Abstract
This study was conducted to evaluate a possible protective role of apricot in apoptotic cell death induced by methotrexate (MTX) and renal damage by different histological and biochemical parameters. Twenty-eight rats were divided into four groups, control, apricot, methotrexate, and apricot + methotrexate. Methotrexate induced renal failure, as shown by significant serum creatinine and urea elevation. Additionally, the results indicated that methotrexate significantly induced lipid peroxidation and reduced antioxidant activities in rats. In contrast, apricot significantly prevented toxic effects of methotrexate via increased catalase, superoxide dismutase, and glutathione levels but decreased formation of malondialdehyde. Also, it was determined that exposure to methotrexate leads to significant histological damage in kidney tissue such as glomerulosclerosis and apoptosis. On the other hand, these effects can be eliminated with apricot diet. These data indicate that apricot may be useful in preventing undesirable effects of MTX such as nephrotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epidemiologic evidence increasingly supports the concept that diets rich in fruits and vegetables promote health and may attenuate various conditions or delay their onset [20, 47]. Some studies show that the health benefits of fruits and vegetables are related to their antioxidant constituents, including vitamins, carotenoids, and flavonoids. Dietary antioxidant compounds are believed to protect against chronic disease by mitigating free radical damage to proteins, lipids, and DNA [15].
The apricot used in this study is widely produced in the province of Malatya in eastern Turkey. Recent statistics indicate that about 80 % of world apricot production originates in this area; 95 % is marketed as dry fruit [2]. Since apricot contains polyphenols, flavonoids, β-carotene, and vitamins A and C, there is a foundation for assuming that it may have antioxidant properties [28, 39, 40].
Methotrexate (MTX) is widely used in the treatment of clinical conditions such as breast cancer, lymphoma, stomach cancer, urinary bladder cancer, psoriasis, and rheumatoid arthritis [3]. It has been shown to promote cell death through apoptosis of both cancerous and non-transformed cells [27]. Apoptotic cell death, a well-recognized phenomenon in various circumstances including renal disease, is frequently observed in acute renal failure; it might play a pathogenic role in renal dysfunction [18]. Multiple factors are known to induce renal tubular cell apoptosis. These factors can be divided into several categories, one of which is also related to reactive oxygen species (ROS) [23]. It has been established that MTX inhibits cytosolic nicotinamide adenosine diphosphate (NADP)-dependent dehydrogenase and NADP-malic enzyme, resulting in decreased availability of NADPH in cells. NADPH is normally used by glutathione reductase (GSH-Rd) to maintain cell glutathione (GSH), known as an important protective agent against ROS, in its reduced state. Thus, when the antioxidant defense system is reduced, cells begin to sensitize to ROS-related cellular injury [4].
There is increasing evidence to support the view that the kidneys are susceptible to oxidative stress through exogenous drugs and chemicals. MTX, primarily cleared by renal excretion, may also lead directly to renal dysfunction and damage [13]. Because of the close association between MTX and oxidative stress in renal dysfunction, we hypothesized that dietary antioxidants may contribute to the prevention of MTX-induced renal damage, which several studies based on a similar hypothesis have shown to be prevented by different agents including melatonin [19], caffeic acid [29], and proanthocyanidin [13].
There is no published report to date regarding the protective effect of apricot diet on MTX-induced renal damage. The aims of this study were therefore to assess the role of oxidative stress and apoptosis in MTX-induced nephrotoxicity and to investigate the possible protective effects of apricot diet against renal damage from MTX.
Material and methods
Animals and diets
For this study, 28 male Wistar albino rats weighing 210–310 g were obtained from the Experimental Animal Research Center, Inonu University Faculty of Medicine. The animals were kept in a room controlled for temperature (21 ± 2 °C) and humidity (60 ± 5 %), in which a 12–12-h light–dark cycle was maintained. They had free access to food and water. Experiments were performed in accordance with the National Institute of Health guidelines for animal research and approved under number 2006/002 by the Inönü University Committee on Animal Research in Malatya, Turkey.
The animals were randomized into four groups of seven rats each, as follows:
-
Group I (control): This group received volumes of saline intraperitoneally (ip) equivalent to those of MTX and was fed with standard pellet diet (Elazig, Turkey) ad libitum.
-
Group II (AP): Rats were fed diets containing 10 % dried apricot ad libitum for 24 days.
-
Group III (MTX): MTX (David Bull Laboratories, Mulgrave, Victoria, Australia) was administered as a single ip dose (in saline, 20 mg/kg) [2] on day 21 of the experiment. Animals were fed with standard pellet diet ad libitum.
-
Group IV (AP + MTX): Before MTX application, a diet containing 10 % dried apricot was started and continued until the animals were sacrificed on day 24.
The composition of the standard diet and that with dried apricot is given in Table 1. Metabolic energy for each composition was adjusted to 2,650 kcal/kg.
Organic sun-dried apricots of the Kabaaşi variety were obtained from the region of Akçadag in the province of Malatya, a major apricot-producing area of Turkey. This variety was chosen because of its higher radical scavenging power and total phenolic contents ascertained in our preliminary studies.
All animals were killed on day 24, or 3 days after the MTX injection. Their kidneys were quickly removed; one of them was used for histological examination by light microscopy and the second part stored at −20 °C until it was assayed for catalase (CAT), superoxide dismutase (SOD), GSH, and malondialdehyde (MDA) contents.
Determination of the antioxidant capacities of chow extracts
Extract preparation
Animal diet and apricot samples (10 g) were mixed with 90 mL ethanol (70 %) and crushed in an Ultra-Turrax homogenizer (Ultra-Turrax, Model T25, IKA-Works, Inc., Cincinnati, OH, USA) at 20,000 rpm. The suspension was kept in the refrigerator (+4 °C) for 48 h and filtered through Whatman no. 1 filter paper. Extracts were used in 1,1-diphenyl–2-picrylhydrazyl (DPPH) radical scavenging tests and total phenolic content analysis.
DPPH radical scavenging power assay
Radical scavenging power (RSP) of rat feed extracts was assessed by the method of Shimada et al. [41] with slight modifications. Every 2 mL of reaction mixture contained 2.9 mmol DPPH (1.8 mL 1 × 10−4 M DPPH) and 0.2 mL extracts. To control, ethanol (70 %) was used in place of sample. Cuvettes were left 10 min in the dark at room temperature and the resulting color measured spectrophotometrically at 520 nm against blanks. Decreasing intensity of purple color was related to a higher RSP percentage, calculated using the following equation: \( \mathrm{RSP}=\left[ {1-\left( {\frac{{{A_{S:10 }}}}{{{A_{B:10 }}}}} \right)} \right]\times 100 \), where A S:10 is the absorbance of samples and A B:10 is the absorbance of the blank at the 10th minute of the reaction period.
Determination of total phenolic content
Total phenolic contents of the samples were determined by Folin & Ciocalteu’s reagent method [10]. Sample extracts (0.1 mL) diluted to 1 mL with ethanol (70 %) and 1 mL of Folin & Ciocalteu’s reagent were added. The mixture was left to stand for 3 min after which 1 mL of 2 % sodium carbonate was added. After 5 min incubation with shaking, at room temperature, the resulting absorbance was measured at 760 nm. The calibration curve was produced with gallic acid, and the results were expressed as micrograms of gallic acid equivalents (μg GAE/g dw).
Histological analysis
The kidney tissue was fixed in 10 % formalin and embedded in paraffin. Sections of the tissue were cut at 5 μm, mounted on slides, and stained with periodic acid-Schiff (PAS). For immunohistochemical analysis, thick sections were taken onto polylysine-coated slides. After rehydrating, the samples were transferred to citrate buffer (pH 7.6) and heated in a microwave oven for 20 min. After cooling for 20 min at room temperature, the sections were washed with phosphate-buffer saline (PBS). They were then kept 7 min in 0.3 % H2O2 after which they were washed with PBS. The sections were incubated with mouse monoclonal antibody poly ADP-ribose polymerase (PARP) antibody (Thermo, USA) for 30 min, rinsed in PBS, and incubated 10 min with biotinylated goat anti-polyvalent and streptavidin peroxidase also for 10 min at room temperature. This staining procedure was completed with chromogen + substrate for 15 min after which the slides were counter-stained with Mayer’s hematoxylin for 1 min, rinsed in tap water, and dehydrated. PARP kit was used according to the manufacturer’s instructions with only minor revision. The sections were examined under a Leica DFC 280 light microscope, by a histopathologist unaware of the status of the respective animals.
Semiquantitative evaluation
A semiquantitative morphometric score index was used to evaluate the degree of glomerulosclerosis. Sclerosis was defined as obliteration of glomerular capillary tuft and decrease of Bowman’s space. Glomerulosclerosis scoring was performed by taking the severity and extent of the sclerotic lesion into consideration for each glomerulus. Thus, each glomerulus was assigned a score between 0 and 4 in the following way: 0 for normal glomeruli, 1 for sclerosis in ≤25 % of the total area, 2 for sclerosis in 25–50 % of the total area, 3 for sclerosis in 50–75 % of the total area, and 4 for sclerosis in ≥80 % of the total area. This analyzed method of glomerulus was modified from Fujihara [11]. One hundred glomeruli were evaluated in each kidney, and the arithmetic mean of the sclerosis scores was accepted as the mean glomerulosclerosis score for that rat.
Tubular injury was defined as tubular apoptosis. PARP stainings were also evaluated semiquantitatively as follows: weak (+), moderate (++), and strong (+++) according to intensity of staining and stained cell quantity. PARP-positive cell nuclei are stained as brown color. Stained nuclei with PARP were counted using Leica Q Win Image Analysis System (Leica Micros Imaging Solution Ltd, Cambridge, UK) and classified according to three intensity categories. For each specimen, 10 microscopic fields were analyzed under a ×40 objective per animals.
Biochemical assessment
Homogenization
Tissues were homogenized (PCV Kinematica Status Homogenizator) in ice-cold phosphate-buffered saline (pH 7.4). The homogenate was sonified with an ultrasonifier (Bronson sonifier 450) by 3 cycles (20-s sonications and 40-s pause on ice). The homogenate was centrifuged (15,000×g, 10 min, 4 °C), and cell-free supernatant was subjected to enzyme assay immediately.
CAT assay
CAT activity was measured at 37 °C by following the rate of disappearance of hydrogen peroxide (H2O2) at 240 nm (ε 240 = 40 M−1 cm−1) [25]. One unit of CAT activity is defined as the amount of enzyme catalyzing the degradation of 1 μmol of H2O2 per min at 37 °C and specific activity corresponding to transformation of substrate (micromoles of H2O2) per minute per milligram protein.
SOD assay
SOD activity was assayed using the nitroblue tetrazolium (NBT) method [44]. The samples were subjected to ethanol–chloroform (62.5:37.5 %) extraction prior to the assay. NBT was reduced to blue formazan by the superoxide ion (O2 −), which has a strong absorbance at 560 nm. One unit of SOD is defined as the amount of protein that inhibits the rate of NBT reduction by 50 %. The calculated SOD activity was expressed as units per milligram tissue protein.
Total GSH assay
The formation of 5-thio-2-nitrobenzoate is followed spectrophotometrically at 412 nm [46]. The amount of GSH in the extract was determined as nanomoles per milligram protein utilizing a commercial GSH as the standard. The results are expressed as nanomoles per milligram protein.
Lipid peroxidation assay (MDA)
The analysis of lipid peroxidation was carried out as described previously [5] with a minor modification. The absorbance of the supernatant was recorded at 532 nm. MDA results were expressed as nanomoles per milligram protein in the homogenate. The protein content of the samples was determined by the colorimetric method of Lowry et al. [24]. The absorbance measurement was taken at 595 nm using a UV–VIS spectrophotometer (Shimadzu UV-1601). Bovine serum albumin was used as protein standard.
Creatinine and urea levels
Creatinine and serum urea levels were assayed spectrophotometrically according to the standard procedures, using commercially available diagnostic kits (Biolabo Reagents, Maizy, France).
Statistical analysis
A computer program (SPSS 15.0) was used for statistical analysis. Results were compared by Kruskal–Wallis variance analysis. Where differences among the groups were detected, group means were compared using the Mann–Whitney U test. Values of p < 0.05 were considered significant. All results were expressed as means ± standard error.
Results
Antioxidant capacities of chow extracts
DPPH radical scavenging power and total phenolic contents of chow extracts were assessed in vitro to determine the latter’s antioxidant capacities. Antioxidant capacity of rat chow (pellet diet) was significantly increased after supplementation with dried apricot. The radical scavenging power and total phenolic content of apricot and pellet diet were presented in Figs. 1 and 2.
Biochemical results
Creatinine and urea
Creatinine and urea serum levels are shown in Table 2. Briefly, serum creatinine level, an index of kidney function, was higher (0.7 ± 0 mg/dL) in the MTX group compared to normal values (0.1 mg/dL). We also measured serum blood urea, a secondary index of kidney function, which was also increased in the MTX group (60.0 ± 9.4 mg/dL). Apricot diet preceding MTX treatment significantly prevented an increase in serum creatinine and urea.
Kidney MDA, SOD, CAT, and GSH levels
MDA, SOD, CAT, and GSH levels are shown in Table 3. Kidney MDA was significantly increased compared to the control group. However, kidney CAT, SOD, and GSH levels were significantly decreased in MTX group when compared to the control group (p < 0.05).
On the other hand, apricot diet before MTX application provided a significant decrease in MDA level and an increase in SOD, CAT, and GSH activity in the kidney, when compared to MTX alone. SOD, CAT, and GSH levels in AP + MTX group were similar to control, whereas MDA level was found as significantly different.
Histological results
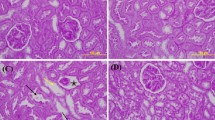
Essentially, the glomeruli of control group did not show any detectable histological abnormalities (Fig. 3), and the AP group was similar to the control (Fig. 4). However, MTX application caused injury in renal corpuscles. The most remarkable histological damage was glomerulosclerosis. Glomeruli revealed obliteration of glomerular capillary tuft and presence of glomerular tuft capsular adhesion (Fig. 5). On the other hand, although glomerulosclerosis was recognized as alleviated also in AP + MTX group, the lesions did not completely ameliorate (Fig. 6). Using semiquantitative scoring methods, the mean glomerulosclerosis was determined and was given in Table 4.
Control and apricot groups showed few apoptotic cells, according to examination after PARP staining, in the proximal convoluted tubules (data not shown). However, in the MTX group, PARP-positive cells were found to be significantly increased when compared to the control group (Fig. 7). An apricot diet administration before MTX treatment significantly reduced the expression of apoptotic cells (Fig. 8). The coloring density and the number of PARP stained tubular cells were lower in the AP + MTX group than in the MTX group (p < 0.05). The results of semiquantitative histological examination after staining with PARP are shown in Table 5.
Discussion
The results clearly indicated that MTX causes oxidative renal damage, as shown by increased lipid peroxidation and decreased SOD, CAT, and GSH contents in the kidney. Serum creatinine and urea levels and histological analysis confirm the presence of MTX-induced renal damage. Given the fact that multiple factors are involved in the pathogenesis of MTX-induced renal damage, it appears that oxidative injury is closely related to the toxic effects of MTX on tubular epithelial cells, evidenced by an increased number of apoptotic cells as determined by PARP immunohistochemical staining. This study also shows that an apricot diet can reduce oxidative stress, improve the recovery of renal function, and prevent apoptotic cell death.
Evidence accumulated to date indicates that naturally occurring antioxidant compounds in fruits have protective effects in biological systems [12]. Antioxidants help maintain the oxidant-antioxidant balance and inhibit the damage caused by ROS. The antioxidant capacity of common fruit and vegetables has attracted much attention and has been the object of studies by many researchers in the last years [6, 36, 49]. These studies demonstrated a wide diversity of antioxidant capacity among common fruit and vegetables. The reason of such diversity has been attributed mainly to phenolic components, such as phenolic acids, flavonoids, and carotenoids [15, 34]. These substances have a protective effect against diseases resulting from free radical-mediated damage on proteins, lipids, and DNA [3].
With its flavonoids and carotenoid content, apricot is considered to be a good source of dietary antioxidant. Flavonoids are a large group of polyphenolic antioxidants exhibiting a wide range of biological activity, including the inhibition of lipid peroxidation, capillary permeability, and platelet aggregation [35]. Flavonoids also inhibit enzymes responsible for O2 − production through xanthine oxidase and protein kinase C and by ROS generation via the cyclooxygenase, lipoxygenase, and NADH oxidase pathways [33]. As for β-carotene, which was found to be the principal carotenoid in studies of the carotenoid content characterization of apricot [40], it is known to have provitamin A activity and antioxidant properties. Its ability to scavenge free radicals, especially against singlet oxygen and the peroxyl radical, has already been described [43].
A diet supplemented with dried apricots was used against MTX-induced renal damage in this study. DDPH radical scavenging power and total phenolic contents of chow extracts were assessed first. The DPPH radical is a stable lipophilic free radical, which has been generally used to estimate the antioxidant activities of food and drugs [7]. Since the total phenolic level has been shown to be closely related to the antioxidant capacity of a sample, this was also determined [17, 34]. DPPH radical scavenging capacity and total phenolic contents of rat chow were significantly increased following dietary supplementation with dried apricots.
ROS are generally considered to be key mediators of MTX-induced damage to the kidney [1, 8]. Excessive ROS formation causes lipid peroxidation of cell membrane proteins and enzyme oxidation [22]. Tissue MDA content is a reliable marker for breakdown during the major chain reactions, leading to significant oxidation of polyunsaturated fatty acids [30]. In the present study, MDA levels were found to be significantly increased in the MTX group. Our results are in agreement with several reports of MTX-induced oxidative stress in tissues following an increase in MDA levels [13, 19, 30, 47]. Apricot diet had a clearly protective effect against lipid peroxidation and reduced renal MDA production. Their radical-scavenging potential seems to be contributing to the inhibition of lipid peroxidation. GSH, the major cytosolic thiol, which serves as a cofactor for several detoxifying enzymes (GP-x, glutathione-S-transferases) is involved in the reduction of protein disulfides; it additionally scavenges ROS, being oxidized to GSSH [9]. This tripeptide and other major enzymes such as CAT and SOD that are directly involved in the detoxification of ROS are present in high concentration in the kidney tissue [31]. In the present study, MTX treatment significantly reduced SOD, CAT, and GSH levels in a fashion similar to that of available published studies [29, 47]. An apricot diet were also able to prevent the fall in SOD, CAT, and GSH contents in the rat kidney. This action may be due to an improvement in the antioxidant status and the scavenging of excessive free radicals such as O2 − and the peroxyl radical. Therefore, these factors can protect cell or tissue from oxidative stress. Similar results were obtained with different antioxidants such as melatonin [19] and proanthocyanidin [13], which preserve antioxidant enzymes in MTX-induced nephropathy.
MTX is primarily cleared by renal excretion. MTX toxicity may also result in acute renal failure due to precipitation of its metabolite 7-OH-MTX in renal tubules [42, 48]. The result of this study confirmed that MTX at a dose of 20 mg/kg causes nephrotoxicity, as evidenced by an elevation of serum creatinine and serum urea levels. MTX-induced increases in serum creatinine, and urea levels were prevented by an apricot diet in this study.
Serum Cr concentration is more significant than the BUN level in the earlier phases of kidney disease. On the other hand, BUN begins to rise only after a marked renal parenchymal injury occurs [32]. ROS is known to induce mesangial cell contraction, altering the filtration surface area and modifying the ultrafiltration coefficient factors that affect glomerular function. [26]. Moreover, decreased SOD activity favors the increase in O2 −.concentration, and this radical can in its turn react with nitric oxide (NO) to form peroxynitrite (ONOO−), a cytotoxic oxidant radical species. Inactivation of NO by O2 − also could lead to glomerular damage [38]. These data indicate that ROS could be involved in the decrease of GFR in MTX-induced renal damage and that the protective effect, especially that of apricot, could be related to an ability to preserve the activity of antioxidant enzymes such as SOD, CAT, and GSH.
Apoptotic cells were found in the renal tissue by staining for PARP activity. Apoptosis is a complex process; it involves many different steps before resulting in DNA fragmentation. PARP is activated in response to DNA damage, and it participates in DNA repair and cell death [45]. Our study showed that PARP activity was remarkably increased after MTX treatment. Many investigators including Herman et al. [14] and Mazur et al. [27] have shown that MTX causes programmed cell death in T lymphocytes, human uterine cervix cancer, and the normal fibroblastic rat kidney. MTX is believed to very probably induce apoptosis through oxidative stress, with a high reactivity that results in damage to DNA [14]. We also demonstrated that pretreatment apricot diet significantly decreased the apoptotic cell ratio when compared to the MTX-treated group. In parallel with our findings, Huang et al. [16] reported that following treatment with phenolic acids against methylglyoxal-induced apoptosis, phenolic acids markedly decreased the activation of PARP protein, probably as a result of their antioxidant features. Phenolic compounds and carotenoids have been shown to exert direct antioxidant effects by acting as ROS scavengers and singlet oxygen quenchers [21, 37]. The researches by our research group based on these features have more recently indicated that β-carotene exhibited a protective effect on MTX-induced apoptosis in testicular cells. This beneficial effect could largely be attributed to phenolic compounds or carotenoids.
Our results indicate that lipid peroxidation and impairment of antioxidant status may be involved in the sequence of events leading to MTX-induced renal damage. Additionally, increased serum creatinine and urea levels may reflect renal dysfunction and an activation of apoptotic cell markers, such as PARP, which also possibly contribute to MTX-caused kidney injury. Prophylactic administration of apricot may provide new therapeutic implications for the treatment of kidney diseases, which are characterized by apoptotic cell death and renal failure.
References
Abraham P, Kolli VK, Rabi S (2010) Melatonin attenuates methotrexate-induced oxidative stress and renal damage in rats. Cell Biochem Funct 28:426–433
Akinci MB, Olmez HA (2004) Kayısı. In: Malatya Tarim Il Mudurlugu, Malatya, Turkey
Al-Saleh E, Al-Harmi J, Nandakumaran M, Al-Shammari M, Al-Jassar W (2009) Effect of methotrexate administration on status of some essential trace elements and antioxidant enzymes in pregnant rats in late gestation. Gynecol Endocrinol 25:816–822
Babiak RMV, Campello AP, Carnieri EGS, Oliveira MBM (1998) Methotrexate: pentose cycle and oxidative stress. Cell Biochem Funct 16:283–293
Buege AJ, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Cao G, Sofic E, Prior RL (1998) Antioxidant capacity of tea and common vegetables. J Agric Food Chem 44:3426–3431
Chen C, Tang HR, Sutcliffe LH, Belton PS (2000) Green tea polyphenols react with 1, 1-diphenyl-2-picrylhydrazyl free radicals in the bilayer of liposomes: direct evidence from electron spin resonance studies. J Agric Food Chem 48:5710–5714
Devrim E, Cetin R, Kılıncoglu B (2005) Methotrexate causes oxidative stress in rat kidney tissues. Ren Fail 27:771–773
Diplock AT, Chaleux JL, Crozier-Willi G, Kok FJ, Rice-Evans C, Roberfroid M, Stahl W, Vina-Ribes J (1998) Functional food science and defence against reactive oxidative species. Br J Nutr 80:77–112
Durmaz G, Alpaslan M (2007) Antioxidant properties of roasted apricot (Prunus armeniaca L.) kernel. Food Chem 100:1177–1181
Fujihara CK, Malherios DMAC, Zatz R, Noronha IL (1998) Mycophenolate mofetil attenuates renal injury in the rat remnant kidney. Kidney Int 54:1510–1519
Graziani G, Argenio GD, Tuccillo C, Loguercio C, Ritieni A, Morisco F, Del Vecchio Blanco C, Fogliano V, Romano M (2005) Apple polyphenol extract prevent damage to human gastric epithelial cells in vitro and to rat gastric mucosa in vivo. Gut 54:193–200
Gulgun M, Erdem O, Oztas E, Kesik, Balamtekin N, Vurucu S, Kul S, Kismet E, Koseoğlu V (2010) Proanthocyanidin prevents methotrexate-induced intestinal damage and oxidative stress. Exp Toxicol Pathol 62:109–115
Herman Z, Zurgil N, Deutsch M (2005) Low dose methotrexate induces apoptosis with reactive oxygen species involvement in T lymphocytic cell lines to a greater extent than in monocytic lines. Inflamm Res 54:273–280
Howard LR, Pandjaitan N, Morelock T, Gil MI (2002) Antioxidant capacity and phenolic content of as affected by genetics and growing season. J Agric Food Chem 50:5891–5896
Huang SM, Chuang HC, Wu CH, Yen GC (2008) Cytoprotective effects of phenolic acids on methylglyoxal-induced apoptosis in Neuro-2A cells. Mol Nutr Food Res 52:940–949
Huang Z, Wang B, Eaves DH, Shikany JM, Pace RD (2007) Total phenolics and antioxidant capacity of indigenous vegetables in the southeast United States: Alabama Collaboration for Cardiovascular Equality Project. Int J Food Sci Nutr 18:1–9
Jo SK, Yun SY, Chang KH, Cha DR, Cho YW, Kim HK, Won NH (2001) α-MSH decreases apoptosis in ischaemic acute renal failure in rats: possible mechanism of this beneficial effect. Nephrol Dial Transplant 16:1583–1591
Johovic N, Cevik H, Sehirli OA, Yegen BC, Sener G (2003) Melatonin prevents methotrexate-induced hepatorenal oxidative injury in rats. J Pineal Res 34:282–287
Keen CL, Holt RR, Oteiza PI, Fraga CG, Schmitz HH (2005) Cocoa antioxidants and cardiovascular health. Am J Clin Nutr 81:298–303
Kennedy TA, Lieber DC (1992) Peroxyl radical scavenging by beta-carotene in lipid-bilayer effect of oxygen partial-pressure. J Appl Biol Chem 267:4658–4663
Kunduzova OR, Escourrou G, Seguelas MH, Delagrange P, De La Frage F, Cambon C, Parini A (2003) Prevention of apoptotic and necrotic cell death, caspase-3 activation, and renal dysfunction by melatonin after ischemia/reperfusion. FASEB J 17:872–874
Lieberthal W, Koh JS, Levine JS (1998) Necrosis and apoptosis in acute renal failure. Semin Nephrol 18:505–518
Lowry O, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurements with the Folin phenol reagent. J Biol Chem 193:265–275
Luck H (1963) Methods of enzymatic analysis. Verlag Chemie, New York, pp 885–888
Martinez-Salgado C, Eleno N, Tavares P, Rodriguez-Barbero A, Garcia-Criado J, Bolanos JP, Lopez-Novoa JM (2002) Involvement of reactive oxygen species on gentamicin-induced mesangial cell activation. Kidney Int 62:1682–1692
Mazur AJ, Nowak D, Mannherz HG, Malicka-Blaszkiewicz M (2009) Methotrexate induces apoptosis in CaSki and NRK cells and influences the organization of their actin cytoskeleton. Eur J Pharmacol 613:24–33
Munzuroğlu O, Karatas F, Geckil H (2003) The vitamin and selenium contents of apricot fruit of different varieties cultivated in different geographical regions. Food Chem 83:205–212
Oktem F, Yilmaz HR, Ozguner F, Olgar S, Ayata A, Uzar E, Uz E (2006) Methotrexate- induced renal oxidative stress in rats: the role of a novel antioxidant caffeic acid phenethyl ester. Toxicol Ind Health 22:241–247
Özen S, Akyol Ö, Iraz M, Söğüt S, Özuğurlu F, Özyurt H, Odacı E, Yıldırım Z (2004) Role of caffeic acid phenethyl ester, an active component of propolis, against cisplatin-induced nephrotoxicity in rats. Fundam Appl Toxicol 24:27–35
Paller MS (1988) Renal work, glutathione and susceptibility to free radical-mediated postischemic injury. Kidney Int 33:843–849
Parlakpinar H, Tasdemir S, Polat A, Bay-Karabulut A, Vardi N, Ucar M, Acet A (2005) Protective role of caffeic acid phenethyl ester (cape) on gentamicin-induced acute renal toxicity in rats. Toxicology 207:169–177
Pietta PG (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042
Prior RL, Cao GH, Martin A, Sofic E, McEwen J, O’Brien C, Lishner N, Ehlenfeldt M, Kalt W, Krewer G, Mainland CM (1998) Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J Agric Food Chem 46:2686–2693
Ramos S, Alia M, Bravo L, Goya L (2005) Comparative effects of food-derived polyphenols on the viability and apoptosis of a human hepatoma cell line (Hep G). J Agric Food Chem 53:1271–1280
Rangkadilok N, Sitthimonchai S, Worasuttayangkurn L, Mahidol C, Ruchirawat M, Satayavivad J (2007) Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem Toxicol 45:328–336
Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB (1995) The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res 9:35–37
Rivas-Cabonero L, Rodriguez-Lopez AM, Martinez-Salgado C, Saura M, Lamas S, Lopez-Novoa JM (1997) Gentamicin treatment increases mesangial cell nitric oxide production. Exp Nephrol 5:23–30
Ruiz D, Egea J, Gil MI, Barberan FAT (2005) Characterization and quantitation of phenolic compounds in new apricot (Prunus armeniaca L.) varieties. J Agric Food Chem 53:9544–9552
Ruiz D, Egea J, Tomas-Barberan FA, Gil MI (2006) Characterization from apricot (Prunus armeniaca L.) varieties and their relationship with flesh and skin color. J Agric Food Chem 53:6368–6374
Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40:945–948
Smeland E, Bremnes RM, Anderson A, Jaeger R, Eide TJ, Huseby NE, Aabakke J (1994) Renal and hepatic toxicity after high-dose 7-hydroxymethotrexate in the rat. Cancer Chemother Pharmacol 34:119–124
Stahl W, Sies H (2003) Antioxidant activity of carotenoids. Mol Asp Med 24:345–351
Sun Y, Oberley LW, Li YA (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
Szabó C, Dawson VL (1998) Role of poly(ADP-ribose) synthetase in inflammation and ischaemia–reperfusion. Trends Pharmacol Sci 19:287–298
Theodorus P, Akerboom M, Sies H (1981) Assay of glutathione, glutathione disulfide and glutathione mixed disulfides in biological samples. Methods Enzymol 77:373–383
Vardi N, Parlakpinar H, Ates B, Cetin A, Otlu A (2009) Antiapoptotic and antioxidant effects of β-carotene against methotrexate-induced testicular injury. Fertil Steril 92:2028–2033
Widemann BC, Adamson PC (2006) Understanding and managing methotrexate nephrotoxicity. Oncologist 11:694–703
Wu X, Beecher GR, Holden JM, Haytowita DB, Gebhardt SE, Prior RL (2004) Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J Agric Food Chem 52:4026–4037
Acknowledgments
This study was supported by a grant from the Scientific Research Fund of Inonu University (project number 2005/78) and the Apricot Research Foundation of Malatya Province. Total caloric composition of the standard diet and diet with dried apricot was assayed by Prof. Dr. Kazim Sahin, who was on duty in Animal Nutrition and Nutritional Diseases department at Firat University in Elazig/Turkey.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vardi, N., Parlakpinar, H., Ates, B. et al. The protective effects of Prunus armeniaca L (apricot) against methotrexate-induced oxidative damage and apoptosis in rat kidney. J Physiol Biochem 69, 371–381 (2013). https://doi.org/10.1007/s13105-012-0219-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-012-0219-2