Abstract
Methotrexate (MTX), as a folic acid antagonist, is an effective drug in treating a wide range of malignancies and autoimmune diseases. However, the clinical use of MTX has been limited due to its side effects, the most common of which is hepatotoxicity. In this study, rats were randomly divided into six groups: three treatment groups received methotrexate and different doses of astaxanthin (AX) for 14 days. At the end of the study, blood samples were collected to determine serum levels of ALT, AST, ALP, and LDH. Also, liver tissues were isolated to evaluate antioxidant enzymes and markers of oxidative stress, histopathological damage, and expression of NF-E2-related transcription factor (Nrf2) and Heme oxygenase-1 (HO-1) genes. The results showed that administration of MTX significantly increased the levels of ALT, AST, ALP, and LDH in the blood, markers of oxidative stress, and histopathological damage in liver tissue and significantly reduced the levels of antioxidant enzymes and the expression of Nrf2 and HO-1 genes. On the other hand, treatment with AX decreased blood levels of ALT, AST, ALP, and LDH and oxidative stress markers and remarkably raises the activity of antioxidant enzymes and expression of Nrf2 and HO-1 genes in liver tissue. In addition, histopathological lesions were improved with AX administration. The findings of this study indicated that AX may be useful for the prevention of MTX-induced hepatotoxicity by improving oxidative and inflammatory changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methotrexate (MTX) is an anti-cancer and immunosuppressive drug, a folic acid antagonist that competitively inhibits the enzyme dihydrofolate reductase, thus interfering with nucleic acid synthesis (Ali et al. 2014; Kuduban et al. 2016). MTX is widely used in the treatment of several malignancies including multiple sclerosis, dermatomyositis, sarcoidosis, psoriasis, and rheumatoid arthritis, along a variety of inflammatory diseases (Abo-Haded et al. 2017). However, its clinical use has several side effects, the most important of which are hepatotoxicity from mild hepatitis and cholestasis to fibrosis, cirrhosis, and renal toxicity. Although the main side effect of MTX is hepatotoxicity, the exact mechanisms of MTX-induced hepatotoxicity are not fully understood. However, several studies have confirmed that MTX administration increases lipid peroxidation and oxidative stress, which increases the production of reactive oxygen species (ROS), nitric oxide (NO) synthesis, and lipoperoxidation in the liver. This rise in ROS production can lead to cellular degradation of proteins, lipids, and DNA (Kremer 2004; Al Maruf et al. 2018; Farzaei et al. 2018; Mehrzadi et al. 2018; Pınar et al. 2018). On the other hand, MTX reduces the antioxidant defense of the cell. Hence, the activity of superoxide dismutase (SOD), catalase (CAT), and total antioxidant capacity (TAC) are noticeably reduced (Kremer 2004; Badary et al. 2005). Antioxidants play an important role in preventing oxidative damage by neutralizing the effect of free radicals on cellular components (Halliwell 1991; Goudarzi et al. 2017). Many studies have been conducted to overcome the side effects of anti-cancer drugs using natural products with antioxidant and anti-inflammatory properties (Ueki et al. 2013; Pınar et al. 2018). Astaxanthin (3,3′-dihydroxy-b, b'-carotene-4,4′-dione, AX) is a natural carotenoid with antioxidant and anti-inflammatory properties derived from Haematococcus pluvialis microalgae and acts as one of the best potent antioxidants available in nature (Higuera-Ciapara et al. 2006; Choi et al. 2011). This carotenoid reduces oxidative stress by scavenging oxygen-free radicals and preventing the release of chain reactions that may be caused by free radicals (Chen et al. 2003; Pınar et al. 2018). Astaxanthin is widely used as a dietary supplement and antioxidant due to its great potential protecting organisms against a wide range of diseases (Guerin et al. 2003; Higuera-Ciapara et al. 2006; Heidari Khoei et al. 2019). To date, no studies have been conducted to evaluate the protective effects of astaxanthin against MTX-induced hepatotoxicity. Therefore, the present study was designed to investigate the antioxidant effects of astaxanthin against MTX-induced hepatotoxicity based on serum biochemical parameters, oxidative stress markers, expression of NF-E2-related transcription factor (Nrf2) and Heme oxygenase-1 (HO-1) genes, and histopathological changes.
Materials and methods
Chemicals
Astaxanthin was purchased from Jingzhou Natural Astaxanthin Inc. (Hubei, China). The methotrexate used in this study was manufactured by the American pharmaceutical company Mylan. RNA extraction kit was obtained from Cinacolone, Iran. cDNA synthesis kit and SYBR Green PCR Master Mix were obtained from Biofact (Biofact, Korea). All other chemicals and reagents were supplied by reputable and standard companies.
Animals
In this study, forty-two male Wistar rats with an approximate weight of 200 to 250 g were provided by the Animal Care Center of Isfahan University of Medical Sciences (Isfahan, Iran). Rats were kept in an animal care center at room temperature (25 ± °C), relative humidity of 50 ± 5%, and a 12-h light/dark cycle. They were allowed free access to water and a standard diet. The entire process of the experimental study was approved by the ethics committee (approval ID: IR.MUI.RESEARCH.REC.1398.213) and under the ethics standards of “Principles of Laboratory Animal Care.”
Study design
After examining the toxic effects of astaxanthin administration (75 mg/kg) once a day (14 consecutive days) based on liver function tests and liver histopathology, this study was designed. So, adult male Wistar rats were randomly divided into six groups and each group containing seven rats. The duration of the experimental study was 14 days. Group 1 (normal control group; Normal.Ctrl): the rats were injected with normal saline (intra-peritoneal; i.p.) only on the 8th day. Group 2 (olive oil control group; olive oil): received 400 μl of olive oil (astaxanthin vehicle) by gavage for 2 weeks (Wu et al. 2018). Group 3 (methotrexate group; MTX.Ctrl): administered a dose of 20 mg/kg by intraperitoneal injection only on the 8th day. Groups 4, 5, and 6, in addition to receiving methotrexate on the 8th day of the study (i.p.), were also gavage daily with concentrations of 25 mg/kg, 50 mg/kg, and 75 mg/kg of astaxanthin, respectively. Therefore, these groups included methotrexate + 25 mg/kg/day astaxanthin (MTX + AX25), methotrexate + 50 mg/kg/day astaxanthin (MTX + AX50), and methotrexate + 75 mg/kg/day astaxanthin (MTX + AX75) (Akca et al. 2018; Mehrzadi et al. 2018). At the end of the fourteenth day, rats in all groups were anesthetized with 50 mg/kg ketamine hydrochloride and 10 mg/kg xylazine hydrochloride intraperitoneally, and blood samples were taken from their hearts to prepare sera. Liver tissues were also removed to determine the activities of catalase (CAT), superoxide dismutase (SOD), total antioxidant capacity (TAC), nitric acid (NO), malondialdehyde (MDA), expression of Nrf2 and HO-1 genes, and histopathological lesions.
Biochemical analysis
Serum evaluation
Serum biochemical parameters ALT, AST, ALP, and LDH were determined using commercial kits (Parsazmoon, Iran).
Evaluation of antioxidant enzymes
CAT activity was determined using the method of Aebi et al., based on the rate of decomposition of H2O2 at a wavelength of 240 nm and expressed as a unit/mg of protein (Shahzad et al. 2020). SOD enzyme activity was measured using commercial kits (Byrex, Shiraz, Iran), and the results were expressed in U/ml. Total antioxidant capacity (TAC) was measured by the method described by Janaszewska et al. and was reported as percentage based on DPPH radical scavenging (Janaszewska and Bartosz 2002). Total protein content was determined using the Bradford method (Mohammadalipour et al. 2019).
Evaluation of oxidative stress parameters
The amount of lipid peroxidation was determined by measuring the level of MDA in the liver tissue and using spectrophotometric technique based on our previous study (Kazemi et al. 2020). The concentration of NO (nitric oxide) in the liver tissue was assessed using commercial kits (Kiazist, Iran).
Determination of Nrf2 and HO-1 gene expression
Total RNA of liver tissues was extracted using RNA extraction kit (Cinacolone, Iran). Total RNA was transcribed into cDNA using BioFact™—RT kit (BIOFACT Germany). Specific PCR primers were designed, and published primers are shown in Table 1. Rat beta-actin (β-actin) was used as an internal control gene to normalize the data. The amplification of the selected gene was performed by the applied biosystems instrument (ABI7500Real-time PCR). The results were analyzed by 2−∆∆CT method (Golestaneh et al. 2023).
Histopathological evaluation
For histopathological evaluation, appropriate tissue samples were collected from the livers, then fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 μm thickness, and stained with hematoxylin-eosin staining for light microscopic examination.
Statistical analysis
The results were expressed as mean ± SD, and data analysis was performed using one-way analysis of variance (ANOVA) by GraphPad Prism 8.4.0 software. Tukey post-test was used to compare the mean values of the groups. For all analyses, p < 0.05 was considered statistically significant.
Results
Effects of astaxanthin on serum ALT, AST, ALP, and LDH
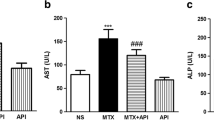
Figure 1 shows the effects of MTX and astaxanthin on serum ALT, AST, ALP, and LDH. In MTX.Ctrl group, the administration of MTX significantly increased the levels of ALT, AST, ALP, and LDH compared to those of the normal control group (Normal.Ctrl) (p < 0.05). Treatment with astaxanthin in groups MTX + AX25, MTX + AX50, and MTX + AX75 for 14 consecutive days resulted in a significant reduction in serum ALT, AST, ALP, and LDH in a dose-dependent manner compared to those of methotrexate group (MTX.Ctrl) (p < 0.05). In addition, the administration of olive oil to normal rats in olive oil control group (olive oil) did not change serum ALT, AST, ALP, and LDH levels compared to those of the normal control rats (Normal.Ctrl) (p > 0.05).
The effect of astaxanthin on serum ALT, AST, ALP, and LDH levels. The data were expressed as means ± SD (n = 7) and analyzed by one-way ANOVA and then Tukey post hoc test was used to compare the mean values of the groups. ??? p < 0.05 compared to the normal control group. ## and ### p < 0.05 compared to the MTX. Ctrl group
Effects of astaxanthin on antioxidant enzymes
The effects of astaxanthin and MTX on the activities of CAT and SOD antioxidant enzymes as well as the level of total hepatic antioxidant capacity (TAC) are shown in Fig. 2. MTX administration led to a remarkable reduction (p < 0.05) in CAT and SOD activities and liver TAC levels in methotrexate group (MTX.Ctrl) compared to those of the normal control group (Normal.Ctrl) (Fig. 2A–C). Whereas, in groups treated with astaxanthin (MTX + AX25, MTX + AX50 and MTX + AX75), SOD activity and liver TAC level significantly (p < 0.05) increased compared to those of methotrexate group (MTX.Ctrl) (Fig. 2B and C).
The effect of astaxanthin on CAT and SOD activity and TAC level of liver tissues. The data were expressed as means ± SD (n = 7) and analyzed by one-way ANOVA and then Tukey post hoc test to compare the mean values of the groups. ??? p < 0.05 compared to the normal control group. ## and ### p < 0.05 compared to the MTX. Ctrl group
Moreover, in groups treated with astaxanthin at doses of 50 and 75 mg/kg for 14 consecutive days (MTX + AX50 and MTX + AX75), the activity of CAT enzyme in liver tissue increased significantly (p < 0.05) compared to those of methotrexate group (MTX.Ctrl) (Fig. 2A). In addition, the administration of olive oil to normal rats in olive oil control group (olive oil) did not change the level of these indices compared to that of normal control rats (Normal.Ctrl) (p > 0.05).
Effects of astaxanthin on oxidative stress parameters
The effects of astaxanthin and MTX on the concentration of oxidative stress parameters such as MDA and NO in liver tissue are depicted in Fig. 3. In methotrexate group (MTX.Ctrl), MTX resulted in a significant increase in the concentration of nitric oxide and MDA, the final product of lipid peroxidation, compared to those of the normal control group (Normal.Ctrl) (p < 0.05). In contrast, treatment with astaxanthin at doses of 25, 50, and 75 mg/kg (MTX + AX25, MTX + AX50, and MTX + AX75) led to a significant reduction (p < 0.05) in liver MDA concentration compared with those of methotrexate group (MTX.Ctrl) (Fig. 3A). Also, treatment with astaxanthin at doses of 50 and 75 for 14 consecutive days (MTX + AX50 and MTX + AX75) remarkably reduced NO concentration compared with that of MTX group (MTX.Ctrl) (Fig. 3B). In addition, the administration of olive oil to normal rats in olive oil control group (olive oil) did not change the amount of oxidative stress parameters compared to those of the control rats (Normal.Ctrl) (p > 0.05).
Effect of astaxanthin on MDA and NO levels of liver tissue. The data were expressed as means ± SD (n = 7) and analyzed by one-way ANOVA and then Tukey post hoc test was used to compare the mean values of the groups. ??? p < 0.05 compared to the normal control group. #, ##, and ### p < 0.05 compared to the MTX. Ctrl group
Effects of astaxanthin on the expression of Nrf2 and HO-1 genes in liver tissue
The mRNA expression of Nrf2 and HO-1 genes in liver tissue was analyzed by RT-PCR. The administration of MTX in methotrexate group (MTX.Ctrl) significantly reduced the expression of Nrf2 and HO-1 genes compared to that of the normal control group (Normal.Ctrl) (p < 0.05). Treatment with astaxanthin at doses of 50 and 75 mg/kg in groups 5 and 6 for 14 consecutive days (MTX + AX50 and MTX + AX75) induced a significant increase (p < 0.05) in the expression of Nrf2 and HO-1 genes compared to those of methotrexate group (MTX.Ctrl). However, no significant changes in the expression of Nrf2 and HO-1 genes were observed in the group receiving 25 mg/kg astaxanthin for 14 consecutive days (MTX + AX25) (p > 0.05). Furthermore, the administration of olive oil to normal rats in olive oil control group (olive oil) did not significantly change the expression of Nrf2 and HO-1 genes compared to that of normal rats in control group (Normal.Ctrl) (p > 0.05) (Fig. 4A and B).
The effect of astaxanthin on the expression of Nrf2 and HO-1 genes in liver tissues. The data were expressed as means ± SD (n = 7) and analyzed by one-way ANOVA and then Tukey post hoc test was used to compare the mean values of the groups. ??? p < 0.05 compared to the normal control group. #, ##, and ### p < 0.05 compared to the MTX. Ctrl group
Effects of astaxanthin on changes in liver histopathology
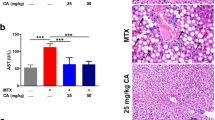
On histopathological examination, liver tissues of rats in the normal control group (Normal.Ctrl) and the olive oil control group (olive oil) had a normal structure containing central veins, radiating hepatic cords and the portal triads containing portal vein, hepatic artery, and bile duct, while the livers in other experimental groups showed a considerable histopathological changes from mild to moderate lesions (MTX + AX25, MTX + AX50, and MTX + AX75) (Fig. 6) to severe (MTX.Ctrl) (Fig. 5). Administrating 20 mg/kg methotrexate on the 8th day (MTX.Ctrl) produced moderate vacuolar degeneration in the hepatocytes, severe congestion and dilation of sinusoids and central veins, infiltration of mononuclear cells in the liver parenchyma, Kupffer cell hyperplasia, and focal necrosis (Fig. 5). Treatment with astaxanthin in different doses (MTX + AX25, MTX + AX50, and MTX + AX75) considerably attenuated these lesions, mainly the extent and severity of necrosis and inflammation (Fig. 6). In comparison between treated groups, the most therapeutic effect was observed with the high dose of astaxanthin (MTX + AX75), and except the presence of a few degenerated hepatocytes with pyknotic nuclei, other hepatocytes were almost normal (Fig. 6). Furthermore, there was no evidence of sinusoids and central veins congestion as well as inflammation in this group (MTX + AX75), while the rats treated with 25 and 50 mg/kg astaxanthin (MTX + AX50 and MTX + AX75) showed a mild necrosis and mononuclear cell infiltration in the hepatic parenchyma (Fig. 6).
Histological sections of liver from methotrexate intoxicated rats (H&E; Bar = 50 μm). A Focal necrosis with many hepatocytes showing ballooning degeneration; B vacuolar degeneration in the hepatocytes (arrows); C infiltration of mononuclear cells in the liver parenchyma (arrows); D severe congestion and dilation of sinusoids
Histological sections of liver from methotrexate intoxicated rats treated with astaxanthin (H&E; Bar = 50 μm). A Methotrexate + astaxanthin (25 mg/kg), mild necrosis and hepatocytes degeneration (stars); B methotrexate + astaxanthin (25 mg/kg), mild infiltration of mononuclear cells around the portal area (arrows); C methotrexate + astaxanthin (50 mg/kg), mild necrosis and hepatocytes degeneration (stars); D methotrexate + astaxanthin (75 mg/kg), well-organized radiating hepatic cords, and the portal triads and central veins comparable with the normal group
Discussion
Methotrexate is an immunosuppressive drug and its high doses, commonly used for acute leukemia or severe psoriasis, are associated with organ toxicity, including hepatotoxicity, liver fibrosis, and cirrhosis (Abo-Haded et al. 2017; Pınar et al. 2018). So far, many studies have demonstrated the effectiveness of natural products with antioxidant activity, other than astaxanthin, in protecting against methotrexate-induced hepatotoxicity (Mehrzadi et al. 2018; Abdelaziz et al. 2020). In the present study, attempts were made to demonstrate the hepatoprotective effects of astaxanthin against methotrexate-induced hepatotoxicity by regulating the Nrf2/HO1 pathway. This is the first study investigating the effect of astaxanthin on MTX-induced hepatotoxicity in rats. According to our results, a significant increase in serum levels of ALT, AST, ALP, and LDH, which indicates acute hepatotoxicity, was observed in rats receiving methotrexate (Fig. 1) compared with rats in the normal control group. These findings are in line with the finding of other studies (Abo-Haded et al. 2017; Zakaria et al. 2019). Transaminases are cytosolic enzymes that are the best indicators of liver necrosis, and their leakage into the bloodstream indicates a disruption in the integrity of the liver membrane and cell damage (Abo-Haded et al. 2017). MTX-induced hepatotoxicity was confirmed by histological studies including increased moderate vacuolar degeneration with mononuclear cell infiltration and focal hepatic necrosis in the methotrexate group. These biochemical and histopathological changes were remarkably attenuated by astaxanthin pretreatment in all doses (Fig. 1). These findings suggest that astaxanthin can effectively inhibit the leakage of transaminases into the bloodstream, indicating membrane stabilization and the hepatic protective effects of astaxanthin. Significant repair of histopathological lesions in AX-treated groups also supports the protective role of AX against MTX-induced hepatotoxicity. This finding is consistent with Liu Anjun et al.’s report on the hepatoprotective effect of astaxanthin on carbon tetrachloride-induced liver damage (Liu et al. 2010). Astaxanthin seems to have different effects on these parameters at doses of 25, 50, and 75 mg/kg. Therefore, it can be claimed that the effect of astaxanthin on these biochemical parameters is dose-dependent. MTX also increases lipid peroxidation by increasing the amount of MDA. MDA is an indicator of oxidative stress and the metabolite of lipid peroxidation caused by the generation of free radicals (Pınar et al. 2018). Another effect of methotrexate-induced hepatotoxicity is the production of nitric oxide (NO), which leads to nitrous stress and liver tissue damage. Nitric oxide is a free radical that is synthesized in the body by the enzyme nitric oxide synthase (iNOS), the amino acid L-arginine, and is associated with many physiological processes in the body, including cell growth, apoptosis, and the body’s defense mechanism. Although NO plays an important role in maintaining homeostasis and host defense, it is also involved in the pathogenesis of various diseases. This molecule reacts with reactive oxygen species and thiol groups, which can have a protective or toxic effect depending on its concentration and location (Leitão et al. 2011). These results are in line with those of previous studies conducted by Ayman M. Mahmoud and Ayat O.S. Montasser (Mahmoud et al. 2017; Montasser et al. 2017). In the current study, MTX-induced oxidative damage was observed with a noticeable increase in liver tissue MDA and NO levels in the group receiving only methotrexate, compared to those of the normal control group (Fig. 3), which is consistent with the findings of other studies (Bu et al. 2018). On the other hand, astaxanthin reduced the levels of MDA and NO in the treated groups compared with the toxic control group (Fig. 3), which may be due to its free radicals scavenging properties. The reduction of NO in the liver tissue of astaxanthin-treated groups showed that this compound protects the liver against methotrexate-induced damage by reducing NO and also nitrous stress. In addition, various studies have shown that high oxidative stress impairs liver function, followed by increased production of ROS, which rapidly reduces the body’s enzymatic and non-enzymatic antioxidant content (Goudarzi et al. 2018). Therefore, the activity of antioxidant enzymes including superoxide dismutase (SOD), catalase (CAT), and the content of total antioxidant capacity (TAC) is significantly reduced (Kremer 2004; Al Maruf et al. 2018). The main role of antioxidant enzymes such as catalase and superoxide dismutase is to regulate cellular antioxidant systems. The function of SOD and CAT is to scavenge oxygen free radicals and they are the most effective enzymes in neutralizing ROS products (Pınar et al. 2018). The SOD enzyme converts O2− anion to O2 and H2O2; then, the H2O2 produced by CAT is converted to H2O (Bresciani et al. 2015). Inhibition of enzymes such as catalase, which play a role in removing free radicals from the body, leads to the accumulation of H2O2 and high lipid peroxidation, as well as DNA modulation, gene expression changes, and cell death (Tayeb et al. 2010). The cooperation between different antioxidants provides greater protection against the attack of oxygen and nitrogen free radicals. Total antioxidant capacity (TAC) is an analyte that shows the effect of all antioxidants in the body and is used to assess the antioxidant status of biological samples that can determine the antioxidant response to free radicals produced in a particular disease. Therefore, measuring the total antioxidant capacity (TAC) can provide more information than measuring each of the antioxidant components by itself (Suresh et al. 2009). Previous studies have shown that the administration of MTX remarkably decreases the activity of SOD, CAT, and TAC levels in rats (Ueki et al. 2013; Abo-Haded et al. 2017). In this study, the activity of SOD and CAT and the level of TAC in the MTX group decreased compared to the normal control group.
Moreover, treatment with astaxanthin at doses of 25, 50, and 75 mg/kg significantly reversed SOD enzyme activity and TAC levels. Also, treatment with astaxanthin at doses of 50 and 75 mg/kg significantly decreased CAT activity (Fig. 2). The findings of several studies have shown that astaxanthin can react with hydroxyl radicals, protect tissues against oxidative stress, and can also inhibit the activity of antioxidant enzymes (Liu et al. 2010; Abo-Haded et al. 2017; Heidari Khoei et al. 2019). It can be concluded that the increase in the activity of SOD, CAT, and TAC levels can be caused by the antioxidant activity of astaxanthin, which seems to have different effects on these parameters at doses of 25, 50, and 75 mg/kg of astaxanthin. Therefore, it can be claimed that the effect of astaxanthin on these biochemical parameters is dose-dependent.
Nrf2 is a transcription factor that plays an important role in regulating genes involved in the metabolism of antioxidants and xenobiotics. The transcription of about 50 antioxidant genes is performed under Nrf2 control. The Keap1-Nrf2 signaling pathway is the major key regulator of cell-protective responses to ROS and electrophilic stresses (Kansanen et al. 2012). Under normal conditions, Nrf2 is degraded by binding to keap1 in the cytosol. Oxidative stress, on the other hand, releases Nrf2 from keap1 which, in turn, transports Nrf2 to the nucleus, causing to regulate the activation of defensive genes and induction of antioxidant enzymes such as SOD and CAT (Sporn and Liby 2012; Chang et al. 2013). According to prior experimental studies, the administration of 20 mg/kg methotrexate to rats decreased the expression of Nrf2 and HO-1 genes in their livers (Bu et al. 2018; Abdelaziz et al. 2020). The present study indicated that the expression of Nrf2 and HO-1 in the liver tissues of rats in the methotrexate group was significantly reduced compared to those of the normal control group. In this study, treatment with astaxanthin significantly increased the expression of Nrf2 and HO-1 genes in the rats of the groups receiving astaxanthin. Several studies confirm the findings of the present study in this respect (Abo-Haded et al. 2017; Soliman et al. 2020). Therefore, increased expression of Nrf2 and HO-1 is evidence that confirms the hepatoprotective effects of astaxanthin due to its antioxidant properties.
Conclusion
An overview of these results suggests that astaxanthin administration may confer protection against MTX-induced hepatotoxicity, which could be partially attributed to modulation of Nrf2 signaling pathways. Our results demonstrated that although astaxanthin does not completely prevent liver damage at lower doses, it can particularly play a beneficial and protective role, especially by preventing changes in the activity of antioxidant enzymes at higher doses. Moreover, astaxanthin is effective in reducing oxidative tissue damage and functional disorders caused by methotrexate in rats by inhibiting free radicals and increasing the body’s antioxidant stores. However, further experimental and clinical studies can clarify our understanding of these findings.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to [REASON(S) WHY DATA ARE NOT PUBLIC] but are available from the corresponding author on reasonable request.
References
Abdelaziz RM, Abdelazem AZ, Hashem KS, Attia YA (2020) Protective effects of hesperidin against MTX-induced hepatotoxicity in male albino rats. Naunyn Schmiedebergs Arch Pharmacol 393:1405–1417. https://doi.org/10.1007/s00210-020-01843-z
Abo-Haded HM, Elkablawy MA, Al-Johani Z, Al-Ahmadi O, El-Agamy DS (2017) Hepatoprotective effect of sitagliptin against methotrexate induced liver toxicity. PLoS ONE 12: e0174295. https://doi.org/10.1371/journal.pone.0174295
Akca G, Eren H, Tumkaya L, Mercantepe T, Horsanali MO, Deveci E, Dil E, Yilmaz A (2018) The protective effect of astaxanthin against cisplatin-induced nephrotoxicity in rats. B Biomed Pharmacother 100:575–582. https://doi.org/10.1016/j.biopha.2018.02.042
Al Maruf A, O’Brien PJ, Naserzadeh P, Fathian R, Salimi A, Pourahmad J (2018) Methotrexate induced mitochondrial injury and cytochrome c release in rat liver hepatocytes. Drug Chem Toxicol 41:51–61. https://doi.org/10.1080/01480545.2017.1289221
Ali N, Rashid S, Nafees S, Hasan SK, Sultana S (2014) Beneficial effects of chrysin against methotrexate-induced hepatotoxicity via attenuation of oxidative stress and apoptosis. Mol Cell Biochem 385:215–223. https://doi.org/10.1007/s11010-013-1830-4
Badary OA, Abdel-Maksoud S, Ahmed WA, Owieda GH (2005) Naringenin attenuates cisplatin nephrotoxicity in rats. Life Sci 76:2125–2135. https://doi.org/10.1016/j.lfs.2004.11.005
Bresciani G, da Cruz IBM, González-Gallego J (2015) Manganese superoxide dismutase and oxidative stress modulation. Adv Clin Chem 68:87–130. https://doi.org/10.1016/bs.acc.2014.11.001
Bu T, Wang C, Meng Q, Huo X, Sun H, Sun P, Zheng S, Ma X, Liu Z, Liu K (2018) Hepatoprotective effect of rhein against methotrexate-induced liver toxicity. Eur J Pharmacol 834:266–273. https://doi.org/10.1016/j.ejphar.2018.07.031
Chang L-C, Fan C-W, Tseng W-K, Chen J-R, Chein H-P, Hwang C-C, Hua C-C (2013) Immunohistochemical study of the Nrf2 pathway in colorectal cancer: Nrf2 expression is closely correlated to Keap1 in the tumor and Bach1 in the normal tissue. Appl Immunohistochem Mol Morphol 21:511–517. https://doi.org/10.1097/PAI.0b013e318282ac20
Chen Y, Li D, Lu W, Xing J, Hui B, Han Y (2003) Screening and characterization of astaxanthin-hyperproducing mutants of Haematococcus pluvialis. Biotechnol Lett 25:527–529. https://doi.org/10.1023/a:1022877703008
Choi HD, Kim JH, Chang MJ, Kyu-Youn Y, Shin WG (2011) Effects of astaxanthin on oxidative stress in overweight and obese adults. Phytother Res 25:1813–1818. https://doi.org/10.1002/ptr.3494
Farzaei MH, Zobeiri M, Parvizi F, El-Senduny FF, Marmouzi I, Coy-Barrera E, Naseri R, Nabavi SM, Rahimi R, Abdollahi M (2018) Curcumin in liver diseases: a systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients 10:855. https://doi.org/10.3390/nu10070855
Golestaneh E, Aslani A, Aghaei M, Hashemnia M, Aarabi MH (2023) Preparation and characterisation of a new form of silymarin as a potential antidiabetic agent in the adult male rat. Arch Physiol Biochem 129:799–809. https://doi.org/10.1080/13813455.2021.1874018
Goudarzi F, Mohammadalipour A, Bahabadi M, Goodarzi MT, Sarveazad A, Khodadadi I (2018) Hydrogen peroxide: a potent inducer of differentiation of human adipose-derived stem cells into chondrocytes. Free Radic Res 52:763–774. https://doi.org/10.1080/10715762.2018.1466121
Goudarzi M, Khodayar MJ, Hosseini Tabatabaei SMT, Ghaznavi H, Fatemi I, Mehrzadi S (2017) Pretreatment with melatonin protects against cyclophosphamide-induced oxidative stress and renal damage in mice. Fundam Clin Pharmacol 31:625–635. https://doi.org/10.1111/fcp.12303
Guerin M, Huntley ME, Olaizola M (2003) Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol 21:210–216. https://doi.org/10.1016/S0167-7799(03)00078-7
Halliwell B (1991) Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am J Med 91:S14–S22. https://doi.org/10.1016/0002-9343(91)90279-7
Heidari Khoei H, Fakhri S, Parvardeh S, Shams Mofarahe Z, Baninameh Z, Vardiani M (2019) Astaxanthin prevents the methotrexate-induced reproductive toxicity by targeting oxidative stress in male mice. Toxin Rev 38:248–254. https://doi.org/10.1080/15569543.2018.1452263
Higuera-Ciapara I, Felix-Valenzuela L, Goycoolea F (2006) Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci Nutr 46:185–196. https://doi.org/10.1080/10408690590957188
Janaszewska A, Bartosz G (2002) Assay of total antioxidant capacity: comparison of four methods as applied to human blood plasma. Scand J Clin Lab Invest 62:231–236. https://doi.org/10.1080/003655102317475498
Kansanen E, Jyrkkänen H-K, Levonen A-L (2012) Activation of stress signaling pathways by electrophilic oxidized and nitrated lipids. Free Radic Biol Med 52:973–982. https://doi.org/10.1016/j.freeradbiomed.2011.11.038
Kazemi M, Mohammadifar M, Aghadavoud E, Vakili Z, Aarabi MH, Talaei SA (2020) Deep skin wound healing potential of lavender essential oil and licorice extract in a nanoemulsion form: biochemical, histopathological and gene expression evidences. J Tissue Viability 29:116–124. https://doi.org/10.1016/j.jtv.2020.03.004
Kremer JM (2004) Toward a better understanding of methotrexate. Arthritis Rheum 50:1370–1382. https://doi.org/10.1002/art.20278
Kuduban O, Mazlumoglu MR, Kuduban SD, Erhan E, Cetin N, Kukula O, Yarali O, Cimen FK, Cankaya M (2016) The effect of Hippophae rhamnoides extract on oral mucositis induced in rats with methotrexate. J Appl Oral Sci 24:423–430. https://doi.org/10.1590/1678-775720160139
Leitão RF, Brito GA, Oriá RB, Braga-Neto MB, Bellaguarda EA, Silva JV, Gomes AS, Lima-Júnior RC, Siqueira FJ, Freire RS (2011) Role of inducible nitric oxide synthase pathway on methotrexate-induced intestinal mucositis in rodents. BMC Gastroenterol 11:1–10. https://doi.org/10.1186/1471-230X-11-90
Liu A, Ma T, Chen Y, Wang Y, Tao D, Zhang H (2010) Protective effect of astaxanthin on the acute liver injury induced by CCl4 in mice. Mod Food Sci Technol 26:1197–1199. https://doi.org/10.3390/toxins14090628
Mahmoud AM, Hussein OE, Hozayen WG, Abd el-Twab SM, (2017) Methotrexate hepatotoxicity is associated with oxidative stress, and down-regulation of PPARγ and Nrf2: protective effect of 18β-glycyrrhetinic acid. Chem Biol Interact 270:59–72. https://doi.org/10.1016/j.cbi.2017.04.009
Mehrzadi S, Fatemi I, Esmaeilizadeh M, Ghaznavi H, Kalantar H, Goudarzi M (2018) Hepatoprotective effect of berberine against methotrexate induced liver toxicity in rats. Biomed Pharmacother 97:233–239. https://doi.org/10.1016/j.biopha.2017.10.113
Mohammadalipour A, Hashemnia M, Goudarzi F, Ravan AP (2019) Increasing the effectiveness of tyrosine kinase inhibitor (TKI) in combination with a statin in reducing liver fibrosis. Clin Exp Pharmacol Physiol 46:1183–1193. https://doi.org/10.1111/1440-1681.13157
Montasser AO, Saleh H, Ahmed-Farid OA, Saad A, Marie M-AS (2017) Protective effects of Balanites aegyptiaca extract, melatonin and ursodeoxycholic acid against hepatotoxicity induced by methotrexate in male rats. Asian Pac J Trop Med 10:557–565. https://doi.org/10.1016/j.apjtm.2017.06.003
Pınar N, Kaplan M, Özgür T, Özcan O (2018) Ameliorating effects of tempol on methotrexate-induced liver injury in rats. Biomed Pharmacother 102:758–764. https://doi.org/10.1016/j.biopha.2018.03.147
Shahzad S, Mateen S, Kausar T, Naeem SS, Hasan A, Abidi M, Nayeem SM, Faizy AF, Moin S (2020) Effect of syringic acid and syringaldehyde on oxidative stress and inflammatory status in peripheral blood mononuclear cells from patients of myocardial infarction. Naunyn Schmiedebergs Arch Pharmacol 393:691–704. https://doi.org/10.1007/s00210-019-01768-2
Soliman MM, Aldhahrani A, Alkhedaide A, Nassan MA, Althobaiti F, Mohamed WA (2020) The ameliorative impacts of Moringa oleifera leaf extract against oxidative stress and methotrexate-induced hepato-renal dysfunction. Biomed Pharmacother 128: 110259. https://doi.org/10.1016/j.biopha.2020.110259
Sporn MB, Liby KT (2012) NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer 12:564–571. https://doi.org/10.1038/nrc3278
Suresh D, Annam V, Pratibha K, Prasad BM (2009) Total antioxidant capacity–a novel early bio-chemical marker of oxidative stress in HIV infected individuals. J Biomed Sci 16:1–4. https://doi.org/10.1186/1423-0127-16-61
Tayeb W, Nakbi A, Trabelsi M, Attia N, Miled A, Hammami M (2010) Hepatotoxicity induced by sub-acute exposure of rats to 2, 4-dichlorophenoxyacetic acid based herbicide “Désormone lourd.” J Hazard Mater 180:225–233
Ueki M, Ueno M, Morishita J, Maekawa N (2013) Curcumin ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in mice. J Biosci Bioeng 115:547–551. https://doi.org/10.1016/j.jhazmat.2010.04.018
Wu L, Qiu W, Sun J, Wang J (2018) SENP1 attenuates the liver fibrosis through down-regulating the expression of SMAD2. Biochem Biophys Res Commun 495:755–760. https://doi.org/10.1016/j.bbrc.2017.11.047
Zakaria ZA, Mahmood ND, Omar MH, Taher M, Basir R (2019) Methanol extract of Muntingia calabura leaves attenuates CCl4-induced liver injury: possible synergistic action of flavonoids and volatile bioactive compounds on endogenous defence system. Pharm Biol 57:335–344. https://doi.org/10.1080/13880209.2019.1606836
Acknowledgements
This study was supported by a grant (No: 298058) from Isfahan University of Medical Sciences, Iran. The authors would like to extend their deep and sincere gratitude to each and every one of those who helped them.
Author information
Authors and Affiliations
Contributions
A: Razieh Azadian, B: Adel Mohammadalipour, C: Mohammad Reza Memarzadeh, D: Mohammad Hashemnia, E: Mohammad Hosein Aarabi. A.B. and B.C. carried out the experiment. A.E. wrote the manuscript with support from B. D.E. fabricated the AB sample. E conceived the original and supervised the project. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
All measures of experimental study were approved by the Ethics Committee of Isfahan University of Medical Sciences (Isfahan, Iran), ethical code IR.MUI.RESEARCH.REC.1398.213.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azadian, R., Mohammadalipour, A., Memarzadeh, M.R. et al. Examining hepatoprotective effects of astaxanthin against methotrexate-induced hepatotoxicity in rats through modulation of Nrf2/HO-1 pathway genes. Naunyn-Schmiedeberg's Arch Pharmacol 397, 371–380 (2024). https://doi.org/10.1007/s00210-023-02581-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02581-8