffith and Newberry 2008). Similar morphological variations are found among populations living in habitats with contrasting water movement conditions (Kaandorp 1999). In addition, in the family Campanulariidae, variations in colony size, perisarc thickness, length of the hydrotheca and gonotheca, and number of branches and annulations are found among populations subjected to contrasting water flow, temperature, and substrate type (Naumov 1969; Ralph 1956; Cornelius 1975, 1982, 1990b; Lindner and Migotto 2002). Many of these variations, however, may also occur within a single colony (Fig. 1). Although the amplitude of variation of morphological characters at the colony level is not the same as at the population level (Fig. 2), there may be an overlap of morphological variation produced by different levels of intraspecific variation.
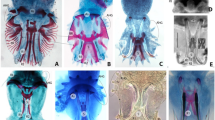
Intracolony variation in Orthopyxis sargassicola (Nutting, 1915) based on three polyps randomly sampled from a single colony (MZUSP4079, see Online Resource 1). a Measurements (in μm) of total length of trophosome (Tr), length of pedicel (Pd), hydrothecal length (Hd), and diameter at margin (Diam); b measurements (in μm) of maximum perisarc (Ps) thickness (Thick) of hydrotheca and pedicel at medial portion, as well as maximum number of sinuosities (NS) in pedicel and number of hydrothecal cusps (NC ); c, d polyps of O. sargassicola from a single colony (both polyps are at the same position of maximum perisarc thickness). Note the differences in size and shape of the pedicels (Pd) and hydrotheca (Hd), as well as the perisarc thickness (Ps) and sinuosities of the pedicel (S)
Mean values (μm) (±standard deviation) of a total length of trophosome (Tr), length of pedicel (Pd), hydrothecal length (Hd), and diameter at margin (Diam); and b maximum perisarc (Ps) thickness (Thick) of hydrotheca and pedicel at medial portion, maximum number of sinuosities in pedicel (NS), and number of hydrothecal cusps (NC). Measurements were taken from polyps of the same colony of Orthopyxis sargassicola (intracolony variation, MZUSP4161, see Online Resource 1) and from polyps of different populations of the same species from the states of São Paulo, Espírito Santo, Rio de Janeiro and Santa Catarina, Brazil (variation among populations, Online Resource 1)
Assessing morphological variations between populations may be a difficult task since it involves the presupposition that populations are conspecific, when they might, in fact, represent different species. Although many studies have reported intraspecific morphological variation in the Medusozoa, only a few of them have attempted to assess the identity of the populations studied, using phylogenetic inferences or other methods for detecting reproductive isolation (e.g. Dawson 2005a; Galea and Leclère 2007). For instance, many studies have reported variations in the symmetry of medusae (Scyphozoa and Hydrozoa, Navas-Pereira 1984; Gershwin 1999; Silva et al. 2003; Nogueira and Haddad 2006) and stauromedusae (Zagal 2008). Although these variations are known to originate at the clonemate level in Aurelia (Gershwin 1999) and may also be a response to variation in physical factors (e.g., temperature and salinity, Zamponi and Genzano 1989), the underlying causes of these variations are still unclear, and they may be different depending on the group and populations studied. As a result, studies of morphological variation may end in questioning the taxonomic affinities of the populations sampled (e.g., Bolton and Graham 2004). Considering the complicated taxonomic history of many groups within the Medusozoa, it is not always easy to delimit conspecific individuals or populations based on morphological characters alone. Individuals from different species may frequently be regarded as conspecific, particularly since intraspecific morphological variation may extend from individuals to populations. Obviously, in order to be confident of the taxonomic level investigated, it is important to know whether one is dealing with intraspecific or interspecific variations.
Macroevolutionary morphological variation: interspecific variation
Difficulties related to variations in morphological characters have led taxonomists to search for additional characters that could contribute to species delimitation. Characters of the cnidome in many hydroid species (e.g., Östman 1982, 1987; Marques 1995, 1996; Morandini and Marques 2010), as well as ecological and behavioral patterns in medusae (e.g., Dawson and Martin 2001; Dawson 2005b) have contributed to the diagnosis of species in these groups, but the difficulties in assessing and describing these characters have limited their use in species delimitation. Similarly, advances in molecular techniques have introduced many new approaches for studying species relationships and have improved our understanding of the evolutionary history and diversification of the Medusozoa (Collins et al. 2006; Leclère et al. 2007, 2009; Cartwright and Nawrocki 2010; Kayal et al. 2013; Cunha et al. 2015). Species delimitation, however, still remains difficult, and many studies adopt integrative approaches, recognizing species based not only on genetic divergence but also on additional characters, such as morphological, ecological, and behavioral, that could contribute evidence for species boundaries (Dayrat 2005; Padial et al. 2010).
The combination of morphological and molecular data for studying species boundaries contributed to the reevaluation of several morphological diagnostic characters in Medusozoa, leading to the description of many new species (e.g., Schierwater and Ender 2000; Collins and Daly 2005; Bayha and Dawson 2010; Collins et al. 2011; Cunha et al. 2015) and the revalidation of formerly synonymized species (e.g., Dawson 2003, 2005c; Schuchert 2005; Miglietta et al. 2007, 2009; Fritz et al. 2009; Lindner et al. 2011; Moura et al. 2012). Reassessment of morphological characters is showing that many “species” previously considered cosmopolitan are in fact geographically isolated lineages, which often can be delimited morphologically (Dawson 2003; Miglietta et al. 2007; Bentlage et al. 2010). This means that the underestimation of species diversity in the Medusozoa, in most cases, results from misinterpretations of species diagnostic characters, which may explain the historical splitting and lumping of species that have been common in several groups. The paucity of morphological characters and poor descriptions in some groups, as well as the wide morphological variation in others, have certainly contributed to these misinterpretations.
Morphological variation can be misleading when there is an overlap between intraspecific and interspecific variations. Considering the phenotypic plasticity in colony form shown by species of Millepora in different water movement conditions (e.g., De Weerdt 1981; Kaandorp 1999, see previous section), molecular and morphological data proved that this variation is also interspecific and resulted in the delimitation of two different lineages based on colony growth form (Meroz-Fine et al. 2003). Moreover, branched and unbranched forms of species of Aglaophenia, commonly thought to be a result of phenotypic plasticity (e.g., Andrade and Migotto 1999), were shown to represent different species in the North Atlantic (Thorpe et al. 1992). It is clear from these findings that interspecific variations may easily be misinterpreted as intraspecific variation.
Once again, the family Campanulariidae is a good example of the historical splitting and lumping of species owing to misinterpretations of morphological characters. The validity of the genus Orthopyxis L. Agassiz, 1862, for instance, is a frequent source of disagreement among taxonomists, since the perisarc thickness, regarded by some authors as one of the diagnostic characters of the genus (Calder 1991; Cornelius 1995; Bouillon et al. 2004), is also thought to be phenotypically plastic (Millard 1975; Galea et al. 2009). This common belief prevents the use of perisarc thickness as a diagnostic character in Orthopyxis, although it may have taxonomic value for delimiting other species of the family (e.g., Obelia geniculata (Linnaeus, 1758), Cornelius 1975). Molecular and morphological data clearly show that intraspecific variation in perisarc thickness occurs, but the perisarc also shows interspecific variation, which makes it a reliable character for species delimitation within Orthopyxis (Cunha et al. 2015). Additionally, this approach supported the validity of the species Orthopyxis caliculata (Hincks, 1853) (Cunha et al. 2015), which was long regarded as a synonym of the widespread species Orthopyxis integra (MacGillivray, 1842) (Cornelius 1982, 1995). The evidence that characters previously regarded as intraspecifically variable may be diagnostic of different species in Orthopyxis, support the idea that O. integra might not have as wide a geographic range as presently thought, and that the morphological variation assumed for this species is overestimated (e.g., shape of the gonotheca; see Cornelius 1995; Cunha et al. 2015).
Macroevolutionary phylogenetic signal with no morphological variation: cryptic species
The existence of species that are morphologically indistinguishable has always intrigued taxonomists. These species were originally termed “sibling species” and defined as “sympatric forms which are morphologically very similar or indistinguishable, but which posses specific biological characteristics and are reproductively isolated” (Mayr 1964: 200). This morphological indistinctness, however, may prove to be a result of previously overlooked morphological differences (Mayr 1976). This may explain the majority of the cryptic diversity found among the Medusozoa (e.g., Aurelia, Dawson and Jacobs 2001; Schroth et al. 2002; Dawson 2003; Nemertesia, Moura et al. 2008, 2012; Acryptolaria, Lafoea, Moura et al. 2011), although in some cases, a reassessment of morphological characters has not proved useful for delimiting species (e.g., Cassiopea, Holland et al. 2004; Stylactaria, Miglietta et al. 2009; Cryptolaria pectinata, Moura et al. 2011).
Current estimates of the total global species richness indicate that the Hydrozoa as a group has an increasing rate of species discovery and a high proportion of cryptic species, probably due to the paucity of morphological diagnostic characters (Appeltans et al. 2012). Indeed, our estimate showed that the amount of cryptic diversity within nominal species of Hydrozoa is significant (R 2 adj = 27.2 %, p < 0.001, Fig. 3). The positive association between geographical and genetic distances (F = 21.0417, p < 0.01) indicate that many species of Hydrozoa may contain cryptic lineages, especially if samples from different geographical localities are considered. Schuchert (2014), for instance, showed that Plumularia setacea is a species complex that is mostly composed of geographically circumscribed lineages, and the same is true for other species of hydrozoans (e.g., Obelia geniculata, Govindarajan et al. 2005; Clytia gracilis and Obelia dichotoma, A.F. Cunha pers. obs.).
A Linear Model (LM) showing the relationship between haplotype genetic distance (n ≥ 2) and geographical distance (Km) of specimens with COI sequences deposited in GenBank (up to January 2013), in accordance with their life cycle strategy (holoplanktonic/pleustonic or benthic/meroplanktonic). Both geographical distance and life cycle strategy were included as explanatory variables. Similarity was calculated using BLAST by pairwise comparisons of all haplotypes of a single species, and the inverse of the minimum value of similarity recorded was used as its genetic distance. The geographical distance was calculated based on geographical information provided with the metadata for the sequences. See Online Resource 2 for more details
Additionally, the occurrence of species complexes in the Hydrozoa has frequently been associated with limited dispersal abilities of species that lack a long-lasting pelagic phase (Moura et al. 2011, 2012; Schuchert 2005, 2014). Hydrozoans with holopelagic life cycle stages were shown to be more widely distributed and have lower species richness than benthic and meroplanktonic species (Gibbons et al. 2009), corroborating the prediction that a relatively short period in the plankton is associated with limited dispersal (Palumbi 1992; Bradbury et al. 2008). Following these predictions, we also found a significant relationship when considering different life cycle strategies, with meroplanktonic/benthic species showing higher genetic distance between haplotypes than did holoplanktonic species (F = 4.5027, p = 0.0379, Fig. 3). This is evidence that benthic/meroplanktonic taxa have potential for the discovery of cryptic lineages even over short geographical distances, probably because of their limited dispersal ability. Studies investigating species boundaries among taxa with different life cycle strategies are important to corroborate this hypothesis. Nevertheless, our results provide evidence that the number of species in the Hydrozoa, and probably in all the Medusozoa, is underestimated. Increased sampling, integrative approaches, and careful investigations of morphological variations will inevitably uncover this hidden species diversity.
Conclusion
It is clear that morphological characters have many different levels of variation or may not vary at all in some cases (summarized in Fig. 4). When morphological variation is present, it may frequently overlap between microevolutionary and macroevolutionary scales, hampering their use at different hierarchical and inclusive taxonomic levels. The widespread morphological variation of the Medusozoa, as well as the frequent overlapping between intraspecific and interspecific variation, indicate that phenotypic plasticity may play an important role in the diversification of the group (see West-Eberhard 1989; Pfennig et al. 2010). However, whether alternative phenotypes are, indeed, environmentally induced or are genetically controlled is an important question (see Schwander and Leimar 2011) which needs further investigation. Nevertheless, at the taxonomic level, morphological variation leads to misinterpretations of diagnostic characters and difficulties in species delimitation. Importantly, however, the level of variation and amount of overlap may be different depending on the group studied and its general biology and life history. In order to minimize the possibility of misinterpretations of morphological characters, generalizations should be avoided, and morphological variation should be interpreted within the context of each taxon, taking into account its phylogenetic relationships and evolutionary history.
Schematic summary of levels of morphological variation found in medusozoans, including the absence of variation (cryptic species). Clades indicate different lineages, colors and shades represent the phenotype in current time, and the circles represent individuals. Note that there is individual variation (arrows) and it can parallel intraspecific variation. The same occurs with interspecific variation, which can parallel intraspecific variation in b
References
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410.
Andrade, L. P., & Migotto, A. E. (1999). Variação morfológica de Aglaophenia latecarinata (Cnidaria, Hydrozoa, Aglaopheniidae) da região do Canal de São Sebastião, São Paulo, Brasil. VIII Congreso Latinoamericano sobre Ciencias del Mar, Trujillo, 1, 412–413.
Appeltans, W., Ahyong, S. T., Anderson, G., Angel, M. V., Artois, T., Bailly, N., et al. (2012). The magnitude of global marine species diversity. Current Biology, 22, 1–14.
Badyaev, A. V., & Foresman, K. R. (2000). Extreme environmental change and evolution: stress-induced morphological variation is strongly concordant with patterns of evolutionary divergence in shrew mandibles. Proceedings of the Royal Society of London, Biological Sciences, 267, 371–377.
Bavestrello, G., Puce, S., Cerrano, C., & Sarà, M. (2000). Phenotypic plasticity in hydrozoans: morph reversibility. Rivista di Biologia, 93, 283–294.
Bayha, K. M., & Dawson, M. N. (2010). New family of allomorphic jellyfishes, Drymonematidae (Scyphozoa, Discomedusae), emphasizes evolution in the functional morphology and trophic ecology of gelatinous zooplankton. Biological Bulletin, 219, 249–267.
Bentlage, B., Cartwright, P., Yanagihara, A. A., Lewis, C., Richards, G. S., & Collins, A. G. (2010). Evolution of box jellyfish (Cnidaria: Cubozoa), a group of highly toxic invertebrates. Proceedings of the Royal Society Biological Sciences, 277, 493–501.
Boardman, R. S., & Cheetham, A. H. (1973). Degrees of colony dominance in Stenolaemate and Gymnolaemate Bryozoa. In R. S. Boardman, A. H. Cheetham, & W. A. Oliver Jr. (Eds.), Animal colonies: development and function through time (pp. 121–220). Stroudsburg: Dowden, Hutchinson & Ross Inc.
Boero, F., & Bouillon, J. (1989). An evolutionary interpretation of anomalous medusoid stages in the life cycles of some Leptomedusae (Cnidaria). In J. S. Ryland & P. A. Tyler (Eds.), Reproduction, genetics and distributions of marine organisms, 23rd European Marine Biology Symposium (pp. 37–41). Fredensborg: Olsen & Olsen.
Boero, F., & Bouillon, J. (1993). Zoogeography and life cycle patterns of Mediterranean hydromedusae (Cnidaria). Biological Journal of the Linnean Society, 48, 239–266.
Boero, F., & Bouillon, J. (1994). Ecological and evolutionary implications of medusozoan (mainly hydromedusae) life cycles. UNEP/FAO: final reports on research projects dealing with the effects of pollutants on marine organisms and communities. MAP Technical Reports Series, 80, 1–24.
Boero, F., & Sarà, M. (1987). Motile sexual stages and evolution of Leptomedusae (Cnidaria). Bollettino di Zoologia, 54, 131–139.
Boero, F., Bouillon, J., & Piraino, S. (1992). On the origins and evolution of hydromedusan life cycles (Cnidaria, Hydrozoa). In R. Dallai (Ed.), Sex origin and evolution (pp. 59–68). Modena: Selected Symposia and Monographs U.Z.I.
Boero, F., Bouillon, J., Piraino, S., Schmid, V. (1997). Diversity of hydroidomedusan life cycles: ecological implications and evolutionary patterns. Proceedings of the 6th International Conference on Coelenterate Biology, 53–62.
Boero, F., Bouillon, J., & Piraino, S. (1998). Heterochrony, generic distinction and phylogeny in the family Hydractiniidae (Hydrozoa, Cnidaria). Zoologische Verhandelingen, Leiden, 323, 25–36.
Boero, F., Bouillon, J., & Gravili, C. (2000). A survey of Zanclea, Halocoryne and Zanclella (Cnidaria, Hydrozoa, Anthomedusae, Zancleidae) with description of new species. Italian Journal of Zoology, 67, 93–124.
Bolton, T. F., & Graham, W. M. (2004). Morphological variation among populations of an invasive jellyfish. Marine Ecology Progress Series, 278, 125–139.
Boschma, H. (1948). The species problem in Millepora. Zoologische Verhandelingen, Leiden, 1, 1–115.
Bouillon, J., & Boero, F. (2000). Synopsis of the families and genera of the hydromedusae of the world, with a list of worldwide species. Thalassia Salentina, 24, 47–296.
Bouillon, J., Boero, F., & Fraschetti, S. (1991). The life cycle of Laodicea indica (Laodiceidae, Leptomedusae, Cnidaria). Hydrobiologia, 216(217), 151–157.
Bouillon, J., Medel, M. D., Pagès, F., Gili, J. M., Boero, F., & Gravili, C. (2004). Fauna of the Mediterranean Hydrozoa. Scientia Marina, 68(2), 1–449.
Bradbury, I. R., Laurel, B., Snelgrove, P. V. R., Bentzen, P., & Campana, S. E. (2008). Global patterns in marine dispersal estimates: the influence of geography, taxonomic category and life history. Proceedings of the Royal Society B: Biological Sciences, 275, 1803–1809.
Braverman, M. (1974). The cellular basis for colony form in Podocoryne carnea. American Zoologist, 14, 673–698.
Brewer, R. H., & Feingold, J. S. (1991). The effect of temperature on the benthic stages of Cyanea (Cnidaria, Scyphozoa), and their seasonal distribution in the Niantic River estuary, Connecticut. Journal of Experimental Marine Biology and Ecology, 152, 49–60.
Bumann, D., & Buss, L. W. (2008). Nutritional physiology and colony form in Podocoryna carnea (Cnidaria : Hydrozoa). Invertebrate Biology, 127(4), 368–380.
Calder, D. R. (1988). Shallow-water hydroids of Bermuda: the Athecatae. Life Sciences Contributions, 148, 1–107.
Calder, D. R. (1991). Shallow-water hydroids of Bermuda: the thecatae, exclusive of Plumularioidea. Life Science Contributions Royal Ontario Museum, 154, 1–140.
Calder, D. R. (1997). Shallow-water hydroids of Bermuda: superfamily Plumularioidea. Life Sciences Contributions, 161, 1–85.
Cartwright, P., & Nawrocki, A. M. (2010). Character evolution in Hydrozoa (phylum Cnidaria). Integrative and Comparative Biology, 50(3), 456–472.
Clark, W. C. (1976). The environment and the genotype in polymorphism. Zoological Journal of the Linnean Society, 58, 255–262.
Collins, A. G., & Daly, M. (2005). A new deepwater species of Stauromedusae, Lucernaria janetae (Cnidaria, Staurozoa, Lucernariidae), and a preliminary investigation of stauromedusan phylogeny based on nuclear and mitochondrial rDNA data. Biological Bulletin, 208(3), 221–230.
Collins, A. G., Schuchert, P., Marques, A. C., Jankowski, T., Medina, M., & Schierwater, B. (2006). Medusozoan phylogeny and character evolution clarified by new large and small subunit rDNA data and an assessment of the utility of phylogenetic mixture models. Systematic Biology, 55(1), 97–115.
Collins, A. G., Bentlage, B., Gillan, W. B., Lynn, T. H., Morandini, A. C., & Marques, A. C. (2011). Naming the Bonaire banded box jelly, Tamoya ohboya, n. sp. (Cnidaria: Cubozoa: Carybdeida: Tamoyidae). Zootaxa, 2753, 53–68.
Cornelius, P. F. S. (1975). The hydroid species of Obelia (Coelenterata, Hydrozoa: Campanulariidae), with notes on the medusa stage. The Bulletin of the British Museum (Natural History), 5, 251–293.
Cornelius, P. F. S. (1982). Hydroids and medusae of the family Campanulariidae recorded from the eastern North Atlantic, with a world synopsis of genera. Bulletin of the British Museum of Natural History (Zoology series), 42(2), 37–148.
Cornelius, P. F. S. (1990a). Evolution in leptolid life-cycles (Cnidaria: Hydroida). Journal of Natural History, 24(3), 579–594.
Cornelius, P. F. S. (1990b). European Obelia (Cnidaria, Hydroida): systematics and identification. Journal of Natural History, 24, 535–578.
Cornelius, P. F. S. (1992). Medusa loss in leptolid Hydrozoa (Cnidaria), hydroid rafting, and abbreviated life-cycles among their remote-island faunae: an interim review. Scientia Marina, 56(2–3), 245–261.
Cornelius, P. F. S. (1995). North-west European thecate hydroids and their medusae. Part 2. Sertulariidae to Campanulariidae. Synopses of the British Fauna New Series, 50, 1–386.
Courtney, R., & Seymour, J. (2013). Seasonality in polyps of a tropical cubozoan: Alatina nr moderns. PLoS One, 8(7), e69369.
Cunha, A. F., Genzano, G. N., & Marques, A. C. (2015). Reassessment of morphological diagnostic characters and species boundaries requires taxonomical changes for the genus Orthopyxis L. Agassiz, 1862 (Campanulariidae, Hydrozoa) and some related campanulariids. PLoS One, 10(2), e0117553.
Darwin, C. (1859). On the origin of species by means of natural selection. London: John Murray.
Dawson, M. N. (2003). Macro-morphological variation among cryptic species of the moon jellyfish Aurelia (Cnidaria, Scyphozoa). Marine Biology, 143, 369–379.
Dawson, M. N. (2005a). Morphological variation and systematics in the Scyphozoa: Mastigias (Rhizostomeae, Mastigiidae)—a golden unstandard? Hydrobiologia, 537, 185–206.
Dawson, M. N. (2005b). Five new subspecies of Mastigias from marine lakes, Palau, Micronesia. Journal of the Marine Biological Association of the United Kingdom, 85, 679–694.
Dawson, M. N. (2005c). Cyanea capillata is not a cosmopolitan jellyfish: morphological and molecular evidence for C. annaskala and C. rosea (Scyphozoa: Semaeostomeae: Cyaneidae) in south-eastern Australia. Invertebrate Systematics, 19, 361–370.
Dawson, M. N., & Jacobs, D. K. (2001). Molecular evidence for cryptic species of Aurelia aurita (Cnidaria, Scyphozoa). Biological Bulletin, 200, 92–96.
Dawson, M. N., & Martin, L. E. (2001). Geographic variation and ecological adaptation in Aurelia (Scyphozoa, Semaeostomeae): some implications from molecular phylogenetics. Hydrobiologia, 451, 259–273.
Dayrat, B. (2005). Towards integrative taxonomy. Biological Journal of the Linnean Society, 85, 407–415.
De Weerdt, W. H. (1981). Transplantation experiments with Caribbean Millepora species (Hydrozoa, Coelenterata), including some ecological observations on growth forms. Bijdragen tot de Dierkunde, 51, 1–19.
De Weerdt, W. H. (1984). Taxonomic characters in Caribbean Millepora species (Hydrozoa, Coelenterata). Bijdragen tot de Dierkunde, 54, 243–262.
Dudgeon, S. R., & Buss, L. W. (1996). Growing with the flow: on the maintenance and malleability of colony form in the hydroid Hydractinia. The American Naturalist, 147(5), 667–691.
Dunn, C. W., & Wagner, G. P. (2006). The evolution of colony-level development in the Siphonophora (Cnidaria: Hydrozoa). Development Genes and Evolution, 216, 743–754.
Forsman, Z. H., Barshis, D. J., Hunter, C. L., & Toonen, R. J. (2009). Shape-shifting corals: molecular markers show morphology is evolutionary plastic in Porites. BMC Evolutionary Biology, 9, 45.
Fritz, G. B., Pfannkuchen, M., Reuner, A., Schill, R. O., & Brümmer, F. (2009). Craspedacusta sowerbii, Lankester 1880—population dispersal analysis using COI and ITS sequences. Journal of Limnology, 68(1), 46–52.
Galea, H. R., & Leclère, L. (2007). On some morphologically aberrant, auto-epizootic forms of Plumularia setacea (Linnaeus, 1758) (Cnidaria: Hydrozoa) from southern Chile. Zootaxa, 1484, 39–49.
Galea, H. R., Häussermann, V., & Försterra, G. (2009). New additions to the hydroids (Cnidaria: Hydrozoa) from the fjords region of southern Chile. Zootaxa, 2019, 1–28.
Gershwin, L. A. (1999). Clonal and population variation in jellyfish symmetry. Journal of the Marine Biological Association of the United Kingdom, 79, 993–1000.
Gershwin, L. A. (2001). Systematics and biogeography of the jellyfish Aurelia labiata (Cnidaria: Schypozoa). Biological Bulletin, 201, 104–119.
Gibbons, M. J., Janson, L. A., Ismail, A., & Samaai, T. (2009). Life cycle strategy, species richness and distribution in marine Hydrozoa (Cnidaria: Hydrozoa). Journal of Biogeography, 37, 441–448.
Gili, J., & Hughes, R. G. (1995). The ecology of marine benthic hydroids. Oceanography and Marine Biology: An Annual Review, 33, 351–426.
Gomez-Mestre, I., & Buchholz, D. R. (2006). Developmental plasticity mirrors differences among taxa in spadefoot toads linking plasticity and diversity. Proceedings of the National Academy of Sciences, 103(50), 19021–19026.
Govindarajan, A. F., Halanych, K. M., & Cunningham, C. W. (2005). Mitochondrial evolution and phylogeography in the hydrozoan Obelia geniculata (Cnidaria). Marine Biology, 146, 213–222.
Gravier-Bonnet, N. (2004). Hydroid nematophores: morphological, structural and behavioural variety from old knowledge and new data. Hydrobiologia, 530(531), 199–208.
Gravier-Bonnet, N. (2008). Polymorphism in hydroids: the extensible polyp of Halecium halecinum (Cnidaria: Hydrozoa: Haleciidae). Journal of the Marine Biological Association of the United Kingdom, 88(8), 1731–1736.
Griffith, K. A., & Newberry, A. T. (2008). Effect of flow regime on the morphology of a colonial cnidarian. Invertebrate Biology, 127(3), 259–264.
Harvell, C. D. (1994). The evolution of polymorphism in colonial invertebrates and social insects. The Quarterly Review of Biology, 69(2), 155–185.
Hirano, Y. M. (1997). A review of a supposedly circumboreal species of stauromedusa, Haliclystus auricula (Rathke, 1806). Proceedings of the 6th International Conference on Coelenterate Biology, 247–252.
Holland, B. S., Dawson, M. N., Crow, G. L., & Hofmann, D. K. (2004). Global phylogeography of Cassiopea (Scyphozoa: Rhizostomeae): molecular evidence for cryptic species and multiple invasions of the Hawaiian Islands. Marine Biology, 145, 1119–1128.
Holst, S., & Jarms, G. (2010). Effects of low salinity on settlement and strobilation of Scyphozoa (Cnidaria): is the lion’s mane Cyanea capillata (L.) able to reproduce in the brackish Baltic Sea? Hydrobiologia, 645, 53–68.
Holst, S., Sötje, I., Tiemann, H., & Jarms, G. (2007). Life cycle of the rhizostome jellyfish Rhizostoma octopus (L.) (Scyphozoa, Rhizostomeae), with studies on cnidocysts and statoliths. Marine Biology, 151, 1695–1710.
Hughes, R. G. (1986). Differences in the growth, form and life history of Plumularia setacea (Ellis and Solander) (Hydrozoa: Plumulariidae) in two contrasting habitats. Proceedings of the Royal Society of London Biological Sciences, 228(1251), 113–125.
Hughes, R. N. (1989). A functional biology of clonal animals. London: Chapman and Hall.
Hughes, R. N. (2005). Lessons in modularity: the evolutionary ecology of colonial invertebrates. Scientia Marina, 69(1), 169–179.
Kaandorp, J. A. (1999). Morphological analysis of growth forms of branching marine sessile organisms along environmental gradients. Marine Biology, 134, 295–306.
Kawamura, M., & Kubota, S. (2008). Influences of temperature and salinity on asexual budding by hydromedusa Proboscidactyla ornata (Cnidaria: Hydrozoa: Proboscidactylidae). Journal of the Marine Biological Association of the United Kingdom, 88(8), 1601–1606.
Kayal, E., Roure, B., Philippe, H., Collins, A. G., & Lavrov, D. V. (2013). Cnidarian phylogenetic relationships as revealed by mitogenomics. BMC Evolutionary Biology, 13, 5.
Kosevich, I. A. (2006). Changes in the patterning of a hydroid colony. Zoology, 109, 244–259.
Leclère, L., Schuchert, P., & Manuel, M. (2007). Phylogeny of the Plumularioidea (Hydrozoa, Leptothecata): evolution of colonial organisation and life cycle. Zoologica Scripta, 36, 371–394.
Leclère, L., Schuchert, P., Cruaud, C., Couloux, A., & Manuel, M. (2009). Molecular phylogenetics of Thecata (Hydrozoa, Cnidaria) reveals long-term maintenance of life history traits despite high frequency of recent character changes. Systematic Biology, 58(5), 509–526.
Lindner, A., & Migotto, A. E. (2002). The life cycle of Clytia linearis and Clytia noliformis: metagenic campanulariids (Cnidaria: Hydrozoa) with contrasting polyp and medusa stages. Journal of the Marine Biological Association of the United Kingdom, 82, 541–553.
Lindner, A., Govindarajan, A.F., & Migotto, A.E. (2011). Cryptic species, life cycles, and the phylogeny of Clytia (Cnidaria: Hydrozoa: Campanulariidae). Zootaxa, 2980, 23–36.
Lucas, C. H. (2001). Reproduction and life history strategies of the common jellyfish, Aurelia aurita, in relation to its ambient environment. Hydrobiologia, 451, 229–246.
Ma, X., & Purcell, J. E. (2005). Effects of temperature, salinity and predators on mortality of and colonization by the invasive hydrozoan Moerisia lyonsi. Marine Biology, 147, 215–224.
Marfenin, N. N. (1997). Adaptation capabilities of marine organisms. Hydrobiologia, 355, 153–158.
Maronna, M. M., Miranda, T. P., Peña Cantero, A. L., Barbeitos, M. S., & Marques, A. C. (2016). Towards a phylogenetic classification of Leptothecata (Cnidaria, Hydrozoa). Scientific Reports, 6, 18075.
Marques, A. C. (1995). Eudendrium pocaruquarum n. sp. (Hydrozoa, Eudendriidae) from the southeastern coast of Brazil, with remarks on taxonomic approaches to the family Eudendriidae. Contributions to Zoology, 65, 35–40.
Marques, A. C. (1996). A critical analysis of a cladistic study of the genus Eudendrium (Cnidaria: Hydrozoa), with some comments on the family Eudendriidae. Journal of Comparative Biology, 1(3/4), 153–162.
Marques, A. C., & Collins, A. G. (2004). Cladistic analysis of Medusozoa and cnidarians evolution. Invertebrate Biology, 123(1), 23–42.
Mayer, A. G. (1910). Medusae of the world (I, Vol. The hydromedusae). Washington: Carnegie Institution of Washington.
Mayr, E. (1964). Systematics and the origin of species from the viewpoint of a zoologist (2nd ed.). New York: Dover Publications.
Mayr, E. (1973). Animal species and evolution (5th ed.). Cambridge: The Belknap Press of Harvard University Press.
Mayr, E. (1976). Principles of systematic zoology. New Delhi: Tata McGraw-Hill Publishing Company LTD.
McFadden, C. S., McFarland, M. J., & Buss, L. W. (1984). Biology of Hydractiniid hydroids. 1. Colony ontogeny in Hydractinia echinata (Flemming). Biological Bulletin, 166, 54–67.
Menezes, N. M., Neves, E. G., Barros, F., Kikuchi, R. K. P., & Johnsson, R. (2013). Intracolonial variation in Siderastrea de Blainville, 1830 (Anthozoa, Scleractinia): taxonomy under challenging morphological constraints. Biota Neotropica, 13(1), 108–116.
Meroz-Fine, E., Brickner, I., Loya, Y., & Ilan, M. (2003). The hydrozoan coral Millepora dichotoma: speciation or phenotypic plasticity? Marine Biology, 143, 1175–1183.
Miglietta, M. P., & Cunningham, C. W. (2012). Evolution of life cycle, colony morphology, and host specificity in the family Hydractiniidae (Hydrozoa, Cnidaria). Evolution, 66(12), 3876–3901.
Miglietta, M. P., Piraino, S., Kubota, S., & Schuchert, P. (2007). Species in the genus Turritopsis (Cnidaria, Hydrozoa): a molecular evaluation. Journal of Zoological Systematics and Evolutionary Research, 45(1), 11–19.
Miglietta, M. P., Schuchert, P., & Cunningham, C. W. (2009). Reconciling genealogical and morphological species in a worldwide study of the family Hydractiniidae (Cnidaria, Hydrozoa). Zoologica Scripta, 38, 403–430.
Millard, N. A. H. (1975). Monograph on the Hydroida of Southern Africa. Annals of the South African Museum, 68, 1–513.
Mills, C. E., Marques, A. C., Migotto, A. E., Calder, D. R., & Hand, C. (2007). Hydrozoa: polyps, hydromedusae, and Siphonophora. In J. T. Carlton (Ed.), The Light and Smith manual: intertidal invertebrates from Central California to Oregon (pp. 118–168). California: University of California Press.
Miranda, L. S., Morandini, A. C., & Marques, A. C. (2009). Taxonomic review of Haliclystus antarcticus Pfeffer, 1889 (Stauromedusae, Staurozoa, Cnidaria), with remarks on the genus Haliclystus Clark, 1863. Polar Biology, 32, 1507–1519.
Miranda, L. S., Collins, A. G., & Marques, A. C. (2010). Molecules clarify a cnidarian life cycle—the “Hydrozoan” Microhydrula limopsicola is an early stage of the Staurozoan Haliclystus antarcticus. PLoS One, 5(4), e10182.
Morandini, A. C., & Marques, A. C. (2010). Revision of the genus Chrysaora Péron & Lesueur, 1810 (Cnidaria: Scyphozoa). Zootaxa, 2464, 1–97.
Moura, C. J., Harris, D. J., Cunha, M. R., & Rogers, A. D. (2008). DNA barcoding reveals cryptic diversity in marine hydroids (Cnidaria, Hydrozoa) from coastal and deep-sea environments. Zoologica Scripta, 37, 93–108.
Moura, C. J., Cunha, M. R., Porteiro, F. M., & Rogers, A. D. (2011). Polyphyly and cryptic diversity in the hydrozoan families Lafoeidae and Hebellidae (Cnidaria: Hydrozoa). Invertebrate Systematics, 25, 454–470.
Moura, C. J., Cunha, M. R., Porteiro, F. M., Yesson, C., & Rogers, A. D. (2012). Evolution of Nemertesia hydroids (Cnidaria: Hydrozoa, Plumulariidae) from the shallow and deep waters of the NE Atlantic and western Mediterranean. Zoologica Scripta, 41, 79–96.
Namikawa, H., Mawatari, S. F., & Calder, D. R. (1992). Role of the tentaculozooids of the polymorphic hydroid Stylactaria conchicola (Yamada) in interactions with some epifaunal space competitors. Journal of Experimental Marine Biology and Ecology, 162, 65–75.
Naumov, D. V. (1969). Hydroids and hydromedusae of the USSR. Jerusalem: Israel Program for Scientific Translations.
Navas-Pereira, D. (1984). On the morphological variability of Phialucium carolinae (Mayer, 1900) (Leptomedusae, Phialuciidae). Dusenia, 14, 51–53.
Nawroth, J. C., Feitl, K. E., Colin, P., Costello, J. H., & Dabiri, J. O. (2010). Phenotypic plasticity in juvenile jellyfish medusa facilitates effective animal-fluid interaction. Biology Letters, 6, 389–393.
Nogueira, M., Jr., & Haddad, M. A. (2006). Variações morfológicas em Olindias sambaquiensis (Cnidaria, Hydrozoa, Limnomedusae) no litoral de Guaratuba, Paraná, Brasil. Revista Brasileira de Zoologia, 23, 879–882.
Ong, C. W., Reimer, J. D., & Todd, P. A. (2013). Morphologically plastic responses to shading in the zoanthids Zoanthus sansibaricus and Palythoa tuberculosa. Marine Biology, 160, 1053–1064.
Östman, C. (1982). Nematocysts and taxonomy in Laomedea, Gonothyraea and Obelia (Hydrozoa, Campanulariidae). Zoologica Scripta, 11(4), 227–241.
Östman, C. (1987). New techniques and old problems in hydrozoan systematics. In J. Bouillon, F. Boero, F. Cicogna, & P. F. S. Cornelius (Eds.), Modern trends in the systematics, ecology and evolution of hydroids and hydromedusae (pp. 67–82). Oxford: Oxford University Press.
Padial, J. M., Miralles, A., Riva, I. D., & Vences, M. (2010). The integrative future of taxonomy. Frontiers in Zoology, 7, 16.
Palumbi, S. R. (1992). Marine speciation on a small planet. Trends in Ecology and Evolution, 7(4), 114–118.
Pfennig, D. W., Wund, M. A., Snell-Rood, E. C., Cruickshank, T., Schlichting, C. D., & Moczek, A. P. (2010). Phenotypic plasticity’s impacts on diversification and speciation. Trends in Ecology and Evolution, 25(8), 459–467.
Pigliucci, M. (2007). Do we need an extended evolutionary synthesis? Evolution, 61(12), 2743–2749.
Piraino, S., Boero, F., Aeschbach, B., & Schmid, V. (1996). Reversing the life cycle: medusa transforming into polyps and cell transdifferentiation in Turritopsis nutricula (Cnidaria, Hydrozoa). Biological Bulletin, 190, 302–312.
Piraino, S., De Vito, D., Schmich, J., Bouillon, J., & Boero, F. (2004). Reverse development in Cnidaria. Canadian Journal of Zoology, 82, 1748–1754.
Price, T. D., Qvarnström, A., & Irwin, D. E. (2003). The role of phenotypic plasticity in driving genetic evolution. Proceedings of the Royal Society of London Biological Sciences, 270, 1433–1440.
Pugh, P. R. (1999). Siphonophorae. In D. Boltovskoy (Ed.), South Atlantic zooplankton (pp. 467–511). Leiden: Backhuys Publishers.
Purcell, J. E. (2007). Environmental effects on asexual reproduction rates of the scyphozoan Aurelia labiata. Marine Ecology Progress Series, 348, 183–196.
Purcell, J. E., White, J. R., Nemazie, D. A., & Wright, D. A. (1999). Temperature, salinity and food effects on asexual reproduction and abundance of the scyphozoan Chrysaora quinquecirrha. Marine Ecology Progress Series, 180, 187–196.
Purcell, J. E., Atienza, D., Fuentes, V., Olariaga, A., Tilves, U., Colahan, C., & Gili, J. M. (2012). Temperature effects on asexual reproduction rates of scyphozoan species from the northwest Mediterranean Sea. Hydrobiologia, 690, 169–180.
R CORE TEAM. (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org. Accessed 16 Feb 2016.
Ralph, P. M. (1956). Variation in Obelia geniculata (Linnaeus, 1758) and Silicularia bilabiata (Coughtrey, 1875) (Hydroida, F. Campanulariidae). Transactions of the Royal Society of New Zealand, 84(2), 279–296.
Russell, F. S. (1953). The medusae of the British Isles. Cambridge: Cambridge University Press.
Schierwater, B., & Ender, A. (2000). Sarsia marii n. sp. (Hydrozoa, Anthomedusae) and the use of 16S rDNA sequences for unpuzzling systematic relationships in Hydrozoa. Scientia Marina, 64(1), 117–122.
Schierwater, B., & Hadrys, H. (1998). Environmental factors and metagenesis in the hydroid Eleutheria dichotoma. Invertebrate Reproduction and Development, 34(2–3), 139–148.
Schlichting, C. D. (2004). The role of phenotypic plasticity in diversification. In T. J. DeWitt & S. M. Scheiner (Eds.), Phenotypic plasticity, functional and conceptual approaches (pp. 191–200). New York: Oxford University Press.
Schroth, W., Jarms, G., Streit, B., & Schierwater, B. (2002). Speciation and phylogeography in the cosmopolitan marine moon jelly, Aurelia sp. BMC Evolutionary Biology, 2, 1.
Schuchert, P. (2004). Revision of the European athecate hydroids and their medusa (Hydrozoa, Cnidaria): families Oceanidae and Pachycordylidae. Revue Suisse de Zoologie, 111(2), 315–369.
Schuchert, P. (2005). Species boundaries in the hydrozoan genus Coryne. Molecular Phylogenetics and Evolution, 36, 194–199.
Schuchert, P. (2008). The European athecate hydroids and their medusae (Hydrozoa, Cnidaria): Filifera part 3. Revue Suisse de Zoologie, 115(2), 221–302.
Schuchert, P. (2014). High genetic diversity in the hydroid Plumularia setacea: a multitude of cryptic species or extensive population subdivision? Molecular Phylogenetics and Evolution, 76, 1–9.
Schwander, T., & Leimar, O. (2011). Genes as leaders and followers in evolution. Trends in Ecology and Evolution, 26(3), 143–151.
Silva, C. A., Aguirre, S. G., & Arce, M. G. M. (2003). Variaciones morfológicas en Blackfordia virginica (Hydroidomedusae: Blackfordiidae) en lagunas costeras de Chiapas, México. Revista de Biología Tropical, 51(2), 409–412.
Silveira, F. L., & Migotto, A. E. (1991). The variation of Halocordyle disticha (Cnidaria, Athecata) from the Brazilian coast: an environmental indicator species? Hydrobiologia, 216(217), 437–442.
Stampar, S. N., Silveira, F. L., & Morandini, A. C. (2008). Food resources influencing the asexual reproductive cycle of coronate Scyphozoa. Cahiers de Biologie Marine, 49, 247–252.
Stefani, R. (1959). Sulla variabilità ecologica di un idrozoo (Campanularia caliculata Hincks). Estratto dal Bollettino di Zoologia, 26(2), 115–120.
Straehler-Pohl, I., & Jarms, G. (2011). Morphology and life cycle of Carybdea morandinii, sp. nov. (Cnidaria), a cubozoan with zooxanthellae and peculiar polyp anatomy. Zootaxa, 2755, 36–56.
Thein, H., Ikeda, H., & Uye, S. (2013). Ecophysiological characteristics of podocysts in Chrysaora pacifica (Goette) and Cyanea nozakii Kishinouye (Cnidaria: Scyphozoa: Semaeostomeae): effects of environmental factors on their production, dormancy and excystment. Journal of Experimental Marine Biology and Ecology, 446, 151–158.
Thorpe, J. P., Ryland, J. S., Cornelius, P. F. S., & Beardmore, J. A. (1992). Genetic divergence between branched and unbranched forms of the thecate hydroid Aglaophenia pluma. Journal of the Marine Biological Association of the United Kingdom, 72(4), 887–894.
Van Winkle, D. H., Longnecker, K., & Blackstone, N. W. (2000). The effects of hermit crabs on hydractiniid hydroids. Marine Ecology, 21(1), 55–67.
Vogt, K. S. C., Geddes, G. C., Bross, L. S., & Blackstone, N. W. (2008). Physiological characterization of stolon regression in a colonial hydroid. The Journal of Experimental Biology, 211, 731–740.
Vogt, K. S. C., Harmat, K. L., Coulombe, H. L., Bross, L. S., & Blackstone, N. W. (2011). Causes and consequences of stolon regression in a colonial hydroid. The Journal of Experimental Biology, 214, 3197–3205.
West-Eberhard, M. J. (1986). Alternative adaptations, speciation, and phylogeny (A Review). Proceedings of the National Academy of Sciences, 83, 1388–1392.
West-Eberhard, M. J. (1989). Phenotypic plasticity and the origins of diversity. Annual Review of Ecology and Systematics, 20, 249–278.
West-Eberhard, M. J. (2003). Developmental plasticity and evolution. New York: Oxford University Press.
West-Eberhard, M. J. (2005). Developmental plasticity and the origin of species differences. Proceedings of the National Academy of Sciences, 102(1), 6543–6549.
Widmer, C. L. (2004). The hydroid and early medusa stages of Mitrocoma cellularia (Hydrozoa, Mitrocomidae). Marine Biology, 145, 315–321.
Zagal, C. J. (2008). Morphological abnormalities in the stauromedusa Haliclystus auricula (Cnidaria) and their possible causes. Journal of the Marine Biological Association of the United Kingdom, 88(2), 259–262.
Zamponi, M. O., & Genzano, G. N. (1989). Variaciones de algunas estructuras de valor taxonómico en la familia Geryonidae (Cnidaria; Trachymmedusae) y su relación con la temperature y salinidad. Iheringia Série Zoologia, 69, 31–47.
Zamponi, M. O., & Girola, C. V. (1989). Variaciones morfológicas y estructurales de los juveniles de Olindias sambaquiensis Müller, 1861 (Cnidaria, Limnomedusae, Olindiidae). Iheringia Séria Zoologia, 69, 19–30.
Acknowledgments
The authors thank all their colleagues from Laboratory of Marine Evolution (LEM) and Laboratory of Molecular Evolution (LEMol) of the University of São Paulo, Brazil, for their valuable help and support during the course of this study. We are also very grateful to P.K. Maruyama and two anonymous reviewers for their helpful comments and suggestions on previous versions of this manuscript. This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (AFC, ACM), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grant no. 490348/2006-8, 562143/2010-6, 563106/2010-7, 477156/2011-8, 305805/2013-4, 445444/2014-2—ACM) and São Paulo Research Foundation (FAPESP) (grant no. 2006/56211-6—MMM, 2010/52324-6, 2011/50242-5, 2013/50484-4—ACM, 2011/22260-9, 2013/25874-3—AFC).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
OnlineResource 1
(PDF 99.8 kb)
OnlineResource 2
(PDF 102 kb)
Rights and permissions
About this article
Cite this article
Cunha, A.F., Maronna, M.M. & Marques, A.C. Variability on microevolutionary and macroevolutionary scales: a review on patterns of morphological variation in Cnidaria Medusozoa. Org Divers Evol 16, 431–442 (2016). https://doi.org/10.1007/s13127-016-0276-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-016-0276-4